Abstract
Background
With the growing use of second-generation antipsychotics for the treatment of a spectrum of psychiatric illnesses in pregnancy, concerns have been raised about the long-term impact of these medications on offspring neurodevelopment. However, preclinical and clinical evidence on the lasting effects of prenatal antipsychotic exposure is still sparse.
Methods
Risperidone, a widely used second-generation antipsychotic, and haloperidol, a representative first-generation antipsychotic, were administered to pregnant C57BL/6N mice from embryonic day 6 to 16. Behavioral tests, immunohistochemical staining, Golgi-Cox technique, and western blot were used to determine the effects of prenatal antipsychotic exposure on the plasticity of the dentate gyrus and related behavior in adult male mice.
Results
Both prenatal haloperidol- and risperidone-exposed mice showed recognition memory deficits but had no anxiety-related behavior. In addition, both prenatal haloperidol and risperidone exposure impaired the proliferation and maturation of adult-born dentate granule cells. We found that haloperidol exposure decreased dendritic length of dentate granule cells, while risperidone had no effect. However, both drugs inhibited dendrite branching in granule cells. Haloperidol exposure also significantly reduced total spine density in the middle dendritic segment of dentate gyrus. Prenatally risperidone-exposed mice only displayed a loss in thin and mushroom spines of infrapyramidal blade of dentate gyrus. Collectively, prenatal haloperidol exposure exerted more robust negative effects than risperidone.
Conclusion
These data provide evidence for the long-term programming effects of early-life exposure to antipsychotics on hippocampal plasticity and behavior.
Keywords: risperidone, haloperidol, prenatal, dentate gyrus, plasticity
Significance Statement
During the past decades, the use of second-generation antipsychotics (SGA) in pregnant women has progressively increased. Although cumulative data suggest that SGA is unlikely to raise the risk of major malformations, there is an extreme paucity of studies regarding the long-term neurodevelopmental safety of these compounds. Using animal models of prenatal exposure to antipsychotic drugs can help examine the subtler and more long-lasting neurodevelopmental impacts of such exposure. Here, we report prenatal exposure to haloperidol, a representative first-generation antipsychotic, or risperidone, a widely used SGA, induced memory deficits in adult mice. Also, prenatal exposure to haloperidol or risperidone disrupted the plasticity of dentate granule cells. Prenatal haloperidol exposure exerted more robust negative effects than risperidone. Thus, these current data provide important experimental evidences for the long-term programming effects of prenatal exposure to antipsychotics on brain and behavior.
Introduction
Recently, reports from different countries indicate that there is an increase in second-generation antipsychotic (SGA) use during pregnancy (Sadowski et al., 2013; Toh et al., 2013). Although cumulative data suggest that SGA is unlikely to raise the risk of major malformations, there is an extreme paucity of studies regarding the neurodevelopment safety of these compounds (Ennis and Damkier, 2015; Tosato et al., 2017). Up to now, the only 2 prospective studies found an association between prenatal SGA exposure and delayed neuromotor performance during infancy (Johnson et al., 2012; Peng et al., 2013). The latter study has also found delayed development in cognitive, social-emotional, and adaptive behavior at 2 months of age (Peng et al., 2013). A recent register study from Denmark has reported that prenatal exposure to psychotropic drugs, including SGA, is associated with cognitive impairment later in life (Wibroe et al., 2017). However, it is difficult to draw a conclusion about the long-term risk of detrimental neurodevelopmental outcome for SGA use in pregnancy based on the very limited evidence, and clinical studies with longer follow-up periods and more subtle assessment of neurodevelopmental outcomes are required.
Using animal models of prenatal exposure to SGA can help examine the subtler and more long-lasting neurodevelopmental impacts of such exposure and may shed light on the mechanisms underlying developmental dysfunctions. Relative to the limited data of SGA, slightly more evidence shows cognitive abnormalities in adult rats after prenatal exposure to first-generation antipsychotics (FGA), especially haloperidol (Archer and Fredriksson, 1992; Archer, 1993; Rosengarten and Quartermain, 2002). The possible mechanism is the alteration in development of the dopamine system in various brain areas (Scalzo and Spear, 1985; Williams et al., 1992; Zhang et al., 1996). Compared with FGAs, SGAs have a higher ratio of 5-HT2 receptor blockade than that of D2 (Meltzer et al., 1989), but it is unclear whether SGA exposure in uterus produces different neurochemical and behavioral changes from FGA. Some studies show that developmental exposure to SGAs, including risperidone, clozapine, and quetiapine but not olanzapine, also exerts long-lasting detrimental effects on cognitive function (Rosengarten and Quartermain, 2002; Singh and Tripathi, 2015; Singh et al., 2016; Singh and Singh, 2017). In contrast, a conflicting result showed prenatal risperidone-exposed rats did not show spatial memory deficits (Zuo et al., 2008). In addition to the behavioral changes, Singh et al. reported in utero exposure to risperidone and quetiapine induces fetal neurotoxicity and apoptotic neurodegeneration in neocortical region, hippocampus, and striatum (Singh and Tripathi, 2015; Singh et al., 2016; Singh and Singh, 2017). As a remarkable brain structure, hippocampal development starts from mid-embryogenesis (embryonic day 8.5 [E8.5]) until postnatal stages. Noteworthy, regional differentiation of the hippocampal formation substantially precedes that of the neocortex (Khalaf-Nazzal and Francis, 2013). However, there is no evidence for the long-term programming effects of early-life exposure to SGA on hippocampal plasticity and function.
The dentate gyrus (DG) is a hippocampal region essential for the storage of information. Noteworthy, plasticity is one of its remarkable characteristics, including neurogenesis throughout the lifespan (Angevine, 1965; Parent, 2007). Emerging evidence suggest neurotransmitters including dopamine and serotonin, which are affected by antipsychotics, have important roles in hippocampus development in addition to neurotransmission (Emerit et al., 1992; Money and Stanwood, 2013; Brummelte et al., 2017). Antipsychotics can easily cross the placental and blood-brain barriers, and lower levels of protein in fetal plasma induce a greater portion of the drug unbound, facilitating entry into the fetal brain (Seeman, 2004; Newport et al., 2007). It is therefore possible that DG may be a key and sensitive location where prenatal antipsychotics exert influence to disrupt cognition.
In this study, we aimed to investigate and compare the long-lasting programming effects of prenatal exposure to an SGA, risperidone, and an FGA, haloperidol, on hippocampal DG plasticity and related behavior.
