Abstract
Severe pulmonary fibrosis such as idiopathic pulmonary fibrosis (IPF) is characterized by the accumulation of extracellular matrix and fibroblast activation. Targeting fibroblast activation has contributed to the development of antifibrotic therapeutics for patients with IPF. Mitogen-activated protein kinase–activated protein kinase 2 (MK2), downstream in the transforming growth factor-β/p38 mitogen-activated protein kinase pathway, has been implicated in inflammatory and fibrosing diseases. Increased concentrations of activated MK2 were expressed in IPF lung and in the mouse bleomycin model of lung fibrosis. The aim of the present study was to determine the role and the mechanisms of MK2 in fibroblast invasion and lung fibrosis. Our results showed that an MK2 inhibitor (MMI-0100) was able to inhibit the invasive capacity of lung fibroblasts isolated from patients with IPF, as well as fibroblasts isolated from both wild-type mice and mice with overexpressing hyaluronan synthase 2 (HAS2) in the myofibroblast compartment. We previously showed that hyaluronan and HAS2 regulate fibroblast invasion and lung fibrosis in vivo. The results of the present study showed that MMI-0100 reduced transforming growth factor-β–induced hyaluronan production in human and mouse fibroblasts in vitro and that HAS2 mediated MK2 activation, suggesting a feed-forward loop in fibroblast activation. More importantly, MK2 inhibition attenuated hyaluronan accumulation and reduced collagen content in bleomycin-injured mouse lungs in vivo. Conditional deletion of MK2 in fibroblasts attenuated bleomycin-induced lung fibrosis. These data provide evidence that MK2 has a role in fibroblast invasion and fibrosis and may be a novel therapeutic target in pulmonary fibrosis.
Keywords: lung fibrosis, idiopathic pulmonary fibrosis, fibroblast invasion, mitogen-activated protein kinase, hyaluronan synthase 2
Clinical Relevance
Progressive pulmonary fibrosis is an increasing cause of morbidity and mortality worldwide with limited therapeutic options. This study provides evidence that mitogen-activated protein kinase–activated protein kinase 2 has a role in fibroblast invasion and fibrosis and may be a novel therapeutic target in pulmonary fibrosis.
Progressive pulmonary fibrosis is an increasing cause of morbidity and mortality worldwide with limited therapeutic options. Idiopathic pulmonary fibrosis (IPF) is a particularly severe form of lung fibrosis with no known etiology and a survival rate of 3 to 5 years without treatment (1–4). Histopathologically, IPF is characterized by fibroblastic foci in the lung interstitium resulting from excessive fibroblast proliferation and myofibroblast activation (5). Hyaluronan (HA) is an extracellular glycosaminoglycan that has been reported to be elevated in the lungs of patients with IPF, and the concentrations of HA correlate with the severity of the disease (6). HA fragments that accumulate during inflammation stimulate inflammatory cells to release multiple proinflammatory and fibrogenic mediators, such as transforming growth factor (TGF)-β and IL-13, which in turn trigger myofibroblast proliferation and recruitment (7, 8). Fibroblasts are one of the major HA-producing cell types in the lung (9, 10). We showed that an invasive phenotype of fibroblasts is required for the development of severe lung fibrosis and that fibroblast invasion depends on expression of hyaluronan synthase 2 (HAS2) and CD44 (11) as well as β-arrestins (12). Fibroblasts isolated from IPF patient lung showed increased invasive capacity (11). Mice with overexpression of Has2 in fibroblasts had increased invasive capacity and worsened lung fibrosis, whereas mice with targeted deletion of Has2 in fibroblasts showed reduced fibroblast invasion and decreased lung fibrosis after bleomycin challenge (11). This phenotype was confirmed by our subsequent studies (13–15) as well as studies by other groups (16–21).
Pirfenidone (22, 23) and nintedanib (24) have been approved for patients with IPF, and they have been shown to reduce the loss of lung function in patients with IPF (22, 23). Pirfenidone showed an antifibrotic effect in bleomycin-induced lung fibrosis (25) and in other animal models (26). The antifibrotic effect of pirfenidone is attributed to its ability to block multiple fibrotic signaling pathways, including TGF-β, platelet-derived growth factor (PDGF), and TNF-α (26). Therefore, it is plausible to target these signaling pathways to block fibroblast activation. Mitogen-activated protein kinase–activated protein kinase 2 (MK2) is an intracellular serine/threonine kinase and is activated by p38 mitogen-activated protein kinase (27). MK2 contains an N-terminus proline-rich region that interacts with SH3 (SRC homology 3) domain, a well-conserved catalytic domain, a nuclear export sequence, and a nuclear localization sequence. Thr222 and Thr334 are the main phosphorylation sites (28). MK2 targets the TGF-β pathway distal to p38 (27). MK2 was required for TGF-β–induced α-smooth muscle actin expression and myofibroblast differentiation (29). Elevated MK2 activation has been reported in lung tissues of patients with IPF and in bleomycin-challenged mouse lungs (30). In addition, MK2 deficiency in mice results in downregulation of inflammatory cytokine expression, including TNF-α, IL-1β, and IL-6 (31, 32).
