Abstract
Sepsis is a life-threatening disease resulted from a dysregulated host immune response to bacterial infections, continuing to cause high morbidity and mortality worldwide. Despite discoveries of many potential therapeutic targets, effective treatments of sepsis are lacking. Here, a strategy is reported to target infectious microenvironments (IMEs) via bioresponsive nanoparticles that simultaneously eliminate bacteria and alleviate the host inflammation response, thus managing sepsis in mice. The nanoparticle is made of copolymers sensitive to pH and bacterial enzymes to self-assemble into a micelle loaded with both an antibiotic (ciprofloxacin) and an anti-inflammatory agent ((2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide). In addition, the nanoparticle is conjugated with intercellular adhesion molecule-1 antibodies to target IMEs. Nanoparticle targeting to IMEs and local cues as triggers to deliver therapeutics in on-demand manners is demonstrated using an acute lung bacterial infection mouse model. In the sepsis mouse model induced by peritonitis at a lethal dose of bacterial invasion, it is shown that concurrently targeting pathogens and excessive inflammation pathways is valuable to manage the sepsis. The study illustrates not only the development of a new delivery system but also the mechanism-based therapy of nanomedicine for infectious diseases.
Keywords: infectious microenvironments, nanoparticle, sepsis, targeted drug delivery
Sepsis, defined as systemic inflammatory response syndrome, is a life-threatening organ dysfunction caused by a dysregulated host response to infections, particularly for bacterial infections.[1–3] The incidence of sepsis continues to rise worldwide, and it involves a prolonged stay in the intensive care unit with a high mortality.[4,5] Currently, the supportive care is a primary option for sepsis management. Despite the discovery of many potential therapeutic targets,[4,6] effective drug treatments of sepsis are lacking in clinic, partially because it remains challenging to differentially deliver therapeutics in the lesions induced by invading pathogens in on-demand manners.
While the pathogenesis of sepsis remains poorly understood,[2,5] there are two factors involved: invading pathogens and activation of the host immune system. When bacteria invade in the body, the host immune system is activated to eradicate invading bacteria.[1] For example, immune cells (such as macrophages) recognize lipopolysaccharide on a Gram-negative bacterium by Toll-like receptors,[7,8] and release proinflammatory cytokines (such as TNF-α, IL-1β, and IL-6), which activate endothelial cells lining blood vessels to increase the expression of adhesion molecules for leukocyte recruitment to eliminate bacteria.[8] However, uncontrolled and excessive inflammation responses could cause sepsis, precipitating a collapse of cardiovascular functions with multiple organ failure.[1] Antibiotics or anti-inflammatory agents are often used to control bacterial dissemination or alleviate inflammation responses.[9,10] There are several common issues in administering small molecules in vivo, such as rapid metabolism, poor bioavailability, and high toxicity. Despite recent advances in nanomedicine have brought new tools and technologies to overcome these issues,[11] most nanocarriers are designed to target either bacteria[12] or inflammation pathways.[13–15] Furthermore, these nanocarriers cannot specifically target infectious tissues.
Co-delivery of an antibiotic and anti-inflammatory agent to the site of infection may be a novel strategy for sepsis therapy as it may concurrently prevent bacterial dissemination and manage inflammation induced by bacteria, therefore averting systemic bacteremia and excessive inflammation that otherwise cause sepsis. However, this task presents various challenges. For example, bacterial infections usually occur in the tissues outside vasculature,[16] so intravenous administration of drug carriers needs to overcome the vessel barrier to arrive at a location of bacteria. In addition, concurrent delivery of multiple drugs requires a novel carrier that can specifically target tissue lesions induced by bacteria, enabling the simultaneous sequestration of spreading of pathogens and inflammation responses.
It has been shown that an infection lesion forms a unique microenvironment comprised of low pH,[17] bacterial enzymes,[18] and activated blood vessels.[10] Endothelium lining the lumen of blood vessels rapidly expresses several cell adhesion molecules in response to infections, such as intercellular adhesion molecule-1 (ICAM-1).[10,13] Inspired by the features of infectious microenvironments (IMEs), we designed a novel polymeric nanoparticle that is coated with ICAM-1 antibody for targeting of infectious tissues. The nanoparticle is sensitive to acidity and bacterial enzymes present in IMEs as triggers for drug release. Using this nanoparticle, we are able to co-deliver an antibiotic and an anti-inflammatory agent in IMEs for concurrent control of bacterial burden and host inflammation response to prevent the mouse sepsis induced by a lethal dose of bacterial invasion.
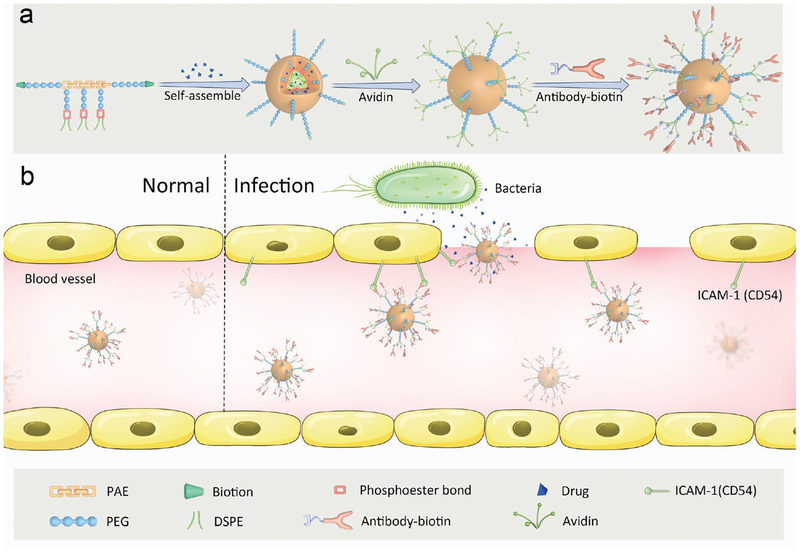
Scheme 1 shows a principle concept of design of nanotherapeutics targeted to IMEs. A multi-functional block copolymer composed of pH/enzyme-sensitive moieties and biotin linker can self-assemble into nanoparticles. An antibiotic (ciprofloxacin, CIP)[19] and an anti-inflammatory agent (2-[(amino-carbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide, TPCA-1)[20] are co-loaded in IMEs-responsive nanoparticles, followed by coating of anti-mouse ICAM-1 antibody via the binding of biotin to avidin (Scheme 1a). As a result, the nanoparticles may deliver two drugs in infectious tissues. In an infectious lesion, activated endothelium highly expresses ICAM-1 molecules and vascular permeability is increased.[21] When antibody-coated nanoparticles bind to activated vasculature at the site of infection, acidic[22] and enzymatic[23,24] cues might trigger the dissembling of nanoparticles for drug release (Scheme 1b). To examine this concept, we studied the physicochemical properties of drug delivery system co-loaded with CIP and TPCA-1, drug delivery mechanism, and their therapeutic efficacy in management of sepsis induced by a lethal dose of Pseudomonas aeruginosa in a mouse model of peritonitis.[1,25]
Scheme 1.
