Graphical abstract
Novel 1-hydroxy-naphthyl substituted heterocycles were synthesized and tested in vitro as antioxidant and antimicrobial agents. In silico molecular docking were performed in order to understand the binding interactions of the molecules with the receptors.
Keywords: Hydroxyacetyl, heterocycle; Naphthyl; Biological; Docking; 1DXO active site
Abstract
The versatile precursor 2-acetyl-4-allyl-1-hydroxy naphthalene was synthesized efficiently via Claisen rearrangement 2-acetyl-1-allyloxynaphthalene. The Claisen-Schmidt condensation of latter precursor afforded the corresponding chalcones which were exploited to synthesize a series of potential heterocycles such as pyrazoline, isoxazoline, benzocoumarin and benzoflavone. The synthesized products showed potent antioxidant and antimicrobial activities. Chalcone 3c, naphthyl pyrazoline 6b and hydroxycoumarin 13 exhibited the highest activity as antioxidants. Their binding mode showed specialized recognition of hydroxycoumarin 13 with the triad key amino acids at the active site of the oxidoreductase enzyme (PDB code 1DXO). 1-Hydroxynaphth-2-yl pyrazoline (6b) revealed the highest efficacy against both Gram positive and negative bacterial species. In silico molecular docking of pyrazoline 6b endorsed its proper binding at the active site of the 2EX6 enzyme which explains its potent antibacterial activity in comparison with standard ampicillin.
1. Introduction
Microbial diseases are considered as one of the most serious health problems in the world because of the increasing number of drug resistant microbes (Karad et al., 2017, Sapariya et al., 2017, Thakkar et al., 2017a, Thakkar et al., 2017b). Increasing the number of multi-drug resistant microbial strains leads to continuous demand for new drugs (Karad et al., 2017, Sapariya et al., 2017, Thakkar et al., 2017a, Thakkar et al., 2017b). The solution for this observed drug resistance of microorganisms is to develop new classes of potent antimicrobial agents possessing different action mechanisms. In addition, the human antioxidant defense system plays a crucial role to protect the physiological processes in the bodies via counteracting the harmful effects of free radicals and other oxidants. In this regard, there are various free radical induced diseases such as cardiovascular problems (Singh and Jialal, 2006) as well as alzheimer (Smith et al., 2000) and cancer (Kinnula and Crapo, 2004). As a result, the antioxidants became very important in disease prevention and therapy.
On the other hand, chalcones are important natural products as well as being prescusors for various flavonoids possessing broad pharmacological activities (Anto et al., 1995, Nakamura et al., 2002, Bhat et al., 2005). From the synthetic point of view, α,β-unsaturated carbonyl system enables chalcones and their heteroanalogs to undergo conjugative addition reactions in the presence of Lewis acids and bases affording various medicinally interesting heterocycles (Wang et al., 2003). Additionally, naphthalene is important motif in many bioactive compounds such as antimicrobial, anticancer and anti-inflammatory (Pandya et al., 2012). Moreover, various naphthalene containing drugs are widely used, such as nafacillin (A), naftifine (B), tolnaftate (C), terbinafine (D), etc (Fig. 1). which play crucial role in the treatement of different microbial infections (Wilson et al., 2004).
Fig. 1.
Naphthyl substituted antimicrobial drugs.
In view of the preceding facts and in continuation of our research program (Abdel-Rahman et al., 2005, Al-Omary et al., 2010, El-Desoky et al., 2013a, El-Desoky et al., 2013b, El-Desoky et al., 2014, Abdel-Aziz et al., 2016), we disclose our findings in the design new types of hybrid motifs that combine 1-hydroxy-naphthyl moiety and different biologically active heterocyclic pharmacophores with the aim to produce promising antioxidant and antimicrobial agents.
2. Results and discussion
2.1. Chemistry
2-Acetyl-1-allyloxynaphthalene (1) underwent Claisen rearrangement yielding 2-acetyl-4-allyl-1-hydroxynaphthalene (2) in good yield (Scheme 1). Claisen-Schmidt condensation of 5-allyl-2-hydroxy-3,4-benzoacetophenone (2) with different aromatic aldehydes namely; benzaldehyde, 4-methoxybenzaldehyde and 3-hydroxy-4-methoxybenzaldehyde (vanillin) yielded the corresponding 4-allyl-1-hydroxy-naphthyl substituted chalcones 3a-c (Scheme 1).
Scheme 1.
After that, the cinnamoyl naphthalene 3a,b were allowed to react with hydrazine hydrate or phenyl hydrazine in ethanol to give only the corresponding pyrazoline derivatives 6a-d via 1,2-addition (Aziz et al., 1976) followed by dehydration to give the corresponding hydrazones and then pyrazolines 6a-d via Michael addition. The reduction of the allyl group in compounds 6a,b is similar to the reduction of propen-2-yl group previously reported by our group (El-Desoky et al., 2014). It is notable that the strong heating the naphthyl pyrazoline derivative 6b afforded the corresponding pyrazole 7 via dehydrogenation (Scheme 2). Additionally, chalcone 3a,b reacted with hydroxylamine hydrochloride to yield the corresponding phenylisoxazolines 8a,b in very good yields (Scheme 2).
