Abstract
The olfactory receptor neurons lining the nasal cavity have a remarkable capacity to regenerate throughout life. They are replenished continuously and their axons make new connections within the olfactory bulb. However, some factors such as head trauma and skull base surgery damage the olfactory nerve which lead to olfactory dysfunction. Losing the sense of smell has considerable effects on quality of life and life-expectancy. Therefore, there is a clear need to find a treatment for olfactory dysfunction. One such potential treatment is growth factor therapy which showed promising results in the spinal cord and brain injuries. The aim of the present study was to investigate whether combined delivery of two growth factors, vascular endothelial growth factor and platelet-derived growth factor treatment can improve the olfactory neurons regeneration in mice. The degeneration of the olfactory neurons was induced by unilateral bulbectomy. The treatment group received 1.5 µg of the combined growth factors intranasally, while the control injured group received saline. Growth factor treatment significantly increased the number of immature neurons at 5 and 7 days post injury and also the number of mature olfactory neurons at 10 and 14 days post bulbectomy. Regenerating axons extended over a larger volume in the operated cavity in the treatment group compared to control group at 14 days post bulbectomy. The growth factor treatment also significantly reduced astrocytic glia scar in the operated cavity. The results indicate that the combined delivery of the growth factors has the potential to improve olfactory dysfunction.
Keywords: astrocytes, olfactory bulb, glial scar, axon, growth factors, neuron
Introduction
The mammalian olfactory system is unique as the primary olfactory neurons that reside in nasal cavity continuously turnover throughout life. After injury, the primary olfactory neurons degenerate, however they are rapidly replaced by new neurons arising from stem cells which line the basal layer of the olfactory epithelium. Unfortunately, this ability reduces with age, inflammation, and chronic sinus infection; and tumour extraction of the anterior skull can cause scarring or tissue disruption which prevents effective axon targeting and subsequently leads to olfactory dysfunction (Doty et al., 1984; Deems et al., 1991; Schiffman, 1997; Wu and Davidson, 2008). One of the major causes of olfactory dysfunction in humans is head trauma, which results in 5-26% incidence of olfactory dysfunction (Temmel et al., 2002; de Kruijk et al., 2003) and only 10–38% of individuals recover from the condition (Temmel et al., 2002; de Kruijk et al., 2003; London et al., 2008). A recent study has reported that anosmia increases the mortality of adult individuals up to four times (Pinto et al., 2014). Therefore, finding a treatment to improve olfactory dysfunction has been considered by several studies, but effective treatments are not yet available (Kobayashi and Costanzo, 2009; Reden et al., 2011).
Growth factors play critical roles in olfactory neurogenesis. Various studies have shown that neurotrophic factors, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and basic fibroblast growth factor (bFGF) can stimulate regeneration of primary olfactory neurons (Aiba et al., 1993; Nishikawa et al., 2009; Nota et al., 2013). Vascular endothelial growth factor (VEGF) has showed its association in cell proliferation, survival and migration of neural/stem progenitor cells and its receptor VEGFR-1 regulated adult olfactory bulb neurogenesis in mice (Jin et al., 2002; Wittko et al., 2009). Platelet-derived growth factor (PDGF-BB) also implicated as an essential factor in the regulation of stem/progenitor cells and it has promoted proliferation and migration of new born neurons into striatum and the substantia nigra of brain (Mohapel et al., 2005). In our previous study, we showed that combined delivery of two VEGF and PDGF into the injured spinal cord significantly reduced tissue loss and scar formation, and improved axonal regeneration and locomotor function (Lutton et al., 2012; Chehrehasa et al., 2014). Similar results were reported with combined delivery of PDGF and VEGF after stab brain injury (Norazit et al., 2011).
The aim of this study was to investigate the effect of combined VEGF and PDGF treatment in early regeneration of primary olfactory neurons and glial scar formation after unilateral bulbectomy in neonatal mice.
Materials and Methods
Animals
We used a transgenic reporter mouse line which we produced: OMP-ZsGreen mice, in which the olfactory marker protein (OMP) drives expression of the bright fluorescent protein ZsGreen in primary olfactory sensory neurons (Windus et al., 2010; Ekberg et al., 2011). This allows easy visualisation of these neurons in vivo and in vitro, and also distinguishes olfactory nerve fascicles from bundles of the intranasal branch of the trigeminal nerve (in which neurons do not express OMP). Overall, we used 45 mice at postnatal day 4 (P4)–P18 (weight between 3–10 g). All procedures were carried out with the approval of the Griffith University Animal Ethics Committee (ESK/01/16/AEC) and QUT Animal Research Ethics Committee (1600000364) under the guidelines of the National Health and Medical Research Council of Australia and the Australian Commonwealth Office of the Gene Technology Regulator.
Surgical ablation of olfactory bulb (bulbectomy)
Unilateral surgical ablation of olfactory bulb was performed in OMP-ZsGreen transgenic mice at P4 age as described previously (Chehrehasa et al., 2008). Pups were anaesthetized by putting them on ice for 7 min. To expose the cranial bones to access the olfactory bulb, a midline skin incision was made and the bone was removed using a surgical drill with a 0.5 mm burr. The olfactory bulb was aspirated using a glass pipette attached to a vacuum. The surgical wound was sutured and the animals were injected subcutaneously with analgesic drug Carprofen (4 mg/kg; Parchem, New York, NY, USA). For each time point, six littermates were used with three operated control pups and three operated with growth factor treatment.