The MK2 inhibitor (MK2i), known as MMI-0100, is a synthetic, 22–amino acid, cell-permeant peptide. MMI-0100 inhibits MK2 activity once it is entered into the cells (33). Recent reports demonstrated promising antifibrotic effects of this peptide. MMI-0100 inhibited fibrosis after vascular graft and abdominal surgeries (34), prevented postoperative ileus in mice (35), reduced bleomycin-induced lung fibrosis (30), and reduced ischemic heart injury and cardiac fibrosis (36–38). In the present study, we explored the role of MMI-0100 in regulating HAS2 expression and invasiveness of fibroblasts, and we further determined the molecular mechanism by which MMI-0100 inhibits pulmonary fibrosis. We identified a novel mechanism of a feed-forward loop between MK2 activation and HAS2 expression in fibrogenesis. We demonstrated that MK2 inhibition reduced the invasive capacity of lung fibroblasts from patients with IPF and bleomycin-treated fibrotic mice. In vivo administration of MMI-0100 was able to attenuate bleomycin-induced lung fibrosis.
Methods
Mice
α-Smooth muscle actin–human HAS2–transgenic mice (SMA-HAS2) were described previously (11, 39). Mice with MK2 deletion in collagen, type I, α2 chain (Col1a2)-expressing fibroblasts (Col1a2-CreER;Rosa-Tomato;MK2flox/flox or MK2ΔCol) were generated by cross-breeding of Col1a2-CreER (40) and MK2flox/flox (full name Mapkapk2tm1.1Yaff/J; The Jackson Laboratory) mice (41) on a Rosa-Tomato background to facilitate cell sorting. All mice were housed in a pathogen-free facility at Cedars-Sinai Medical Center. All animal experiments were permitted by the institutional animal care and use committee at Cedars-Sinai Medical Center (protocols IACUC004722 and IACUC004751).
Mouse Lung Fibrosis Model
Bleomycin (Hospira) at 2.5 U/kg was instilled as described previously (11, 42). For Col1a2-CreER;Rosa-Tomato;MK2flox/flox mice and littermate control Col1a2-CreER;Rosa-Tomato mice, four doses of tamoxifen were injected 2 weeks before bleomycin administration. MMI-0100 at 37.5 μg/kg was injected daily intraperitoneally at designated starting points after bleomycin injection.
Fibroblast Isolation and Culture
Human lung fibroblasts were isolated from lung transplant explants of patients with IPF and healthy donors as previously reported (43). Mouse lung fibroblasts were isolated from bleomycin-treated Day 10 mouse lungs (11). Cells from passages 4 to 7 were used for experiments. All experiments were approved by the Cedars-Sinai Institutional Review Board (IRB Pro00035396) and carried out in accordance with the guidelines outlined by the board.
Matrigel Invasion Assay
Fibroblast invasion assay was performed as described previously (11, 14). Equal numbers of fibroblasts were plated into the top chamber of Matrigel invasion Transwells, and Dulbecco’s modified Eagle’s medium containing 10 ng/ml PDGF (R&D Systems) for human fibroblasts or 10% FBS for mouse fibroblasts, respectively, was added to the bottom chamber. After 24 hours, the invading cells were counted, and invasive and noninvasive fibroblasts were harvested from the bottom and top of the Transwells, respectively.
RNA Interference Assay
Transfection with siRNA duplexes was performed as described previously (44). siRNA duplexes designed to target nucleotide sequences of the human HAS2 gene (National Center for Biotechnology Information GenBank accession no. NM_005328) (HAS2 small interfering RNA (si), CCAGCTAGTAGGTCTCATAAA), as well as a control siRNA (control small interfering RNA (si) with sense sequence UUCUCCGAACGUGUCACGUdTdT) were obtained from Qiagen.
Quantification of mRNA Expression
Gene expression was examined with SYBR Green fluorescent dye, enabling real-time detection of PCR products. The primers used were as follows: human HAS2 (National Center for Biotechnology Information GenBank accession no. NM_005328) forward: 5′-TCG CAA CAC GTA ACG CAA T; human HAS2 reverse: 5′-ACT TCT CTT TTT CCA CCC CAT TT; human GAPDH (National Center for Biotechnology Information GenBank accession no. NM_002046) forward: 5′-CCC ATG TTC GTC ATG GGT GT; human GAPDH reverse: 5′-TGG TCA TGA GTC CTT CCA CGA TA. The fold change of the target genes was calculated by using the comparative cycle threshold method.
Western Blot Analysis
Proteins were assessed with Western blotting as previously described (44). The membranes were probed with antibodies against phospho-Thr334MK2 and total MK2 (Cell Signaling Technology). β-actin or GAPDH was used as a loading control. Quantitative densitometric analysis relative to β-actin or GAPDH was used to aid clarity.
