Abstract
Aims
We assessed the potential mutual interaction of oral macitentan (cytochrome P450 (CYP) 3A4 substrate) at steady‐state with single‐dose oral rivaroxaban (CYP3A4 and P‐glycoprotein substrate) and evaluated the effect of the CYP3A and P‐glycoprotein inducer St John's wort (SJW) on the pharmacokinetics of these drugs in healthy volunteers.
Methods
Twelve healthy volunteers completed this open‐label, monocentre, two‐period, one‐sequence phase I clinical trial. The pharmacokinetics of macitentan (10 mg) was assessed on study days 3 (single dose), 15 (steady‐state), 16 (impact of rivaroxaban) and 29 (after induction by oral SJW), and of rivaroxaban on days 2 (single dose), 16 (impact of macitentan at steady‐state) and 29 (after induction by SJW). Concurrently, we quantified changes of CYP3A activity using oral microdoses of midazolam (30 μg).
Results
Rivaroxaban and macitentan did not significantly change the pharmacokinetics of each other. After induction with SJW, CYP3A activity increased by 272% and geometric mean ratios of macitentan AUC decreased by 48% and of Cmax by 45%. Concurrently, also geometric mean ratios of rivaroxaban AUC and Cmax decreased by 25%.
Conclusions
There is no evidence for a relevant pharmacokinetic interaction between macitentan and rivaroxaban suggesting that these two drugs can be combined without dose adjustment. SJW strongly increased CYP3A activity and substantially reduced rivaroxaban and macitentan exposure while estimated net endothelin antagonism only decreased by 20%, which is considered clinically irrelevant. The combination of SJW with rivaroxaban should be avoided.
Keywords: macitentan, pharmacokinetic interaction, rivaroxaban, Saint John's wort
What is Already Known about this Subject
In pulmonary arterial hypertension, the endothelin antagonist macitentan is highly effective. In these patients, comorbidities requiring treatment (anticoagulation, antidepressants) are frequent.
The interaction between macitentan and rivaroxaban has not been studied.
As an inducer of several important pharmacokinetic pathways, St John's wort (SJW) is expected to affect macitentan and rivaroxaban pharmacokinetics.
What this Study Adds
Macitentan does not alter rivaroxaban pharmacokinetics.
SJW moderately, but variably decreases rivaroxaban exposure, suggesting that this combination should be avoided.
SJW substantially decreases exposure with the CYP3A substrate macitentan but because the more prevalent active metabolite (aprocitentan) is unchanged, net endothelin‐antagonistic activity is only slightly reduced.
Introduction
Oral long‐term anticoagulation is a cornerstone of the supportive therapy of various forms of pulmonary hypertension (PH) 1, 2, even though prospective trials are still lacking and epidemiological evidence from registries on the potential impact of anticoagulation on survival is equivocal 3, 4. Whereas substantial benefits of direct oral anticoagulants (DOAC) over vitamin K antagonists (VKA) have been reported for established treatment indications such as atrial fibrillation and venous thromboembolism 5, evidence for efficacy in pulmonary hypertension is lacking. Indirect evidence suggests that a considerable fraction of patients will require dose modifications due to renal impairment, low body weight or drug interactions caused by targeted therapies 6.
Data on the interaction of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=21 antagonists with VKA indicate a substantial interaction with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3494 7, 8, 9 but no interaction with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3951 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7352 2, 9, 10, 11. In contrast, there are no data available on the potential pharmacokinetic interaction of DOACs with macitentan, a nonselective http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=21 antagonist with dose‐dependent beneficial effects in patients with pulmonary arterial hypertension 12, 13. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6388 is a possible therapeutic option in patients with pulmonary hypertension not least because of its established efficacy in venous thromboembolism 14 and nonvalvular atrial fibrillation 15 and also because the 5‐year incidence of atrial fibrillation in PH patients is considerable and often associated with clinical worsening 16. Hence, information on the interaction between rivaroxaban and macitentan would be very useful.
Another important and very frequent comorbidity in PH patients is depression 17, 18, which has substantial impact on the patients' quality of life 19 and often requires pharmacological treatment 20. As a nonprescription drug, St John's wort (SJW, Hypericum perforatum L.) is frequently used as an antidepressant and often not reported by the patients 21. However, this can be disadvantageous because of the many serious drug interactions linked to induction of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=768 (P‐gp) 22 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=242&familyType=ENZYME (CYP) such as http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1337 23, both of which are substantially involved in the pharmacokinetics of rivaroxaban 24 and macitentan 25, 26 or inhibited by macitentan in vitro 27.
We therefore conducted a clinical trial assessing the impact of macitentan on the pharmacokinetics of rivaroxaban and the impact of CYP3A4 induction by SJW on the pharmacokinetics of macitentan and its active metabolite http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10070 as well as on the single‐dose pharmacokinetics of rivaroxaban. Concurrently, we used low http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3342 doses for CYP3A phenotyping to assess the relationship between SJW‐induced CYP3A4 activity changes and pharmacokinetic changes of macitentan and rivaroxaban, and to assess the acute effect of rivaroxaban and macitentan on CYP3A activity.
Methods
Quality standards
All procedures performed in this trial involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This trial (Eudra‐CT 2016–002300‐61) was approved by the responsible Ethics Committee of the Medical Faculty of Heidelberg University (Germany) and the Federal Institute for Drugs and Medical Devices (BfArM, Bonn, Germany). The trial followed the standards of Good Clinical Practice, and the specific legal requirements in Germany. It was conducted at the Clinical Research Centre (KliPS) of the Department of Clinical Pharmacology and Pharmacoepidemiology, which is certified according to DIN EN ISO 9001: 2015. Before inclusion, written informed consent was obtained from each participant.