Tissue fixation and preparation
Mice were sacrificed by cervical decapitation at 3, 5, 7, 10 and 14 days and the heads fixed by immersion in 4% paraformaldehyde in phosphate buffered saline (PBS) at room temperature for 24 hours. Following fixation, the skulls of the animals over 10 days old were decalcified in 20% disodium ethylene diaminetetraacetic acid (EDTA) in PBS. They were then embedded in Optimal Cutting Temperature (O.C.T) compound (Miles Scientific, Naperville, IL, USA) and snap frozen by immersion in iso-pentane that had been cooled with liquid nitrogen. Cryostat sections (30 μm) of the nasal cavity and brain were sectioned, mounted on to gelatinized slides and stored at –20°C before immunohistochemistry.
Immunohistochemistry
Immunohistochemistry was performed as previously described (Chehrehasa et al., 2009). Cryostat sections were incubated with the following antibodies: polyclonal rabbit anti-GFAP (1:500; Dako, Glostrup, Denmark, ZO334), polyclonal rabbit anti-GAP43 (1:1000, AB-16053, Abcam, Melbourne, VIC, Australia) overnight at room temperature and incubated with the appropriate secondary antibodies Alexa Fluor 647 (1:200; Life Technologies, Pty Ltd, Melbourne, Australia) for 3 hours at room temperature. The sections were counterstained using Vectashield 4’,6-diamidino-2-phenylindole mounting medium (Vector Labs, Burlingame, CA, USA) to label cell nuclei.
Quantification of axonal extension into operated cavity
Within the cavity left by the bulbectomy, the regenerating axons were present and formed a network structure termed “regenerating plexus”. To quantify the length of regenerating axon, the first and the last coronal sections of the operated cavity in which regenerating olfactory axons were detectable were considered the starting and ending points respectively. The length of the region was determined by the number of serial coronal sections between two points.
Quantification of olfactory receptor neurons and astrocytes
Quantifications of olfactory receptor neurons were performed by counting number of neurons in 300 µm spans of the olfactory epithelium of coronal sections of each animal (three sections from each animal [three animals in two groups], similar regions of the epithelium). Three sections from specific regions of the olfactory epithelium from each animal were chosen. The number of olfactory sensory neurons was quantified in each section by counting the number of OMP-ZsGreen positive neurons or growth associated protein 43 (GAP-43) neurons in the field of view using a 40× objective (Olympus FV1000 laser scanning confocal microscope, Australia Pty, Ltd., VIC, Australia) (300 µm; length of the epithelium in each section, 900 µm in each animal. The quantification were performed after bulbectomy in both treated and control injured and no injury animals (injured animals = 30, control non-injured = 15). For the olfactory epithelium thickness quantification, the same regions of the olfactory epithelium were measured in the same animal groups (as explained above).
For quantification of astrocytes within the region of operated cavity, three sections from each animal were chosen (three sections from each animal [three animals in two groups]) and the number of DAPI-stained nuclei co-labelled with glial fibrillary acidic protein (GFAP) antibody was counted (3.09 mm2 areas in each animal).
Intranasal delivery of combined VEGF and PDGF
Animals were randomly divided into control injured (n = 15) and combined VEGF/PDGF growth factors groups (n = 15). The growth factors were dissolved in normal saline to a final concentration of 100 μg/100 μL. The pup was held in prone position and a droplet was placed by pipette tip on each nostril to inhale. Pups remained in the prone position until no liquid was present around the nose. The growth factor treatment group received 1.5 μg of each growth factor intranasally for three consecutive days post bulbectomy (VEGF-165, Cat # PMG0041; PDGF-BB, Cat # PHC939, Life Technologies, Melbourne, VIC, Australia). The control injured group received the same volume (3 μL) of normal saline. A group of control animals with no injury (n = 15) was added to provide base lines for data analysis.
Image capture and image preparation
Images were captured using an Olympus FV1000 laser scanning confocal microscope (Olympus Australia Pty Ltd.). Captured images were colour balanced uniformly across the field of view with Adobe Photoshop CC 2015 (Creative Cloud All Apps, VIC, Australia) and compiled into panels with Adobe Illustrator CC 2015 (Creative Cloud All Apps).
Statistical analysis
Comparisons were performed using the two-tailed t-test; P values of < 0.05 were considered statistically significant. All data are expressed as mean ± SEM. Data analysis was performed by AcaStat software 10, Winter Garden, Florida, USA).