Methods
Animals
Both adult male and female C57BL/6N mice (8 weeks old) were purchased from Vital River Laboratories (Beijing, China). After 1 week of acclimation, pairs of male and female mice (1:1) were caged together overnight for mating; pregnancy was determined by observation of vaginal plugs (E0). The pregnant mice were single-housed until delivery. All animals were maintained on a 12-h-light/-dark cycle (lights on at 8:00 am) in a temperature- and humidity-controlled room (23°C±1°C, 45%–55%) with free access to food and water. All procedures were performed in accordance with the National Institute of Health’s Guide for the Use and Care of Laboratory Animals and were approved by the Peking University Committee on Animal Care and Use.
Experimental Design and Drug Treatment
Both risperidone (Ris) and haloperidol (Hal) were obtained from Sigma-Aldrich (St. Louis, MO) and were dissolved in acetic acid and diluted with saline to the desired concentration. The pregnant dams were randomly assigned to 3 groups and were exposed to Ris (2.0 mg/kg), Hal (2.5 mg/kg), or vehicle (Veh) daily from E6 to E16, which is a sensitive and critical period for brain development (Costa et al., 2004). To better control the amount of administered drug, all substances were given i.p. in a volume of 10 mL/kg. Acclimatization was used to reduce stress during drug delivery. The experimental doses of antipsychotics were selected based on previous studies and clinically relevant doses (Singh and Singh, 2002; Singh et al., 2016). Body weights of pregnant mice were recorded daily during drug treatment. The day of birth was defined as postnatal day 0 (P0). Pups were weighed once per week and weaned on P28. For behavioral tests, both male and female offspring were examined. Only male offspring were included in western blot, immunohistochemistry, and morphology tests.
Experiment 1 examined the effects of prenatal antipsychotics on anxiety-related behavior and recognition memory on the same animals from P90 to P97. At 24 hours after behavioral tests, mice were killed for western blot or immunohistochemistry (Figure 1A). In Experiment 2, effects of prenatal antipsychotics on dendritic and spine morphology of DG granule cells were evaluated (Figure 4A). For experiment 1, 2 to 3 male offspring and 1 to 3 female offspring were selected randomly from each litter (4–6 litters in each experimental group) for behavioral observations. For experiment 2, 2 male offspring were selected randomly from each litter (4 litters in each experimental group).
Figure 1.
Long-term effects of prenatal risperidone (Ris) or haloperidol (Hal) treatment on offspring bodyweight and recognition memory. (A) The experimental timeline of prenatal antipsychotic administrations, behavioral, western blot, and immunohistochemistry procedures. (B) Hal and Ris administration during pregnancy had no significant effect on maternal body weight. (C) Weekly mean body weights (g)±SEM from birth to 91 days, n=9~15. Prenatal Hal exposure significantly stunted the body weight growth in female offspring. (D) In the spatial object recognition (SOR) test, both the Hal and Ris groups failed to discriminate the displaced object from the nondisplaced one in both genders; only male offspring performed worse than the controls. (E) In the novel object recognition (NOR) test, males in the Hal group and females in both antipsychotic treatment groups failed to discriminate the novel object from the familiar one. Moreover, male and female offspring in both the Hal and Ris groups performed significantly worse than the controls. *P<.05, **P<.01, Ris group vs vehicle (Veh) group. #P<.05, ##P<.01, Hal group vs Veh group. &P<.05, &&P<.01, &&&P<.001, for comparison between the displaced and nondisplaced objects or between the novel and familiar objects within the same group. In this and subsequent figures, the number of animals in each group is indicated in the bar groups.
Figure 4.
Effects of prenatal exposure to antipsychotics on dendritic architecture of dentate gyrus (DG) granule cells in adult mice. (A) The experimental timeline of prenatal antipsychotics administration and Golgi-Cox staining procedures. (B,C) Representative photomicrographs of Golgi-impregnated granule cells (left) and corresponding tracings reconstructed by Neurolucida (right) in the 3 groups in both blades. Scale bar=50 µm. (D,F) Prenatal haloperidol (Hal) but not risperidone (Ris) exposure significantly reduced the length of dendrites both in the suprapyramidal (DGsp) and infrapyramidal blades (DGip). (E,G) Both antipsychotic groups reduced the complexity of dendrites in both blades. *P<.05, **P<.01, Ris group vs Veh group. #P<.05, ##P<.01, Hal group vs Veh group.
Western Blot
Using Western blot, we examined the protein levels of NMDAR (GluN1, GluN2A, and GluN2B) and 2 apoptosis-related molecules (Bcl-2 and Bax). Mice were deeply anesthetized with isoflurane, and brains were rapidly removed and dissected to obtain the dorsal hippocampus (dHC) and ventral hippocampus (vHC) as previously described (Gearhart et al., 2006). Tissue was immediately homogenized on ice in ice-cold lysis buffer, sonicated, and centrifuged. The supernatants were stored at −80°C until required. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Samples containing 20 µg of protein were resolved by 10% acrylamide gels using Laemmli–SDS-PAGE (Schägger, 2006) and electro-transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). Membranes were labeled with primary antibodies overnight at 4°C. The following primary antibodies were used: GluN1 (rabbit anti-GluN1, 1:2000, ab109182, Abcam, Cambridge, UK), GluN2A (rabbit anti-GluN2A, 1:2000, 4205S, Cell Signaling Technology, Danvers, MA), GluN2B (rabbit anti-GluN2B, 1:2000, 4207S, Cell Signaling Technology), Bcl-2 (rabbit anti-Bcl-2, 1:1000, ab7973, Abcam), Bax (rabbit anti-Bax, 1:1000, ab32503, Abcam), and β-actin (mouse anti-β-actin, 1:20000, 3700S, Cell Signaling Technology). Following incubation with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse secondary antibodies (1:5000–10000, Santa Cruz Biotechnology, Dallas, TX) for 2 hours at room temperature, bands were visualized using an enhanced chemiluminescence system (Pierce) and quantified by densitometry (Quantity One 4.2, Bio-Rad, Hercules, CA). All results were normalized by taking the value of the vehicle group as 100%.
Immunohistochemistry
Immunohistochemistry was performed to quantify the density of the endogenous proliferative markers Ki-67, a nuclear protein that is expressed during all active phases of the cell cycle (Kee et al., 2002), and doublecortin (DCX), a brain-specific microtubule-associated protein and expressed in migrating neuroblasts and young neurons (von Bohlen Und Halbach, 2007). Mice were deeply anaesthetized with pentobarbital (200 mg/kg, i.p.) and perfused transcardially with cold saline solution, followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4). Extracted brains were kept in 4% paraformaldehyde overnight at 4°C and then transferred to 30% sucrose for dehydration. The brains were quenched in cold N-hexane at −60°C for 20 seconds and stored at −80°C until required.