HA Quantification
The HA contents in conditioned media from fibroblast culture and mouse BAL were measured as described previously (9).
Hydroxyproline Assay
Collagen content in the lung tissue was measured with the conventional hydroxyproline method as previously described (11).
Statistical Analysis
Data are presented as the mean ± SEM. Student’s t tests were used for two-group comparisons. One-way or two-way ANOVA with the Bonferroni or Tukey posttest was performed for multiple comparisons. Results were considered statistically significant at P ≤ 0.05. Statistical analysis was done with Prism software (GraphPad Software).
Results
MK2 Regulates Fibroblast Invasion and HA Production
We reported previously that fibroblasts isolated from IPF lungs were more invasive than fibroblasts from healthy human lungs (11). Because pirfenidone has been shown to be antifibrotic by inhibiting fibroblast biology, we tested if pirfenidone affects fibroblast invasion. Pirfenidone significantly reduced invasion of fibroblasts from three patients with IPF (see Figures E1A–E1C in the data supplement). The inhibitory effect of pirfenidone on fibroblast invasion was in a dose-dependent manner (Figure E1C). However, pirfenidone did not affect proliferation of IPF fibroblasts at these experimental conditions (Figure E1D). Therefore, pirfenidone inhibited the invasive capacity of IPF fibroblasts, and this inhibitory effect was not due to inhibition of cell proliferation.
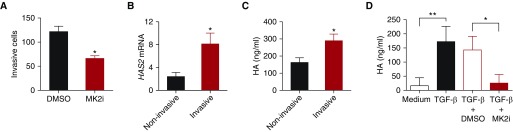
Pirfenidone has been shown to inhibit multiple kinase pathways, including PDGF and TGF-β. MK2 is a p38-associated kinase and has been shown to transmit multiple signals. MK2 activation was also elevated in the lung tissues of patients with IPF (30). We further demonstrated that MK2 activation was increased in IPF fibroblasts (Figure E2). To discern the role of MK2 in fibroblast activation and invasion, we treated fibroblasts isolated from IPF lungs with MMI-0100 (an MK2i) in vitro. MMI-0100 treatment significantly reduced the invasive capacity of IPF fibroblasts (Figure 1A). We previous reported that HA accumulation was increased in IPF patient lungs (6) and that HA and HAS2 mediate fibroblast invasion (11). In the present study, we confirmed that IPF fibroblasts expressed high amounts of HAS2 mRNA (Figure E3A) and released more HA into culture media (Figure E3B). Furthermore, we confirmed that invasive fibroblasts isolated from IPF patient lungs had increased HAS2 expression (Figure 1B) and produced increased HA compared with noninvasive fibroblasts (Figure 1C). Importantly, MMI-0100 treatment reduced TGF-β–induced HA production of IPF fibroblasts (Figure 1D). These data indicated that elevated MK2 activation in IPF fibroblasts might cause an increase in invasion capacity by mediating HAS2 gene expression and HA production. MK2 inhibition also blocked TGF-β–induced connective tissue growth factor (33). However, connective tissue growth factor had no effect on HA production or invasiveness of fibroblasts (Figures E3C and E3D).
Figure 1.
Mitogen-activated protein kinase–activated protein kinase 2 inhibitor (MK2i, MMI-0100) inhibition reduced hyaluronan (HA) production and invasion of idiopathic pulmonary fibrosis (IPF) fibroblasts. Fibroblasts were isolated from lung tissues of patients with IPF and healthy donors. (A) IPF fibroblasts were subjected to invasion assay with and without MMI-0100 treatment. The number of invasive cells per view was counted (n = 3 wells per treatment). *P < 0.05. (B) HAS2 (hyaluronan synthase 2) mRNA was determined with RT-PCR in invasive and noninvasive fibroblasts from 11 patients. *P < 0.05. (C) Invasive and noninvasive IPF fibroblasts were plated into multiple well plates. HA in conditioned media was measured (n = 4). *P < 0.05. (D) Equal numbers of IPF fibroblasts were plated into multiple-well plates and serum starved overnight. Cells were then treated with transforming growth factor (TGF)-β and with or without MMI-0100. HA concentrations were measured in the medium collected 24 hours after treatment (n = 3 or 4 wells per treatment). *P < 0.05; **P < 0.01.
HAS2 Regulates MK2 Activation in IPF Fibroblasts
To further explore the relationship between MK2 activation and HAS2 expression in IPF fibroblasts, we performed siRNA transfection to knock down HAS2 expression in lung fibroblasts isolated from patients with IPF. The efficiency of HAS2 siRNA interference was confirmed with a reduction in HAS2 expression (Figure 2A) and HA production of the transfected cells (Figure 2B). Knocking down HAS2 caused significant reduction of MK2 activation (Figures 2C and 2D), suggesting a feed-forward loop between MK2 activation and HAS2 expression.