Results
VEGF and PDGF growth factor improved olfactory axonal regeneration
To determine the effect of VEGF and PDGF on olfactory nerve regeneration, we induced a large-scale injury to the olfactory nervous system and assessed the effects of the growth factors in stimulating regeneration. Our injury model was unilateral bulbectomy (removal of one olfactory bulb) (Figure 1A) which is a well-established model of olfactory nervous system injury (Chehrehasa et al., 2010). To allow for easy visualisation of primary olfactory sensory axons, we used neonatal OMP-ZsGreen transgenic mice, in which the OMP promoter drives expression of the bright fluorescent protein ZsGreen in primary olfactory neurons (Figure 1A). When unilateral bulbectomy is performed in neonatal mice, the neurons whose axons have already reached the olfactory bulb die off and are rapidly replaced by new neurons that arise from stem cells lining the basal layer of the olfactory epithelium. As expected, three days after bulbectomy, there was a widespread ipsilateral death of the primary olfactory neurons within the nasal cavity (Figure 1A, C) and ZsGreen expression on the bulbectomized side revealed a dramatic loss of olfactory receptor neuron populations (Figure 1C). However, olfactory receptor neurons in the contralateral (unoperated) side of nasal cavity remained intact (Figure 1B) which was similar to the uninjured (no injury) olfactory epithelium (Figure 1D, E).
Figure 1.
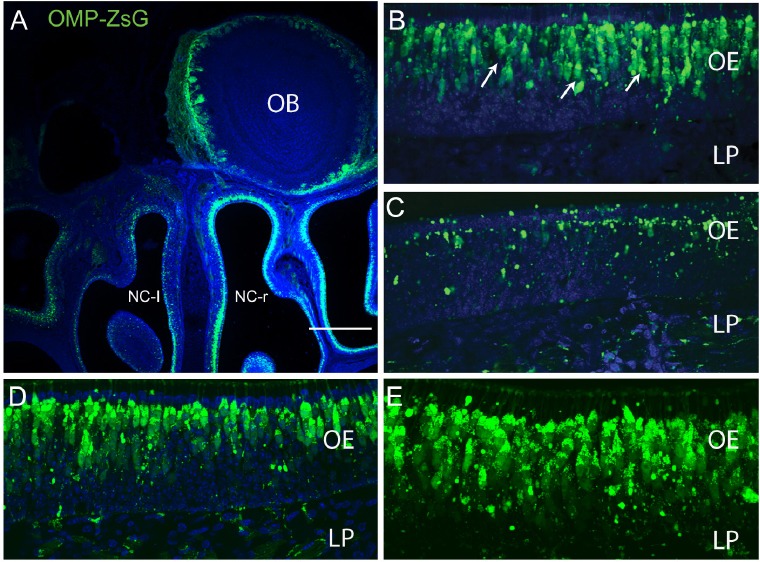
Widespread death of primary olfactory neurons in response to unilateral bulbectomy.
Panels are coronal sections through the nasal cavity of postnatal OMP-ZsGreen (OMP-ZsG) mice, dorsal is to the top. Primary olfactory neurons are green and nuclei are blue (DAPI). (A) Three days after unilateral bulbectomy, the olfactory receptor neurons expressing ZsGreen on the bulbectomy side (left nasal cavity, NC-l) underwent degeneration and the number of neurons was reduced compared to the epithelium lining the nasal cavity on right control unoperated side (NC-r). Higher magnification views of the primary olfactory neurons (indicating by arrows) in unoperated side (B) and in the operated (C) indicated degenerating neurons at 3 days after bulbectomy. The olfactory epithelium of control animal with no injury depicted in D. E is higher magnification of D. Scale bar: 500 μm for A, 50 μm for B–D and 30 μm for E. OE: Olfactory epithelium; OB: olfactory bulb; LP: lamina propria.
We assessed effect of the growth factors treatment on early stage of olfactory neuron regeneration by comparing the thickness of the olfactory epithelium on the bulbectomized side of growth factor treated (1.5 μg each of VEGF and PDGF delivered intranasally over 3 consecutive days after bulbectomy) and control injured (saline-treated) animals. At five days post bulbectomy, the thickness of the olfactory epithelium on the bulbectomized side was significantly reduced in both groups due to degeneration of primary olfactory neurons after injury (indicated by low ZsGreen expression; Figure 2A, B). After 10 days, the epithelium started to replenish in both groups (Figure 2E–G), however the thickness of the olfactory epithelium increased significantly in growth factor treated animals at 10 days and 14 days post bulbectomy (P < 0.05) compared with control injured group (Figure 2G). We chose 14 days post bulbectomy as for the regeneration period as our previous paper demonstrated that after methimazole destruction of the nasal mucosa, the olfactory epithelium regenerates to near-maximal levels by this time (Chehrehasa et al., 2010). To confirm the thickness increase was due to regenerated neurons, we quantified the number of ZsGreen positive neurons which represent mature olfactory neurons (Ekberg et al., 2011). The olfactory neuron quantifications confirmed that at 10 and 14 days post bulbectomy, there was a significant increase in number of ZsGreen positive cells in the olfactory epithelium of treated animals compared to control injured animals (P < 0.05) (Figure 2H), which coincides with increase thickness of the epithelium during this period. At 14 days post bulbectomy, the number of olfactory receptor neurons in the treated animals reached to 70% of the control uninjured animals compared to 56% in the control injured group.
Figure 2.