Serial coronal sections of the hippocampus (30 µm thick) were collected on a cryostat (Leica, Wetzlar, Germany). Slices were washed in 0.1 M phosphate-buffered saline and treated with 3% hydrogen peroxide for 10 minutes. After incubation with 1% normal goat serum for 90 minutes, slices were labeled with primary antibodies overnight at 4°C: Ki-67 (rabbit anti-Ki-67, 1:1000; ab15580, Abcam), DCX (rabbit anti-DCX,1:5000; 4604S, Cell Signaling Technology) for 24 hours. All slices were rinsed and incubated with secondary goat anti-rabbit or anti-mice antibodies (Zhongshan Gold Bridge Biotechnology, China) for 90 minutes at room temperature. After washing, the slices were visualized with 3, 3’-Diaminobenzidine Horseradish Peroxidase Color Development Kit, mounted on slides, and coverslipped.
The total number of Ki-67 and DCX-positive cells was counted in the DG in 12 sections/each animal using an Olympus BX51 microscope equipped with a charge-coupled device camera (CoolSNAP MP5, Roper Scientific Corporation) and analyzed by the NIH ImageJ Software (National Institutes of Health, Bethesda, MD).
Golgi-Cox Staining and Analysis of Dendrites and Spines
Adult brains were extracted and immediately immersed in the Golgi-Cox solution (Glaser and Van der Loos, 1981) for 14 days, followed by immersion in 30% sucrose solution for 5 days in the dark at room temperature. Serial coronal sections of 120 µm thick were cut on a Microm HM 650V vibratome (Thermo Scientific, Walldorf, Germany), mounted on Superfrost plus slides (Thermo Scientific), and processed as described previously (Liu et al., 2016). Darkly and consistently stained granule neurons in the suprapyramidal blade and infrapyramidal blade of DG were selected for structural analysis (6–8 neurons per region per animal). Neurons were traced at 400× using the Neurolucida software (MicroBrightField Bioscience, Williston, VT). Total dendritic length was measured using a Sholl analysis, and the number of intersections at concentric circles (20 µm apart) was counted using the NeuroExplorer software (MicroBrightField).
Dendritic branches of DG were divided into 3 different segments according to distances from the cell body: an inner segment, middle segment, and outer segment, which receive innervations from associational-commissural path, medial perforant path, and lateral perforant path, respectively. Of note, the medial perforant path is involved in transferring spatial information from the medial entorhinal cortex and a critical site of memory processing into the hippocampus (Amaral et al., 2007). Thus, protrusions on the dendrites located in the middle segment were captured using the aforementioned charge-coupled device camera, digitized at 1000×. Dendritic spines were identified and categorized as: (1) thin spines, (2) mushroom spines, and (3) stubby spines based on established criteria (Rochefort and Konnerth, 2012). Various dendritic protrusions were counted manually using the NIH Image J software, and the data were presented as the number of protrusions per 10 µm of dendrite.
Behavioral Testing
The following tests were conducted between 9:00 am and 2:00 pm and scored by ANY-maze 4.98 (Stoelting, Wood Dale, IL).
Open Field Test
Mice were placed in the open field arena (50×50×50 cm3) made of gray polyvinyl chloride and evenly illuminated at 60 lux. During the test, mice were individually placed in the corner of the arena and allowed to freely explore the apparatus for 10 minutes. The total distance traveled and time spent in the center zone was recorded.
Elevated Plus-Maze Test
The maze was elevated 50 cm above the floor. The experimental animals were placed individually in the center of the maze facing an enclosed arm. Testing duration was 5 minutes. The time spent in the open arms was recorded.
Light-Dark Box
Mice were placed in the dark chamber (15×20×25 cm3, 10 lux), backing against the brightly illuminated chamber (30×20×25 cm3, 650 lux). During the 5-minute test, the time spent in the light chamber was recorded.
Spatial Object Recognition Task
The animals were habituated to the empty open field arenas for 10 minutes on 2 trials (with 1-hour interval) on 2 consecutive days before testing. In this test, 2 identical objects (cylinders) were put in 2 adjacent corners, 10 cm apart from the arena walls. In the acquisition trial, animals were left to explore the objects for 10 minutes, and exploration time for each of the objects was measured. One hour later, during the retrieval trial, one of the objects was displaced to a diagonal corner and mice were allowed to explore for 10 minutes again. The position of the relocated object was counterbalanced among animals. Exploration time for each object was recorded. The discrimination index (DI) was calculated as: 100%×time with the displaced object/time with both objects. Mice with a total exploring time <10 seconds in the retrieval phase were excluded from statistical analysis.
Novel Object Recognition Task
Two different metal-made objects (in duplicate, 7–8 cm high) were used: cubes and a triangular prism. On the testing day, a 10-minute acquisition trial was firstly given; animals were allowed to freely explore 2 identical objects. One hour later, one of the familiar objects was replaced by a novel object, and the animal was reintroduced into the chamber to explore the 2 objects for 10 minutes. The exploration time of each object was recorded. The DI was calculated as: 100%×time with the novel object/time with both objects. Mice with a total exploring time <10 seconds in the retrieval phase were excluded from statistical analysis.
Statistical Analysis
SPSS 17.0 (SPSS, Chicago, IL) was used to perform statistical analysis. Because the interaction between gender and prenatal treatment was rarely observed, data from male or female offspring were analyzed separately. Body weight and the number of dendritic intersections were analyzed using 1-way repeated-measures ANOVA, with treatment as the between-subject factor and day or distance from soma as the within-subject factor. The results of behavior, the number of positive cells, dendritic length, spine density, and protein levels were analyzed by 1-way ANOVA followed by Dunnett posthoc test when appropriate. Data are reported as mean±SEM. Statistical significance was defined at P<.05.