Population
Fourteen healthy Caucasian nonsmokers (four females) aged ≥18 years were included into the trial (age ± standard deviation, 37 ± 10 years). Two volunteers (one female) dropped out, due to concurrent viral infection and non‐compliance, respectively, leaving 12 participants who all finished the trial. They were not allowed to have clinically relevant findings in pretrial examinations (medical history, physical examination and 12‐lead electrocardiogram) and laboratory testing (haematology, blood chemistry, urinalysis with screening of illicit drugs and negative pregnancy tests for females). Furthermore, females had to agree to use reliable contraception. Volunteers were excluded if they received regular drug treatment within the last 2 months (except for oral contraceptives in female volunteers and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2635), had taken enzyme inhibiting or inducing substances in the last 2 weeks or within a period of <10 times the elimination half‐life of the respective substance (whatever was longer), or had participated in a clinical trial within the last month before inclusion. Further exclusion criteria were physical disorders that could interfere with the participant's safety during the clinical trial or study objectives (e.g. any condition that could modify absorption, distribution, metabolism or excretion of the drug regimen under investigation), regular smoking, blood donation within 6 weeks before the first trial day, excessive alcohol drinking (more than approximately 20 g alcohol per day), known or planned pregnancy or breast feeding, and any contraindication against trial medication. During the entire trial, alcohol, beverages containing grapefruit juice and smoking were not allowed. Furthermore, on trial days and 24 h before trial days, volunteers were not allowed to consume caffeinated food or beverages.
Clinical trial design
This was a phase 1, single‐centre, open‐label, two‐period, one‐sequence trial (see Figure 1), assessing the effect of macitentan (10 mg Opsumit, Actelion, Allschwil, Switzerland) before and during SJW on the pharmacokinetics of rivaroxaban (20 mg Xarelto, Bayer AG, Leverkusen, Germany). Therefore, the single‐dose pharmacokinetics of rivaroxaban was assessed alone and at macitentan steady‐state before and during combination with SJW. Concurrently, we also monitored acute and chronic effects of macitentan on CYP3A activity, using midazolam phenotyping with microdosed midazolam (30 μg Dormicum; 5 mg/5 ml solution for infusion; Roche Pharma AG, Grenzach‐Wyhlen, Germany, dissolved in 200 ml tap water) on six occasions. Using a limited sampling strategy 28, 29, midazolam pharmacokinetics were analysed after the first macitentan single dose, at macitentan steady‐state, and before and after a 12‐day combination phase with SJW (300 mg three times daily; Jarsin; Casella‐med GmbH & Co KG, Köln, Germany). All drugs were administered orally and concomitantly after an overnight fast and volunteers were served a standardized lunch not earlier than 4 h after drug administration. Compliance of the participants was monitored with a drug diary.
Figure 1.

Partial metabolic clearance of oral midazolam (1 × 30 μg) in 12 healthy volunteers alone and during oral coadministration of rivaroxaban (1 × 20 mg), macitentan (1 × 10 mg) and macitentan (10 mg day–1) with St John's Wort (3 × 300 mg day–1). +: loading with 30 mg macitentan on day 12. *P < 0.05; **P < 0.01 compared to all other values
Blood and urine sampling
Plasma samples for rivaroxaban pharmacokinetics were taken on trial days 2, 16 and 29 (predose, and for 24 h postdose). Macitentan plasma samples were drawn on trial days 3 (predose and for 120 h postdose), 15, 16, and 29 (predose and for 24 h postdose). Midazolam plasma samples were taken on trial days 1, 2, 3, 15, 16, and 29 (predose and 2 h to 4 h postdose; Figure 1). Blood was withdrawn into lithium heparin tubes, centrifuged within 20 min after collection (2835 g, 10 min, 4°C), and frozen at –20°C until analysis. Urine was collected for 24 h postdose on trial days with macitentan or rivaroxaban administration. The volume of urine was documented and samples were frozen at –20°C until analysis.
Safety assessments
Throughout the trial, adverse events (AE) were monitored by physical examinations, 12‐lead electrocardiograms and laboratory testing (haematology, blood chemistry, coagulation and urinalysis).
Bioanalysis
Quantification of plasma samples
Macitentan, its active metabolite aprocitentan (ACT‐132577), midazolam and rivaroxaban concentrations in plasma were analysed with high‐performance liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) according to previously described assays 30, 31, 32. The lower limits of quantification were 1.2 ng ml–1 for macitentan, 5 ng ml–1 for aprocitentan, 0.093 pg ml–1 for midazolam and 1 ng ml–1 for rivaroxaban. All assays were validated according to the Food and Drug Administration and European Medicines Agency guidelines for bioanalytical method validation 33, 34 and fulfilled the respective criteria for accuracy/precision, linearity, recovery, matrix effects, and stability.
Quantification of urine samples
Urine analyses of macitentan, aprocitentan and rivaroxaban were performed according to the respective plasma protocols 31, 32 with slight modifications. For the analysis of macitentan and its metabolite, urine samples were treated with and without https://www.uniprot.org/uniprot/P08236 (200 U ml–1) and incubated overnight at room temperature. After incubation, urine samples were diluted with plasma (1/8, v/v %) and further processed and analysed according to the published plasma protocol 31. For the quantification of rivaroxaban, urine samples (100 μl) were processed and analysed according to the published plasma protocol 32 without dilution. Rivaroxaban in urine samples could be precisely (overall precision ≤6.9%) and accurately (overall accuracies between 106–111%) quantified with an expanded linear calibration range of 1–2500 ng ml–1 (R2 ≥ 0.99). Also, the urine assays were validated according to the Food and Drug Administration and European Medicines Agency guidelines for bioanalytical method validation 33, 34 and fulfilled all pertinent quality criteria.
Quantification of the unbound fraction
The unbound fraction of macitentan (at steady‐state) and rivaroxaban (single dose) was determined at Cmax using rapid equilibrium dialysis (Thermo Fisher Scientific, Karlsruhe, Germany). The two chambers, separated by a dialysis membrane, were filled with plasma or Dulbecco's phosphate buffered saline (Sigma Aldrich, Taufkirchen Germany). For a complete equilibration between the unbound and bound fraction, samples were kept on an orbital shaker for 24 h at 37°C. For the subsequent LC–MS/MS analysis, equal volumes of plasma and buffer were prepared and analysed according to the published plasma assays 31, 32.
