Abstract
Purpose
To determine if there is added benefit of using iodine maps from dual-energy (DE) CT in addition to conventional CT angiography images to diagnose pulmonary embolism (PE).
Materials and Methods
In this retrospective analysis, 1144 consecutive dual-energy CT angiography examinations performed from January through September 2014 at an oncologic referral center to evaluate for PE were reviewed. The 1144 examinations included 1035 patients (mean age, 58.7 years; range, 15–99 years). First, the location, level, and type (occlusive vs nonocclusive) of PEs on conventional CT angiograms were recorded. Iodine maps were then reviewed for defects suggestive of PE. Last, CT angiograms were rereviewed to detect additional PEs suggested by the iodine map. Consensus reviews were performed for examinations with PEs. The confidence interval of percentages was calculated by using the Clopper-Pearson method.
Results
On 147 of 1144 (12.8%) CT angiograms, a total of 372 PEs were detected at initial review. After review of the DE CT iodine map, 27 additional PEs were found on 26 of 1144 CT angiograms (2.3%; 95% confidence interval [CI]: 1.5%, 3.3%). Of the 27 additional PEs, six (22.2%) were segmental, 21 (77.8%) were subsegmental, 24 (88.9%) were occlusive, and three (11.1%) were nonocclusive. Eleven of 1144 (1.0%; 95% CI: 0.5%, 1.7%) CT angiograms had a new diagnosis of PE after review of the DE CT iodine maps.
Conclusion
Dual-energy CT iodine maps show a small incremental benefit for the detection of occlusive segmental and subsegmental pulmonary emboli.
© RSNA, 2018
Introduction
Pulmonary embolism (PE) is one of the leading causes of cardiovascular-related mortality in the United States, with an incidence of 112.3 per 100 000 (1) and a 3-month all-cause mortality rate of 3.9%–15.3% (2,3). Early diagnosis and treatment of PE are essential for optimizing clinical outcomes. Multidetector CT angiography is the current reference standard for the detection of PE, allowing visualization of contrast material within the pulmonary vasculature, and has been shown to be comparable to conventional angiography for diagnosis of PE (4). A recent meta-analysis reported the overall sensitivity of conventional CT angiography to be 82% (95% confidence interval [CI]: 78.5%, 85.5%) (5).
Dual-energy (DE) CT angiography allows differentiation of materials based on their energy absorption characteristics and has been proposed as a method to aid in the detection of PE (6–10). In the lungs, the pattern of iodine enhancement at DE CT angiography has been shown to correspond to lung blood volume at planar scintigraphy and single photon-emission CT (11,12). Thus, DE CT angiography allows the simultaneous assessment of pulmonary vasculature and parenchymal iodine distribution without excess radiation dose, compared with conventional CT angiography (8,13–16).
Several studies have reported the feasibility of using DE CT angiography for detection of PE in vivo (6–8,11,15). Moreover, an animal study by Zhang et al found DE CT angiography to improve the detection of small PEs compared with conventional CT angiography alone (10). However, larger human studies of type and location of PE detected at DE CT angiography have not been performed. We hypothesized that DE CT angiography will improve the detection of small (segmental and subsegmental) PEs that may otherwise be missed at conventional CT angiography. The purpose of our study was to determine the type of PE identified on iodine maps and to evaluate their added benefit over conventional CT angiography in the detection of PE.
Materials and Methods
Study Population and Imaging Data
Our retrospective study was approved by the institutional review board and was compliant with the Health Insurance Portability and Accountability Act. Informed consent was waived.
All patients who underwent DE CT angiography at an oncologic referral center during a 9-month period (January through September 2014) were included. DE CT angiography was performed as part of the standard clinical care for suspicion of PE, either as DE CT angiography of the chest only or as DE CT angiography of the chest with concurrent abdominopelvic imaging. A picture archiving and communication system inquiry was performed by a study investigator (E.W.) to capture all DE CT angiography examinations performed during our study period. No DE CT angiography examination was excluded, and no patient was excluded. All DE CT angiography examinations were performed on a single-tube DE CT scanner (Discovery CT750 HD; GE Healthcare, Chicago, Ill) with rapid kilovoltage peak switching with tube voltage of 130 and 80 kVp, tube current of 600 mA, pitch of 0.984, and rotation time of 0.8 seconds.
Patients who underwent CT angiography of only the chest received intravenously 120 mL iohexol (Omnipaque 300; GE Healthcare, Princeton, NJ), while those who underwent CT angiography of the chest with concurrent abdominopelvic imaging received 150 mL iohexol. Contrast material was infused at 3.0–4.0 mL/sec. A region of interest was placed over the pulmonary artery at the level of the carina (central venous access) or proximal abdominal aorta at the level of the celiac artery and infused via a peripheral intravenous catheter injection.