Scheme 2.
Yahiaoui et al., designed a potent aromatase inhibitors via introducing of a phenyl fragment at position C-7 and C-8 of the flavanone skeleton (Yahiaoui et al., 2008). These flavanone based aromatase inhibitors were found to be more effective than aminoglutethimide (Cytadren®) which is an antisteroid drug and used clinically in the treatment of Cushing's syndrome and metastatic breast cancer (Gross et al., 2007). Therefore, we constructed allylbenzoflavones 9a,b via cyclization of 2-hydroxybenzochalcones 3a,b by refluxing in basic medium such as triethylamine. The 1H NMR of compounds 9a,b showed three signals in the aliphatic region corresponding to the heterocyclic ring protons H-2, H-3eq and H-3ax. In the case of flavanone 9a, the protons H-3ax and H-3eq are approximately coupled with 16.8 Hz corresponding to geminal coupling constant. The coupling constant (J2,3ax) equal to 13.5 Hz due to the trans-diaxial coupling leads to a conclusion that H-2 is axial and the phenyl group is equatorial. Finally, the coupling constant between H-2ax and H-3eq is about 3.3 Hz which confirms the conformation of pyranone heterocycle (Yahiaoui et al., 2008).
As an attempt to construct pyrimidine or thiopyrimidine derivatives 10a-d, The reaction of compounds 3a,b with urea or thiourea in presence a catalytic amount of triethylamine afforded the same previously prepared 7,8-benzoflavanone derivatives. 9a,b in good yields. Additionally, flavones 11a,b were prepared in good yields via treatment cinnamoyl naphthalene 3a,b with iodine in DMSO (Scheme 3).
Scheme 3.
Claisen condensation of 5-allyl-2-hydroxy-3,4-benzoacetophenone (2) with diethyl carbonate afforded the open claisen product ethyl-1-hydroxy-4-allyl-2-naphthoylacetate (12) as a major product (Scheme 4). In addition, 6-allyl-4-hydroxy benzo[h]coumarin (13) was detected as a very minor product by GC/MS. Moreover, refluxing of compound 12 with hydrazine hydrate gave hydroxycoumarin 13 instead of the expected 3-(4-allyl-1-hydroxynaphth-2-yl)-1H-pyrazol-5-(4H)-one (14).
Scheme 4.
2.2. Biological evaluation
2.2.1. Antioxidant activity
The antioxidant activity of the synthesized compounds was evaluated using ABTS free radical scavenging assay (El-Gazzar et al., 2009). The results of antioxidant screening were depicted in Table 1.
Table 1.
Antioxidant activity of by ABTS scavenging assay.
| Comp. | Aa | Inhibition (%) | Comp. | Aa | Inhibition (%) |
|---|---|---|---|---|---|
| 2 | 0.186 | 67.65 | 8b | 0.557 | 3.13 |
| 3a | 0.333 | 42.08 | 9a | 0.488 | 15.13 |
| 3b | 0.401 | 30.26 | 9b | 0.568 | 1.21 |
| 3c | 0.143 | 75.13 | 11b | 0.520 | 9.56 |
| 6b | 0.147 | 74.43 | 13 | 0.084 | 85.39 |
| 8a | 0.557 | 3.13 | LAAb | 0.067 | 88.34 |
A: Absorbance of the sample.
LAA: L-Ascorbic acid.
As seen from Table 1, compounds 3c, 6b and 13 exhibited the highest activity however compounds 2, 3a and 3b showed a moderate antioxidant activity.
2.2.2. Antimicrobial activity
Eleven compounds were evaluated for their in vitro antimicrobial activities against Staphylococcus aureus (Gram-positive bacteria) and Escherichia coli (Gram-negative bacteria). They were also evaluated for their antifungal activities against Candida albicans (Table 2). Agar-diffusion method was used for the preliminary screening of the antibacterial and antifungal activities. Ampicillin and coltrimazole were used as reference drugs. The results were recorded for each tested compound as the average diameter of inhibition zones (D) of microbial growth around the disks in mm and were attributed to the original tested concentration (1 mg/mL) as a preliminary test. The minimum inhibitory concentration (MIC) measurements were determined using twofold serial dilution method.
Table 2.