Data analysis and statistics
Pharmacokinetic parameters were calculated using standard noncompartmental equations (Kinetica 5.0; Thermo Fisher Scientific, Waltham, MA, USA). Data are given as geometric mean with 90% confidence intervals (CI) unless indicated otherwise. Peak plasma concentration (Cmax) and time to reach Cmax (tmax) were directly obtained from individual data. The area under the plasma concentration–time curve of the dosing interval (AUC0–τ), and the area under the plasma concentration–time curve from the time of dosing extrapolated to infinity (AUC0–∞) were determined using the mixed log‐linear rule. The terminal slope of the concentration–time curve λ was calculated by linear regression of the time–log‐concentration data and used to estimate the terminal half‐life of macitentan and rivaroxaban. Cl/F of macitentan (after the first and repetitive administration) and rivaroxaban (after single dose administration) was calculated as dose of macitentan or rivaroxaban divided by plasma AUC0–∞ (single dose) or macitentan dose at steady‐state divided by AUC0–τ. Renal clearance (Clren) of rivaroxaban was calculated as amount excreted unchanged into urine (AE) divided by AUC during the collection interval. Partial midazolam AUC2–4h was calculated using Prism 7.02 (GraphPad Software Inc., La Jolla, USA) and partial metabolic midazolam clearance was calculated using the previously validated equation: CLmet = 5668/ [AUC2–4h/dose (mg)] 35, 36, 37. Spearman rank correlation and linear regression were used to calculate the relationship between absolute changes of midazolam, rivaroxaban and macitentan clearances. The parametric paired t test was used to evaluate differences between two samples of independent observations using Prism 7.02. A P‐value <0.05 was considered significant.
Individual net endothelin‐antagonistic activity of macitentan and aprocitentan was calculated considering that the metabolite has only 20% of the activity of the parent compound 38:
Sample size calculation was conducted for the main study objective: non‐inferiority of rivaroxaban AUC ratios with and without macitentan administration was to be demonstrated with a paired t test (one‐sided) on log transformed AUC values against a non‐inferiority margin of 1.25. Assuming a coefficient of variation of 23% and an intrasubject correlation coefficient of 0.5 39, the sample size of n = 12 yields a power of 88% with a type I error of α = 0.025. Sample size calculation and corresponding inferential tests on the main study objective were performed in SAS 9.4 (SAS Institute, Cary, USA).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 40, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 41, 42, 43.
Results
Rivaroxaban pharmacokinetics were highly variable (Figure 2) and interindividual Cl/F differed substantially (range from 130–306 ml min–1). After 5 days of macitentan administration, Cl/F and exposure of rivaroxaban were not influenced by macitentan but combining macitentan with rivaroxaban resulted in a 25% increase in rivaroxaban Cmax (P = 0.06) and a significant shortening of 31.5% of rivaroxaban tmax compared to baseline without significant alterations of AUC or half‐life of rivaroxaban (Table 1). The GMR of the rivaroxaban AUC was 0.98 with confidence intervals excluding both the non‐inferiority margin (P = 0.001) and also the standard bioequivalence limits (P = 0.003; Table 2). SJW increased Cl/F of rivaroxaban by 33% (P < 0.05), and decreased AUC and Cmax by 25% (P < 0.05; Tables 1 and 2). Renal clearance and half‐life were unchanged (Table 1) suggesting that these changes are caused by changes in bioavailability. Individual rivaroxaban Cl/F and partial metabolic midazolam clearance before and during induction with SJW were significantly correlated explaining 22% of the observed variability of rivaroxaban Cl/F (r 2 = 0.22, P = 0.02, Figure 3).
Figure 2.

Plasma concentration‐time curve [with linear y‐axis (A) and log transformed y‐axis (B)] of rivaroxaban after administration of single, oral 20 mg doses in the presence and absence of macitentan (at steady‐state) and St John's wort (SJW; at steady‐state) in 12 healthy volunteers
Table 1.
Pharmacokinetics (geometric means; 90% CI) of macitentan, aprocitentan and rivaroxaban before and after induction with St John's wort (SJW) in 12 healthy volunteers
| Macitentan pharmacokinetics | Rivaroxaban pharmacokinetics | ||||||
|---|---|---|---|---|---|---|---|
| First dose (alone) | Steady‐state (alone) | Steady‐state + rivaroxaban | Steady‐state + rivaroxaban + SJW (steady‐state) | Alone | + Macitentan (steady‐state) | + Macitentan (steady‐state) + SJW (steady‐state) | |
| AUC τ [h ng ml –1 ] | 4180a (3751–4658) | 4397 (3785–5109) | 4259 (3799–4776) | 2198 (2024–2387)g , i | 1940a (1716–2194) | 1911a (1602–2278) | 1438a (1261–1640)g , i |
| C min [ng ml –1 ] | ‐ | 125 (103–151) | 109 (94–126) | 53.7 (47–62)i j | ‐ | ‐ | ‐ |
| C max [ng ml –1 ] | 105 (91–121) | 256 (217–301)g | 241 (211–276)g | 133 (121–145)f , i | 223 (189–262) | 279 (213–364) | 209 (164–267)h |
| Cl/F [ml min –1 ] | 40 (36–44) | 38 (33–44) | 39 (35–44) | 76 (70–82)g , i | 172 (152–194) | 175 (146–208) | 232 (203–264)g , i |
| T 1/2 [h] | 19.3 (17.9–20.9) | 22.4 (18.7–26.9) | 21.8 (16.5–28.8) | 16.3b (13.9–19.2) | 7.90 (6.68–9.28) | 6.81 (5.65–8.22) | 5.82 (4.78–7.09) |
| T max [h] | 9.31 (7.93–10.9) | 5.85 (4.44–7.18)f | 5.32 (4.36–6.48)g | 5.50 (4.49–6.74)g | 2.32 (1.78–3.02) | 1.59 (1.33–1.89)f | 2.20 (1.73–2.81)h |
| Fraction unbound [%] | ‐ | 0.16 (0.1–0.25) | ‐ | ‐ | 5.76 (5–6.64) | 5.26 (4.68–5.92) | 5.01 (3.98–6.3) |
| AUC τ (aprocitentan) [h ng ml –1 ] | 34478a , c (26 026–45 675) | 27 433 (23 975–31 391)f | 29 068 (25 236–33 482)h | 29 191 (26005–32 768) | |||
| A E [mg] | n.d. | n.d. | n.d. | n.d. | 5.37e (4.72–6.11) | 5.51e (4.76–6.39) | 3.76e , f , i (3.32–4.27) |
| Cl ren [ml min –1 ] | 53.9 (48.7–59.6) | 53.4 (46.4–61.4) | 48.2 (40.3–57.5) | ||||
| A E (aprocitentan) [ng] | 20584d , e (17 068–24 823) | 35060b (26 329–46 686) | 36 811 (30 178–44 903) | 29267b (21974–38 981) | |||
AE, amount excreted into urine; AUC, area under the plasma concentration‐time curve; Cl/F, apparent oral clearance of macitentan; Cmax, peak plasma concentration; n.d. = not detectable; SD, single dose; SS, steady‐state; t1/2, elimination half‐life; tmax, time to reach peak plasma concentration
AUC0–inf;
n = 11;
n = 5;
n = 10;
only collected for 24 h,
P < 0.05 vs. corresponding (single dose),
P < 0.01 vs. corresponding (single dose),
P < 0.05 vs. corresponding value of macitentan (steady‐state),
P < 0.01 vs. corresponding value of macitentan (steady‐state),
P < 0.01 vs. corresponding value of macitentan (steady‐state) + rivaroxaban
Table 2.