Imaging Assessment
Consensus reviews were performed because DE CT angiography is a relatively recent technology without an established standardized reading pattern. DE CT angiograms were retrospectively reviewed by one of three junior thoracic radiologists (2−4 years of attending thoracic radiology experience; D.H., S.H., A.P.) and then in consensus with a senior thoracic radiologist (21 years of attending thoracic radiology experience; M.G.) (Fig 1). All radiologists in our study were blinded to the clinical report. A junior thoracic radiologist first reviewed CT angiograms for the presence of PE (cutoff of contrast opacification in a pulmonary artery, visualization of thromboembolic clot). If a PE was present, the location, level (main, lobar, segmental, or subsegmental pulmonary artery), and extent (occlusive vs nonocclusive) of PE was recorded. PEs were classified as occlusive if no contrast material was visualized distal to the clot. For each examination, the iodine map was reviewed after review of the CT angiogram. Image quality on the iodine maps was recorded as either excellent (no artifacts), good (minor artifacts), moderate (still able to assess iodine distribution), or poor (impossible to assess iodine distribution). The iodine map images were then reviewed for the presence of any defect. Iodine map defects were characterized as either consistent with PE (if peripheral and wedge shaped) (Fig 2), not consistent with PE if they appeared to be caused by tumors or consolidations (Fig 3a), or band-like defects consistent with artifact, often due to cardiac motion or beam hardening from contrast material within the superior vena cava or innominate vein (Fig 3b).
Figure 1:
Dual-energy CT angiography (CTA) review scheme. Flowchart demonstrates method of consensus review.
Figure 2:
Pulmonary embolism (PE) at CT angiography (CTA) with, A, B, and without, C, D, corresponding finding on iodine map. A, Dual-energy (DE) CT angiogram in a 49-year-old man suspected of having PE. CT angiogram (left) with subsegmental occlusive PE in right lower lobe (arrow) with peripheral wedge-shaped defect (arrows) on iodine map (right). B, DE CT angiogram (left) in a 54-year-old woman suspected of having PE. CT angiogram with segmental occlusive PE in left lower lobe (arrow) with corresponding peripheral wedge-shaped defect (arrows) on iodine map (right). C, DE CT angiogram in a 60-year-old man suspected of having PE. CT angiogram (left) with segmental nonocclusive PE in right lower lobe (arrow) without corresponding finding on iodine map (right). D, DE CT angiogram (left) in a 66-year-old woman suspected of having PE. CT angiogram with subsegmental nonocclusive chronic-appearing PE in right lower lobe (arrow) without finding on iodine map (right).
Figure 3a:
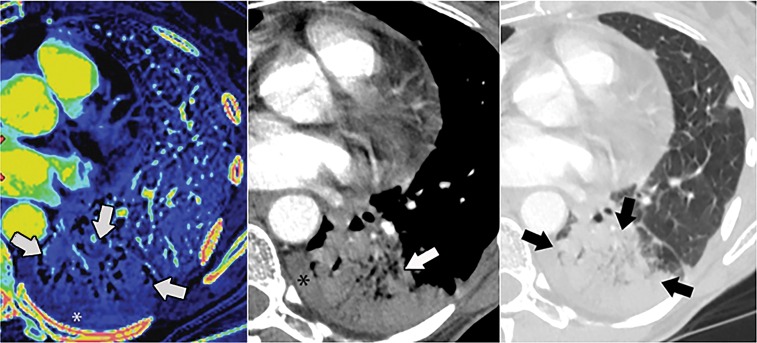
Iodine map findings not reflecting pulmonary embolism (PE). (a) Dual-energy (DE) CT angiogram in a 44-year-old woman suspected of having PE. Peripheral defect (arrows) on iodine map (left) corresponds to consolidation (arrows) in soft-tissue (middle) and lung (right) windows. * = pleural effusion. (b) DE CT angiogram in a 64-year-old woman suspected of having PE. Defect (arrows) on iodine map reflects artifact from dense contrast opacification of the right atrium.
Figure 3b:

Iodine map findings not reflecting pulmonary embolism (PE). (a) Dual-energy (DE) CT angiogram in a 44-year-old woman suspected of having PE. Peripheral defect (arrows) on iodine map (left) corresponds to consolidation (arrows) in soft-tissue (middle) and lung (right) windows. * = pleural effusion. (b) DE CT angiogram in a 64-year-old woman suspected of having PE. Defect (arrows) on iodine map reflects artifact from dense contrast opacification of the right atrium.