Antimicrobial activity assay and minimal inhibitory concentrations (MIC, µg/ml, between brackets).
| Comp. |
S. aureus (µg/ml) |
E. coli (µg/ml) |
C. albicans (µg/ml) |
|||
|---|---|---|---|---|---|---|
| Da (mm) | AIb (%) | D (mm) | AIb (%) | D (mm) | AIb (%) | |
| 2 | 16 (187.5) | 64 | 23 (125) | 85.5 | 14 (250) | 46.7 |
| 3a | NAc (–) | NAc | 26 (15.6) | 96.3 | 15 (250) | 50 |
| 3b | 26 (15.6) | 104 | 22 (187.5) | 81.5 | 22 (125) | 73.3 |
| 3c | 20 (125) | 80 | 27 (93.7) | 100 | 17 (250) | 65.7 |
| 6b | 30 (3.9) | 120 | 31 (15.6) | 114.8 | NAc (–) | NAc |
| 8a | 22 (93.7) | 88 | 30 (15.6) | 111.1 | 21 (187.5) | 70 |
| 8b | NAc (–) | NAc | NAc (–) | NAc | NAc (–) | NAc |
| 9a | NAc (–) | NAc | 28 (15.6) | 103.7 | 13 (250) | 43.3 |
| 9b | NAc (–) | NAc | NAc (–) | NAc | 15 (250) | 50 |
| 11b | NAc (–) | NAc | 29 (7.8) | 107.4 | NAc (–) | NAc |
| 13 | 13 (250) | 52 | 20 (125) | 74.1 | 16 (250) | 53.3 |
| Ampicillin | 25 (187.5) | 100 | 27 (125) | 100 | NAc (–) | NAc |
| Clotrimazole | NAc (–) | NAc | NAc (–) | NAc | 30 (5.8) | 100 |
D: Diameter of inhibition zone
AI: Activity index.
NA: No activity.
From Table 2, 4-allyl-1-hydroxynaphth-2-yl pyrazoline (6b) revealed highest efficacy against both bacterial species and this is compatible with the well known biological profile of pyrazoline scaffold (Yusuf and Jain, 2014). As well as, the same compound 6b exhibited the lowest minimal inhibitory concentration (3.9 µg/ml) compared to the reference antibiotic ampicillin (125 or 187.5 µg/ml) against both Gram positive and Gram negative bacteria. However chalcone 3b showed almost similar antibacterial activity (S. aureus) compared to the reference drug, chalcone 3c showed less activity with smaller MIC (125 µg/ml). Although chalocne 3a has no activity against S. aureus or similar activity to ampicillin in case of E. coli, the introduction of isooxazoline pharmacophore improved the activity and the MIC as shown in 8a (Mandawad et al., 2014). By comparing the activities of chalcones 3a-c against E. coli, similar activities were observed for 3a and 3c, but chalcone 3b gave the lowest activity. The structure modification of 3b to the corresponding flavonone 9b destroyed the activity but the further the modification to flavone 11b strongly improved the activity beyond the reference antibiotic. Additionally, flavone 11b showed the lowest MIC against E. coli. As shown in both bacterial species, courmarin pharmacophore 13 showed the lowest antibacterial activity.
The synthesized compounds didn't show excellent activities against the fungal species C. albicans. p-Anisyl chalcone 3b showed the highest antifungal activity among the synthesized compounds. Also, chalocne 3c, isoxazoline 8a revealed good activity compared to the reference drug clotrimazole. In contrast, compound 6b has no antifungal activity at all (C. albicans).
2.3. In Silico docking studies
Molecular docking provides a powerful tool in understanding the degree of recognition between the tested compounds and the amino acids of the enzyme active site.
2.3.1. Antioxidant activity
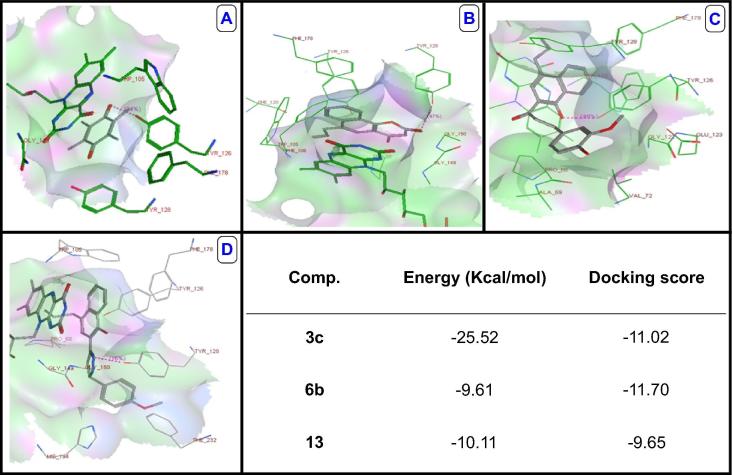
NQO1; NAD(P)H dehydrogenase (quinone) family is FAD-binding protein forms homodimers that reduces quinones to hydroquinones. This protein's enzymatic activity prevents quinonoid compounds from generating reactive oxygen species (ROS); performing its antioxidant activity (Siegel et al., 1997, Faig et al., 2000, Bian et al., 2015). In sillico docking study was performed based on the crystal structure of NQO1 (PDB code 1DXO) (Fig. 2A). The benzocoumarin ring of compound 13 laid in one plane parallel to FAD isoalloxazine ring performing high degree of π-stacking interactions in the same manner as the parent duroquinone. Also, the three oxygen atoms of the 4-hydroxycoumarin fragment performed proper recognition with the triad key amino acids; Tyr126, Tyr128 and Phe178. Additionally, there is H-bond interaction with Tyr-128 resulting in promising antioxidant activity that in agreement with the ABTS antioxidant assay (Fig. 2B).