Figure 2.
Knockdown of HAS2 reduced MK2 activation of IPF fibroblasts. IPF fibroblasts were serum starved after cultured to 80% confluence overnight. The cells were then transfected with HAS2 siRNA and control (Ctl) siRNA. (A) HAS2 siRNA interference reduced HAS2 expression and (B) HA production. (C) Total cell lysates were harvested 48 hours after transfection and subjected to Western blotting for phospho-MK2 (pMK2) and total MK2. (D) Densitometric analyses of individual band intensities of expression of activated Thr334-MK2 when normalized to β-actin (n = 3). **P < 0.01. hHAS2 = human HAS2. Complete images of uncropped gels are shown in Figure E4 in the data supplement.
Enhanced MK2 Activation in Mouse Lung Fibroblasts Overexpressing HAS2
To investigate the role of MK2 activation in lung fibrosis, we used a bleomycin-induced lung injury model with mice overexpressing HAS2 in myofibroblasts (SMA-HAS2 mice) (11). HA production of fibroblasts isolated from bleomycin-treated SMA-HAS2 mouse lungs were dramatically increased, both at baseline and after TGF-β treatment (Figure 3A). Consistent with our previous findings (11), fibroblasts isolated from SMA-HAS2 mouse lungs also had increased invasive capacity (Figure 3B). Next we looked at MK2 activation in SMA-HAS2 fibroblasts using Western blot analysis and found that phospho-MK2 was increased in the total protein of bleomycin-treated SMA-HAS2 lung tissue (Figures 3C and 3D) and fibroblasts (Figures 3E and 3F) compared with that of wild-type (WT) mice. These data indicated that HAS2 overexpression enhanced MK2 activation in fibroblasts.
Figure 3.
MK2 activation was correlated with HA production and fibroblast invasion. Lung tissues were harvested from mice overexpressing HAS2 in the myofibroblast compartment (SMA-HAS2–transgenic mice) and littermate controls 10 days after bleomycin treatment, and lung fibroblasts were isolated. (A) HA concentration in 24-hour culture medium of fibroblasts treated with TGF-β for 24 hours (n = 3). ***P < 0.001. (B) Invasive capacity of fibroblasts from SMA-HAS2–transgenic mice and littermate controls (n = 3 or 4). ***P < 0.001. (C) Total lung tissue proteins were subjected to Western blot analysis for p-MK2 and total MK2. (D) Densitometric analyses of individual band intensities of activated Thr334-MK2 expression when normalized to GAPDH (n = 4). **P < 0.01. Complete images of uncropped gels are shown in Figure E5 in the data supplement. (E) Total proteins of lung fibroblasts treated with and without MK2i were subjected to Western blot analysis for p-MK2 and total MK2. (F) Densitometric analyses of individual band intensities of activated Thr334-MK2 expression when normalized to GAPDH (n = 4). ***P < 0.001; ****P < 0.0001. Complete images of uncropped gels are shown in Figure E6 in the data supplement. WT = wild type.
MK2 Inhibition Reduced HA Production and Invasive Capacity of Fibroblasts
To further study the role of MK2 activation in regulating matrix production and the invasive phenotype of fibroblasts, we isolated lung fibroblasts from bleomycin-injured SMA-HAS2 and WT mice. We treated the cells with MK2i together with TGF-β in the culture and measured HA concentration in the culture medium. MK2 inhibition reduced TGF-β–induced HA production of fibroblasts isolated from bleomycin-treated SMA-HAS2 mice as well as those from WT mice (Figures 4A and 4B). Next we looked at the effect of MK2 inhibition on fibroblast invasion and found that MMI-0100 treatment reduced the invasive capacity of the fibroblasts from bleomycin-injured SMA-HAS2 and WT mouse lungs (Figures 4C and 4D). A higher dose (6 μM) of MK2i was required to reduce the invasive capacity of fibroblasts from bleomycin-treated SMA-HAS2 mice (Figure 4D), whereas a lower dose (4 μM) was effective for inhibition of the invasive capacity of WT fibroblasts (Figure 4C).
Figure 4.
MK2i reduced HA production and fibroblast invasion. Mouse lung fibroblasts were isolated from mice overexpressing HAS2 in the myofibroblast compartment (SMA-HAS2 mice) and WT mice 10 days after bleomycin treatment. Equal numbers of cells were plated into 24-well plates. The cells were starved overnight before being treated with TGF-β, and with or without MK2 inhibition with MMI-0100. HA concentrations were measured in 24-hour culture media of fibroblasts from (A) WT mice and (B) SMA-HAS2 mice (n = 4). **P < 0.01; ****P < 0.0001. (C and D) Fibroblasts from bleomycin-treated SMA-HAS2 and control mice with and without MMI-0100 treatment at either (C) 4 μM or (D) 6 μM were subjected to invasion assay. Invasive capacity (invaded cells per view) of primary lung fibroblasts from (C) control mice and (D) SMA-HAS2 mice was compared (n = 3). *P < 0.05; **P < 0.01.