For examinations with iodine map defects characteristic for PE, CT angiograms were rereviewed to determine if iodine map defects corresponded to any CT angiogram findings. If a PE was present on a CT angiogram, it was noted whether it had been detected on the initial review of the CT angiograms (Fig 2) or if the PE was newly detected based on the iodine map findings (Fig 4). Patient age and sex were recorded from the picture archiving and communication system. The senior thoracic radiologist (M.G.) then reviewed the DE CT angiograms with PEs to confirm PE and iodine map findings. For any discrepancies, the senior thoracic radiologist’s reading was accepted as the standard. Chart review was performed (R.P.J.) to determine cancer diagnosis (categorized) (17). For patients who had a new diagnosis of PE based on iodine map findings, additional chart review was performed (R.P.J., E.W.).
Figure 4:
Iodine map suspicious for pulmonary embolism (PE) with, A, B, and without, C, D, CT angiography (CTA) correlate. A, B, PE initially detected on iodine map with correlate on CT angiogram on rereview. C, D, PE suspected on iodine map without correlate on CT angiogram on rereview. A, Dual-energy (DE) CT angiogram in a 64-year-old man suspected of having PE. Peripheral wedge-shaped defect (arrows) found on iodine map (left) suspicious for PE, confirmed upon rereview of CT angiogram (right, arrow). B, DE CT angiogram in a 61-year-old woman suspected of having PE. Small defect (arrows) on iodine map (left) with subsegmental PE (arrow) found on rereview on CT angiogram (right). C, DE CT angiogram in a 60-year-old woman suspected of having PE. Peripheral defects (arrows) on iodine map (left) with patent regional subsegmental pulmonary arteries (arrow) on CT angiogram (right). D, DE CT angiogram in a 40-year-old woman suspected of having PE. Peripheral defects (arrows) on iodine map (left) with patent regional subsegmental pulmonary arteries (arrows) on CT angiogram (right). * = liver dome.
Statistical Analysis
The total number of PEs detected at CT angiography was determined. The proportion of PEs by lobar location, level, and extent was calculated by a biostatistician (J.Z.). The proportion of PEs on CT angiograms with matched defects on iodine maps was calculated separately for occlusive and nonocclusive PEs. For new PEs detected on iodine maps (not detected prospectively on CT angiograms), the proportion of PEs by location, level, and extent was also calculated separately. Proportions were compared by using a McNemar paired test. The confidence interval of percentages was calculated by using the Clopper-Pearson (exact) method. Age was compared between men and women by using a Student t test. A test with a P value greater than .05 was considered to be indicative of a statistically significant difference. All statistical analyses were performed with software packages SAS 9.4 (SAS Institute, Cary, NC) by a biostatistician (J.Z., C.M.).
Results
Patient Population
A total of 1144 DE CT angiography examinations in 1035 patients were performed during our study period. For all patients, the mean age was 58.7 years (median, 60 years; range, 15–99 years); for men, the mean age was 59.1 years (range, 16–99 years); and for women, the mean age was 58.4 years (range, 15–93 years) (P = .48). All 1144 DE CT angiography examinations were included in our study and reviewed; no DE CT angiography examination was excluded. Patient demographic and clinical characteristics are described in Table 1.
Table 1:
Clinical and Demographic Characteristics of Study Group

Note.—PE = pulmonary embolism.
*Patients categorized as having “PE” or “No PE” based on study reviews including CT angiogram and iodine map interpretation.
†Data are number of patients with percentages in parentheses.
DE CT Angiography Findings
A total of 372 PEs were found in 147 of 1144 (12.8%; 95% CI: 10.9%, 15.1%) CT angiograms. Among these 372 PEs, 20 (5.4%) were in a main pulmonary artery, 76 (20.4%) were at the lobar level, 149 (40.1%) were at the segmental level, and 127 (34.1%) were at the subsegmental level; 40.9% (152) were occlusive, and 59.1% (220) were nonocclusive. Table 2 shows the distribution of PEs by location, level, and extent.
Table 2:
Characteristics of Pulmonary Embolisms Detected at CT Angiography

Note.—Data in parentheses are percentages.
Iodine Map Findings
Of 1144 iodine maps, 421 (36.8%) were of excellent quality without artifacts, 627 (54.8%) were of good quality with minor artifacts, 89 (7.8%) were of moderate quality with considerable artifacts but assessment of iodine distribution was still possible, and seven (0.6%) were of poor quality with severe artifacts precluding assessment of iodine distribution. A total of 2238 defects were found from the total 1144 iodine maps. Of these 2238 defects, 1555 (69.5%) were band-like and consistent with artifact, 513 (22.9%) were not consistent with PE and were explained by other abnormalities at CT (eg, consolidation, tumor, pleural effusion), and 170 (7.6%) were peripheral and wedge shaped and appeared consistent with PE on the iodine maps. Of the 170 defects that appeared consistent with PE on the iodine maps, 137 (80.6%) had a corresponding thromboembolism on CT angiogram and 33 (19.4%) did not have a corresponding CT angiogram finding (Fig 4C).