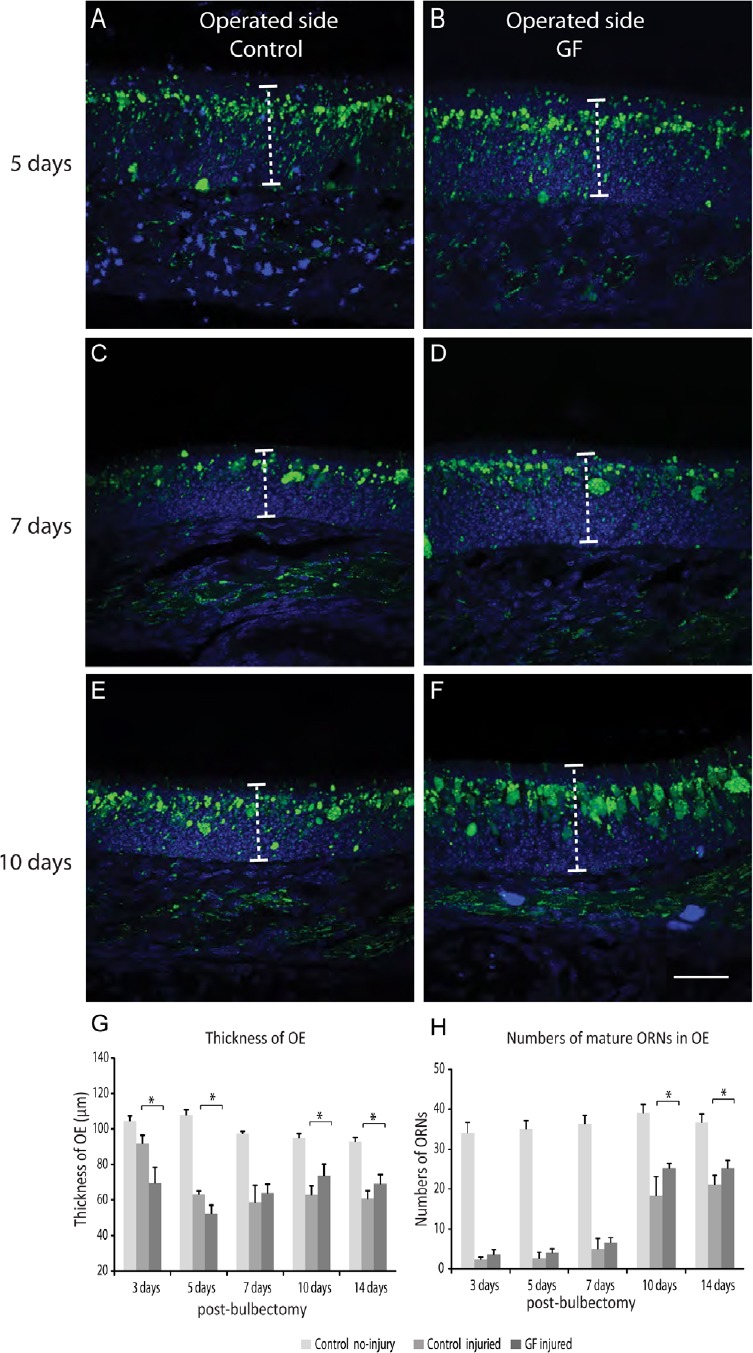
Combined vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) treatment enhanced olfactory neurons regeneration after unilateral bulbectomy.
Panels are coronal sections through the nasal cavity of postnatal olfactory marker protein (OMP)-ZsGreen mice, dorsal is to the top. Primary olfactory neurons are green and nuclei are blue (DAPI or 4′,6-diamidino-2-phenylindole). (A, C, E) factory epithelium of the operated side of the control injured group and (B, D, F) the growth factor treatment group post bulbectomy. Dotted line shows the thickness of the epithelium. (G) The thickness of olfactory epithelium at 3 days and 5 days post injury (n = 6, each time points) was significantly reduced in growth factor animals (GF) compared to control injured animals, however there was a significant increase (*P < 0.05) in the thickness of epithelium at 10 days and 14 days post bulbectomy in the growth factor treatment vs. control injured group. (H) Growth factor treatment significantly increased the number of mature olfactory neurons (300 µm, length of epithelium) at 10 and 14 days post injury (n = 6 at each time points; *P < 0.05, vs. control injured group). There was a significant difference in number of olfactory receptor neurons (H) and the olfactory epithelium thickness (G) between both injured animal groups and animals with no injury across all time points (P < 0.01), indicating the successful unilateral bulbectomy which led to the degeneration of the olfactory epithelium (two asterisks were not shown in the graphs to prevent over-crowding). Scale bar is 50 μm for A–F. OE: Olfactory epithelium; ORN: olfactory receptor neuron.