Results
Prenatal Exposure to Hal or Ris Impaired Recognition Memory in Adult Mice
Hal and Ris administration during pregnancy had no significant effect on maternal body weight (treatment, F(2, 27)=0.940, P=.403; day, F(9243)=52.236, P<.001; treatment×day interaction, F(18, 243)=1.372, P=.146; Figure 1B). Figure 1C showed the body weights of vehicle-, Hal-, and Ris-treated offspring from P1 to P91. In male offspring, there were no significant differences in body weight gain among the 3 groups (treatment, F(2, 35)=1.524, P=.232; week, F(11385)=1555.515, P<.001; treatment×week interaction, F(22, 385)=0.455, P=.883). However, in female offspring, repeated-measures ANOVA of body weight gain across development revealed significant main effects of group (F (2, 28)=5.277, P=.011) and week (F(10, 280)=1651.01, P<.001) as well as the group-week interaction (F(20, 280)=3.497, P<.001). Posthoc tests further showed that prenatal hal exposure significantly stunted the body weight growth in female offspring (P=.020).
In the spatial object recognition task, both Hal and Ris groups failed to discriminate the displaced object from the nondisplaced one in male (Veh: t9=3.736, P=.005; Ris: t9=0.616, P=.553; Hal: t6=0.884, P=.411) and female offspring (Veh: t8=3.234, P=.012; Ris: t10=0.722, P=.487; Hal: t6=0.369, P=.723; Figure 1D). Both antipsychotic treatment groups performed worse than the controls in males (DI, F(2, 24)=8.856, P=.001), but not in females (DI, F(2, 25)=0.579, P=.568; Figure 1D). The total distance traveled during the retrieval phase of 3 groups were similar in male (Veh: 25.2±2.5 m; Ris: 31.2±1.9 m; Hal:26.4±3.2 m; F(2, 24)=0.469, P=.631) and female offspring (Veh: 31.4±1.9 m; Ris: 39.3±8.2 m; Hal:26.3±2.2 m; F(2, 25)=1.288, P=.294). In the novel objection recognition test, the Hal group failed to discriminate the novel object from the familiar one in male mice (Veh: t9=4.917, P=.001; Ris: t10=4.218, P=.001; Hal: t6=0.725, P=.096), while both Hal group and Ris group failed to present a novelty preference in females (Veh: t8=7.750, P<.001; Ris: t11=1.305, P=.219; Hal: t7=1.757, P=.129; Figure 1E). Moreover, both antipsychotic treatment groups performed significantly worse than the controls in male (DI, F(2, 25)=13.526, P<.001) and female offspring (DI, F(2, 25)= 5.276, P=.012; Figure 1E). The total distance traveled during the retrieval phase of 3 groups was similar in male (Veh: 25.7±1.8 m; Ris: 28.3±2.3 m; Hal: 26.1±2.1 m; F(2, 25)=1.632, P=.217) and female mice (Veh: 28.9±2.9 m; Ris: 30.2±3.0 m; Hal: 23.5±2.0 m; F(2, 25)=1.588, P=.225).
To assess the anxiety level in prenatally antipsychotics-exposed offspring, open field, elevated-plus maze, and light dark box tests were used. The total distance traveled and center time in the open field test was similar among Hal, Ris, and control groups in both genders (Figure 2A,B). Likewise, anxiety level evaluated in the other 2 tasks was comparable among the 3 groups in male and female offpring (Figure 2C,D). Therefore, prenatal exposure to Hal or Ris did not affect anxiety-related behaviors in the adult offspring.
Figure 2.
Long-term effects of prenatal risperidone (Ris) or haloperidol (Hal) treatment on offspring anxiety-related behaviors. (A,B) In the open field test, prenatal Ris and Hal administration did not alter total distance traveled (A) and the time in the center zone (B) in offspring of both genders. (C,D) Anxiety level evaluated in the elevated plus maze (EPM) (C) and light dark box (LDB) (D) was comparable among the 3 groups in both genders. OF, open field.
Together, these data indicate prenatal exposure to Hal or Ris induces recognition memory deficits in adult offspring of both genders, and male offspring may be more sensitive. Therefore, the following investigation on hippocampal morphology, adult hippocampal neurogenesis, levels of NMDA receptors, and apoptosis-related proteins focused on the male offspring.
Prenatal Exposure to Hal or Ris Impaired Proliferation and Maturation of Adult-Born Dentate Granule Cells in Adult Male Mice
To investigate the effects of prenatal antipsychotics on adult hippocampal neurogenesis in the offspring, Ki-67 (a marker for proliferation) and DCX (a specific marker for early phases of neurogenesis) were immunostained to quantify proliferating cells and young neurons (Figure 3A,B). We found that hippocampal cell proliferation was significantly decreased in Hal and Ris groups compared with the control group (F(2, 13)=23.615, P<.001; Posthoc: Veh vs Ris, P=.004; Veh vs Hal, P<.001; Dunnett test; Figure 3C). Due to the functional distinction between the suprapyramidal blade (DGsp) and infrapyramidal blade (DGip) of the DG (Gallitano et al., 2016), the number of Ki-67+ cells was counted separately along its transverse axis. The data showed a similar significant reduction both in DGsp and DGip (DGsp: F(2, 13)=49.413, P<.001; DGip: F(2, 13)=10.536, P=.002; Figure 3D,E). As for the total number of DCX+ cells in DG, both antipsychotic groups exhibited a significant decrease (F(2, 12)=4.324, P=.039; Posthoc: Veh vs Ris, P=.028; Veh vs Hal, P=.024; Dunnett test; Figure 2F). In addition, both antipsychotic groups showed a decreasing trend in DGsp and had no effects in DGip (F (2, 12)= 2.543, P=.120; Figure 3G,H).
Figure 3.
Effects of prenatal antipsychotic exposure on adult hippocampal neurogenesis. (A,B) Representative sections show immunoreactivity of Ki-67- (A) and doublecortin (DCX)- (B) positive neurons in dentate gyrus (DG) of the 3 groups. Scale bar=200 µm (left) or 100 µm (right). (C–E) Both prenatal risperidone (Ris) and haloperidol (Hal) treatment significantly decreased hippocampal cell proliferation in suprapyramidal blade and infrapyramidal blade. (F–H) Both antipsychotic groups exhibited a significant decrease in the total number of DCX+ cells in DG. Only Hal prenatal exposure significantly decreased the number of DCX+ cells in infrapyramidal blade (DGip) after counting separately across the blades. *P<.05, **P<.01, ***P<.001, Ris group vs vehicle (Veh) group. #P<.05, ##P<.01, ###P<.001, Hal group vs Veh group. DGsp, suprapyramidal blade.