Schemes for design of an IME-responsive and biofunctional nanoparticle (NP), and targeted delivery of nanotherapeutics at a site of infection. a) Schematic for design of multi-functional NPs. A drug-loaded polymeric micelle is self-assembled from pH/enzyme-responsive amphiphilic block copolymers and drugs, followed by coating with antibody for targeting infection sites. The poly (ß-amino ester) (PAE) segment is pH-sensitive, and enzyme-responsive. 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)] (PEGylated DSPE) on the pendants of PAE is designed for drug loading. Biotins on the surface of a micelle are used for biofunctionalization. The chemical structure and synthesis process are presented in Figure S1 (Supporting Information). b) Schematic shows that drug-loaded NPs-anti-ICAM-1 specifically target activated endothelial cells at a site of infection after intravenous (i.v.) injection. Drug-loaded NPs-anti-ICAM-1 bind to activated endothelial cells in IMEs, crossing the blood vessel and releasing drugs triggered by the local infectious cues.
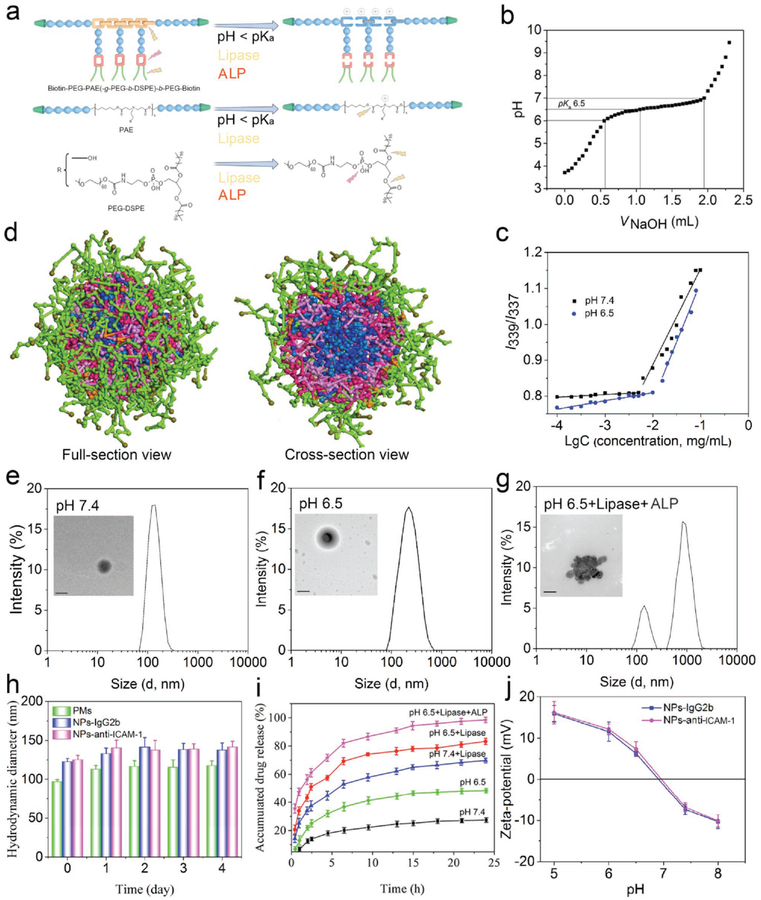
Herein, we designed and synthesized a pH/enzyme-responsive amphiphilic block copolymer consisting of biotinylated poly(ethylene glycol)-b-poly(β-amino ester)-b-poly(ethylene glycol) grafted with PEGylated lipid (Biotin-PEG-b-PAE(-g-PEG-b-DSPE)-b-PEG-Biotin) via the Michael-type polymerization. In a polymer chain, PAE (tertiary amines and ester bonds) and PEG-DSPE (phosphoester bonds) were pH-sensitive and enzyme-responsive (Figure 1a and Figure S1, Supporting Information). The structures of precursors and copolymer were analyzed by nuclear magnetic resonance, and the molecular weight of the polymer was examined by gel permeation chromatography (GPC) (Figures S2–S8 and Table S1, Supporting Information). The results demonstrate that the copolymer was successfully synthesized as designed. The acid–base titration was used to determine the pKa value of copolymer, and it was ≈6.5 which was close to the pH in IMEs (Figure 1b). GPC was used to study the copolymer cleavage in response to enzymes (Figure S8, Supporting Information). Only one peak was observed in the GPC spectrum when the copolymer was incubated in phosphate buffer solution (PBS) at pH 7.4, but two or more peaks appeared when a lipase enzyme was added, implying that the copolymer was cleaved by the enzyme. Collectively, the results indicate that the copolymer responds to acidic and enzymatic cues.
Figure 1.
Characterization of NPs and drug release profile of CIP-NPs-Abs. a) Structure and pH/enzyme-sensitive amphiphilic block copolymer. PAE moieties are pH/bacterium lipase-responsive segments, resulting from tertiary amine residues and ester bonds of a polymer chain. Under acid/lipase conditions, tertiary amine residues are protonated and become positive accompanied by cleavage of ester bonds. PEG-DSPE moieties are lipase and ALP-responsive blocks. Green trapezoids represent biotin groups; Blue balls represent PEG units; Yellow shackles represent PAE segments; Red shackles represent phosphoester bonds. b) pKa of a copolymer was measured by acid–base titration. c) Copolymer CMC at pH 7.4 or 6.5 was determined by pyrene fluorescence spectroscopy. d) Coarse-grained simulations of self-assembly of CIP-PMs using a DPD method. A copolymer chain is divided into five types of beads: peach, HDD (1,6-hexanediol diacrylate) units in PAE and DSPE; PEG (light green); biotin (dark green); AP (3-amino-1-propanol) units in PAE (pink); CIP molecule (blue). Figure S10 in the Supporting Information shows the detail about the simulation process. e–g) Representative DLS size and TEM images of NPs-anti-ICAM-1 incubated in PBS at pH 7.4 (e), pH 6.5 (f) or pH 6.5 with enzymes (g) (Pseudomonas lipase, 1 mg mL−1 and ALP, 500 unit mL−1) for 2 h. Scale bars: 100 nm. h) Serum stability of PMs and NPs-anti-ICAM-1/IgG2b incubated in PBS with 20% FBS at pH 7.4 at 37°C. i) In vitro drug release of CIP-NPs-anti-ICAM-1 in different buffers (Pseudomonas lipase, 1 mg mL−1 and ALP, 500 unit mL−1). j) Zeta-potential of CIP-NPs-anti-ICAM-1/IgG2b in PBS as the function of pH. The data are shown as mean ± s.d. (n = 3 independent experiments).