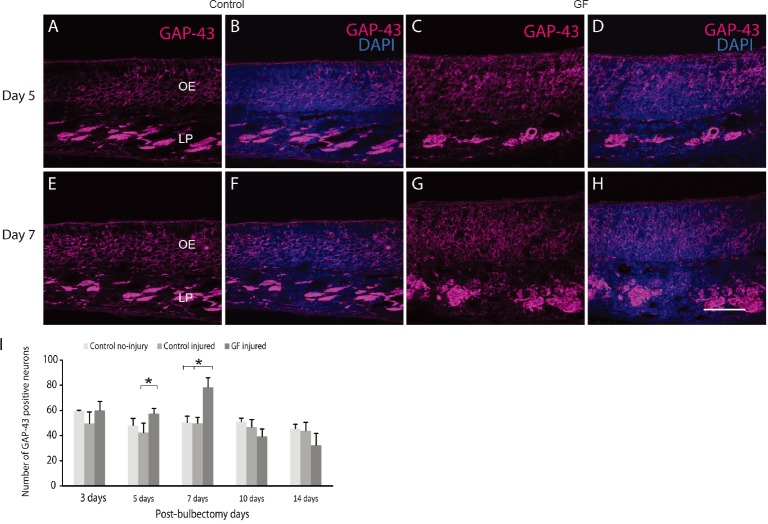
To determine whether the combined growth factor treatment affected generation of immature olfactory receptor neurons in the bulbectomized olfactory epithelium, we immunolabelled the olfactory epithelium with antibodies against GAP-43 which is a marker of immature neurons. We found that the expression of GAP-43 significantly increased at five days (Figure 3C, D) and seven days post bulbectomy (Figure 3G, H) in growth factor treatment animals (P < 0.05) (Figure 3I) compared to control injured and uninjured group (only 7 days), indicating the growth factor treatment increased the number of immature neurons before mature ZsGreen neurons can be detected. Overall these results confirmed the combined growth factor increased regeneration of olfactory neurons after bulbectomy.
Figure 3.
Combined VEGF and PDGF treatment enhanced immature olfactory neurons generation.
Panels are coronal sections through the nasal cavity, illustrating operated side of olfactory epithelium of OMP-ZsGreen mice; dorsal is to the top. Immunolabeling of the olfactory epithelium with anti-growth associated protein-43 (GAP-43) (magenta) showed the treatment group had more GAP-43 positive neurons at 5 days (C, D) and 7 days (G, H) compared to the control injured group (5 days: A, B; 7 days: E, F). (I) There were significantly more GAP-43 positive neurons (300 µm, length of epithelium) at 5 and 7 days post bulbectomy (*P < 0.05) (n = 6 at each time point) in the treatment group vs. control injured, and the treatment vs control no injury groups (only at 7 days). Nuclei are stained with DAPI (4′,6-diamidino-2-phenylindole). Scale bar is 50 µm for A–H.
Combined VEGF and PDGF growth factor reduced glial scar formation by astrocytes
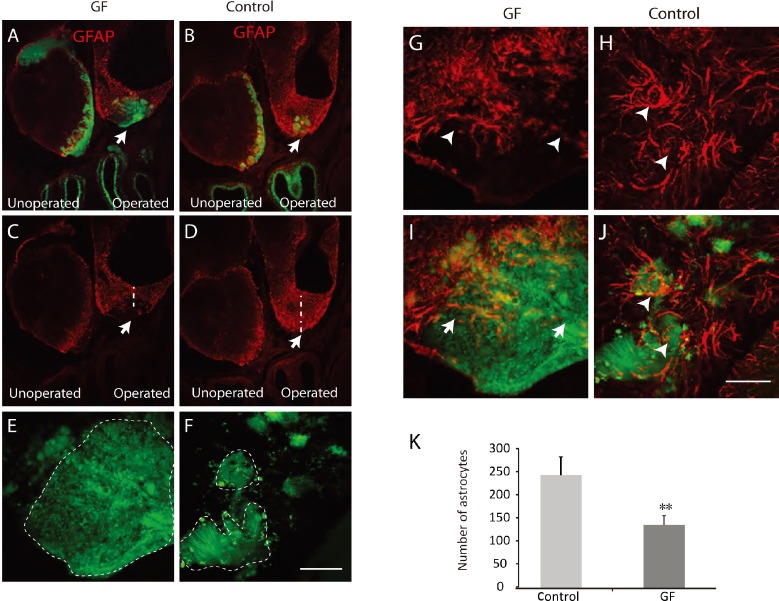
We next examined the cavity left by the olfactory bulbectomy to determine whether the growth factor treatment affected the arrangement of the regenerating plexus and the subsequent axon extension. We have previously shown that following bulbectomy in neonatal animals, olfactory axons enter the cavity left by bulbectomy and form a regenerating plexus (Chehrehasa et al., 2005). At 14 days after bulbectomy, the axons had entered the cavity left by bulbectomy and occupied the rostral half of the cavity in both growth factors treated and control injured animals (Figure 4A, B), however, the regenerated plexus was much larger and formed larger axon bundles in growth-factor treated animals (Figure 4A, E) in comparison to control injured animals where the axon bundles were much smaller (Figure 4B, F). To examine the regenerating plexus further, we immunolabelled the cranial cavity sections with GFAP antibody which is a marker of astrocytes. At 14 days post bulbectomy, GFAP positive cells were present in the operated cavity and contralateral unoperated side in both animal groups (Figure 4A–D). However, GFAP positive cells were distributed mostly around periphery of the regenerating plexus in growth factor treated animals and not many were present inside the plexus (Figure 4A, C). In the control injured animals, the GFAP positive cells were present inside the plexus and tightly wrapped smaller axon bundles (Figure 4B, D). It was also noticeable the astrocytic glial layer was much thinner over the regenerating plexus in growth factor treated compared to control injured animals (Figure 4C, D). In growth factor treated animals, astrocytes covering the superior part of the regenerating plexus were more spread out with bigger gaps between them than in control injured animals (Figure 4G, I). In contrast, in control injured animals, astrocytes were tightly wrapped around axon bundles (Figure 4H, J). We then quantified the number of astrocytes in both animal groups and found that there were significantly less astrocytes in the operated cavity of growth-factor treated animals in comparison to control injured animals (P < 0.01; Figure 4K). Overall, these data show that treatment with VEGF/PDGF reduced the astrocytic scar after olfactory nervous system injury.