Fig. 2.
(A) 3D crystal structure of duroquinone in the binding pocket of 1DXO. (B) Binding mode for compound 13 docked and minimized in the 1DXO binding pocket, showing the H-bond with residue Tyr128 with conserved amino acid residues and the cofactor FAD involved in its recognition. (C) Binding mode for compound 3c docked and minimized in the 1DXO binding pocket, showing the H-bond with residue Tyr126 with conserved amino acid residues and the cofactor FAD involved in its recognition. (D) Binding mode for compound 6b docked and minimized in the 1DXO binding pocket, showing the H-bond with residue Tyr128 with conserved amino acid residues.
However; compound 3c showed moderate antioxidant activity relative to compound 13 that may refer to recognition with only one of the triad key amino acid residues; Tyr126 via H-bond interaction. The α,β-unsaturated keto bridge oriented at the lowest energy configuration (trans isomer) where the carbonyl oxygen oriented away from neighbor amino acids. As a result of the trans configuration of the double bond, the 4-hydroxy and 3-methoxy phenyl ring protruded away from the conserved amino acids of the active site leading to dropping the chance of any H-bond formation (Fig. 2C). However; compound 6b showed less antioxidant activity relative to compound 13 that may refer to recognition with only one of the triad key amino acid residues (Tyr128) via H-bonding with N-pyrazole. The p-methoxy phenyl ring oriented upright to the surface of the naphthyl ring avoiding the clash with Phe232 (Fig. 2D).
2.3.2. Antimicrobial activity
Virtual screening was performed against penicillin-binding protein (PDB code: 2EX6) (Zervosen et al., 2012) which are implicated in maturation of bacterial cell wall and formation of cell shape. The crystallographic structure of 2EX6 has ampicillin as the docked ligand at the binding pocket that showed proper recognition with the conserved amino acid residues (Fig. 3A). The molecular docking study of all the compounds was performed to unveil the correlation between their antibacterial effect and their complementarily pattern at the active site. Compound 6b showed H-bond formation with conserved triad residues; Ser62, Ser306 and Ser398 (Fig. 3B).
Fig. 3.
(A) The binding mode and residues involved in the recognition of ampicillin docked and geometrically optimized in the 2EX6 binding pocket. (B) The binding mode and residues involved in the recognition of compounds 6b and 8a docked and geometrically optimized in the 2EX6 binding pocket.
2.4. Structure activity relationship (SAR)
The molecular docking studies of the newly synthesized compounds come in agreement with the corresponding experimental antibacterial results. It was found that α-naphthol fragment is the main core carrying two essential arms; namely n-propyl chain and the pyrazoline ring that carried 3-methoxy phenyl motif. Compound 6b is the most active lead that exhibited antibacterial effect greater than the standard reference drug (Ampicillin). By considering the structural differences between pyrazoline 6b and isoxazoline 8a, the replacement of pyrazoline heterocycle by isoxazoline, the presence of allyl substituent in addition to the removal of the p-methoxy group reduce both of the docking and the antibacterial activities. However 8a remains to express proper activity over the ampicillin itself due to the main structural elements represented by (i) α-naphthol ring (ii) five membered ring as a separator between the core naphthyl core and the terminal phenyl group. However the replacement of five membered ring with the six pyranone ring, compounds 9a and 11b revealed proper antibacterial activity and this can be interpreted via the presence of the main structural component; central naphthyl core with both terminals (cf. 6b & 8a). The fused benzocoumarin ring is rigid enough to reduce conformational stability of the entire compound 13 that went clear in the docking study where the rigidity of the three fused ring weakened the recognition with the amino acid residues of the active site. Chalcone 3c contains the main structural elements represented by (i) α-naphthol ring (ii) terminal methoxy phenyl ring. However the three atoms of α,β-unsaturated carbonyl spacer mimics the three atoms of the pyrazoline ring (cf. 6b) that allow the compound laid in the active site. But due to the stable trans configuration of the double bond, it was found that the ring C directed in the other plan away from the conserved amino acid residues of the active site that may explain the difference in the antibacterial efficacy of compound 3c relative to the superior antibacterial lead 6b.
3. Conclusions
The present study reports a convenient protocol for the design and synthesis of new heterocyclic compounds incorporating 1-hydroxy-naphthyl fragment. The versatile precursor 2-acetyl-4-allyl-1-hydroxy naphthalene was subjected to Claisen-Schmidt condensation to give the corresponding chalcones 3. The reactivity of chalcones 3 allowed the construction of diverse heterocyclic systems such as pyrazoline, isoxazoline, benzoflavone and benzocoumarin. The structures of the newly synthesized compounds were elucidated via different spectral and chemicals analyses. Compounds 3c and 6b and 13 are considered as promising leads with dual biological activity as antibacterial and antioxidant. Compounds 13 have potent antioxidant effect that is promising to be potential lead in treatment of cancer. Compounds 8a, 9a, 11b are outstanding in their antibacterial over-expression activity relative to the well-known antibiotic antibacterial drug; Ampicillin. The isoalloxazine ring of the cofactor FAD is essential to the recognition with the compound that has proper antioxidant activity. Benzocoumarin ring is essential for the recognition with the amino acid residues. Allyl chain improves the π stacking interaction while the n-propyl chain attenuates the overall interaction. Naphthyl ring needs to be substituted with short bridge to avid the ring extended away from the pocket.
