Abstract
Background:
Neisseria gonorrhea is known to have developed a high level of resistance against different classes of antimicrobials. Patients with coagulation disorders where intramuscular injections are contraindicated, oral cefixime in combination therapy can be utilized as an alternative regimen. Cefixime in combination with another macrolide might be considered as an alternative treatment option. The aim of this systematic review is to assess the efficacy of 400 mg cefixime against a range of comparator drugs.
Methodology:
Extensive literature search for randomized controlled trials was performed using Medline, Cochrane Registry of Controlled Trials, Embase, and Clinical trials registers. The trials assessed the efficacy of cefixime against a range of comparator drugs. Primary outcome of the study was the clinical resolution of signs and symptoms and negative culture at the end of follow-up period.
Results:
After screening for a total of 1184, only 8 studies were eligible for a meta-analysis. Risk ratio random effects model was used with a 95% confidence interval (CI). The pooled efficacy of Cefixime was at 97% at 95 CI 1.01 (0.98, 1.05). No statistically significant difference was found between oral cefixime and comparator drugs.
Conclusion:
A total of 11 studies were included following a review of 1184 publications. 8 randomized controlled trials for 400 mg oral cefixime were included in meta-analysis. Despite a high grade of evidence, a high risk of bias was found among studies. Hence, more high quality randomized controlled trials on cefixime needs to be performed in future to guide the treatment of gonococcal infections.
Keywords: Cefixime, efficacy, meta-analysis, Neisseria gonorrhea, sexually transmitted disease, sexually transmitted infections, systematic review
Introduction
Gonorrhea is reported to be the second most commonly reported communicable disease.[1] In 2008, a survey conducted by the World Health Organization (WHO) estimated that there were around 106 million new cases of gonorrhea worldwide. Targeted microbiologic diagnosis of this infection with Neisseria gonorrhea (N.G.) should be conducted in all individuals at risk or susceptible to acquire N.G. A specific and prompt diagnosis could possibly reduce the percentage of complications, transmissions, or reinfections. Due to the specificity and sensitivity, a Gram Strain of urethral secretions that show polymorphonuclear leukocytes with intracellular Gram-negative diplococci can be considered as diagnostic for infection with N.G in symptomatic individuals.[2]
The treatment of N.G is further complicated due to the tendency of N.G to develop resistance to antimicrobials.[3] The evolution of resistance to antimicrobials agents in N.G. isolates is a global burden in the treatment toward gonococcal infections. The ability of this specific disease to resist significant levels of penicillins, tetracycline, and fluoroquinolones and oral cephalosporins[4–7] have recently escalated in far East Asia.[8] In the 1990s, it was internationally recommended to use the third generation cephalosporins. However, investigations conducted in the past have reported on the treatment failures with cefixime. Nevertheless, it is still recommended as the drug of choice for N.G infections in certain countries.[9] Hence, a regimen of parenteral cephalosporin such as ceftriaxone is generally prescribed as the first line of treatment for uncomplicated gonococcal infections. Cefixime in combination with another macrolide, such as azithromycin might be considered as an alternative oral treatment option.[2] The recommendations made by the Clinical and Laboratory Standards Institute the minimum inhibitory concentration breakpoint for oral cephalosporins cefixime and cefpodoxime susceptibility were <0.25 mg/L and 0.5 mg/L.[10]
The objective of this systematic review is to assess the efficacy of a single oral dose of cefixime against a range of comparator drugs used to treat uncomplicated gonococcal infections, such as ceftriaxone, fluoroquinolones, and amoxicillin. The authors also want to assess whether the efficacy of cefixime for treating uncomplicated gonococcal infections is superior to comparators drugs or not?.
Materials and Methods
Overview of methodology
An extensive review of the literature was done on patients with uncomplicated gonorrhea treated with oral cefixime.
Search strategy
Literature search for randomized controlled trials was performed using Medline, Cochrane registry of controlled trials, Embase, and Clinical trials registers. The studies filtered had no restrictions on dates when using Medline. The studies included in this review ranged from the timeline of 1946 to December 2017, randomized controlled trials and observational comparative studies were included in this review.