Figure 4.
The growth factor (GF) treatment reduced astrocytic glial scars and improved axonal fasciculation.
Panels are coronal sections through the olfactory bulb of operated animals. Astrocytes were immunolabelled with anti-glial fibrillary acidic protein (GFAP) (red); axons are green. At 14 days after bulbectomy, the regenerating axons entered the bulbar cavity with larger axon bundles in treated animals (A, B, arrows). The operated cavity contained less astrocytes in the growth factor treatment animals (A, C) compared to the control injured animals (B, D, arrows), the vertical dotted line indicated the thickness of the astrocytic scar. (E–J) Higher magnification of the area highlighted with arrows in A, B. At 14 days post bulbectomy, the regenerating axons formed a large axon bundle in the treatment group (E, dashed line) compared to the control injured animals with a few smaller axon bundles (F, dashed line). The astrocytes were tightly wrapped around the axon bundles in the control injured animals (H, J, arrowheads), while they were distributed more in the peripheral part of the plexus with more gaps between cells allowing axonal extension (G, I, arrowheads and arrows). (K) There were significantly less astrocytes in the operated cavity of the treated animals compared to control injured animals (n = 6, **P < 0.01). Scale bars: 500 μm for A–D and 50 μm for E–J.
Combined VEGF and PDGF growth factor increased olfactory axon extension and fasciculation in the bulbar cavity
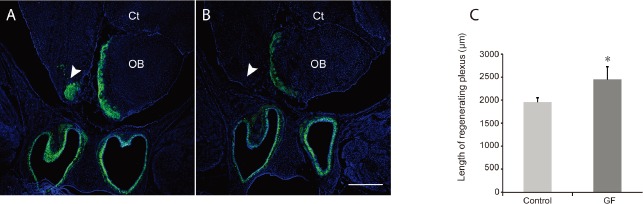
The reduction of the astrocytic glial scar in the operated cavity led us to further examine the distribution of regenerating axons throughout the cavity in the operated animals. We chose the time-point of 14 days post bulbectomy when axonal regeneration is at its peak rate (Chehrehasa et al., 2010). At 14 days post bulbectomy, regenerating axons in growth factor treated animals extended to very caudal region of the cavity at the plane equivalent to forebrain cortex (Ct) on the unoperated contralateral side (Figure 5A). However, in control injured animals, regenerating olfactory axons did not reach at the plane equivalent to forebrain on the unoperated contralateral side (Figure 5B). Next, to confirm whether the treatment had an effect on extension of regenerating neuron axons into the operated bulbar cavity, we quantified the distribution of regenerating axons within the operated cavity of growth factor treated and control injured animals by measuring the rostral-caudal length in which the axons were present. At 14 days post bulbectomy, the axons in growth factor treated animals all projected significantly deeper into the cavity left by the bulbectomy than in the control injured group (P < 0.05; Figure 5C).
Figure 5.
Combined PDGF and VEGF enhanced olfactory axon extension.
Panels are coronal sections through the bulbar cavity of operated animals. Primary olfactory neurons are green and nuclei are blue (DAPI or 4′,6-diamidino-2-phenylindole). (A) 14 days post bulbectomy, the regenerating plexus (arrowhead) in GF treated animals extended to the caudal region of cavity to the level of forebrain cortex (Ct) on the unoperated side. (B) In control injured animals, the plexus was not observed (arrowhead) at the level of the forebrain cortex on the unoperated side. (C) After 14 days post bulbectomy, the regenerating olfactory axons in the growth factors treatment projected significantly deeper into the cavity left by the bulbectomy in comparison to the control injured animals (*P < 0.05). Nuclei are stained with DAPI (blue). Scale bar is 500 μm for A, B. OB: Olfactory bulb; GF: growth factor.
These results confirmed the regenerating axons in growth factor treated animals extended over a greater volume of the operated cavity in comparison to axons in control injured animals.
Discussion
This study demonstrated that combined VEGF and PDGF treatment significantly improved olfactory axon regeneration and reduced astrocytic scar formation after bulbectomy. Our results showed that in the growth factor treated group, there was an increase of GAP-43 positive neurons (immature neurons) at day 5 and 7 post bulbectomy in the operated olfactory epithelium suggesting the treatment enhanced the turnover of olfactory receptor neurons. At day 10 and 14 post injury, the number of mature olfactory neurons (indicating by OMP-ZsGreen positive cells) also significantly increased which coincided with an increase in the olfactory epithelial thickness at day 10 and 14 post bulbectomy. We also showed that the treatment enhanced axonal extension as they projected significantly deeper into the cavity left by the bulbectomy compared to control injured animals. These results are consistent with effects seen in other regions of the nervous system. Reduced tissue loss, improved axonal regeneration and locomotor function were also reported in previous studies using VEGF and PDGF treatment after spinal cord and stab brain injuries (Norazit et al., 2011; Lutton et al., 2012; Chehrehasa et al., 2014).