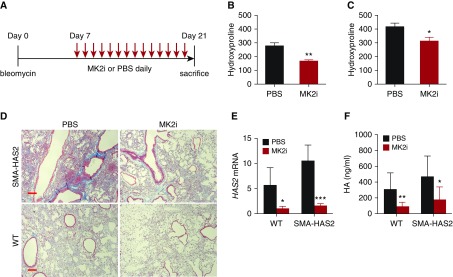
MK2 Inhibition Reduced HA Production and Lung Fibrosis In Vivo
Next we tested to see if our observation on the role of MK2 activation on HA production and fibroblast invasive capacity had biological relevance in vivo. In a bleomycin model, HA accumulation starts at Day 3 and peaks at Day 7, and declines 10–14 days after injury (45). We treated WT mice with bleomycin and then started administration MMI-0100 by intraperitoneal injection daily from Day 3 after bleomycin treatment (Figure 5A). The mice were killed at Day 10, and HA concentrations were measured in the lung tissues. MMI-0100 treatment reduced HA accumulation in the lung compared with that of the control mice receiving PBS (Figure 5B). Next we tested the effect of MMI-0100 treatment on lung fibrosis. We treated SMA-HAS2 mice and littermate controls with bleomycin at Day 0 and started daily MMI-0100 treatment at Day 7 after bleomycin (Figure 6A). The mice were killed at Day 21, and their lungs were harvested for collagen deposition analysis. The results showed that MMI-0100 treatment was able to attenuate bleomycin-induced lung fibrosis of SMA-HAS2 mice and WT controls, as evidenced by reduced collagen contents (Figures 6B and 6C) and reduced trichrome staining in the lungs (Figure 6D). Has2 expression in lung tissues and HA concentrations in the BAL fluid of mice that received MMI-0100 treatment were also reduced (Figures 6E and 6F).
Figure 5.
MK2i reduced HA accumulation in bleomycin-injured mouse lung in vivo. WT C57BL/6J mice were subjected to bleomycin-induced lung injury. From Day 3, mice started to receive intraperitoneal injections of MMI-0100 37.5 μg/kg or PBS daily. (A) Mice were killed on Day 10, and their lung tissues were harvested. (B) HA contents in lung tissues were compared between the MMI-0100 treatment group and the PBS control group (n = 4). **P < 0.01.
Figure 6.
MK2i attenuated mouse lung fibrosis in vivo. Mice overexpressing HAS2 in the myofibroblast compartment (SMA-HAS2–transgenic mice) and littermates were subjected to bleomycin-induced lung injury. From Day 7, the mice started to receive MMI-0100 at 37.5 μg/kg or PBS daily. The mice were killed on Day 21, and BAL fluid and lung tissues were harvested (A). Hydroxyproline contents in lung tissues were compared between the MMI-0100 treatment group and the PBS control group in (B) WT mice and (C) SMA-HAS2–transgenic mice, respectively (n = 7 or 8). *P < 0.05. (D) Trichrome staining of lung slides from bleomycin Day 21 WT and SMA-HAS2–transgenic mice with and without MMI-0100 treatment (scale bars: 100 μm). (E) Has2 mRNA expression (n = 4) and (F) hyaluronan concentrations in BAL fluid in four groups of mice were compared (n = 5–9). *P < 0.05; **P < 0.01; ***P < 0.001.
Conditional Deletion MK2 in Fibroblasts Attenuated Lung Fibrosis in Mice
To further demonstrate the role of MK2 inhibition in pulmonary fibrosis, we generated a mouse line (Col1a2-CreER;Rosa-Tomato;MK2flox/flox or MK2ΔCol) with specific deletion of MK2 in collagen-expressing fibroblasts, in which Cre-mediated Tomato expression facilitated fibroblast isolation. Fibroblasts isolated from bleomycin-treated MK2ΔCol mice showed reduced MK2 phosphorylation (Figures 7A and 7B), reduced Has2 expression (Figure 7C), and decreased invasion capacity (Figure 7D) compared with fibroblasts isolated from littermate control mice. Mice with MK2 deletion in fibroblasts developed less lung fibrosis, as shown by reduced hydroxyproline content in the lungs and reduced trichrome staining (Figures 7E and 7F) on Day 21 after bleomycin treatment. HA concentrations in BAL fluid of bleomycin-treated MK2ΔCol mice were also reduced.
Figure 7.