Prenatal Exposure to Hal or Ris Impaired Dendritic Complexity of DG Granule Cells in Adult Male Mice
To evaluate the long-term consequences of prenatal antipsychotics exposure on the morphology of DG granule cells in adulthood, the Golgi-Cox technique was used (Figure 4B,C). As shown in Figure 4D and F, prenatal Hal but not Ris exposure significantly reduced the length of dendrites both in the DGsp and DGip (DGsp: F(2, 20)=5.046, P=.017; Posthoc: Veh vs Ris, P=.050; Veh vs Hal, P=.006; DGip: F(2, 20)=9.160, P=.001; Posthoc: Veh vs Ris, P=.082; Veh vs Hal, P<.001; Dunnett test) compared with the controls. Sholl analysis further revealed the effects of prenatal antipsychotics on complexity of dendrites (Figure 4E,G). We found a significant main effect of treatment in both blades of DG (DGsp: F(2, 20)=5.271, P=.014; DGip: F(2, 20)=4.748, P=.021) and distance from soma (DGsp: F(8, 160)=156.926, P<.001; DGip: F(2, 140)=270.064, P<.001), but no significant interaction effect was observed (DGsp: F(16, 160)=1.359, P=.250; DGip: F(14, 140)=1.329, P=.259). In the DGsp, the effects of Ris and Hal were prominent at 80 to 120 µm and 20 to 120 µm from the soma, respectively (all P<.05; Figure 4E). In the DGip, the effects of Ris and Hal were most pronounced at 20 µm and 100 tp 120 µm as well as 100 to 120 µm from the soma, respectively (P<.05; Figure 4G). This indicates that the middle segments of dendritic branches were mostly affected by Ris and Hal.
Prenatal Exposure to Hal Showed More Robust Impairments in Dendritic Spine Plasticity of DG Granule Cells in Adult Male Mice
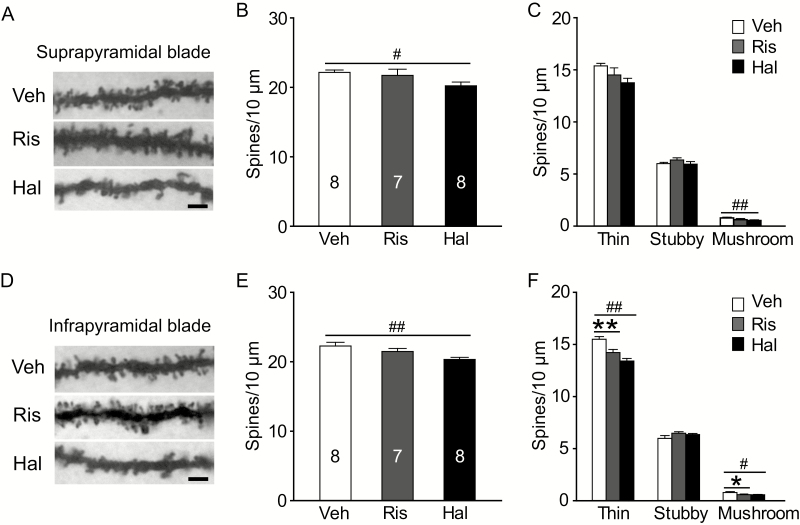
The number of protrusions on the dendrites in middle segments was further quantified (Figure 5A and D). We found that total spine density in the Hal group was significantly decreased in both blades, but not in the Ris group (DGsp: F(2, 20)=6.358, P=.007; Posthoc: Veh vs Ris, P=.3; Veh vs Hal, P=.002; DGip: F(2,20)=5.178, P=.015; Posthoc: Veh vs Ris, P=.222; Veh vs Hal, P=.004; Dunnett test; Figure 4B and E). In the DGsp, the number of mushroom spines was reduced only in Hal group (thin: F(2, 20)=3.210, P=.062; mushroom: F(2, 20)=4.437, P=.025; Posthoc: Veh vs Ris, P=.071; Veh vs Hal, P=.008; Dunnett test; Figure 5C). In the DGip, both prenatal Ris and Hal exposure significantly decreased the density of thin and mushroom spines (thin: F(2, 20)=16.619, P<.001; Posthoc: Veh vs Ris, P=.003; Veh vs Hal, P<.001; mushroom: F(2, 20)=3.463, P=.048; Posthoc: Veh vs Ris, P=.047; Veh vs Hal, P=.027; Dunnett test; Figure 5F).
Figure 5.
Long-term effects of prenatal antipsychotics exposure on spine density in middle dendritic segments of dentate gyrus (DG) granule cells. (A and D) Representative photomicrographs of dendrites segments in the middle molecular layer of both blades. Scale bar=2 µm. (B,C) In the suprapyramidal blade, prenatal haloperidol (Hal) exposure evoked a loss of thin and mushroom spines as well as the total number of spines. (E, F) In the infrapyramidal blade, both risperidone (Ris) and Hal groups induced a loss of thin and mushroom spines, while only the Hal group reduced the total number of spines. *P<.05, **P<.01, Ris group vs vehicle (Veh) group. #P<.05, ##P<.01, Hal group vs Veh group.
Prenatal Exposure to Antipsychotics Did Not Change the Levels of NMDA Receptor Subunits in Adult Male Hippocampus
Because we revealed that prenatal antipsychotics exposure had a long-lasting impact on dendrite morphology and spine density, it is likely that synaptic abnormalities were evoked. To test this possibility, we examined the expression levels of 3 NMDA receptor subunits in the dorsal and ventral hippocampus of adult offspring, considering the critical role of NMDA receptors in synaptic development and plasticity (Ewald and Cline, 2009). We found that prenatal exposure to RIS or Hal did not affect the levels of 3 NMDA receptor subunits in the adult hippocampus (F(2,16)=3.291, P=.064) (Figure 6A–F).
Figure 6.
Effects of prenatal antipsychotics exposure on the expression levels of NMDA receptor subunits and apoptosis-related molecules in the adult hippocampus. (A) GluN1 subunit in the dorsal hippocampus (dHC) and (B) GluN1 subunit in the ventral hippocampus (vHC) were similar among groups. (C) GluN2A subunit in dHC, (D) GluN2A subunit in vHC, (E) GluN2B subunit in dHC, (F) GluN2B subunit in vHC, (G) the ratio of Bax and Bcl-2 in dHC, and (H) the ratio of Bax and Bcl-2 in vHC were similar among groups. *P<.05, Hal group vs Veh group. Hal, haloperidol; Ris, risperidone; Veh, vehicle.