We next systematically addressed the copolymer self-assembly to load a drug (Figure 1c, Figure S9 and Table S2, Supporting Information). The critical micelle concentration (CMC) of the copolymer was 5 μg mL−1 at pH 7.4 determined by pyrene fluorescence spectroscopy. Decreasing pH from 7.4 to 6.5 resulted in increased CMC (≈16 μg mL−1 at pH 6.5), possibly associated with protonation of tertiary amine residues in the copolymer chain (Figure 1c). The antibiotic CIP was used as a model drug. To optimize the drug loading content (LC) and encapsulation efficiency (EE), CIP-loaded polymeric micelles (CIP-PMs) were prepared at different weight ratios of drug to copolymer, and the drug loading efficacy was analyzed by high performance liquid chromatography (HPLC). The LC was increased from 2.18% to 12.84% and the EE was decreased from 85.20% to 58.60% when the drug-to-copolymer ratio was increased from 1:40 to 10:40 (Table S2, Supporting Information). Furthermore, we co-loaded CIP and TPCA-1 in micelles, and the LC of CIP or TPCA-1 was ≈10% by weight when the drug-to-copolymer ratio was 1:8, respectively (Table S3, Supporting Information), indicating that our micelles are good drug carriers.
To further explore the self-assembly of copolymer and drug loading, the formation process of CIP-PMs (Figure 1d and Figure S10, Supporting Information) was studied by coarse-grained simulations using a dissipative particle dynamics (DPD) method. A CIP-PM was formed after 50 000 steps of polymer movement, showing a core-shell structure where CIP was inside the core (Figure S10, Supporting Information). Figure 1d showed a full-section view of a micellar nanoparticle where PAE (pink) and DSPE (red) moieties formed a mixed inner core of the micelle with PEG (light green) moieties on the surface. Interestingly, biotin (dark green) segments were extended on the surface of micelles, allowed for biofunctionalization via the binding of biotin to NeutrAvidin. In a cross-section view, CIP (blue) molecules were also encapsulated in the core owing to hydrophobic interaction between CIP and PAE/DSPE.
Next, we prepared blank and CIP-loaded NPs coated with biotinylated anti-mouse ICAM-1 or biotinylated rat IgG2b (NPs-anti-ICAM-1/IgG2b and CIP-NPs-anti-ICAM-1/IgG2b) using a dialysis method. We systematically studied antibody coating efficiency and effects of pH/enzyme on hydrodynamic size of NPs, distribution, and drug release. Biotinylated antibodies (Abs, biotin-anti-ICAM-1, and biotin-IgG2b) were coated on the surface of polymeric micelles via interaction between biotin and NeutrAvidin. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Figure S11a, Supporting Information) showed that Abs were successfully linked to NPs. The similar coating efficiency for anti-ICAM-1 and IgG2b was confirmed by bicinchoninic acid assay (BCA assay, Figure S11b, Supporting Information).
Next, we addressed how NPs were responsive to pH and enzymes using dynamic light scattering (DLS) and transmission electron microscopy (TEM) in vitro (Figure 1e–g and Figure S11c,d, Supporting Information NPs-IgG2b was not shown). At pH 7.4, a spherically topological structure was observed for Abs-coated NPs (Figure 1e) and it was dense, consistent with the size of 120 nm in diameter measured by DLS. The particles increased to 180 nm in diameter and their polydispersity index was higher at pH 6.5. Similarly, the TEM image showed a spherical particle, but it was swollen in acidic environment (Figure 1f). This pH-responsiveness might be associated with deprotonation/protonation of tertiary amine groups in the PAE segment when pH was lower than pKa of the copolymer. When NPs were incubated with lipase and alkaline phosphatase (ALP), the TEM image showed a large particle with several nanosized structures, and DLS showed a broad range of particle sizes (averaged hydrodynamic diameter 450 ± 100 nm) (Figure 1g, Figure S11d and Figure S12, Supporting Information). The morphological changes of NPs might be ascribed to the cleavage of ester bonds and phosphoester bonds in the copolymer, resulting in heterogeneous aggregation of NPs. Bacterial lipase and ALP are present in infectious environments,[24] so NPs would respond to local cues for their disassembling to release cargos. Figure 1h showed that the conjugation of Abs onto nanoparticle surface slightly increased the particle size, consistent with the result of SDS-PAGE in showing that Abs were linked to particle surface. The size of NPs unlikely changed 4 days after incubation in serum, suggesting that they may have high stability in vivo. When we investigated pH/enzyme-triggered drug release, we found that the drug was rapidly released in several hours at acidic pH with the presence of enzymes compared to that at normal physiological pH without enzymes (Figure 1i). Together, the results are consistent with the morphological changes of NPs measured by TEM (Figure 1e–g). We also measured zeta potential of micellar NPs at different pH, finding that NPs showed negative zeta-potentials (−6.81 mV) at pH 7.4 and positive zeta potentials below pH 6.8 (+7.35 mV at pH 6.5) (Figure 1j). P. aeruginosa strains had negative charges between pH 5.0 and 8.0 (Figure S13, Supporting Information),[26] therefore possibly increasing interactions between NPs and bacteria in the infectious site.
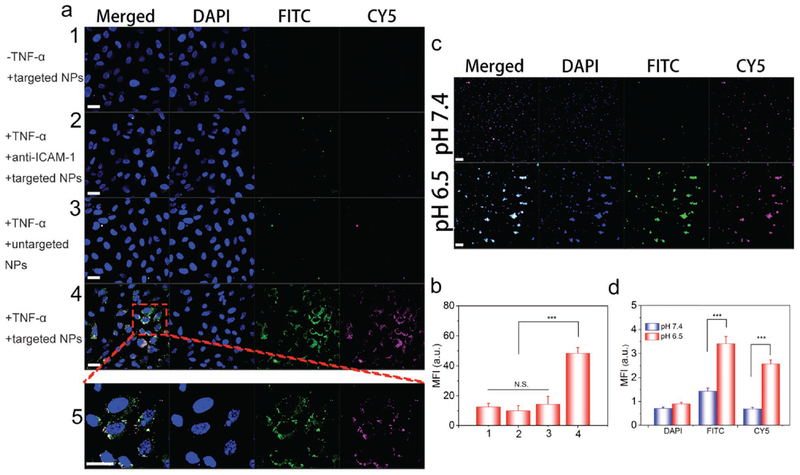
Next, we asked whether Abs-coated NPs specifically bound to inflamed endothelium. In order to visualize this interaction, we chemically labeled CIP and NPs with CY5 and FITC, respectively, and we confirmed fluorescent labeling using mass spectroscopy and UV–vis absorption (Figures S14 and S15, Supporting Information). As shown in Figure 2a and Figure S16 (Supporting Information), when human umbilical vein cells (HUVECs) were not treated with TNF-α, we did not observe the binding of anti-ICAM-1-coated NPs. When TNF-α-treated HUVECs were incubated with blocking anti-ICAM-1 before anti-ICAM-1-coated NPs were added, we observed no binding of anti-ICAM-1-coated NPs either. In another case, where TNF-α-treated HUVECs were incubated with control IgG-coated NPs, there was negligible binding of NPs. In both cases, it was unlikely that NPs bound to inflamed endothelium. However, we observed the remarkable binding of anti-ICAM-1-coated NPs to TNF-α-treated HUVECs, indicating that endothelial activation and anti-ICAM-1 coating were required for the binding of NPs to inflamed endothelium. We also quantified interactions between NPs and endothelium using flow cytometry (Figure 2b and Figure S17, Supporting Information). The mean fluorescence intensity (MFI) per cell was an indicator of interactions between NPs and endothelium. The results indicate that MFI of activated HUVECs treated with CIP-NPs-anti-ICAM-1 was increased compared to those of various controls (Figure 2b). The findings demonstrate that anti-ICAM-1 coating on NPs facilitates the binding of NPs to inflamed endothelium. Furthermore, we examined the penetration of NPs across an endothelial layer using Transwell assay. We found that the particle transport was dramatically enhanced after the endothelia were treated with TNF-α (Figure S18, Supporting Information), suggesting that inflamed endothelia may facilitate the tissue permeability of NPs.