Geometric mean AUC and Cmax ratios (90% confidence interval) of rivaroxaban pharmacokinetics
| Comparisons | AUC ratios | C max ratios |
|---|---|---|
| Rivaroxaban + macitentan (steady‐state) vs. rivaroxaban alone | 0.98 (0.88–1.1) | 1.25 (1.03–1.52) |
| Rivaroxaban + macitentan (steady‐state) + SJW (steady‐state) vs. rivaroxaban alone | 0.74 (0.69–0.8) | 0.94 (0.75–1.18) |
| Rivaroxaban + macitentan (steady‐state) + SJW (steady‐state) vs. rivaroxaban + macitentan (steady‐state) | 0.75 (0.68–0.84) | 0.75 (0.55–1.02) |
AUC, area under the plasma concentration‐time curve; Cmax, peak plasma concentration; SJW, St. John's Wort
Figure 3.
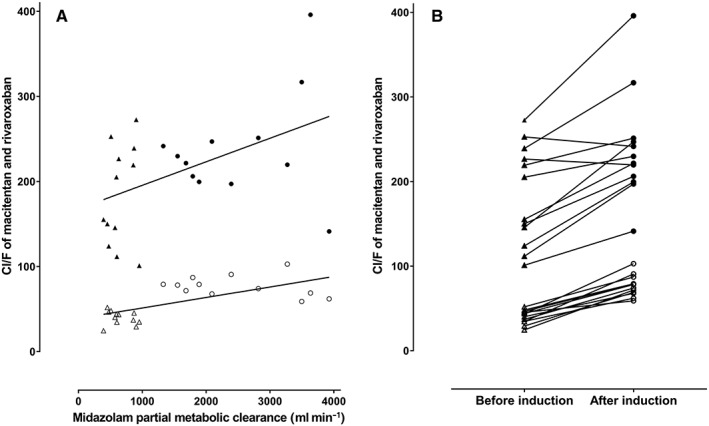
Linear regression of partial metabolic clearance of oral midazolam in 12 healthy volunteers during oral coadministration of rivaroxaban and macitentan before and after induction with St John's Wort (A) and interindividual change of oral clearance of rivaroxaban and macitentan (B). Cl/F, apparent total oral clearance; macitentan before (▵) and after (○) induction; rivaroxaban before (▴) and after (●) induction
Consistent with the absence of auto‐induction, macitentan single dose AUC values did not significantly differ from AUC values at steady‐state. At steady‐state of macitentan and during coadministration with rivaroxaban macitentan exposure was similar (Figure 4, Tables 1, 2, 3). In contrast, SJW substantially decreased the AUC of macitentan by 48% and Cmax by 45% (P < 0.05, Table 3) and macitentan Cl/F and midazolam metabolic clearance were strongly correlated (ρ = 0.66, P = 0.0005; Spearman rank) as were individual clearance changes of the drugs (r2 = 0.43, P = 0.0005, Figure 3). Steady‐state aprocitentan pharmacokinetics were similar before and during comedication of rivaroxaban and SJW (Table 1).
Figure 4.
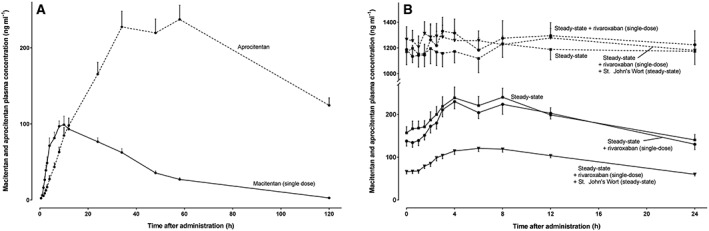
Macitentan and aprocitentan plasma concentration–time curves after the first macitentan dose (A) and at macitentan steady‐state (B) in 12 healthy volunteers
Table 3.