Keywords included “Neisseria gonorrhea,” “Cefixime,” “Cephalosporins,” “Ceftriaxone,” “Gonococcal urethritis,” “Neisseria,” and “Gonococcal infections.” In addition, studies were also retrieved from databases such as “ScienceDirect,” “CINAHL,” and Clinical trial registries in North America and the United Kingdom and also by hand searching research articles for further references.
Other databases
Clinical trial registers of China, Europe, Russia, India, Japan, WHO, and Brazil were also searched extensively.
Participants, intervention, and comparators
Inclusion criteria
The following criteria were included in this study:
Healthy male and female patients above 15 years of age.
Patients with a history of exposure to infected individuals.
Patients who were diagnosed with a gonorrhea infection microbiologically by culture and microscopy or nucleic acid amplification test (NAAT).
AND
Patients who were diagnosed with gonococcal infection on clinical grounds
Patients who went through a proper procedure of informed consent, before they enrolled in the randomized the controlled trial.
Test of cure was performed after follow-up period, either by the culture of NAAT.
Exclusion criteria
Patients with non-gonococcal urethritis were excluded from the study.
Patients not diagnosed microbiologically were also excluded from this review.
Patients with an impaired immune system with conditions such as HIV, Diabetes mellitus, or any other autoimmune disease such as SLE.
Patients with a history of allergy to penicillin or cephalosporin.
Patients with a history of renal failure.
Types of interventions
Studies where cefixime was administered to the patients in the following manner
800 mg × Once daily orally for 1 day.
400 mg × Twice daily orally for 1 day.
200 mg × Once daily orally for 1 day.
400 mg × Once daily orally for 1 day.
Primary outcome measures
Primary outcome measures were defined as:
Microbiological cure (negative culture/microscopy) at the end of the treatment and follow-up.
Clinical resolution of signs and symptoms such as abdominal pain, genital pain, discharge from urethra and dysuria at the end of the follow-up period.
Secondary outcome measure
Secondary outcome measures were defined as:
Adverse reaction related to drug intakes such as diarrhea, loose stools, abdominal pain, headaches, nausea, rashes, or pseudomembranous colitis.
Patients requiring further symptomatic or antimicrobial therapy.
Data collection and analysis
Two authors Syed Bilal Tanvir and Syed Saad bin Qasim independently reviewed the studies for eligibility. Study selection was based on abstracts and titles of research articles. Any conflict regarding inclusion or exclusion was mainly resolved by consensus.
Data sources, studies sections, and data extraction
A customized form was developed and tested for data extraction for the included studies. The form was derived from the template provided by the Cochrane data extraction tool. The form was customized to extract the following data from the research articles Author name.
Year the study was published
Study design
Dosage and route of cefixime
Dosage and route of comparator drug
Types of participants
Primary outcome measures such as cure rate
Adverse events
Length of treatment
Follow-up duration
Method of follow-up
Method of diagnosis.
The data extracted from the studies were rechecked by the other authors for mistakes.
Data analysis
Risk ratio (RR) random effects model was used with a 95% confidence interval (CI). Cochrane Revman 5.0 software was used for this purpose. Studies were grouped according to the class of the drugs used to treat uncomplicated gonococcal infections. The pooled efficacy of cefixime was calculated against a range of comparator drugs at 95 CI 1.01). Studies were assessed for the statistically significant difference between oral cefixime and comparator drugs. Heterogeneity was also assessed among different studies.
Quality assessment of randomized and non-randomized observational studies
A modified downs WW and black method checklist were used for this purpose. The checklist has 27 questions for assessing the quality of the studies and for the assessment of the risk of bias. Confounding, Selection bias, External validity, and reporting bias were included. The last question in the checklist was adapted from another study and assessed the power of the study.
Results
The results concluded that there was a lack of high quality of evidence on the use of oral cefixime for the treatment of both complicated and uncomplicated gonorrhea. A comprehensive literature search was done despite that only 8 RCTs were identified, where patients were treated for both complicated and uncomplicated gonorrhea treated with oral cefixime.