Figure 2.
Abs-coated NPs target inflamed endothelium and bind to bacteria in vitro. a) Confocal laser scanning microscope (CLSM) images of HUVECs treated with Abs-coated NPs under various conditions. (1, unactivated HUVECs treated with CY5/FITC-labeled CIP-NPs-anti-ICAM-1; 2, activated HUVECs coated with anti-ICAM-1 and treated with CY5/FITC-labeled CIP-NPs-anti-ICAM-1; 3, activated HUVECs treated with CY5/FITC-labeled CIPNPs-IgG2b; 4, activated HUVECs treated with CY5/FITC-labeled CIP-NPs-anti-ICAM-1; 5, enlarged image of the box in image 4.) Scale bars, 20 μm. b) Flow cytometry mean fluorescence intensity of HUVECs treated with Abs-coated NPs under various conditions. c) pH-dependent interactions between CY5/FITC-labeled CIP-NPs-anti-ICAM-1 and P. aeruginosa at pH 7.4 or 6.5 imaged by CLSM. Scale bars, 10 μm. d) Fluorescence intensities of FITC and CY5 in bacteria were quantified based on confocal images. Data are shown as mean ± s.d. (n = 6 independent experiments). Statistical analysis was conducted using Student’s t-test of Origin 8.5, p values: ***p < 0.001, N.S. (no significant difference) p > 0.05.
To determine whether NPs bind to bacteria in the acidic condition, we performed fluorescence microscopy. After unbound CIP-NPs-anti-ICAM-1 was removed by centrifugation, bacteria were collected and stained using DAPI. Confocal microscopy visually confirmed that pH-sensitive CIP-NPs-anti-ICAM-1 effectively bound to bacteria (Figure 2c). The strong FITC and CY5 fluorescence intensity were observed in bacteria at pH 6.5 but not at pH 7.4 after CIP-NPs-anti-ICAM-1 were treated. Quantitative analysis demonstrated that fluorescence (FITC and CY5) of samples treated with CIP-NPs-anti-ICAM-1 at pH 6.5 showed a threefold higher than that at pH 7.4 (Figure 2d). Furthermore, flow cytometric analysis demonstrated that the interaction between bacteria and CIP-NPs-anti-ICAM-1 at pH 6.5 was significantly increased in comparison to that at pH 7.4 (Figure S19, Supporting Information). The binding of NPs to bacteria may be due to electrostatic interactions between NPs and bacteria at pH 6.5, as shown in Figure 1j.
Bacterial infection usually occurs outside vasculature[16,27] and activates the host inflammatory response. For example, endothelium on the lumen of blood vessels highly expresses ICAM-1 molecules in response to bacterial infections.[10] We asked whether anti-ICAM-1-coated NPs can specifically target the infection site and subsequently respond to the local cues for delivery of therapeutics outside vasculature. To address this question, we established a local lung infection mouse model and chemically labeled the drug and NPs.
The lung has a unique feature with many tiny air sacs (so-called alveoli) surrounded by blood capillaries to form an interface between bloodstream and airspace (Figure 3a).[13] At the interface, a monolayer of endothelium lines the lumen of a blood vessel, and a monolayer of epithelium exposes to airway. The lung infection usually occurs in airspace. The airway–blood interface is a barrier to deliver NPs to extravascular space. When bacteria invade in airway, inflammatory responses to bacterial invasion include increased lung vascular permeability, cytokine production, and vascular activation (high expression of ICAM-1).[21,28,29] Using an acute lung bacterial infection mouse model, we would address whether our designed NPs can specifically be localized in IMEs and release drugs in response to local cues induced by bacteria.
Figure 3.
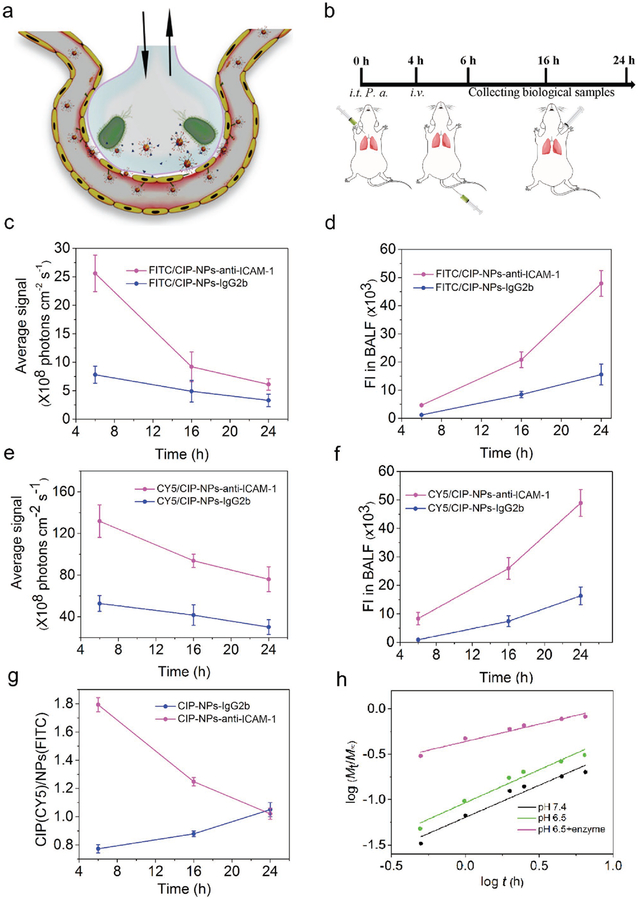
NPs-anti-ICAM-1 targeted to IMEs in vivo. a) Schematic shows an alveolus that is a major component of the lung. The alveolus contains endothelial/epithelial monolayers forming the interface of airway and bloodstream. Bacteria (P. aeruginosa) invade in the lung via airway, and subsequently activate inflammation responses to highly express ICAM-1 on the lumen of blood vessels and enhance lung permeability. NPs-anti-ICAM-1 binds to inflamed vasculature and local infection environments trigger drug release from pH/enzyme-responsive NPs. b) Experimental protocol of the acute lung inflammation model after intratracheal administration of P. aeruginosa (106 CFU per animal). c,d) Fluorescence intensity of FITC (c) and CY5 (e) of lung tissues after BALF removal from mice. d,f) Fluorescence intensity of FITC (d) and CY5 (f) in BALF. g) Fluorescence intensity ratios of CY5 to FITC in BALF. h) Drug release dynamics of CIP from Abs-coated NPs based on in vitro drug release experiments of Figure 2i. Data are shown as mean ± s.d. (n = 3 independent experiments).