We found that in the operated cavity, the astrocytic glial scar surrounding the regenerating plexus was much smaller in the treatment group which may be the reason for which the axons projected over a larger volume in the operated cavity. In contrast, in control injured animals, the astrocytic glial scar was thicker and present around and within the regenerating plexus, where the small axon bundles were tightly wrapped by astrocytes. At this stage, the exact mechanisms underlying presence of a smaller glial scar in growth factor treated group remain unclear. It is possible that presence of more regenerating axons in the treatment group occupied the majority of the operated cavity which subsequently pushed the astrocytes to the forebrain. Another possible mechanism might be that the growth factors treatment reduced the glial scar formation similar to the previous studies using combined VEGF and PDGF treatment after spinal cord injury and stab brain injury (Norazit et al., 2011; Lutton et al., 2012; Chehrehasa et al., 2014).
The identities of cells upon which the growth factors act through are not well understood in mice. However the presence of VEGF and its receptor in the olfactory system has been reported in amphibians (Pozzi et al., 2006). VEGFR-1 signalling has been involved in neurogenesis of interneurons of the olfactory bulb in mice (Wittko et al., 2009) and an increase in generation of new neurons after VEGF infusion into lateral ventricle was reported in the subventricular zone and olfactory bulb (Schanzer et al., 2004). VEGFR is also reported in the human neuronal stem cell/progenitor cells derived from the olfactory epithelium which affect the migration of progenitor cells (Ramirez-Rodriguez et al., 2017). PDGF also acts on olfactory precursor cells and immature neurons of the olfactory epithelium in vitro and increases their survival rate (Newman et al., 2000). Our data confirmed the combined growth factor treatment increased the number of immature neurons in the olfactory epithelium, an effect likely mediated by PDGF.
While the exact mechanisms which the growth factors act upon the olfactory epithelium cells remains unclear, it is likely that VEGF stimulated proliferation of the stem cells/progenitor cells of the olfactory epithelium to form new neurons and replenish the injured olfactory epithelium and it seems PDGF enhanced maturation of immature olfactory neurons.
In summary, combined VEGF and PDGF treatment improved regeneration of the mature and immature olfactory receptor neurons after unilateral bulbectomy and the regenerating axons formed larger bundles and extended over larger volume to the operated cavity. The astrocytic glial scar within the operated cavity was smaller which could be due to presence of a larger regenerating plexus in the treated animals. Although the cellular mechanisms remain undetermined, the beneficial effects of VEGF and PDGF to restore sense of smell warrant future investigation.
Additional file: Open peer review report 1 (129.5KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: This work was supported by Queensland University of Technology Start Up Grant to FC and by a grant by the Clem Jones Foundation to JASJ. The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: All procedures were approved by the Griffith University Animal Ethics Committee (ESK/01/16/AEC) and QUT Animal Research Ethics Committee (1600000364) and performed under the guidelines of the National Health and Medical Research Council of Australia and the Australian Commonwealth Office of the Gene Technology Regulator.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Yike Li, Vanderbilt University, USA.
Funding: This work was supported by Queensland University of Technology Start Up Grant (to FC) and a grant from the Clem Jones Foundation (to JASJ).
References
- Aiba T, Mori J, Nakai Y. Nerve growth factor (NGF) and its receptor in rat olfactory epithelium. Acta Otolaryngol Suppl. 1993;506:37–40. doi: 10.3109/00016489309130238. [DOI] [PubMed] [Google Scholar]
- Chehrehasa F, St John J, Key B. The sorting behaviour of olfactory and vomeronasal axons during regeneration. J Mol Histol. 2005;36:427–436. doi: 10.1007/s10735-006-9015-z. [DOI] [PubMed] [Google Scholar]
- Chehrehasa F, Key B, St John JA. The cell surface carbohydrate blood group A regulates the selective fasciculation of regenerating accessory olfactory axons. Brain Res. 2008;1203:32–38. doi: 10.1016/j.brainres.2008.01.084. [DOI] [PubMed] [Google Scholar]
- Chehrehasa F, Meedeniya AC, Dwyer P, Abrahamsen G, Mackay-Sim A. EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J Neurosci Methods. 2009;177:122–130. doi: 10.1016/j.jneumeth.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Chehrehasa F, Cobcroft M, Young YW, Mackay-Sim A, Goss B. An acute growth factor treatment that preserves function after spinal cord contusion injury. J Neurotrauma. 2014;31:1807–1813. doi: 10.1089/neu.2013.3294. [DOI] [PubMed] [Google Scholar]