Summary of Studies retrieved for the review is included in Figure 1.
Figure 1.
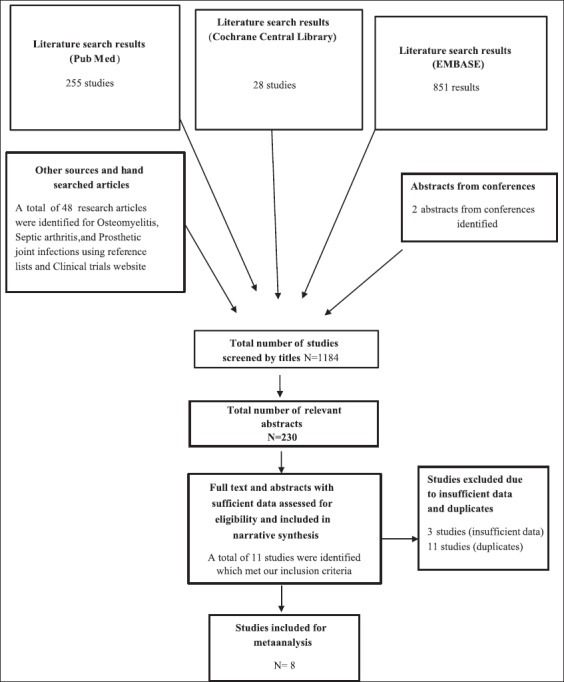
Flow diagram for studies retrieved for the review
Study selection and characteristics of the included studies are mentioned in Table 1
Table 1.
Study selection and characteristics of the included studies
After screening for a total of 1184 studies, using different databases, 230 relevant abstracts were screened for inclusion, in the systematic review and meta-analysis. A total of 255 studies from PubMed, 28 studies from Cochrane Central Register of Controlled Trials, and 851 results were obtained for EMBASE.
Out of the relevant 255 abstracts that were screened for eligibility, only 11 studies were included in the narrative review while only 8 studies were eligible for a meta-analysis.
Out of the 230 abstracts screened for this review, 219 abstracts were excluded from the review because of the following reasons.
Studies where the diagnosis of uncomplicated and complicated gonorrhea, were not made microbiologically by performing, pharyngeal, rectal or urethral swab, or NAAT.
Studies involving patients with hematological malignancies and immunocompromised conditions such as diabetes, HIV, and other systemic diseases.
Studies where cefixime was not being assessed for its efficacy/effectiveness but after pharmacokinetic or pharmacodynamics activity.
Study where the primary outcome of the study was adverse effects of cefixime or comparator drug.
A total of 11 studies were included in this review for final narrative and qualitative review. Out of the 11 studies, 2 studies were excluded. Finally, only 8 studies were included in the meta-analysis as they were randomized controlled trials [Figure 1].
Study selection and characteristics of the included studies
Of the 8 studies included in the review of meta-analysis, 3 studies compared the effectiveness of cefixime versus fluoroquinolone (ciprofloxacin and grepafloxacin)[11-13] while 5 studies compared the efficacy of cefixime versus ceftriaxone.[13-17]
While 2 studies were noncomparative in study design, assessing the efficacy of cefixime in a patient with complicated gonorrhea;[13,18] finally, one study compared the effectiveness of cefixime versus amoxicillin and probenecid.[19]
Data analysis of individual studies [Figure 2]
Figure 2.