Deletion of MK2 in collagen, type I, α2 chain (Col1a2)-expressing fibroblasts attenuated lung fibrosis. Mouse lung fibroblasts were isolated from Col1a2-CreER;Rosa-Tomato;MK2flox/flox mice (MK2△Col) and littermate Col1a2-CreER;Rosa-Tomato mice 10 days after bleomycin treatment. (A) Fibroblast total cell protein lysis was subjected to Western blot analysis for p-MK2 and total MK2. (B) Densitometric analyses of individual band intensities of activated Thr334-MK2 expression when normalized to GAPDH knockdown (n = 4). ***P < 0.001. (C) Murine Has2 (mHas2) gene expression in fibroblasts with and without MK2 knockdown (n = 4). **P < 0.01. (D) Invasive cells per view of fibroblasts isolated from MK△Col mice and littermate control mice (n = 3). *P < 0.05. (E–G) Col1a2-CreER;Rosa-Tomato;MK2flox/flox mice (MK2△Col) and littermate MK2flox/flox mice were subjected to bleomycin for 21 days. (E) Hydroxyproline contents (n = 7–10; *P < 0.05) and (F) trichrome staining (scale bars: 100 μm), as well as (G) HA concentrations in BAL fluid (n = 4–6; *P < 0.05) were determined. Complete images of uncropped gels in A are shown in Figure E7 in the data supplement.
Discussion
In this study, we discovered that MK2 activation mediates HAS2 expression and HA production and that HAS2 in turn regulates MK2 activation, suggesting a feed-forward loop in fibroblast activation. MK2-blocking peptide inhibits fibroblast invasion in vitro and attenuates lung fibrosis in vivo. Thus, the study indicates that MK2 has a role in fibroblast invasion and fibrosis and that blocking MK2 may represent a feasible strategy for patients with severe pulmonary fibrosis.
We previously identified an invasive phenotype of fibroblasts from patients with IPF and from a bleomycin-induced fibrosis model (11). The invasive phenotype of fibroblasts is mediated by HAS2 (11). In the present study, we provide several lines of evidence that the feed-forward regulation of MK2 and HAS2 may contribute to persistent fibroblast activation, augmented invasion, and relentless fibrosis in severe lung fibrosis:
-
1.
MK2 activation mediated fibroblast invasion and HA release.
-
2.
HAS2 activation and upregulation caused MK2 phosphorylation, and knockdown of HAS2 led to reduction in MK2 phosphorylation.
-
3.
MK2 inhibition with a permeable peptide inhibited fibroblast invasion as well as HA production in lung fibroblasts from either patients with IPF or bleomycin-injured mice.
-
4.
MK2 inhibition led to a decrease in HA production and lung fibrosis in a bleomycin-induced fibrosis model.
-
5.
Conditional deletion of MK2 in collagen-expressing fibroblasts attenuated lung fibrosis.
Thus, the study identified a role for MK2 in lung fibrosis and provides additional insights into the mechanism by which MK2 mediates HAS2 activation to influence fibroblast invasion and lung fibrosis. These data are consistent with previous studies on MK2 in lung fibrosis. MK2 targets the TGF-β pathway distal to p38 (27) and is required for TGF-β–induced myofibroblast differentiation (29). Pirfenidone is able to block multiple fibrotic signaling pathways, including TGF-β (26), to elicit its antifibrotic effect. Although we do not know if the mode of action of MK2 inhibition is related to the mechanism of pirfenidone in limiting fibrosis, targeting these profibrotic pathways is a plausible strategy for patients with severe pulmonary fibrosis. Just as pirfenidone showed an antifibrotic effect in multiple-organ fibrosis (46, 47), MK2 inhibition seems to have a wide range of organ fibrosis (30, 36–38). Because TGF-β is a main driver of tissue fibrosis (8), targeting TGF-β has been investigated in patients as well as in animal models. To avoid the potential adverse effect of blocking TGF-β directly, researchers in several studies reported modulation of TGF-β signaling. For example, partial inhibition of integrin αvβ6 or αvβ1 prevented pulmonary and other organ fibrosis without exacerbating inflammation (48, 49). We showed previously that mediating TGF-β signaling by targeting TGF-β/bone morphogenetic protein–associated molecules such as FSTL1 (follistatin-related protein 1) and BMPER (bone morphogenetic protein–binding endothelial regulator) can be used as a strategy for blocking lung fibrosis in cell culture in vitro and animal models in vivo (14, 50). MK2 has been shown to mediate TGF-β signaling and TGF-β–activated cellular events in multiple ways (30). We offer a new mechanism by which MK2 regulates fibroblast invasion through an MK2–HAS2 activation loop. Inhibition of MK2 would effectively block fibrogenesis.
Taken together, these data suggest that there is a feed-forward loop between MK2 activation and HAS2 expression in fibroblasts. MK2 inhibition with a permeable peptide inhibited fibroblast invasion and HA production in lung fibroblasts in vitro, and it also attenuated lung fibrosis in a bleomycin-induced fibrosis model in vivo. Thus, blocking MK2 may represent a feasible strategy for patients with severe pulmonary fibrosis.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Cynthia Lander of Moerae Matrix for providing the MK2i MMI-0100.