Geometric mean ratios (90% confidence interval) of macitentan pharmacokinetics
| Comparisons | AUC ratios | C max ratios | C min ratios |
|---|---|---|---|
| Macitentan (steady‐state) + rivaroxaban (single dose) vs. macitentan (steady‐state) | 0.97 (0.89–1.06) | 0.94 (0.84–1.06) | 0.87 (0.76–1.00) |
| Macitentan (steady‐state) + rivaroxaban (single dose) + SJW (steady‐state) vs. macitentan (steady‐state) | 0.50 (0.43–0.58) | 0.52 (0.45–0.6) | 0.43 (0.34–0.54) |
| Macitentan (steady‐state) + rivaroxaban (single dose) + SJW (steady‐state) vs. macitentan (steady‐state) + rivaroxaban (single dose) | 0.52 (0.46–0.58) | 0.55 (0.48–0.63) | 0.49 (0.41–0.59) |
AUC, area under the plasma concentration‐time curve; Cmax, peak plasma concentration; Cmin, plasma trough concentration; SJW, St. John's Wort
In agreement with the absence of pharmacokinetic changes (Figure 2B), also the predicted mean net endothelin‐antagonistic activity of macitentan and its active metabolite was unchanged by rivaroxaban (Figure 5). In contrast, after induction by SJW, the decrease of macitentan was large enough to reduce the predicted net endothelin‐antagonistic activity by factor 0.80 (95% CI: 0.78–0.82, P < 0.01) even though aprocitentan plasma concentrations were unchanged.
Figure 5.
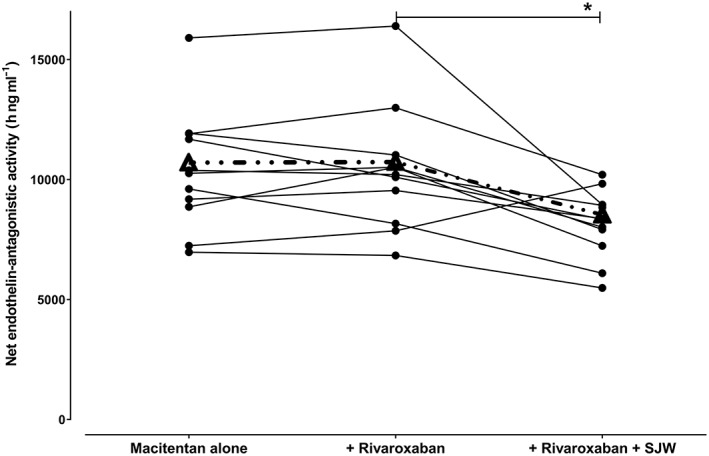
Estimated net endothelin‐antagonistic activity of macitentan and aprocitentan [net activity = AUCmacitentan + (AUCaprocitentan/5)] at macitentan steady‐state, in the presence and absence of single rivaroxaban doses and during induction with St. John's Wort in 12 healthy volunteers. Dashed line = mean curve, *P < 0.01
Macitentan transiently reduced midazolam exposure (day 3 compared to baseline; P = 0.018, Table 4), but this effect disappeared at macitentan steady‐state. SJW substantially induced CYP3A activity as shown in an increase of partial metabolic midazolam clearance by 272% (Figure 1).
Table 4.
Geometric mean ratios (90% CI) of midazolam pharmacokinetics
| Comparisons | AUC 2–4h ratios | Cl met ratios |
|---|---|---|
| Midazolam + rivaroxaban (single dose) vs. midazolam alone | 0.88 (0.76–1.02) | 1.13 (0.98–1.32) |
| Midazolam + macitentan (single dose) vs. midazolam alone | 0.80 (0.69–0.94) | 1.25 (1.07–1.46) |
| Midazolam + macitentan (steady‐state) vs. midazolam alone | 0.89 (0.78–1.02) | 1.12 (0.98–1.28) |
| Midazolam + macitentan (steady‐state) + rivaroxaban (single dose) vs. midazolam alone | 0.84 (0.73–0.98) | 1.19 (1.02–1.37) |
| Midazolam + macitentan (steady‐state) + rivaroxaban (single dose) + SJW (steady‐state) vs. midazolam alone | 0.23 (0.18–0.28) | 4.43 (3.59–5.46) |
AUC2–4h, partial area under the plasma concentration‐time curve; Clmet, partial metabolic clearance; SJW, St. John's Wort
Compliance
On study days, all drug doses were administered by trial personnel. According to the participants' diaries, almost all ambulatory drug doses were taken within ±1 h of the planned intake. Only six SJW doses in five of 12 participants were not documented in the personal drug diary with no more than two forgotten doses per participant. These mistakes were considered negligible because the selected doses were high (900 mg SJW day–1), enough time remained for induction of CYP3A, and midazolam metabolism was accelerated in all participants.
Tolerability and safety
There were no serious AEs and only four mild and transient AEs occurred, which were considered unrelated to the trial medication. One of these AEs (suspected viral infection with decrease of white blood cell count) led to discontinuation of the trial for this participant.
Discussion
Thus far, the interaction between macitentan and oral anticoagulants has only been assessed for the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1326 substrate http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6853 and no interaction was found 10. However, the targets involved in the elimination of warfarin substantially differ from those determining rivaroxaban exposure, whose fate critically depends on CYP3A and P‐gp with only minor contributions of CYP2C9 and numerous other CYP isozymes. Hence, the absence of a pharmacokinetic interaction with warfarin can neither predict nor preclude a potential interaction with rivaroxaban and the multitude of clearance pathways determining rivaroxaban exposure mandate evaluation in a dedicated trial.
Therapeutic doses of the nonselective endothelin receptor antagonist macitentan did not modify the overall exposure with rivaroxaban and the only significant effect on rivaroxaban pharmacokinetics was a shortening of tmax by approximately 44 min, which resulted in slightly higher (25%) rivaroxaban peak concentrations. After intravenous administration, the P‐gp substrate rivaroxaban is actively transported into the gut 44, indicating that a certain fraction of its clearance depends on this pathway. Because macitentan is an inhibitor of P‐gp in vitro 27, concurrent oral administration of both compounds could impair intestinal efflux of rivaroxaban into the gut lumen and thus accelerate its absorption and shorten tmax. High rivaroxaban peak concentrations have been related to bleeding events 45, but the observed pharmacokinetic trend in this study was small and thus unlikely to be of clinical relevance, suggesting that, from a pharmacokinetic point of view, these drugs can probably be combined without dose adjustment. Moreover, the supposed mechanism of interaction suggests that this effect on absorption will be likely to be mitigated if macitentan is only administered after rivaroxaban Cmax is reached (i.e. 2 h after rivaroxaban).