4. Experimental section
4.1. General
All melting points are uncorrected and were recorded on an open glass capillaries using a Gallenkamp apparatus. The IR spectra (ν cm−1) (KBr) were recorded on Perkin Elmer Infrared Spectrophotometer Model 157. The 1H NMR spectra were run on Bruker AC 300 MHz Spectrophotometer or JOEL 500 MHz Spectrophotometer using TMS as an internal reference. CDCl3 or DMSO-d6 were used as solvents and chemical shift (δ) values are recorded in ppm. Mass spectra (MS) were determined at 70 eV on a kratos MS or a Varian MAT 311 a spectrometers. Elemental analysis (C, H and N) were carried out at the microanalytical center at Cairo University, Egypt. Follow-up of the reactions was made by thin layer chromatography (TLC) on Silica gel precoated aluminum sheets (type 60 F254, Merck, Darmstadt, Germany) and the spots were detected by exposure to UV lamp at λ254 nm. Synthesis of compounds 2, 3, 6, 7, 8, 9, 11 were previously reported by our group as shown in the supporting information (El-Desoky et al., 2013a, El-Desoky et al., 2013b). Antioxidant and antimicrobial screening were carried out at faculty of pharmacy, Mansoura University, Mansoura, Egypt. The molecular modeling study was conducted with Hyperchem: Molecular Modeling System Hypercube, Inc. Release 6.03, Florida, USA, 1997. The docking was carried out using Maestro Version 10.1.013, Release 2015-1.
4.2. Synthesis
4.2.1. Ethyl-1-hydroxy-4-allyl-2-naphthoyl acetate (12)
A solution of acetyl naphthol 2 (2.26 g, 10 mmol) in diethylcarbonate (10 ml) was gradually added to a suspension of powdered sodium metal (2 g) [prepared via reflux in dry xylene followed by vigorous shaking under N2] in dry diethyl ether (20 ml). A violent reaction took place which was slowed down by external cooling. The mixture was stirred overnight at room temperature, digested with ethanol and treated with cold water. The reaction mixture was treated with diluted hydrochloric acid and then extracted with ether (3 × 50). The combined organic fractions were dried over anhydrous Na2SO4 then the solvent was evaporated under vacuum. The remaining residue was purified using column chromatography (pet. ether/EtOAc = 7:3) to give the pure 12. Yield (2.24 g, 75%); pale yellow crystals; m.p. 124–126 °C; IR (KBr): ν/cm−1 = 3600–3300 (br., OH), 1742 (CO, ester), 1636 (CO, ketone), 1609,1563,1509 (C C); 1H NMR (CDCl3, 300 MHz) δ (ppm): 1.48 (t, 3H, CH2-CH3, J = 7.6 Hz and J = 6.9 Hz), 3.83 (d, 2H, CH2CH CH2, J = 6.1 Hz), 4.52 (q, 2H, CH2CH3, J = 6.9, 7.6 Hz), 5.11 (dd, 1H, CH2CH CHb, J = 17.6 Hz), 5.17 (dd, 1H, CH2CH CHa, J = 9.95 Hz), 6.11 (m, 1H, CH2CH CH2), 7.68 (d, 1H, 6-H, J = 7.65 Hz), 7.73 (d, 1H, 7-H, J = 7.65 Hz), 7.76 (s, 1H, H-3), 8.02(d, 1H, 5-H, J = 8.4 Hz), 8.61 (d, 1H, 8-H, J = 8.4 Hz), 14.81(s, br., 1H, OH); MS m/z (%) = 297 [M+−1] (0.21), 278 (35.44), 252 (2.8), 210 (78.84), 181 (33.11), 164 (41.98), 152 (100.00), 139 (22.02), 126 (37.54), 101 (14.76), 76 (28.75); Anal. Calcd. for C18H18O4 (298.34): C, 72.47; H, 6.08%. Found: C, 72.54; H, 6.16%.