First, we chemically labeled copolymer chains with FITC and CIP with CY5, respectively. Then we prepared Abs-coated (anti-mouse ICAM-1 and IgG2b) NPs via biotin-avidin interaction. After P. aeruginosa was intratracheally (i.t.) administered in the mouse lung, fluorescent CIP-loaded NPs-anti-ICAM-1/IgG2b were intravenously (i.v.) administered at 4 h post-bacterial infection. After we collected lung bronchoalveolar lavage fluid (BALF) (Figure 3b), the lung was imaged using in vivo imaging system (IVIS). We found that FITC and CY5 fluorescence in lung tissues decreased with time, however, the fluorescence intensity in BALF increased with time (Figure 3c–f). The results indicate that both drug (CY5-CIP) and micellar nanoparticles (FITC-NPs) transported from bloodstream to the lung airspace, consistent with our in vitro nanoparticle transport (Figure S18, Supporting Information). Anti-ICAM-1-coated NPs significantly increased the transport of NPs and drug into the lung compared to IgG2b-coated NPs, indicating that anti-ICAM-1 coating enhanced the deposition of NPs in infectious sites. Furthermore, we analyzed fluorescence ratios of CY5-CIP and FITC-NPs in BALF (Figure 3d,f). When NPs were coated with IgG2b antibody, the fluorescence ratios of CY5-CIP and FITC-NPs were unlikely changed, implying that the drug and NPs transported together from bloodstream to the lung airway (Figure 3g). In contrast, anti-ICAM-1-coated NPs showed more drug in BALF compared to IgG2b-NPs at 2 h after administration of NPs, and the fluorescence ratio decreased with time (Figure 3g). At 24 h, the ratio was close to that of non-targeting NPs (IgG2b-coated NPs). The results suggest that NPs bound to inflamed vasculature first, and the local cues (acids and enzymes) triggered the release of CY5-CIP, and subsequently CY5-CIP diffused in the lung. The fluorescence ratios of CY5-CIP and FITC-NPs decreased over time, implying that more NPs transported into the infected lung after CY5-CIP released from NPs.
We also analyzed the drug release mechanism based on the comprehensive semi-empirical equations,[30] as shown in Figure 3h, Figure S20 and Table S4 (Supporting Information). The release exponent parameter (n value) and the k value represents a drug release process and drug release rate, respectively. The n value is equal to 0.43 for Fickian diffusion-controlled release and 0.85 for a non-Fickian mechanism.[31] When 0.43 <n < 0.85, it indicates that drug release is anomalous transport mechanism. In case of n < 0.43, there is a transition from anomalous transport mechanism to the combination of diffusion–erosion mechanism.[32] At pH 7.4 or 6.5 without enzyme, n value was 0.70 or 0.65, suggesting that the drug release process was anomalous transport-controlled release. While k = 0.13 at pH 6.5 was higher than the value of 0.08 at pH 7.4, indicating accelerated release associated with the acidic condition. When enzymes existed, n values were less than 0.43 (0.40 for pH 6.5 with enzyme), indicating the diffusion–erosion drug release mechanism was dominated because of nanoparticle collapse induced by bacterial enzymes. The disassembling of NPs facilitated drug release. Collectively, the drug release dynamics is consistent with the results of drug release in the local lung infection model.
We first investigated the biodistribution of NPs in mice after lung infected by P. aeruginosa (Figure S21, Supporting Information). While both of CIP-NPs-anti-ICAM-1 and CIP-NPs-IgG2b existed in the liver, kidney and spleen, we found much more NPs-anti-ICAM-1 in the lung compared with NPs-IgG2b. The results indicate that targeting of ICAM-1 at the infection site via nanoparticles is able to enhance drug delivery efficacy. We also assessed the cytotoxicity of NPs in three cell lines (NHF, HUVEC, and HEK 293T). As displayed in Figure S22a–c (Supporting Information), percentages of viable cells after treatment with NPs were negligibly changed, indicating that the copolymers did not show obvious cytotoxicity for these three cell lines. In addition, we studied the inhibitory effect of free CIP and CIP-NPs-anti-ICAM-1 against P. aeruginosa at pH 7.4 or 6.5 for 24 h of incubation in vitro (Figure S22d, Supporting Information). The results show that both free CIP and CIP-loaded NPs can inhibit bacterial growth, suggesting that CIP is able to release from NPs, fully functioning as free drug CIP. At pH 6.5, CIP-NPs were more potent to kill bacteria than those at pH 7.4. This might be associated with NPs in response to acidic conditions to accelerate drug release.