Prenatal Exposure to Antipsychotics Did Not Change the Levels of Apoptosis-Related Proteins in Adult Male Hippocampus
Since recent studies showed the effects of prenatal exposure to Ris or quetiapine on apoptotic neurodegeneration in the fetal hippocampus (Singh and Tripathi, 2015; Singh and Singh, 2017), we further examined the expression levels of Bax, a pro-apoptotic protein, and Bcl-2, an anti-apoptotic protein, in the hippocampus of adult offspring. The results showed that the levels of Bax and Bcl-2 as well as their ratio in both Ris and Hal groups remained unchanged (Figure 6G,H), which suggest that prenatal antipsychotics exposure did not evoke apoptosis in the adult hippocampus.
Discussion
Our main findings suggest that prenatal exposure to Hal and Ris during the sensitive period of brain development left a lasting impact on DG plasticity, accompanied with cognitive deficits in adult male mice. Reduced hippocampal neurogenesis, dendritic retraction, and spine plasticity impairment may be important factors underlying prenatal antipsychotics exposure-induced cognitive impairments. Compared with the SGA Ris, prenatal exposure with the FGA Hal exerted more robust effects in adult offspring.
In the present study, prenatal antipsychotics exposure induced recognition memory impairments in 2 tasks, including object location task and novel object preference task. Recognition memory requires judgments to distinguish novel from familiar stimuli. This ubiquitous form of memory is shared across animal species (Winters et al., 2008). Our results supported previously published data showing FGA or SGA exposure during pregnancy in rodents causes long-term cognitive deficits in other behavioral paradigms (Archer and Fredriksson, 1992; Rosengarten and Quartermain, 2002). However, another study showed that prenatal exposure to Ris in rats fails to elicit deficits in Morris water maze tasks, which might be explained by different species, drug administration paradigms, or cognitive domains measured (Zuo et al., 2008). On the other hand, we did not find an effect of prenatal exposure to Hal or Ris on any anxiety-related measures. To date, results about the long-term effects of prenatal antipsychotics treatment on anxiety-related behavior in rats are mixed from the limited reports (Singh and Singh, 2002; Zuo et al., 2008; Dobryakova et al., 2011; Singh et al., 2016). Noteworthy, only our study used 3 anxiety tests including open field, elevated plus maze, and light-dark box tests to assess emotional behaviors in mice, and the data obtained consistently showed negative effects. Furthermore, it is difficult to make a direct comparison between the current study in mice and previous studies in rats.
Several lines of evidence suggest the hippocampus has a clearly demonstrable role in object recognition memory tasks, especially if a spatial component is involved, such as spatial object recognition assessed in our study. Thus, we examined the long-term effects of prenatal antipsychotics exposure on the plasticity of the hippocampus (Warburton and Brown, 2015). Our study is the first to indicate that prenatal exposure to Ris or Hal led to a persistent impairment in adult hippocampal neurogenesis in male mice. Both the number of Ki-67-positive cells and DCX-positive cells in DG decreased in maternally Ris- or Hal-exposed adult offspring, which suggests proliferation and differentiation steps of neurogenesis were all affected. It is well known that the therapeutic effects of antipsychotics are predominantly based on action on the dopamine and serotonin receptors (Meltzer et al., 1989). On the other hand, both dopamine and serotonin and their receptors are expressed early during embryonic development and are involved in cellular proliferation, neuronal migration, neurite outgrowth, synapse formation, and differentiation of neurons (Emerit et al., 1992; Gaspar et al., 2003; Money and Stanwood, 2013). Speculatively, both Hal and Ris can easily cross the placental and blood-brain barriers, which may interfere with the dopamine and serotonin systems during the critical window of neural development (Newport et al., 2007). Thus, it may cause permanent defects in hippocampal neurogenesis. An impaired capacity for adult neurogenesis may contribute to impairments in synaptic plasticity and cognitive deficits observed in our study and other previous studies (Deng et al., 2010). Future experiments should be conducted to reveal the possible long-lasting effects of prenatal antipsychotics exposure on dopaminergic and serotoninergic pathways.
It is well documented that the hippocampus is capable of structural reorganization. This includes not only modifications in cell numbers within the DG, but also changes in dendritic morphology and spine numbers in all hippocampal subfields (Leuner and Gould, 2010). The current study revealed that prenatal exposure to Hal but not Ris decreased dendritic length of both blades. Noteworthy, both drugs used in the prenatal period inhibited dendrite branching of DG in adult offspring, with a more obvious effect in suprapyramidal blade. Interestingly, recent evidence suggests that there also exists a functional distinction between the suprapyramidal and infrapyramidal blades of the DG in addition to differences in generation time of granule cells during development, size of cell body, etc. (Amaral et al., 2007). Compared with infrapyramidal blade, suprapyramidal blade shows more robust responses during spatial tasks (Gallitano et al., 2016). In the present study, prenatally Hal-exposed mice manifested more detrimental effects in dendritic length than did mice exposed to Ris. This difference might be due to a higher ratio of placental passage in Hal (65.5%) vs Ris (49.2%) and different receptor binding profiles between the drugs (Meltzer et al., 1989; Newport et al., 2007). As discussed before, the possibly perturbed dopamine and serotonin systems induced by prenatal antipsychotics exposure might mediate the effects in dendrite shrinkage, indicating functional impairment contributing to recognition memory deficits (Hamilton et al., 2010; Trakhtenberg and Goldberg, 2012).
Dendritic spines have been thought to represent a morphological basis for synaptic plasticity (Hering and Sheng, 2001). Our results showed prenatal Hal exposure induced a significant reduction in total spine density in both blades of DG, with mushroom spines and thin spines particularly affected. Although prenatal Ris exposure did not alter total spine density, the proportion of thin spines and mushroom spines were decreased significantly in infrapyramidal blade of DG. Mushroom spines may have a more profound effect on neuronal function than other types of spines because they harbor larger postsynaptic densities (Rochefort and Konnerth, 2012). Thin spines are known as “learning spines” for the structural flexibility to enlarge and stabilizing after long-term potentiation (Bourne and Harris, 2007). The alterations in dendritic spine density and morphology of DG may represent a morphological correlate of altered hippocampus functions associated with prenatal antipsychotics exposure-induced cognitive deficits.