4.2.2. 6-Allyl-4-hydroxy-2H-benzo[h]coumarin (13)
Compound 12 and hydrazine hydrate was reacted under the same reaction conditions which described for the synthesis of pyrazolines 6a-d. Yield (2.34 g, 93%); faint yellow crystals; m.p. 260–262 °C; IR (KBr): ν/cm−1 = 1680, 1630 (C O, cyclic lactone), 1598, 1545 (C C, Ar); 1H NMR (CDCl3, 300 MHz) δ (ppm): 3.81 (d, 2H, CH2CH CH2, J = 6.3 Hz), 5.09 (d, 1H, CH2CH CHb), 5.12 (d, 1H, CH2-CH CHa), 6.09 (m, 1H, CH2CH CH2), 6.60 (s, br., 1H, OH, exchangeable with D2O), 7.63 (dd, 1H, H-8), 7.67 (dd, 1H, H-9), 7.80 (s, 1H,H-3), 8.06 (d, 1H, H-7, J = 8.1 Hz), 8.35 (dd, 1H, H-10, J = 8.1 Hz); MS m/z (%) = 252[M+] (2.8), 152 (100.00), 226 (1.69), 210 (78.84), 181 (33.11), 165 (41.98), 139 (22.02), 126 (37.54), 115 (19.27), 101 (14.76), 89 (8.31), 76 (28.75), 69 (38.24); Anal. Calcd. for C16H12O3 (252.26): C, 76.18; H, 4.79%. Found: C, 76.35; H, 4.62%.
4.3. Biological evaluation
4.3.1. Antioxidant activity (ABTS assay)
A solution of 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) [2 ml, 60 µM] was added to MnO2 solution [3 ml, 25 mg/ml], all prepared in [5 ml] aqueous phosphate buffer solution (PH. 7, 0.1 M). The mixture was shaken, centrifuged and filtered. The absorbance (Acontrol) of the resulting green-blue solution (ABTS radical solution) at 734 nm was adjusted to approximately ca. 0.5. The absorbance was measured upon the addition of solution of the tested compound [50 µl of 2 mM] in spectroscopic grade MeOH/phosphate buffer (1:1). The inhibition ratio (%) was calculated using the following equation:
L-ascorbic was used as standard antioxidant (positive control). Blank sample was run without ABTS and using MeOH/phosphate buffer (1:1) instead of tested compound. Negative control was run with ABTS and MeOH/phosphate buffer (1:1) only (El-Gazzar et al., 2009).
4.3.2. Antimicrobial activity
The tested compounds were dissolved in DMSO and solutions of the concentration 1 mg/ml were prepared separately. Paper discs of Whatman filter paper were prepared with standard size (5 cm), were cut and sterilized in autoclave. The paper discs were soaked in the solutions of the tested compounds. After that, the soaked discs were placed aseptically in petri dishes containing nutrient agar media [Agar (20 g) + (Beef extract (3 g) + Peptone (5 g)] seeded with Staphylococcus aureus, Esherichia coli and Candida albicans The petri dishes were incubated at 36 °C and the inhibition zones were recorded after 24 h of the incubation. Each treatment was replicated three times. The activity of the antibiotic ampicillin and the antifungal coltrimazole was recorded as reference drugs under the same conditions and concentrations. The% activity index was calculated using the following equation:
The minimal inhibition concentration (MIC) was determined using the disc diffusion technique by preparing discs containing 1.9–1000 µg/ml of each compound against the selected microorganisms. The twofold dilutions of the solution were prepared. The microorganism suspensions at 10 CFU/ml (colony forming unit/ml) concentrations were inoculated to the corresponding wells. The plates were incubated at 36 °C for 24 h. At the end of the incubation period, the minimal inhibitory concentrations (MIC) values were recorded as the lowest concentration of the substance that had no visible turbidity. Control experiments with DMSO and uninoculated media were run parallel to the test compounds under the same conditions.
4.4. In silico docking studies
4.4.1. Antioxidant activity
Molecular modeling studies of the new analogs in complex with 1DXO have been done to delineate features that differentiate their mode of interaction. Starting coordinate of the X-ray crystal structure of the IDXO pdb enzyme in complex is obtained from the RCSB Protein Data Bank of Brookhaven National Laboratory (Faig et al., 2000). The energy minimization was carried out using the molecular mechanics force field ''AMBER''. The energy-minimized structure was used for molecular dynamics studies. The new analogs were constructed from fragment libraries in the Hyperchem program. The partial atomic charges for each analog were assigned with the semiemperical mechanical calculation method ''AM1'' implemented in Hyperchem 6.03. Conformational search was performed around all the rotatable bonds with an increment of 10° using conformational search module as implemented in HyperChem 6.03. All the conformers were minimized until the RMS deviation was 0.01 Kcal/mol Å. For each of the chosen analogs, energy minimizations (EM) were performed using 1000 steps of steepest descent, followed by conjugate gradient minimization to a RMS energy gradient of 0.01 Kcal/mol Å. The docking was carried out on using a flexible fitting module in Maestro. Each inhibitor was geometrically optimized in the enzyme-binding pocket.
4.4.2. Antimicrobial activity
As the synthesized compounds showed promising in vitro antimicrobial activity, they were virtually docked into the binding pocket of penicillin binding protein from Escherichia coli. The docking was carried out by using Maestro Software (V 10.1.013). The target protein (PDB No: 2EX6) was obtained from Protein Data Bank and further optimized and minimized to obtain a low energy and structural correct target protein. Target grid was generated using ampicillin as the reference ligand. The synthesized compounds were virtually prepared and highly optimized with Hyperchem. The lowest energy conformers were docked against the prepared target grid with extra precision mode. This provides lowest energy ligands, docked into the target pocket with best possible pose. The compounds are quantified using the docking score that used to predict the binding affinity and ranking of the ligands.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jsps.2018.03.013.