Single and multiple dose pharmacokinetics of macitentan revealed slightly higher exposure values than previously reported in healthy volunteers 46, which is possibly caused by the large variability of CYP3A activity in the Caucasian population and in agreement with the observed baseline CYP3A activities (geometric mean midazolam clearance: 524 ml min–1), which also tended to values below the population average when compared with other reports 47, 48. Rivaroxaban did not modify macitentan pharmacokinetics and concurrent assessment of CYP3A activity with midazolam revealed unchanged activity of this important isozyme. Similarly, also during rivaroxaban, midazolam pharmacokinetics was unchanged, thus confirming earlier reports assessing this interaction 24. However, when these drugs were combined with SJW, a potent well‐known inducer of CYP3A and P‐gp 49, exposure with macitentan was reduced to approximately one half, while the concentrations of its active metabolite were unchanged. The fact that the metabolite was unaffected by the inducer is in good agreement with the findings during induction with rifampicin 50. Because metabolite concentrations at macitentan steady‐state are several‐fold higher than the concentrations of the parent compound, the impact of the metabolite on endothelin antagonism is considerable even though its activity is only 20% of macitentan. When estimating the net endothelin antagonism, SJW reduced the activity by 20%, which is not likely to be clinically relevant 51.
SJW is known to cause substantial decreases in plasma concentrations of coadministered drugs via induction of CYP3A4, CYP2C9, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1319, and P‐gp 21, 52, 53, 54, well explaining the observed 3.72‐fold acceleration of midazolam clearance. Clearance and hence exposure of macitentan are mainly determined by CYP3A4 (99%). In contrast, numerous pathways are involved in the metabolic clearance of rivaroxaban; approximately 18% is metabolized by CYP3A, 14% by http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=262, and clearance via CYP‐independent hydrolytic cleavage accounts for approximately 14%, while 36% is eliminated unchanged into urine and 7% into the faeces 24. The efflux pump P‐gp appears to contribute to these latter clearances and our observation that renal clearance of rivaroxaban was unchanged during SJW suggests that AUC changes were not caused by accelerated (P‐gp‐mediated) elimination into urine but rather by reduced bioavailability in the course of P‐gp induction in the gut or by an increase in metabolism. Thus, persistent active rivaroxaban efflux into the gut will prolong its absorption half‐life and tmax while concurrently leading to smaller peak concentrations. This presumed mechanism of interaction is in agreement with the observation in P‐gp knock‐out mice that absorption of rivaroxaban is substantially faster without active P‐gp 42. In sum, induction of P‐gp and CYP3A by SJW leads to slower absorption of rivaroxaban and a likely increase in presystemic elimination. This translates into lower rivaroxaban Cmax, delayed tmax and a reduced bioavailability without significantly shortened elimination t1/2, and suggests that these pharmacokinetic changes are foremost caused by a modification of the absorption. The extent of the observed interaction was highly variable. For a drug such as rivaroxaban whose maintenance doses have to be adjusted in rather small dosing steps, the large variation of exposure reduction is considerable and might thus adversely affect rivaroxaban efficacy at least in some patients. To avoid potential loss of effect, the combination of SJW and rivaroxaban should be discouraged at least in patients on dose regimens requiring small dose adjustments (e.g. 25% reduction from 20 to 15 mg day–1 in atrial fibrillation patients with renal impairment), whose exposure changes are in the same order of magnitude.
Limitations
A limitation of this trial is that we have not evaluated the impact of SJW on macitentan alone but. because in this and all previous interaction trials, rivaroxaban never acted as a perpetrator drug, we believe that the presence of rivaroxaban is unlikely to have modulated the extent of the interaction between SJW and macitentan. Another limitation is that after single dose administration of macitentan urine was collected only for 24 h. While this will not affect the values for macitentan, which is not eliminated into urine, urinary aprocitentan elimination was likely underestimated. However, macitentan first‐dose pharmacokinetics was only of limited importance because it was not part of the interaction assessment.
Conclusion
In conclusion, our trial in healthy volunteers revealed no clinically relevant drug interaction between macitentan and rivaroxaban and a strong but presumably clinically irrelevant reduction of macitentan exposure when it was combined with SJW. In contrast, SJW caused a moderate and highly variable reduction of rivaroxaban that could potentially alter rivaroxaban effects and therefore should best be avoided.
Competing Interests
W.E.H. received fees from Actelion and Bayer for holding lectures not related to this trial. A.H., L.W., A.D.M., K.I.F. and J.B., have nothing to disclose. D.C. received fees from Bayer for holding lectures not related to this trial. G.M. received fees from Bayer for participation in a data safety monitoring board not related to this trial.
The authors would like to thank Marlies Stützle‐Schnetz for her valuable assistance during the trial, Marina Weißenborn and Tanja Wehran for monitoring the trial, and Andrea Deschlmayr and Magdalena Longo for excellent technical support. We also thank all subinvestigators for their excellent support (Mazyar Mahmoudi, Marie‐Louise Lehmann, Nicolas Hohmann and Antje Blank). We are grateful to Actelion Pharmaceuticals Deutschland GmbH (Freiburg, Germany), which provided macitentan tablets for this trial.
Contributors
A.H., L.W., A.D.M., D.C., G.M. and W.E.H. analysed and interpreted the data, and wrote the manuscript; W.E.H., A.H. and L.W. designed the study; L.W. and A.H. performed the trial; J.B. and K.F. developed the analytical methods and analysed the samples. All authors critically reviewed the final version of the manuscript.
Huppertz, A. , Werntz, L. , Meid, A. D. , Foerster, K. I. , Burhenne, J. , Czock, D. , Mikus, G. , and Haefeli, W. E. (2018) Rivaroxaban and macitentan can be coadministered without dose adjustment but the combination of rivaroxaban and St John's wort should be avoided. Br J Clin Pharmacol, 84: 2903–2913. 10.1111/bcp.13757.
The trial was conducted at the Clinical Research Center (KliPS) of the Department of Clinical Pharmacology and Pharmacoepidemiology.