Dendritic spines are sites of excitatory synaptic transmission. On the other hand, NMDA receptors located at excitatory synapses are implicated in synaptic development and plasticity (Arellano et al., 2007; Ewald and Cline, 2009). NMDA receptors are formed by different GluN1-GluN2 subunit combinations. However, the current study showed that in utero exposure to Hal or Ris did not change the expression level of NMDA receptors in hippocampus. The effects on NMDA receptor function in DG by prenatal antipsychotics exposure merit further investigations. As Singh et al. reported that prenatal exposure to Ris leads to apoptotic neurodegeneration in fetal hippocampus (Singh and Singh, 2017), pro-apoptotic protein (Bax) and anti-apoptotic protein (Bcl-2) expression levels in adult offspring hippocampus were further investigated in this study. The present data did not demonstrate the enhanced state of apoptosis in hippocampus until adulthood. This suggested the pro-apoptotic effects of prenatal antipsychotics on fetal brain might not be persistent.
Several pieces of evidence suggest that prenatal exposure to Ris and Hal induces delayed body growth until the adolescent stage or early adulthood (Singh and Singh, 2002; Zuo et al., 2008). This study found prenatal Hal exposure stunted the body weight growth only in female offspring but not in male offspring. This female-selective effect on body weight is not without precedent since Bhanot et at. have described such an influence of Hal in developing rats. They have found prenatal Hal exposure delays female puberty in the offspring, which may correlate with lower body weights (Bhanot and Wilkinson, 1982). Future studies specifically designed to address the mechanism of this gender difference are warranted. In addition, during the exposure period, maternal body weight gain was not obviously influenced. However, Singh’s earlier study reveals Hal-treated dams had a decrease in body weight gain (Singh and Singh, 2002). At this stage, we cannot fully explain the inconsistent results due to paucity of similar studies.
The current study has a few limitations. First, according to previous studies and the equivalent therapeutic doses in humans, only one dosage for Ris and Hal was selected for investigation. Lower or higher dose exposure may differentially influence DG plasticity of adult offspring, which needs further research. Second, the Golgi method provides only the morphology of dendrites of granule cells and is thus unable to reveal direct synaptic function. Future studies using electrophysiological experiments could better reflect the long-term effects of prenatal antipsychotics on synaptic plasticity. Third, due to technical limitations, we only assessed the related protein expression level in dorsal and ventral hippocampus separately instead of dentate gyrus alone. Future accurate analysis in dentate gyrus is warranted. Fourth, more specific behavioral changes related to dopamine and serotonin systems were not investigated.
In conclusion, although the exact mechanisms await further investigations, our findings are the first to show both FGA Hal and SGA Ris exposure in uterus have long-term detrimental effects on hippocampal DG plasticity, thus probably resulting in an impact on DG circuit to support learning and memory. These data shed new light on the cellular and morphological basis for prenatal antipsychotics-induced cognitive deficits observed in our study and other previous studies. Although our results cannot be directly extrapolated to clinical practice, current data provide important experimental evidence for the long-term programming effects of prenatal exposure to antipsychotics on brain and behavior.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81571312, 81630031, 81571321, and 81401129), the National Key Basic Research Program of China (2015CB856400), and Beijing Brain Projects (Z171100000117016).
Statement of Interest
None.
References
- Amaral DG, Scharfman HE, Lavenex P(2007)The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 163:3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevine JB., Jr(1965)Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Exp Neurol Suppl 2:1–70. [PubMed] [Google Scholar]
- Archer T.(1993)Behavioural retardation in the neuropathology of mental retardation. APMIS Suppl 40:35–56. [PubMed] [Google Scholar]
- Archer T, Fredriksson A(1992)Functional changes implicating dopaminergic systems following perinatal treatments. Dev Pharmacol Ther 18:201–222. [PubMed] [Google Scholar]
- Arellano JI, Benavides-Piccione R, Defelipe J, Yuste R(2007)Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci 1:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot R, Wilkinson M(1982)Treatment of pregnant rats with haloperidol delays the onset of sexual maturation in female offspring. Experientia 38:137–139. [DOI] [PubMed] [Google Scholar]
- Bourne J, Harris KM(2007)Do thin spines learn to be mushroom spines that remember?Curr Opin Neurobiol 17:381–386. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander TF(2017)Developmental changes in serotonin signaling: implications for early brain function, behavior and adaptation. Neuroscience 342:212–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano C, Cestari V, Ciamei A(2001)NMDA receptors and learning and memory processes. Curr Drug Targets 2:273–283. [DOI] [PubMed] [Google Scholar]
- Costa LG, Steardo L, Cuomo V(2004)Structural effects and neurofunctional sequelae of developmental exposure to psychotherapeutic drugs: experimental and clinical aspects. Pharmacol Rev 56:103–147. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH(2010)New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory?Nat Rev Neurosci 11:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobryakova YV, Dubynin VA, Luijtelaar Gv(2011)The effect of haloperidol on maternal behavior in WAG/rij rats and its consequences in the offspring. Acta Neurobiol Exp (Wars) 71:339–347. [DOI] [PubMed] [Google Scholar]
- Emerit MB, Riad M, Hamon M(1992)Trophic effects of neurotransmitters during brain maturation. Biol Neonate 62:193–201. [DOI] [PubMed] [Google Scholar]
- Ennis ZN, Damkier P(2015)Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol 116:315–320. [DOI] [PubMed] [Google Scholar]
- Ewald RC, Cline HT(2009)NMDA receptors and brain development. In: Biology of the NMDA receptor (Van Dongen AM, ed), Chapter 1. Boca Raton, FL: CRC Press/Taylor & Francis; Available at: https://www.ncbi.nlm.nih.gov/books/NBK5287/ [PubMed] [Google Scholar]
- Fanselow MS, Dong HW(2010)Are the dorsal and ventral hippocampus functionally distinct structures?Neuron 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallitano AL, Satvat E, Gil M, Marrone DF(2016)Distinct dendritic morphology across the blades of the rodent dentate gyrus. Synapse 70:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L(2003)The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 4:1002–1012. [DOI] [PubMed] [Google Scholar]