Appendix A. Supplementary material
References
- Abdel-Aziz A.A.-M., Abou-Zeid L.A., ElTahir K.E.H., Ayyad R.R., El-Sayed M.A.-A., El-Azab A.S. Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4 (3H)-quinazolinones. Europ. J. Med. Chem. 2016;121:410–421. doi: 10.1016/j.ejmech.2016.05.066. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman A.H., Hammouda M.A.A., El-Desoky S.I. Synthesis of some new azole, azepine, pyridine, and pyrimidine derivatives using 6-hydroxy-4H-4-oxo[1]-benzopyran-3-carboxaldehyde as a versatile starting material. Heteroat. Chem. 2005;16:20–27. [Google Scholar]
- Al-Omary F.A.M., Abou-zeid L.A., Nagi M.N., Habib S.E., Abdel-Aziz A.A.-M., El-Azab A.S., Abdel-Hamid S.G., Al-Omar M.A., Al-Obaid A.M., El-Subbagh H.I. Non-classical antifolates. Part 2: Synthesis, biological evaluation, and molecular modeling study of some new 2, 6-substituted-quinazolin-4-ones. Bioorg. Med. Chem. 2010;18:2849–2863. doi: 10.1016/j.bmc.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Anto R.J., Sukumaran K., Kuttan G., Rao M.N.A., Subbaraju V., Kuttan R. Anticancer and antioxidant activity of synthetic chalcones and related compounds. Cancer Lett. 1995;97:33–37. doi: 10.1016/0304-3835(95)03945-s. [DOI] [PubMed] [Google Scholar]
- Aziz G., Nosseir M.H., Doss N.L., Rizk A.S. Experiments with furochalcones: Addition of nucleophilic reagents, diazomethane, benzonitrile oxide, hydrazine hydrate, phenylhydrazine, hydroxylamine, thiourea and semicarbazide to furochalcones. Indian J. Chem. Sect. B. 1976;14:286–291. [Google Scholar]
- Bhat B.A., Dhar K.L., Puri S.C., Saxena A.K., Shanmugavel M., Qazi G.N. Synthesis and biological evaluation of chalcones and their derived pyrazoles as potential cytotoxic agnts. Bioorg. Med. Chem. Lett. 2005;15:3177–3180. doi: 10.1016/j.bmcl.2005.03.121. [DOI] [PubMed] [Google Scholar]
- Bian J., Xu L., Deng B., Quan X., Fan J., Yang X., Liu F., Xu X., Guo X., Li X., Sun H., You Q., Zhang X. Synthesis and evaluation of (±)-dunnione and its ortho-quinone analogues as substrates for NAD(P)H:quinone oxidoreductase 1 (NQO1) Bioorg. Med. Chem. Lett. 2015;25:1244–1248. doi: 10.1016/j.bmcl.2015.01.057. [DOI] [PubMed] [Google Scholar]
- El-Desoky S.I., Abozeid M.A., Kandeel E.A., Abdel-Rahman A.H. Domino reactions of 3-vinylchromone leading to different heterocyclic compounds. J. Heterocycl. Chem. 2014;51:1270–1276. [Google Scholar]
- El-Desoky S.I., Badria F.A., Abozeid M.A., Kandeel E.A., Abdel-Rahman A.H. Synthesis and antitumor studies of novel benzopyrano-1,2,3-selenadiazole and spiro [benzopyrano]-1,3,4-thiadiazoline derivatives. Med. Chem. Res. 2013;22:2105–2114. [Google Scholar]
- El-Desoky S.I., Keshk E.M., El-Sawi A.A., Abdel-Rahman A.H. Synthesis of new benzochromones compounds with anticipated medicinal activities. Mansoura J. Chem. 2013;40:109–123. [Google Scholar]
- El-Gazzar A.B.A., Youssef M.M., Youssef A.M.S., Abu-Hashem A.A., Badria F.A. Design and synthesis of azolopyrimidoquinolines, pyrimidoquinazolines as anti-oxidant, anti-inflammatory and analgesic activities. Europ. J. Med. Chem. 2009;44:609–624. doi: 10.1016/j.ejmech.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Faig M., Bianchet M.A., Talalay P., Chen S., Winski S., Ross D., Amzel L.M. Structures of recombinant human and mouse NAD(P)H:quinone oxidoreductases: Species comparison and structural changes with substrate binding and release. Proc. Natl. Acad. Sci. USA. 2000;97:3177–3182. doi: 10.1073/pnas.050585797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross B.A., Mindea S.A., Pick A.J., Chandler J.P., Batjer H.H. Diagnostic approach to cushing disease. Neurosurg. Focus. 2007;23:1–6. doi: 10.3171/foc.2007.23.3.2. [DOI] [PubMed] [Google Scholar]