References
- 1. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 2. Hoeper MM, Ghofrani HA, Grunig E, Klose H, Olschewski H, Rosenkranz S. Pulmonary hypertension. Dtsch Arztebl Int 2017; 114: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olsson KM, Delcroix M, Ghofrani HA, Tiede H, Huscher D, Speich R, et al Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Circulation 2014; 129: 57–65. [DOI] [PubMed] [Google Scholar]
- 4. Preston IR, Roberts KE, Miller DP, Sen GP, Selej M, Benton WW, et al Effect of warfarin treatment on survival of patients with pulmonary arterial hypertension (PAH) in the Registry to Evaluate Early and Long‐Term PAH Disease Management (REVEAL). Circulation 2015; 132: 2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Renda G, di Nicola M, De Caterina R. Net clinical benefit of non‐vitamin K antagonist oral anticoagulants versus warfarin in phase III atrial fibrillation trials. Am J Med 2015; 128: 1007–14.e2. [DOI] [PubMed] [Google Scholar]
- 6. Gabriel L, Delavenne X, Bedouch P, Khouatra C, Bouvaist H, Cordier JF, et al Risk of direct oral anticoagulant bioaccumulation in patients with pulmonary hypertension. Respiration 2016; 91: 307–315. [DOI] [PubMed] [Google Scholar]
- 7. Murphey LM, Hood EH. Bosentan and warfarin interaction. Ann Pharmacother 2003; 37: 1028–1031. [DOI] [PubMed] [Google Scholar]
- 8. Spangler ML, Saxena S. Warfarin and bosentan interaction in a patient with pulmonary hypertension secondary to bilateral pulmonary emboli. Clin Ther 2010; 32: 53–56. [DOI] [PubMed] [Google Scholar]
- 9. Weber C, Banken L, Birnboeck H, Schulz R. Effect of the endothelin‐receptor antagonist bosentan on the pharmacokinetics and pharmacodynamics of warfarin. J Clin Pharmacol 1999; 39: 847–854. [DOI] [PubMed] [Google Scholar]
- 10. Sidharta PN, Dietrich H, Dingemanse J. Investigation of the effect of macitentan on the pharmacokinetics and pharmacodynamics of warfarin in healthy male subjects. Clin Drug Investig 2014; 34: 545–552. [DOI] [PubMed] [Google Scholar]
- 11. Walker G, Mandagere A, Dufton C, Venitz J. The pharmacokinetics and pharmacodynamics of warfarin in combination with ambrisentan in healthy volunteers. Br J Clin Pharmacol 2009; 67: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehta S, Sastry BK, Souza R, Torbicki A, Ghofrani HA, Channick RN, et al Macitentan improves health‐related quality of life for patients with pulmonary arterial hypertension: results from the randomized controlled SERAPHIN trial. Chest 2017; 151: 106–118. [DOI] [PubMed] [Google Scholar]
- 13. Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA, et al Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. [DOI] [PubMed] [Google Scholar]
- 14. Bueller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366: 1287–1297. [DOI] [PubMed] [Google Scholar]
- 15. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 16. Olsson KM, Nickel NP, Tongers J, Hoeper MM. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int J Cardiol 2013; 167: 2300–2305. [DOI] [PubMed] [Google Scholar]
- 17. McCollister DH, Beutz M, McLaughlin V, Rumsfeld J, Masoudi FA, Tripputi M, et al Depressive symptoms in pulmonary arterial hypertension: prevalence and association with functional status. Psychosomatics 2010; 51: 339–339.e8. [DOI] [PubMed] [Google Scholar]
- 18. Poms AD, Turner M, Farber HW, Meltzer LA, McGoon MD. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest 2013; 144: 169–176. [DOI] [PubMed] [Google Scholar]
- 19. Harzheim D, Klose H, Pinado FP, Ehlken N, Nagel C, Fischer C, et al Anxiety and depression disorders in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Respir Res 2013; 14: 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loewe B, Grafe K, Ufer C, Kroenke K, Grunig E, Herzog W, et al Anxiety and depression in patients with pulmonary hypertension. Psychosom Med 2004; 66: 831–836. [DOI] [PubMed] [Google Scholar]
- 21. Martin‐Facklam M, Rieger K, Riedel KD, Burhenne J, Walter‐Sack I, Haefeli WE. Undeclared exposure to St. John's Wort in hospitalized patients. Br J Clin Pharmacol 2004; 58: 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwarz UI, Hanso H, Oertel R, Miehlke S, Kuhlisch E, Glaeser H, et al Induction of intestinal P‐glycoprotein by St John's wort reduces the oral bioavailability of talinolol. Clin Pharmacol Ther 2007; 81: 669–678. [DOI] [PubMed] [Google Scholar]
- 23. Haefeli WE, Carls A. Drug interactions with phytotherapeutics in oncology. Expert Opin Drug Metab Toxicol 2014; 10: 359–377. [DOI] [PubMed] [Google Scholar]
- 24. Mueck W, Kubitza D, Becka M. Co‐administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013; 76: 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sidharta PN, Krähenbühl S, Dingemanse J. Pharmacokinetic and pharmacodynamic evaluation of macitentan, a novel endothelin receptor antagonist for the treatment of pulmonary arterial hypertension. Expert Opin Drug Metab Toxicol 2015; 11: 437–449. [DOI] [PubMed] [Google Scholar]
- 26. Sidharta PN, Treiber A, Dingemanse J. Clinical pharmacokinetics and pharmacodynamics of the endothelin receptor antagonist macitentan. Clin Pharmacokinet 2015; 54: 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weiss J, Theile D, Ruppell MA, Speck T, Spalwisz A, Haefeli WE. Interaction profile of macitentan, a new non‐selective endothelin‐1 receptor antagonist, in vitro . Eur J Pharmacol 2013; 701: 168–175. [DOI] [PubMed] [Google Scholar]
- 28. Hohmann N, Kocheise F, Carls A, Burhenne J, Haefeli WE, Mikus G. Midazolam microdose to determine systemic and pre‐systemic metabolic CYP3A activity in humans. Br J Clin Pharmacol 2015; 79: 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katzenmaier S, Markert C, Riedel KD, Burhenne J, Haefeli WE, Mikus G. Determining the time course of CYP3A inhibition by potent reversible and irreversible CYP3A inhibitors using a limited sampling strategy. Clin Pharmacol Ther 2011; 90: 666–673. [DOI] [PubMed] [Google Scholar]
- 30. Burhenne J, Halama B, Maurer M, Riedel KD, Hohmann N, Mikus G, et al Quantification of femtomolar concentrations of the CYP3A substrate midazolam and its main metabolite 1′‐hydroxymidazolam in human plasma using ultra performance liquid chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem 2012; 402: 2439–2450. [DOI] [PubMed] [Google Scholar]
- 31. Enderle Y, Witt L, Wilkens H, Grunig E, Haefeli WE, Burhenne J. Simultaneous quantification of endothelin receptor antagonists and phosphodiesterase 5 inhibitors currently used in pulmonary arterial hypertension. J Pharm Biomed Anal 2017; 143: 291–298. [DOI] [PubMed] [Google Scholar]
- 32. Foerster KI, Huppertz A, Müller OJ, Rizos T, Tilemann L, Haefeli WE, et al Simultaneous quantification of direct oral anticoagulants currently used in anticoagulation therapy. J Pharm Biomed Anal 2018; 148: 238–244. [DOI] [PubMed] [Google Scholar]
- 33. Bioanalytical method validation. Food and Drug Administration; 2001. Available at http://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf (last accessed September 2016).