- Gearhart DA, Middlemore ML, Terry AV(2006)ELISA methods to measure cholinergic markers and nerve growth factor receptors in cortex, hippocampus, prefrontal cortex, and basal forebrain from rat brain. J Neurosci Methods 150:159–173. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H(1981)Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity golgi-nissl stain. J Neurosci Methods 4:117–125. [DOI] [PubMed] [Google Scholar]
- Hamilton TJ, Wheatley BM, Sinclair DB, Bachmann M, Larkum ME, Colmers WF(2010)Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells. Proc Natl Acad Sci U S A 107:18185–18190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, Sheng M(2001)Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci 2:880–888. [DOI] [PubMed] [Google Scholar]
- Johnson KC, LaPrairie JL, Brennan PA, Stowe ZN, Newport DJ(2012)Prenatal antipsychotic exposure and neuromotor performance during infancy. Arch Gen Psychiatry 69:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara TS, Bostrom CA, Ratzlaff A, Thompson L, Cater RM, Gil-Mohapel J, Christie BR(2014)Deletion of the NMDA receptor glun2a subunit significantly decreases dendritic growth in maturing dentate granule neurons. Plos One 9:e103155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM(2002)The utility of ki-67 and brdu as proliferative markers of adult neurogenesis. J Neurosci Methods 115:97–105. [DOI] [PubMed] [Google Scholar]
- Khalaf-Nazzal R, Francis F(2013)Hippocampal development - old and new findings. Neuroscience 248:225–242. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E(2010)Structural plasticity and hippocampal function. Annu Rev Psychol 61:111–140, C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Yang XD, Liao XM, Xie XM, Su YA, Li JT, Wang XD, Si TM(2016)Early postnatal stress suppresses the developmental trajectory of hippocampal pyramidal neurons: the role of CRHR1. Brain Struct Funct 221:4525–4536. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC(1989)Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pki values. J Pharmacol Exp Ther 251:238–246. [PubMed] [Google Scholar]
- Money KM, Stanwood GD(2013)Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci 7:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Calamaras MR, DeVane CL, Donovan J, Beach AJ, Winn S, Knight BT, Gibson BB, Viguera AC, Owens MJ, Nemeroff CB, Stowe ZN(2007)Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. Am J Psychiatry 164:1214–1220. [DOI] [PubMed] [Google Scholar]
- Parent JM.(2007)Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res 163:529–540. [DOI] [PubMed] [Google Scholar]
- Peng M, Gao K, Ding Y, Ou J, Calabrese JR, Wu R, Zhao J(2013)Effects of prenatal exposure to atypical antipsychotics on postnatal development and growth of infants: a case-controlled, prospective study. Psychopharmacology (Berl) 228:577–584. [DOI] [PubMed] [Google Scholar]
- Rochefort NL, Konnerth A(2012)Dendritic spines: from structure to in vivo function. EMBO Rep 13:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten H, Quartermain D(2002)Effect of prenatal administration of haloperidol, risperidone, quetiapine and olanzapine on spatial learning and retention in adult rats. Pharmacol Biochem Behav 72:575–579. [DOI] [PubMed] [Google Scholar]
- Sadowski A, Todorow M, Yazdani Brojeni P, Koren G, Nulman I(2013)Pregnancy outcomes following maternal exposure to second-generation antipsychotics given with other psychotropic drugs: a cohort study. BMJ Open 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo FM, Spear LP(1985)Chronic haloperidol during development attenuates dopamine autoreceptor function in striatal and mesolimbic brain regions of young and older adult rats. Psychopharmacology (Berl) 85:271–276. [DOI] [PubMed] [Google Scholar]
- Schägger H.(2006)Tricine-SDS-PAGE. Nat Protoc 1:16–22. [DOI] [PubMed] [Google Scholar]
- Seeman MV.(2004)Gender differences in the prescribing of antipsychotic drugs. Am J Psychiatry 161:1324–1333. [DOI] [PubMed] [Google Scholar]
- Singh KP, Singh M(2002)Effect of prenatal haloperidol exposure on behavioral alterations in rats. Neurotoxicol Teratol 24:497–502. [DOI] [PubMed] [Google Scholar]
- Singh KP, Singh MK(2017)In utero exposure to atypical antipsychotic drug, risperidone: effects on fetal neurotoxicity in hippocampal region and cognitive impairment in rat offspring. Prog Neuropsychopharmacol Biol Psychiatry 75:35–44. [DOI] [PubMed] [Google Scholar]
- Singh KP, Singh MK, Singh M(2016)Effects of prenatal exposure to antipsychotic risperidone on developmental neurotoxicity, apoptotic neurodegeneration and neurobehavioral sequelae in rat offspring. Int J Dev Neurosci 52:13–23. [DOI] [PubMed] [Google Scholar]
- Singh KP, Tripathi N(2015)Prenatal exposure to a novel antipsychotic quetiapine: impact on neuro-architecture, apoptotic neurodegeneration in fetal hippocampus and cognitive impairment in young rats. Int J Dev Neurosci 42:59–67. [DOI] [PubMed] [Google Scholar]
- Toh S, Li Q, Cheetham TC, Cooper WO, Davis RL, Dublin S, Hammad TA, Li DK, Pawloski PA, Pinheiro SP, Raebel MA, Scott PE, Smith DH, Bobo WV, Lawrence JM, Dashevsky I, Haffenreffer K, Avalos LA, Andrade SE(2013)Prevalence and trends in the use of antipsychotic medications during pregnancy in the U.S., 2001-2007: a population-based study of 585,615 deliveries. Arch Womens Ment Health 16:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato S, Albert U, Tomassi S, Iasevoli F, Carmassi C, Ferrari S, Nanni MG, Nivoli A, Volpe U, Atti AR, Fiorillo A(2017)A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry 78:e477–e489. [DOI] [PubMed] [Google Scholar]
- Trakhtenberg EF, Goldberg JL(2012)The role of serotonin in axon and dendrite growth. Int Rev Neurobiol 106:105–126. [DOI] [PubMed] [Google Scholar]
- von Bohlen Und Halbach O.(2007)Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res 329:409–420. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Brown MW(2015)Neural circuitry for rat recognition memory. Behav Brain Res 285:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibroe MA, Mathiasen R, Pagsberg AK, Uldall P(2017)Risk of impaired cognition after prenatal exposure to psychotropic drugs. Acta Psychiatr Scand 136:177–187. [DOI] [PubMed] [Google Scholar]
- Williams R, Ali SF, Scalzo FM, Soliman K, Holson RR(1992)Prenatal haloperidol exposure: effects on brain weights and caudate neurotransmitter levels in rats. Brain Res Bull 29:449–458. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ(2008)Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev 32:1055–1070. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang L, Pitts DK(1996)Prenatal haloperidol reduces the number of active midbrain dopamine neurons in rat offspring. Neurotoxicol Teratol 18:49–57. [DOI] [PubMed] [Google Scholar]
- Zuo J, Liu Z, Ouyang X, Liu H, Hao Y, Xu L, Lu XH(2008)Distinct neurobehavioral consequences of prenatal exposure to sulpiride (SUL) and risperidone (RIS) in rats. Prog Neuropsychopharmacol Biol Psychiatry 32:387–397. [DOI] [PubMed] [Google Scholar]