- Chehrehasa F, Windus LC, Ekberg JA, Scott SE, Amaya D, Mackay-Sim A, St John JA. Olfactory glia enhance neonatal axon regeneration. Mol Cell Neurosci. 2010;45:277–288. doi: 10.1016/j.mcn.2010.07.002. [DOI] [PubMed] [Google Scholar]
- de Kruijk JR, Leffers P, Menheere PP, Meerhoff S, Rutten J, Twijnstra A. Olfactory function after mild traumatic brain injury. Brain Inj. 2003;17:73–78. doi: 10.1080/0269905021000010221. [DOI] [PubMed] [Google Scholar]
- Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, Kimmelman CP, Brightman VJ, Snow JB., Jr Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117:519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226:1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- Ekberg JA, Amaya D, Chehrehasa F, Lineburg K, Claxton C, Windus LC, Key B, Mackay-Sim A, St John JA. OMP-ZsGreen fluorescent protein transgenic mice for visualisation of olfactory sensory neurons in vivo and in vitro. J Neurosci Methods. 2011;196:88–98. doi: 10.1016/j.jneumeth.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Costanzo RM. Olfactory nerve recovery following mild and severe injury and the efficacy of dexamethasone treatment. Chem Senses. 2009;34:573–580. doi: 10.1093/chemse/bjp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London B, Nabet B, Fisher AR, White B, Sammel MD, Doty RL. Predictors of prognosis in patients with olfactory disturbance. Ann Neurol. 2008;63:159–166. doi: 10.1002/ana.21293. [DOI] [PubMed] [Google Scholar]
- Lutton C, Young YW, Williams R, Meedeniya AC, Mackay-Sim A, Goss B. Combined VEGF and PDGF treatment reduces secondary degeneration after spinal cord injury. J Neurotrauma. 2012;29:957–970. doi: 10.1089/neu.2010.1423. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132:767–776. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Newman MP, Feron F, Mackay-Sim A. Growth factor regulation of neurogenesis in adult olfactory epithelium. Neuroscience. 2000;99:343–350. doi: 10.1016/s0306-4522(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Doi K, Ochi N, Katsunuma S, Nibu K. Effect of intranasal administration of basic fibroblast growth factor on olfactory epithelium. Neuroreport. 2009;20:764–769. doi: 10.1097/WNR.0b013e32832b169e. [DOI] [PubMed] [Google Scholar]
- Norazit A, Nguyen MN, Dickson CG, Tuxworth G, Goss B, Mackay-Sim A, Meedeniya AC. Vascular endothelial growth factor and platelet derived growth factor modulates the glial response to a cortical stab injury. Neuroscience. 2011;192:652–660. doi: 10.1016/j.neuroscience.2011.06.035. [DOI] [PubMed] [Google Scholar]
- Nota J, Takahashi H, Hakuba N, Hato N, Gyo K. Treatment of neural anosmia by topical application of basic fibroblast growth factor-gelatin hydrogel in the nasal cavity: an experimental study in mice. JAMA Otolaryngol Head Neck Surg. 2013;139:396–400. doi: 10.1001/jamaoto.2013.92. [DOI] [PubMed] [Google Scholar]
- Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 2014;9:e107541. doi: 10.1371/journal.pone.0107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi AG, Yovanovich CA, Jungblut L, Heer T, Paz DA. Immunohistochemical localization of vascular endothelial growth factor and its receptor Flk-1 in the amphibian developing principal and accessory olfactory system. Anat Embryol (Berl) 2006;211:549–557. doi: 10.1007/s00429-006-0105-1. [DOI] [PubMed] [Google Scholar]
- Ramirez-Rodriguez GB, Perera-Murcia GR, Ortiz-Lopez L, Vega-Rivera NM, Babu H, Garcia-Anaya M, Gonzalez-Olvera JJ. Vascular endothelial growth factor influences migration and focal adhesions, but not proliferation or viability, of human neural stem/progenitor cells derived from olfactory epithelium. Neurochem Int. 2017;108:417–425. doi: 10.1016/j.neuint.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Reden J, Herting B, Lill K, Kern R, Hummel T. Treatment of postinfectious olfactory disorders with minocycline: a double-blind, placebo-controlled study. Laryngoscope. 2011;121:679–682. doi: 10.1002/lary.21401. [DOI] [PubMed] [Google Scholar]
- Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS. Taste and smell losses in normal aging and disease. JAMA. 1997;278:1357–1362. [PubMed] [Google Scholar]
- Temmel AF, Quint C, Schickinger-Fischer B, Klimek L, Stoller E, Hummel T. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg. 2002;128:635–641. doi: 10.1001/archotol.128.6.635. [DOI] [PubMed] [Google Scholar]
- Windus LC, Lineburg KE, Scott SE, Claxton C, Mackay-Sim A, Key B, St John JA. Lamellipodia mediate the heterogeneity of central olfactory ensheathing cell interactions. Cell Mol Life Sci. 2010;67:1735–1750. doi: 10.1007/s00018-010-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittko IM, Schanzer A, Kuzmichev A, Schneider FT, Shibuya M, Raab S, Plate KH. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J Neurosci. 2009;29:8704–8714. doi: 10.1523/JNEUROSCI.5527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AP, Davidson T. Posttraumatic anosmia secondary to central nervous system injury. Am J Rhinol. 2008;22:606–607. doi: 10.2500/ajr.2008.22.3238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.