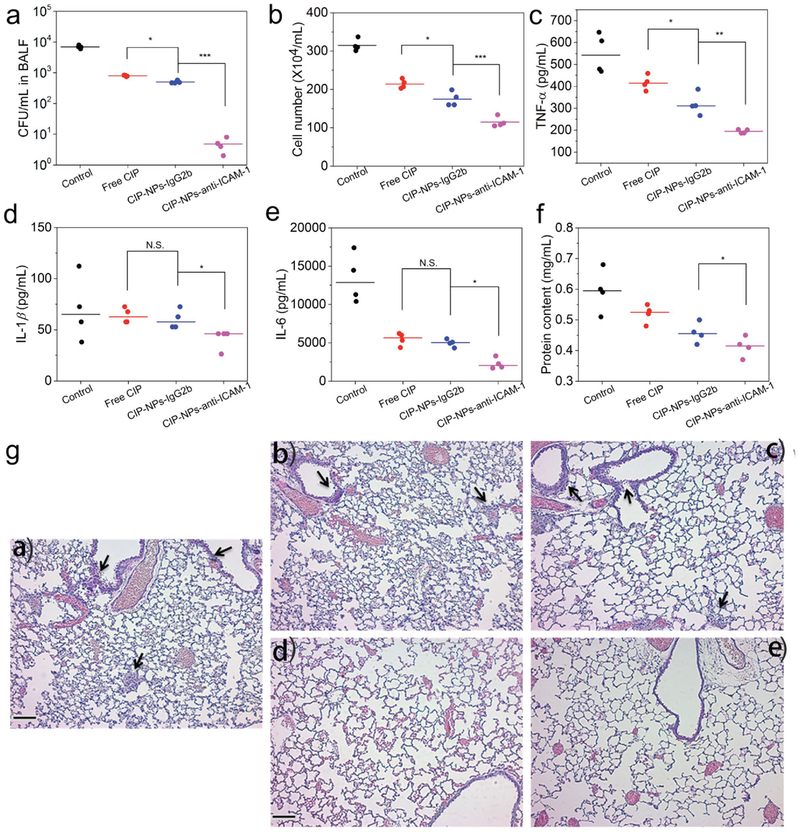
Next, we determined whether the nanoparticles can deliver CIP in infectious mouse lungs to increase bacterial clearance after P. aeruginosa was directly administered to the mouse lungs. 4 h after bacterial administration into the lungs, several therapeutic formulations were i.v. injected to the mice. 20 h later, BALF was collected to analyze bacterial burden and inflammation factors. We observed that CIP-NPs-anti-ICAM-1 remarkably inhibited bacterial proliferation compared to non-targeting NPs and free drug CIP (Figure 4a and Figure S23, Supporting Information), indicating that ICAM-1 targeting improved drug delivery to infectious lungs. The lung infiltrated leukocytes (Figure 4b and Figure S24, Supporting Information) and inflammatory factors (TNF-α, IL-1β, and IL-6) (Figure 4c–e) accordingly decreased. The mitigated inflammation response may be associated with the reduction of bacterial burden in the lungs. Interestingly, when mice were treated with CIP-NPs-anti-ICAM-1, protein concentrations in BALF decreased compared to those in the mice treated with non-targeting NPs and free drug (Figure 4f). Protein permeability in the lungs represents vasculature integrity and is associated with the inflammation. The decreased protein contents in the lung indicated lung vasculature was repaired after bacteria were eliminated.[14,29,33] Together, the results demonstrate that CIP-NPs-anti-ICAM-1 showed the higher reduction of lung bacterial burden and more effectively decreased inflammation responses (cytokines) than free CIP and non-targeting control CIP-NPs-IgG2b, thus improving lung functions. Furthermore, the lung histological studies (Figure 4g) were performed at 20 h post-administration of various CIP formulations, showing that leukocyte infiltration was dramatically decreased compared to control (PBS treatment). This result is similar to the studies in Figure 4b. Collectively CIP-NPs-anti-ICAM-1 improved the efficacy of anti-infection, implying that coating of ICAM-1 on NPs increased drug delivery into infectious tissues. We also evaluated the safety of NPs as drug carriers. The tissue histo-logical analysis (Figure S25, Supporting Information) showed that there was no obvious toxicity for Abs-coated NPs in several mouse organs (liver, heart, spleen, and kidney).
Figure 4.
CIP-NPs-anti-ICAM-1 improves bacterial clearance in the lung. a–f) CFU of bacteria (a), leukocyte number (b), TNF-α (c), IL-1β (d), IL-6 (e), and protein content (f) in BALF of mice infected by P. aeruginosa 20 h after the treatments with PBS, free drug or various drug formulations. g) Hematoxylin and eosin (H&E)-stained tissue sections of lungs of P. aeruginosa-infected mice 20 h after treatments with: a) PBS, b) free CIP, c) CIP-NPs-IgG2b, and d) CIP-NPs-anti-ICAM-1; (e) is the healthy lung. The black arrowheads indicate the infiltration of immune cells (mainly neutrophils). Scale bars: 100 μm. The data are shown as mean ± s.d. (n = 4 independent experiments). p values: *p < 0.05, **p < 0.01, ***p < 0.001, N.S. (no significant difference) p > 0.05.
Since the infectious lung mouse model showed that NPs-anti-ICAM-1 were able to target the site of infection, reducing bacterial burden (Figure 4), we asked whether co-delivery of CIP and TPAC-1 via the NPs can manage sepsis induced by a lethal dose of P. aeruginosa in a peritonitic mouse model. We first co-loaded CIP and TPCA-1 in NPs-anti-ICAM-1/IgG2b, and found that a drug loading capacity was roughly 10 wt% for each drug (Figure S9 and Table S3, Supporting Information).
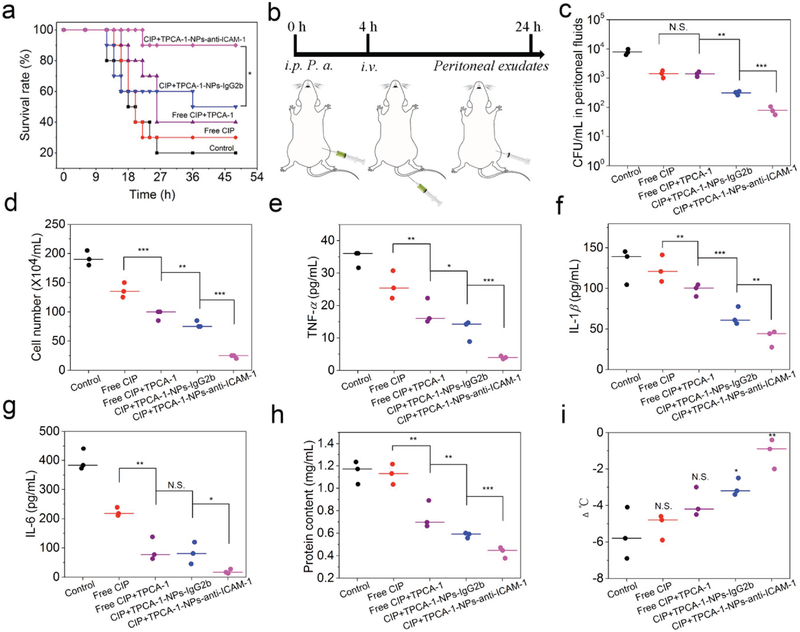
We established a mouse acute peritonitis model by intraperitoneal (i.p.) administration of a lethal dose of P. aeruginosa (1 × 107 CFU per mouse), a model commonly used to mimic sepsis.[1] We monitored the mouse survival after therapeutic treatments (n = 10) (Figure 5a). We observed that 90% mice survived in the CIP+TPCA-1-NPs-anti-ICAM-1-treatment group compared to 50% for the isotype control antibody-coated NPs. Free drug treatments showed 40% mice survived for combined treatment of CIP+TPCA-1 and 30% for free CIP. Thus, the findings reveal that enhanced therapeutic efficacy is dependent on ICAM-1 coating to NPs and the combination of CIP and TPCA-1.