Footnotes
Supported by National Institutes of Health grants R01-HL060539, R01-AI052201, and R01-HL077291 (P.W.N.); R01-HL122068 (D.J.); and P01-HL108793 (P.W.N. and D.J.).
Author Contributions: J.L., D.J., and P.W.N.: conceived of the study; J.L.: performed most of the experiments and analyzed the data; N.L., X.L., J.M.M., T.X., Y.G., C.H., Y.Z., F.T., G.H., A.K., and V.B.: took part in mouse, cell culture, and biochemical experiments; J.L., J.M.M., and D.J.: analyzed data; and J.L., D.J., and P.W.N.: wrote the paper.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0033OC on August 21, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.American Thoracic Society; European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 3.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 4.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 5.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang J, Jiang D, Noble PW. Hyaluronan as a therapeutic target in human diseases. Adv Drug Deliv Rev. 2016;97:186–203. doi: 10.1016/j.addr.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13α2 receptor is involved in induction of TGF-β1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 8.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, et al. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol. 2011;128:403–411.e3. doi: 10.1016/j.jaci.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson TS, Potter-Perigo S, Tsoi C, Altman LC, Wight TN. Pro- and anti-inflammatory factors cooperate to control hyaluronan synthesis in lung fibroblasts. Am J Respir Cell Mol Biol. 2004;31:92–99. doi: 10.1165/rcmb.2003-0380OC. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, et al. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovgren AK, Kovacs JJ, Xie T, Potts EN, Li Y, Foster WM, et al. β-Arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Sci Transl Med. 2011;3:74ra23. doi: 10.1126/scitranslmed.3001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie T, Liang J, Liu N, Huan C, Zhang Y, Liu W, et al. Transcription factor TBX4 regulates myofibroblast accumulation and lung fibrosis. J Clin Invest. 2016;126:3063–3079. doi: 10.1172/JCI85328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huan C, Yang T, Liang J, Xie T, Cheng L, Liu N, et al. Methylation-mediated BMPER expression in fibroblast activation in vitro and lung fibrosis in mice in vivo. Sci Rep. 2015;5:14910. doi: 10.1038/srep14910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng J, Huang X, Li Y, Xu X, Li S, Jiang D, et al. Down-regulation of USP13 mediates phenotype transformation of fibroblasts in idiopathic pulmonary fibrosis. Respir Res. 2015;16:124. doi: 10.1186/s12931-015-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oehrle B, Burgstaller G, Irmler M, Dehmel S, Grün J, Hwang T, et al. Validated prediction of pro-invasive growth factors using a transcriptome-wide invasion signature derived from a complex 3D invasion assay. Sci Rep. 2015;5:12673. doi: 10.1038/srep12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anwar A, Li M, Frid MG, Kumar B, Gerasimovskaya EV, Riddle SR, et al. Osteopontin is an endogenous modulator of the constitutively activated phenotype of pulmonary adventitial fibroblasts in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1–L11. doi: 10.1152/ajplung.00050.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahluwalia N, Grasberger PE, Mugo BM, Feghali-Bostwick C, Pardo A, Selman M, et al. Fibrogenic lung injury induces non-cell-autonomous fibroblast invasion. Am J Respir Cell Mol Biol. 2016;54:831–842. doi: 10.1165/rcmb.2015-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Qu J, Huang X, Kurundkar A, Zhu L, Yang N, et al. Mechanosensing by the α6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis. Nat Commun. 2016;7:12564. doi: 10.1038/ncomms12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghatak S, Hascall VC, Markwald RR, Feghali-Bostwick C, Artlett CM, Gooz M, et al. Transforming growth factor β1 (TGFβ1)-induced CD44V6-NOX4 signaling in pathogenesis of idiopathic pulmonary fibrosis. J Biol Chem. 2017;292:10490–10519. doi: 10.1074/jbc.M116.752469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black M, Milewski D, Le T, Ren X, Xu Y, Kalinichenko VV, et al. FOXF1 inhibits pulmonary fibrosis by preventing CDH2-CDH11 cadherin switch in myofibroblasts. Cell Rep. 2018;23:442–458. doi: 10.1016/j.celrep.2018.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 23.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 24.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 25.Iyer SN, Wild JS, Schiedt MJ, Hyde DM, Margolin SB, Giri SN. Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters. J Lab Clin Med. 1995;125:779–785. [PubMed] [Google Scholar]
- 26.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20:85–97. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberst A. MK2 balances inflammation and cell death. Nat Cell Biol. 2017;19:1150–1152. doi: 10.1038/ncb3619. [DOI] [PubMed] [Google Scholar]
- 28.Gaestel M. MAPKAP kinases — MKs — two’s company, three’s a crowd. Nat Rev Mol Cell Biol. 2006;7:120–130. doi: 10.1038/nrm1834. [DOI] [PubMed] [Google Scholar]