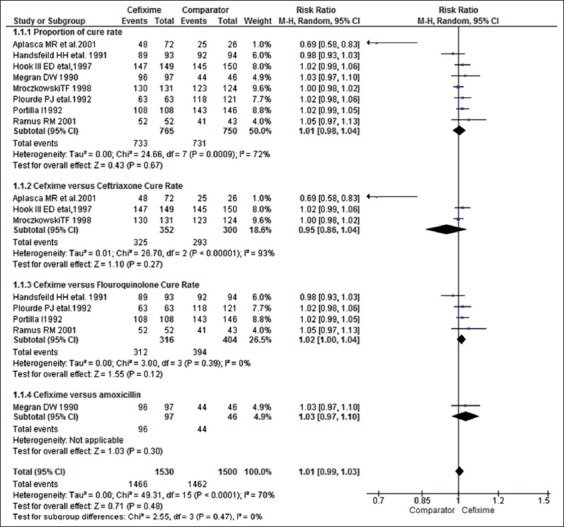
Meta-analysis of the included studies
In trials comparing the cure rate of cefixime versus fluoroquinolone.
Cure rates (3 studies). The cure rate was 92% (325/352 patients in 800 mg cefixime group) compared with 97.6% (293/300 patients in ceftriaxone group). RR (random effects model) 95 CI (0.06, 6.25) P = 0.68. Hence, no statistical significant difference was found between ceftriaxone and cefixime group. There was also a high heterogeneity in this analysis P = 0.03 I2 72%.
In trials comparing the cure rate of cefixime versus ceftriaxone
Cure rate (4 studies). The cure rate was 99% (312/316 in 800mg cefixime group) compared with 97.5% (394/404 in fluoroquinolone group) RR (Random effects model) 95 CI 1.77 (1.00–1.04) P = 0.39. Hence, no statistical significant differences were found in this study analysis. There was a high heterogeneity in this analysis P = 0.12 I2 = 0%.
Trial comparing the cure rate of cefixime versus ceftriaxone:
Cure rate (1 study). The cure rate was 98% (90/97 patients in cefixime 800 mg orally) compared with 95.6% (amoxicillin and probenecid group). RR (Random effects model) 95% CI (1.01, 1.10) P = 0.48. Hence, the comparison was statistically significant. Hence, the statistically significant difference was found between the efficacy of cefixime and amoxicillin and probenecid. Test for heterogeneity was not applicable in this group.
Adverse events
Meta-analysis of adverse events was not performed in this systematic review. The major reason was improper reporting methods for adverse events or absence of data for adverse events.
Patient characteristics
1577 patients were included in this systematic review, of which 1151 patients were included in meta-analysis. The male to female ratio was 1/1.40.
Risk of bias
A modified downs and black method checklist were used to determine the risk of bias. Primarily four parameters were assessed to determine the risk of bias among studies. Confounding, Selection bias, External validity, and reporting bias were included. The last question in the checklist was adapted from another study and assessed the power of the study. Risk of bias is expressed in the form of percentages in Table 2. Questions number 1–10 is related to reporting; number 11–13 related to external validity; 14–20 related to internal validity bias and 21–26 selection; and 27 related to the power of the study.
Table 2.
Risk of bias and quality assessment of the included studies
Discussion
Summary of main findings
This systematic review found out that there is insufficient evidence data to prove or disprove the benefits of cefixime for the treatment of gonorrhea on adult patients. A total of 8 studies of single-dose oral cefixime were identified and included in the meta-analysis. The success rate of the treatment ranged from 92% to 99 %. This systematic review compared the success rate of the treatment at the end of the follow-up period between cefixime and comparator antibiotics. Previously a systematic review and meta-analysis have been conducted to determine the efficacy of intramuscular (IM) ceftriaxone. This systematic review found out ceftriaxone to have better efficacy than cefixime which had a pooled percentage cure rate of 78.1%. All of these studies mentioned in this systematic have met out inclusion criteria.[11,13-17,19] Furthermore, the efficacy of cefixime was also compared with fluoroquinolones such as grepafloxacin and ciprofloxacin and found a pooled cure rate of 97.5% 95% CI 1.02 (1.00, 1.04)[12] while the pool cure rate of cefixime was found to be 99% 95% CI 1.02 (1.00, 1.04).[13,15-17] This systematic review also found out the cure rate of cefixime to be at 98% 95 CI 1.01(0.97, 1.10) when compared with amoxicillin and probenecid, having a cure rate of 95% 95% CI(0.97, 1.10).[19]