Figure 5.
Co-delivery of CIP and TPCA-1 via NPs manages the sepsis induced by bacteria. a) Mouse survival in peritonitis-induced sepsis by i.p. injection of a lethal dose of P. aeruginosa. 4 h after bacterial injection, mice were treated with several drug formulations (PBS, free CIP, free CIP+TPCA-1, CIP+TPCA-1-NPs-IgG2b, and CIP+TPCA-1-NPs-anti-ICAM-1). CIP and TPCA-1 were administered at the same amount for each formulation (1.5 ± 0.1 and 1.4 ± 0.06 mg kg−1, respectively). Statistical analysis was done using Kaplan–Meier method (n = 10). *p < 0.05. b) Experimental design to evaluate pathogen burden and inflammation responses. c–h) CFU of bacteria (c), leukocyte number (d), TNF-α (e), IL-1β (f), IL-6 (g), and protein content (h) in peritoneal fluids, and i) mouse body temperature changes. N.S. (p > 0.05), *p < 0.05, **p < 0.01 versus control. All data are expressed as means ± s.d. (n = 3). p values: *p < 0.05, **p < 0.01, ***p < 0.001, N.S. (no significant difference), p > 0.05.
Next, we addressed whether mouse survival was associated with reduced pathogens and inflammation responses in the peritoneal cavity. After 4 h of i.p. administration of P. aeruginosa to mice, various formulations (PBS, free CIP, free CIP+TPCA-1, CIP+TPCA-1-NPs-IgG2b, and CIP+TPCA-1-NPs-anti-ICAM-1) were i.v. injected. Twenty-four hours later, peritoneal exudates were collected for analysis (Figure 5b). CFU in peritoneal exu-dates for CIP+TPCA-1-NPs-anti-ICAM-1 treatment group was three times lower than that of CIP+TPCA-1-NPs-IgG2b treatment (Figure 5c and Figure S26, Supporting Information). The leukocyte number and inflammatory cytokines (TNF-α, IL-1β, and IL-6) were measured (Figure 5d–g). There was a marked decrease in leukocyte number and inflammatory cytokines after mice were treated with CIP+TPCA-1-NPs-anti-ICAM-1 compared to other control groups. We also studied the systemic inflammation response and bacteria in blood. We found the similar trends of decreased bacteria burden and reduced inflammation (CFU, leukocyte number, TNF-α, IL-1β, and IL-6) after targeted drugs-loaded NPs were delivered (Figure S27, Supporting Information). The results indicate that nanoparticle targeting to IMEs suppressed the peritoneal infection, leading to the reduced systemic inflammation. Protein permeability represents the vascular integrity that is a biomarker of vasculature repair after a therapy to infections.[29] The protein contents in peritoneal exudates decreased after co-delivery of drugs via NPs-anti-ICAM-1, suggesting that inflammation was mitigated and the leakey vasculature was repaired (Figure 5h). Body temperature is associated with the systemic recovery from sepsis.[34] Figure 5i shows that CIP+TPCA-1-NPs-anti-ICAM-1 treatment maintained the normal body temperature, implying sepsis was in control after targeted therapy to IMEs.
Sepsis is one of bacterial infection diseases. Bacterial infections cause many severe and life-threatening diseases, such as endocarditis,[35] pneumonia,[36] and toxic shock syndrome.[37] Spatial and temporal co-existing of bacteria and inflammation responses are a new target to sequester bacterial infection locally, avoiding systemic spreading of bacteria and excessive inflammation responses that lead to sepsis. Our delivery platform and the concept of targeting IMEs for control of multiple signaling pathways could be envisioned in many infectious diseases. Unlikely well-studied tumor microenvironments,[38] IMEs are acute and temporary, depending on the innate immune response. In addition, IMEs possess low pH and bacterial enzymes. The unique local cues at IMEs can be exploited to design bacterium-responsive NPs for drug delivery in on-demand manners.
In summary, we have demonstrated a proof of concept that sepsis can be managed by our drug delivery systems that can specifically target IMEs to simultaneously deliver multiple therapeutics, thus clearing bacteria and alleviating inflammation responses. This study not only shows the development of a new NPs-based drug delivery system but also may shift the current nanomedicine to biology-driven design of nanotherapeutics for improving the therapies of infectious diseases. For translation of our nanoparticles in clinic, we need to formulate new ICAM-1 conjugated NPs, thus avoiding the potential immunogenicity caused by avidin.
Supplementary Material
Acknowledgements
This work was supported by National Institute of Health RO1GM116823 to Z.W. The authors are thankful to Wenjing Lin for assistance in DPD simulation, and Rock J. Mancini and Amy Nielsen for access to GPC instrument. C.Y.Z. and Z.W. conceived the idea. C.Y.Z. and J.G. conducted the experiments. C.Y.Z. and Z.W. analyzed the data and wrote the manuscript. The Washington State University Institutional Animal Care and Use Committee approved all animal care and experimental protocols used in these studies.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG, Nat. Rev. Immunol 2017, 17, 407. [DOI] [PubMed] [Google Scholar]
- [2].Deutschman CS, Tracey KJ, Immunity 2014, 40, 463. [DOI] [PubMed] [Google Scholar]
- [3].Angus DC, van der Poll T, Engl N. J. Med 2013, 369, 840. [DOI] [PubMed] [Google Scholar]
- [4].Mayr FB, Yende S, Angus DC, Virulence 2014, 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, Remick DG, Physiol. Rev 2013, 93, 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Martin GS, Expert. Rev. Anti-Infect. Ther 2012, 10, 701; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li Y, Yu H, Qian Y, Hu J, Liu S, Adv. Mater 2014, 26, 6734. [DOI] [PubMed] [Google Scholar]
- [7].Medzhitov R, Nature 2008, 454, 428. [DOI] [PubMed] [Google Scholar]