- 34. Guideline on bioanalytical methods validation. European Medicines Agency; 2011. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf (last accessed June 2017).
- 35. Fuchs I, Hafner‐Blumenstiel V, Markert C, Burhenne J, Weiss J, Haefeli WE, et al Effect of the CYP3A inhibitor ketoconazole on the PXR‐mediated induction of CYP3A activity. Eur J Clin Pharmacol 2013; 69: 507–513. [DOI] [PubMed] [Google Scholar]
- 36. Katzenmaier S, Markert C, Mikus G. Proposal of a new limited sampling strategy to predict CYP3A activity using a partial AUC of midazolam. Eur J Clin Pharmacol 2010; 66: 1137–1141. [DOI] [PubMed] [Google Scholar]
- 37. Ziesenitz VC, Konig SK, Mahlke NS, Skopp G, Haefeli WE, Mikus G. Pharmacokinetic interaction of intravenous fentanyl with ketoconazole. J Clin Pharmacol 2015; 55: 708–717. [DOI] [PubMed] [Google Scholar]
- 38. Xarelto® drug approval. accessed through http://www.fda.gov in June 2016: Food and Drug Administration; 2011.
- 39. Stampfuss J, Kubitza D, Becka M, Mueck W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther 2013; 51: 549–561. [DOI] [PubMed] [Google Scholar]
- 40. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, et al The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 2017; 174 (Suppl. 1): S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, et al The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 2017; 174 (Suppl. 1): S17–s129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174 (Suppl. 1): S17–s129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gnoth MJ, Buetehorn U, Muenster U, Schwarz T, Sandmann S. In vitro and in vivo P‐glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther 2011; 338: 372–380. [DOI] [PubMed] [Google Scholar]
- 45. Sakaguchi T, Osanai H, Murase Y, Ishii H, Nakashima Y, Asano H, et al Monitoring of anti‐Xa activity and factors related to bleeding events: a study in Japanese patients with nonvalvular atrial fibrillation receiving rivaroxaban. J Cardiol 2017; 70: 244–249. [DOI] [PubMed] [Google Scholar]
- 46. Sidharta PN, Lindegger N, Ulc I, Dingemanse J. Pharmacokinetics of the novel dual endothelin receptor antagonist macitentan in subjects with hepatic or renal impairment. J Clin Pharmacol 2014; 54: 291–300. [DOI] [PubMed] [Google Scholar]
- 47. Hohmann N, Kreuter R, Blank A, Weiss J, Burhenne J, Haefeli WE, et al Autoinhibitory properties of the parent but not of the N‐oxide metabolite contribute to infusion rate‐dependent voriconazole pharmacokinetics. Br J Clin Pharmacol 2017; 83: 1954–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hohmann N, Reinhard R, Schnaidt S, Witt L, Carls A, Burhenne J, et al Treatment with rilpivirine does not alter plasma concentrations of the CYP3A substrates tadalafil and midazolam in humans. J Antimicrob Chemother 2016; 71: 2241–2247. [DOI] [PubMed] [Google Scholar]
- 49. Drug interaction studies ‐ study design, data analysis, implications for dosing, and labeling recommendations. Food and Drug Administration; 2012. Available at http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4078B1_06_BioINequivalence.pdf (last accessed August 2016).
- 50. Bruderer S, Aanismaa P, Homery MC, Hausler S, Landskroner K, Sidharta PN, et al Effect of cyclosporine and rifampin on the pharmacokinetics of macitentan, a tissue‐targeting dual endothelin receptor antagonist. AAPS J 2012; 14: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Krause A, Zisowsky J, Dingemanse J. Modeling of pharmacokinetics, efficacy, and hemodynamic effects of macitentan in patients with pulmonary arterial hypertension. Pulm Pharmacol Ther 2018; 49: 140–146. [DOI] [PubMed] [Google Scholar]
- 52. Durr D, Stieger B, Kullak‐Ublick GA, Rentsch KM, Steinert HC, Meier PJ, et al St John's Wort induces intestinal P‐glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther 2000; 68: 598–604. [DOI] [PubMed] [Google Scholar]
- 53. Henderson L, Yue QY, Bergquist C, Gerden B, Arlett P. St John's wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol 2002; 54: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hennessy M, Kelleher D, Spiers JP, Barry M, Kavanagh P, Back D, et al St Johns wort increases expression of P‐glycoprotein: implications for drug interactions. Br J Clin Pharmacol 2002; 53: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