According to the WHO guidelines and BASHH treatment guidelines, fluoroquinolones are no longer recommended as the mainstay for treating gonococcal infections due to high amounts of resistance.[2] A single dose of 400 mg cefixime orally taken once coupled with 2 g of azithromycin is recommended as an alternative treatment option for uncomplicated gonorrhea.[7] However, in patients where IM injection are contraindicated in conditions such as hemophilia, or patients under therapy with anticoagulants, it might prove as a useful alternative to IM ceftriaxone. Furthermore, in resource poor settings where IM ceftriaxone is not available, it might prove as a useful substitute. Although a steady increase in the prevalence of high cefixime MIC suggests that in future the effectiveness of these drugs might slowly decline. Despite this fact, another oral cephalosporin such as cefuroxime and cefpodoxime cannot be recommended as an adequate substitute to cefixime and ceftriaxone as they possess low efficacy and inadequate pharmacodynamics.[10]
Limitations
Most of the studies included in this systematic review are more than a decade old. Hence, these studies cannot be conclusively relied on by the academics and clinicians for the treatment of patients. Another major limitation of the study is that the adverse effects of different drugs were either not reported or appropriate methods were not used to separately report them. There was a very high level of heterogeneity among studies as well.
Conclusion
Data collected in this systematic review suggest that cefixime might prove to be a useful option for the treatment of gonorrhea infection with a success rate of over 98%. This systematic review and meta-analysis suggest cefixime to be clinically more effective when compared with a fluoroquinolone. However, the efficacy of ceftriaxone is still superior when compared with cefixime, which is in line with the current guidelines of WHO and BASHH. Hence, more high quality randomized controlled trials for cefixime in combination with another macrolide needs to be conducted in future to guide the clinicians in treating uncomplicated gonococcal infections in patients where IM injections are contraindicated.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding
No external funding was provided for this review.
References
- 1.Datta SD, Sternberg M, Johnson RE, Berman S, Papp JR, McQuillan G, et al. Gonorrhea and chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Ann Intern Med. 2007;147:89–96. doi: 10.7326/0003-4819-147-2-200707170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Unemo M. Current and future antimicrobial treatment of gonorrhoea-the rapidly evolvingNeisseria gonorrhoeaecontinues to challenge. BMC Infect Dis. 2015;15:364. doi: 10.1186/s12879-015-1029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Workowski KA, Berman SM, Douglas JM., Jr Emerging antimicrobial resistance inNeisseria gonorrhoeae:Urgent need to strengthen prevention strategies. Ann Intern Med. 2008;148:606–13. doi: 10.7326/0003-4819-148-8-200804150-00005. [DOI] [PubMed] [Google Scholar]
- 4.Barry PM, Klausner JD. The use of cephalosporins for gonorrhea:The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555–77. doi: 10.1517/14656560902731993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lind I. Antimicrobial resistance inNeisseria gonorrhoeae. Clin Infect Dis. 1997;24(Suppl 1):S93–7. doi: 10.1093/clinids/24.supplement_1.s93. [DOI] [PubMed] [Google Scholar]