- Karad S.C., Purohit V.B., Thummar R.P., Vaghasiya B.K., Kamani R.D., Thakor P., Thakkar V.R., Thakkar S.S., Ray A., Raval D.K. Synthesis and biological screening of novel 2-morpholinoquinoline nucleus clubbed with 1,2,4-oxadiazole motifs. Europ. J. Med. Chem. 2017;126:894–909. doi: 10.1016/j.ejmech.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Kinnula V.L., Crapo J.D. Antioxidant enzymes and redox regulating thiol proteins in malignancies of human lung. Free Radic. Biol. Med. 2004;36:718–744. doi: 10.1016/j.febslet.2004.05.045. [DOI] [PubMed] [Google Scholar]
- Mandawad G., Kamble R., Hese S., More R., Gacche R., Kodam K., Dawane B. An efficient synthesis of isoxazoline libraries of thiophene analogs and its antimycobacterial investigation. Med. Chem. Res. 2014;23:4455–4463. [Google Scholar]
- Nakamura C., Kawasaki N., Miyataka H., Jayachandran E., Kim I.H., Kirk K.L., Taguchi T., Takeuchi Y., Hori H., Satoh T. Synthesis and biological activities of fluorinated chalcone derivatives. Bioorg. Med. Chem. 2002;10:699–706. doi: 10.1016/s0968-0896(01)00319-4. [DOI] [PubMed] [Google Scholar]
- Pandya A.B., Prajapati D.G., Pandya S.S. Synthesis of novel naphthalene COX inhibitors for anti-inflammatory activity. J. Appl. Pharm. Sci. 2012;2:226–232. [Google Scholar]
- Sapariya N.H., Vaghasiya B.K., Thummar R.P., Kamani R.D., Patel K.H., Thakor P., Thakkar S.S., Ray A., Raval D.K. Synthesis, characterization, in silico molecular docking study and biological evaluation of a 5-(phenylthio)pyrazole based polyhydroquinoline core moiety. New J. Chem. 2017;41:10686–10694. [Google Scholar]
- Siegel D., Bolton E.M., Burr J.A., Liebler D.C., Ross D. The reduction of α-tocopherolquinone by human NAD(P)H:quinone oxidoreductase: the role of α-tocopherolhydroquinone as a cellular antioxidant. Mol. Pharmacol. 1997;52:300–305. doi: 10.1124/mol.52.2.300. [DOI] [PubMed] [Google Scholar]
- Singh U., Jialal I. Oxidative stress and atherosclerosis. Pathophysiology. 2006;13:129–142. doi: 10.1016/j.pathophys.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Smith M.A., Rottkamp C.A., Nunomura A., Raina A.K., Perry G. Oxidative stress in Alzheimer’s disease. Biochim. Biophys. Acta. 2000;1502:139–144. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- Thakkar S.S., Thakor P., Doshi H., Ray A. 1,2,4-Triazole and 1,3,4-oxadiazole analogues: Synthesis, MO studies, in silico molecular docking studies, antimalarial as DHFR inhibitor and antimicrobial activities. Bioorg. Med. Chem. 2017;25:4064–4075. doi: 10.1016/j.bmc.2017.05.054. [DOI] [PubMed] [Google Scholar]
- Thakkar S.S., Thakor P., Ray A., Doshi H., Thakkar V.R. Benzothiazole analogues: synthesis, characterization, MO calculations with PM6 and DFT, in silico studies and in vitro antimalarial as DHFR inhibitors and antimicrobial activities. Bioorg. Med. Chem. 2017;25:5396–5409. doi: 10.1016/j.bmc.2017.07.057. [DOI] [PubMed] [Google Scholar]
- Wang S., Yu G., Lu J., Xiao K., Hu Y., Hu H. A regioselective tandem reaction between chalcones and 2-acetamidoacetamide promoted by Cs2CO3 for the preparation of 3-unsubstituted 2-pyridones. Synthesis. 2003:487–490. [Google Scholar]
- Wilson C.O., Gisvold O., Block J.H., Beale J.M. Lippincott Williams; Wilkins, Philadelphia: 2004. Wilson and Gisvold's Textbook of Organic Medicinal and Pharmaceutical Chemistry. [Google Scholar]
- Yahiaoui S., Fagnere C., Pouget C., Buxeraud J., Chulia A.-J. New 7,8-benzoflavanones as potent aromatase inhibitors: synthesis and biological evaluation. Bioorg. Med. Chem. 2008;16:1474–1480. doi: 10.1016/j.bmc.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Yusuf M., Jain P. Synthetic and biological studies of pyrazolines and related heterocyclic compounds. Arab. J. Chem. 2014;7:553–596. [Google Scholar]
- Zervosen A., Sauvage E., Frère J.-M., Charlier P., Luxen A. Development of new drugs for an old target—the penicillin binding proteins. Molecules. 2012;17:12478–12505. doi: 10.3390/molecules171112478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.