- 29.Sousa AM, Liu T, Guevara O, Stevens J, Fanburg BL, Gaestel M, et al. Smooth muscle α-actin expression and myofibroblast differentiation by TGFβ are dependent upon MK2. J Cell Biochem. 2007;100:1581–1592. doi: 10.1002/jcb.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vittal R, Fisher A, Gu H, Mickler EA, Panitch A, Lander C, et al. Peptide-mediated inhibition of mitogen-activated protein kinase-activated protein kinase-2 ameliorates bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2013;49:47–57. doi: 10.1165/rcmb.2012-0389OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, et al. MAPKAP kinase 2 is essential for LPS-induced TNF-α biosynthesis. Nat Cell Biol. 1999;1:94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, He H, Ding Y, Liu S, Zhang D, Wang J, et al. MK2 mediates macrophage activation and acute lung injury by regulating let-7e miRNA. Am J Physiol Lung Cell Mol Physiol. 2018;315:L371–L381. doi: 10.1152/ajplung.00019.2018. [DOI] [PubMed] [Google Scholar]
- 33.Lopes LB, Flynn C, Komalavilas P, Panitch A, Brophy CM, Seal BL. Inhibition of HSP27 phosphorylation by a cell-permeant MAPKAP Kinase 2 inhibitor. Biochem Biophys Res Commun. 2009;382:535–539. doi: 10.1016/j.bbrc.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward BC, Kavalukas S, Brugnano J, Barbul A, Panitch A. Peptide inhibitors of MK2 show promise for inhibition of abdominal adhesions. J Surg Res. 2011;169:e27–e36. doi: 10.1016/j.jss.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Wu T, Chi P. Inhibition of MK2 shows promise for preventing postoperative ileus in mice. J Surg Res. 2013;185:102–112. doi: 10.1016/j.jss.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Brown DI, Cooley BC, Quintana MT, Lander C, Willis MS. Nebulized delivery of the MAPKAP kinase 2 peptide inhibitor MMI-0100 protects against ischemia-induced systolic dysfunction. Int J Pept Res Ther. 2016;22:317–324. doi: 10.1007/s10989-015-9507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu L, Yates CC, Lockyer P, Xie L, Bevilacqua A, He J, et al. MMI-0100 inhibits cardiac fibrosis in myocardial infarction by direct actions on cardiomyocytes and fibroblasts via MK2 inhibition. J Mol Cell Cardiol. 2014;77:86–101. doi: 10.1016/j.yjmcc.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng Q, Bhandary B, Osinska H, James J, Xu N, Shay-Winkler K, et al. MMI-0100 inhibits cardiac fibrosis in a mouse model overexpressing cardiac myosin binding protein C. J Am Heart Assoc. 2017;6:e006590. doi: 10.1161/JAHA.117.006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chai S, Chai Q, Danielsen CC, Hjorth P, Nyengaard JR, Ledet T, et al. Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circ Res. 2005;96:583–591. doi: 10.1161/01.RES.0000158963.37132.8b. [DOI] [PubMed] [Google Scholar]
- 40.Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts: a potentially powerful technique for investigating gene function in fibrosis. Am J Pathol. 2002;160:1609–1617. doi: 10.1016/S0002-9440(10)61108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morandell S, Reinhardt HC, Cannell IG, Kim JS, Ruf DM, Mitra T, et al. A reversible gene-targeting strategy identifies synthetic lethal interactions between MK2 and p53 in the DNA damage response in vivo. Cell Rep. 2013;5:868–877. doi: 10.1016/j.celrep.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, et al. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med. 2016;22:1285–1293. doi: 10.1038/nm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meltzer EB, Barry WT, D’Amico TA, Davis RD, Lin SS, Onaitis MW, et al. Bayesian probit regression model for the diagnosis of pulmonary fibrosis: proof-of-principle. BMC Med Genomics. 2011;4:70. doi: 10.1186/1755-8794-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Liang J, Yang T, Monterrosa Mena J, Huan C, Xie T, et al. Hyaluronan synthase 2 regulates fibroblast senescence in pulmonary fibrosis. Matrix Biol. 2016;55:35–48. doi: 10.1016/j.matbio.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Puré E, et al. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 46.Armendáriz-Borunda J, Islas-Carbajal MC, Meza-García E, Rincón AR, Lucano S, Sandoval AS, et al. A pilot study in patients with established advanced liver fibrosis using pirfenidone. Gut. 2006;55:1663–1665. doi: 10.1136/gut.2006.107136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.RamachandraRao SP, Zhu Y, Ravasi T, McGowan TA, Toh I, Dunn SR, et al. Pirfenidone is renoprotective in diabetic kidney disease. J Am Soc Nephrol. 2009;20:1765–1775. doi: 10.1681/ASN.2008090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, et al. Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 49.Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF, et al. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med. 2015;7:288ra79. doi: 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong Y, Geng Y, Li L, Li X, Yan X, Fang Y, et al. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J Exp Med. 2015;212:235–252. doi: 10.1084/jem.20121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.