- 6.Hamasuna R, Yasuda M, Ishikawa K, Uehara S, Hayami H, Takahashi S, et al. The second nationwide surveillance of the antimicrobial susceptibility ofNeisseria gonorrhoeaefrom male urethritis in Japan, 2012-2013. J Infect Chemother. 2015;21:340–5. doi: 10.1016/j.jiac.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Unemo M, Shafer WM. Antibiotic resistance inNeisseria gonorrhoeae:Origin, evolution, and lessons learned for the future. Ann N Y Acad Sci. 2011;1230:E19–28. doi: 10.1111/j.1749-6632.2011.06215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka M, Nakayama H, Huruya K, Konomi I, Irie S, Kanayama A, et al. Analysis of mutations within multiple genes associated with resistance in a clinical isolate ofNeisseria gonorrhoeaewith reduced ceftriaxone susceptibility that shows a multidrug-resistant phenotype. Int J Antimicrob Agents. 2006;27:20–6. doi: 10.1016/j.ijantimicag.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Kropp RY, Latham-Carmanico C, Steben M, Wong T, Duarte-Franco E. What's new in management of sexually transmitted infections? Canadian guidelines on sexually transmitted infections, 2006 edition. Can Fam Physician. 2007;53:1739–41. [PMC free article] [PubMed] [Google Scholar]
- 10.Yu RX, Yin Y, Wang GQ, Chen SC, Zheng BJ, Dai XQ, et al. Worldwide susceptibility rates ofNeisseria gonorrhoeaeisolates to cefixime and cefpodoxime:A systematic review and meta-analysis. PLoS One. 2014;9:e87849. doi: 10.1371/journal.pone.0087849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aplasca De, Los Reyes MR, Pato-Mesola V, Klausner JD, Manalastas R, Wi T, Tuazon CU, et al. Arandomized trial of ciprofloxacin versus cefixime for treatment of gonorrhea after rapid emergence of gonococcal ciprofloxacin resistance in the philippines. Clin Infect Dis. 2001;32:1313–8. doi: 10.1086/319998. [DOI] [PubMed] [Google Scholar]
- 12.Mroczkowski TF, Hook Iii EW, Jones RB, McCormack WM, Martin DH. Grepafloxacin versus cefixime as single-dose therapy for uncomplicated gonorrhea in women. Infect Dis Obstet Gynecol. 1997;5:370–5. doi: 10.1155/S1064744997000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handsfield HH, McCormack WM, Hook EW, 3rd, Douglas JM, Jr, Covino JM, Verdon MS, et al. Acomparison of single-dose cefixime with ceftriaxone as treatment for uncomplicated gonorrhea. The gonorrhea treatment study group. N Engl J Med. 1991;325:1337–41. doi: 10.1056/NEJM199111073251903. [DOI] [PubMed] [Google Scholar]
- 14.Holdcroft C. Cefixime offers effective oral therapy for gonorrhea. Nurse Pract. 1992;17:79–80. doi: 10.1097/00006205-199205000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Plourde PJ, Tyndall M, Agoki E, Ombette J, Slaney LA, D'Costa LJ, et al. Single-dose cefixime versus single-dose ceftriaxone in the treatment of antimicrobial-resistantNeisseria gonorrhoeaeinfection. J Infect Dis. 1992;166:919–22. doi: 10.1093/infdis/166.4.919. [DOI] [PubMed] [Google Scholar]
- 16.Portilla I, Lutz B, Montalvo M, Mogabgab WJ. Oral cefixime versus intramuscular ceftriaxone in patients with uncomplicated gonococcal infections. Sex Transm Dis. 1992;19:94–8. [PubMed] [Google Scholar]
- 17.Ramus RM, Sheffield JS, Mayfield JA, Wendel GD., Jr A randomized trial that compared oral cefixime and intramuscular ceftriaxone for the treatment of gonorrhea in pregnancy. Am J Obstet Gynecol. 2001;185:629–32. doi: 10.1067/mob.2001.117662. [DOI] [PubMed] [Google Scholar]
- 18.Verdon MS, Douglas JM, Jr, Wiggins SD, Handsfield HH. Treatment of uncomplicated gonorrhea with single doses of 200 mg cefixime. Sex Transm Dis. 1993;20:290–3. doi: 10.1097/00007435-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Megran DW, Lefebvre K, Willetts V, Bowie WR. Single-dose oral cefixime versus amoxicillin plus probenecid for the treatment of uncomplicated gonorrhea in men. Antimicrob Agents Chemother. 1990;34:355–7. doi: 10.1128/aac.34.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deguchi T, Yasuda M, Yokoi S, Ishida K, Ito M, Ishihara S, et al. Treatment of uncomplicated gonococcal urethritis by double-dosing of 200 mg cefixime at a 6-h interval. J Infect Chemother. 2003;9:35–9. doi: 10.1007/s10156-002-0204-8. [DOI] [PubMed] [Google Scholar]