- [8].Phillipson M, Kubes P, Nat. Med 2011, 17, 1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Fink MP, Warren HS, Nat. Rev. Drug Discovery 2014, 13, 741; [DOI] [PubMed] [Google Scholar]; b) Kwon EJ, Skalak M, Bertucci A, Braun G, Ricci F, Ruoslahti E, Sailor MJ, Bhatia SN, Adv. Mater 2017, 29, 1701527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dinarello CA, Cell 2010, 140, 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Farokhzad OC, Langer R, ACS Nano 2009, 3, 16; [DOI] [PubMed] [Google Scholar]; b) Wang AZ, Langer R, Farokhzad OC, Annu. Rev. Med 2012, 63, 185; [DOI] [PubMed] [Google Scholar]; c) Wang Z, Li J, Cho J, Malik AB, Nat. Nanotechnol 2014, 9, 204; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wang Z, Tiruppathi C, Cho J, Minshall RD, Malik AB, IUBMB Life 2011, 63, 659; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Torchilin VP, Nat. Rev. Drug Discovery 2014, 13, 813; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Mitragotri S, Burke PA, Langer R, Nat. Rev. Drug Discovery 2014, 13, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) Torge A, Wagner S, Chaves PS, Oliveira EG, Guterres SS, Pohlmann AR, Titz A, Schneider M, Beck RCR, Int. J. Pharm 2017, 527, 92; [DOI] [PubMed] [Google Scholar]; b) Tureli N. Gunday, Torge A, Juntke J, Schwarz BC, Schneider-Daum N, Tureli AE, Lehr CM, Schneider M, Eur. J. Pharm. Biopharm 2017, 117, 363; [DOI] [PubMed] [Google Scholar]; c) Hussain S, Joo J, Kang J, Kim B, Braun GB, She ZG, Kim D, Mann AP, Molder T, Teesalu T, Carnazza S, Guglielmino S, Sailor MJ, Ruoslahti E, Nat. Biomed. Eng 2018, 2, 95; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Pornpattananangkul D, Zhang L, Olson S, Aryal S, Obonyo M, Vecchio K, Huang CM, Zhang LF, J. Am. Chem. Soc 2011, 133, 4132; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Mao D, Hu F, Kenry, Ji S, Wu W, Ding D, Kong D, Liu B, Adv. Mater 2018, 30, e1706831; [DOI] [PubMed] [Google Scholar]; f) Xu H, Fang Z, Tian W, Wang Y, Ye Q, Zhang L, Cai J, Adv. Mater 2018, 30, 1801100. [DOI] [PubMed] [Google Scholar]
- [13].Chu D, Gao J, Wang Z, ACS Nano 2015, 9, 11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Gao J, Chu D, Wang Z, J. Controlled Release 2016, 224, 208; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S, Yazdi IK, Zhao P, De Rosa E, Sherman MB, De Vita A, Furman N. E. Toledano, Wang X, Parodil A, Tasciotti E, Nat. Mater 2016, 15, 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].a) Badri W, Miladi K, Nazari QA, Greige-Gerges H, Fessi H, Elaissari A, Int. J. Pharm 2016, 515, 757; [DOI] [PubMed] [Google Scholar]; b) Thamphiwatana S, Angsantikul P, Escajadillo T, Zhang QZ, Olson J, Luk BT, Zhang S, Fang RH, Gao WW, Nizet V, Zhang LF, Proc. Natl. Acad. Sci. USA 2017, 114, 11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].a) Li X, Kolltveit KM, Tronstad L, Olsen I, Clin. Microbiol. Rev 2000, 13, 547; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, Chaconas G, Kubes P, Nat. Immunol 2010, 11, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC, ACS Nano 2012, 6, 4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xiong MH, Bao Y, Yang XZ, Wang YC, Sun B, Wang J, J. Am. Chem. Soc 2012, 134, 4355. [DOI] [PubMed] [Google Scholar]
- [19].Zeiler H-J, Grohe K, Ciprofloxacin 1986, 14, 14. [Google Scholar]
- [20].Podolin PL, Callahan JF, Bolognese BJ, Li YH, Carlson K, Davis TG, Mellor GW, Evans C, Roshak AK, J. Pharmacol. Exp. Ther 2005, 312, 373. [DOI] [PubMed] [Google Scholar]
- [21].Matthay MA, Ware LB, Zimmerman GA, J. Clin. Invest 2012, 122, 2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].a) Horev B, Klein MI, Hwang G, Li Y, Kim D, Koo H, Benoit DS, ACS Nano 2015, 9, 2390; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xiong M, Bao Y, Xu X, Wang H, Han Z, Wang Z, Liu Y, Huang S, Song Z, Chen J, Peek RM Jr., Yin L, Chen LF, Cheng J, Proc. Natl. Acad. Sci. USA 2017, 114, 12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xiong MH, Li Y-J, Bao Y, Yang X-Z, Hu B, Wang J, Adv. Mater 2012, 24, 6175. [DOI] [PubMed] [Google Scholar]
- [24].Xiu Z, Zhang Q, Puppala HL, Colvin VL, Alvarez PJJ, Nano Lett. 2012, 12, 4271. [DOI] [PubMed] [Google Scholar]
- [25].El Solh AA, Alhajhusain A, J. Antimicrob. Chemother 2009, 64, 229. [DOI] [PubMed] [Google Scholar]
- [26].Shephard J, McQuillan AJ, Bremer PJ, Appl. Environ. Microbiol 2008, 74, 6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rupp J, Hellwig-Burgel T, Wobbe V, Seitzer U, Brandt E, Maass M, Proc. Natl. Acad. Sci. USA 2005, 102, 3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Matthay MA, Zemans RL, Annu. Rev. Pathol 2011, 6, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mehta D, Malik AB, Physiol. Rev 2006, 86, 279. [DOI] [PubMed] [Google Scholar]
- [30].a) Siepmann J, Peppas NA, Adv. Drug Delivery Rev. 2001, 48, 139; [DOI] [PubMed] [Google Scholar]; b) Kamaly N, Yameen B, Wu J, Farokhzad OC, Chem. Rev 2016, 116, 2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ritger PL, Peppas NA, Controlled Release J 1987, 5, 37. [Google Scholar]
- [32].a) Li Y, Li H, Wei M, Lu J, Jin L, Chem. Eng. J 2009, 151, 359; [Google Scholar]; b) Costa P, Lobo J. M. Sousa, Eur. J. Pharm. Sci 2001, 13, 123. [DOI] [PubMed] [Google Scholar]
- [33].Gao J, Wang S, Wang Z, Biomaterials 2017, 135, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Peres Bota D, Lopes Ferreira F, Mélot C, Vincent JL, Intensive Care Med. 2004, 30, 811. [DOI] [PubMed] [Google Scholar]
- [35].Peters PJ, Harrison T, Lennox JL, Lancet Infect. Dis 2006, 6, 742. [DOI] [PubMed] [Google Scholar]
- [36].Madhi SA, Klugman KP, The Vaccine Trialist Group, Nat. Med 2004, 10, 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fischer M, Bhatnagar J, Guarner J, Reagan S, Hacker JK, Van Meter SH, Poukens V, Whiteman DB, Iton A, Cheung M, Dassey DE, Shieh W-J, Zaki SR, Engl N. J. Med 2005, 353, 2352. [DOI] [PubMed] [Google Scholar]
- [38].a) Quail DF, Joyce JA, Nat. Med 2013, 19, 1423; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Turley SJ, Cremasco V, Astarita JL, Nat. Rev. Immunol 2015, 15, 669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.