Comparing CT Angiograms and Iodine Maps
Among the 152 occlusive PEs found on CT angiograms, 113 (74.3%) had a matched defect on iodine maps (Fig 2) and 39 (25.7%) did not (Table 3). In comparison, 25 (11.4%) of the 220 nonocclusive PEs found on CT angiograms had a matched defect on iodine maps and 195 (88.6%) did not.
Table 3:
Pulmonary Embolisms with Matched Iodine Map Defect

Note.—Data in parentheses are percentages.
Altogether, 27 new PEs (2.3% of 1144 DE CT angiograms; 95% CI: 1.5%, 3.3%) were found in 26 examinations based on the iodine map findings and a rereview of the CT angiogram. Six (22.2%) of the 27 new PEs were segmental and 21 (77.8%) were subsegmental; 24 (88.9%; 95% CI: 70.8%, 97.6%) were occlusive and three (11.1%; 95% CI: 2.4%, 29.2%) were nonocclusive. For 15 of the 26 examinations with newly found PEs on iodine maps, PE had already been found at CT angiography examination in another lobe; for 11 of the 26 examinations (1.0% of overall DE CT angiograms; 95% CI: 0.5%, 1.7%), PE was newly diagnosed. Of the 11 patients newly diagnosed with PE, nine (81.8%) had subsegmental PEs and two (18.2%) had segmental PEs; nine (81.8%) had occlusive PEs and two (18.2%) had nonocclusive PEs; three had PEs in the right upper lobe, three had PEs in the right lower lobe, two had PEs in the left lower lobe, and three had PEs in the left upper lobe. The clinical report for the examination (both CT angiograms and iodine maps were available at time of clinical interpretation) was reviewed and the DE CT angiogram was reported as positive for PE in two of 11 cases and negative for PE in four of 11 cases. The examination was read as limited in five of 11 cases. For one of the examinations interpreted as positive for PE, the examination was initially reported as negative for PE by the on-call resident, and the attending radiologist subsequently diagnosed the PE. Treatment and outcome data for these 11 patients with a new diagnosis of PE based on iodine map findings are presented in Table 4. Comparing proportions of patients diagnosed with PE, 127 of 1035 (12.3%) patients had PE detected on conventional CT angiograms alone, compared with 138 of 1035 (13.3%) patients with diagnosis of PE at DE CT angiography (both CT angiograms and iodine maps available) (P = .001).
Table 4:
Clinical Outcome of 11 Patients with New Diagnosis of PE Based on Iodine Map Findings
Note.—PE = pulmonary embolism, AC = anticoagulation, DE = dual energy, CTA = CT angiography, DVT = deep vein thrombosis, CHF = congestive heart failure.
*Outpatient DE CT angiograms; patient not admitted following scan.
†Patients already on anticoagulation for pre-existing condition.
‡Patient transferred to outside hospital or hospice.
§On-call resident read as negative; attending radiologist read as positive for PE.
Thirty-three of 1144 (2.9%) iodine maps in 33 of 1035 (3.2%) patients had peripheral wedge-shaped defects suspicious for PE without a corresponding finding on CT angiograms. Of these 33 patients, 18 (54.5%) were treated with anticoagulation due to concurrent PE in an alternate location or other clinical indication; 20 (60.6%) died within a 6-month follow-up period; and two (6.1%) died within 14 days of the DE CT angiography examination (both of these patients had PEs detected elsewhere with CT angiography and were treated with anticoagulation).
Discussion
Our study demonstrated only a small difference in using DE CT angiography over conventional CT angiography in the detection of PE, with new PEs found using iodine maps in 2.3% of examinations and with new diagnosis of PE in 1.1% of patients. All of the additional PEs found on iodine maps were segmental or subsegmental in location, and the majority (89%) were occlusive PEs. These findings are similar to those of animal studies, which have described higher sensitivity using DE CT angiography compared with conventional CT angiography using histopathologic reference standards. For example, a rabbit study by Zhang et al found that the sensitivity for detecting PE increased from 67% to 89% using iodine maps compared with conventional CT angiography alone, and the authors highlighted the ability of iodine maps to help correctly identify subsegmental PE (10). Similarly, a canine study by Tang et al reported DE CT angiography with iodine maps to have sensitivity of 90% for subsegmental PE and 96% for subsegmental and more distal PE, compared with conventional CT angiography sensitivities of 89% and 24%, respectively (18). Segmental and subsegmental PEs may be difficult to detect anatomically due to size and frequent peripheral location. This is the first large-scale human study to report a higher number of PEs detected on DE CT angiograms compared with CT angiograms alone.
The clinical significance of small (segmental and subsegmental) PEs is controversial. It is the standard of care at our institution, a tertiary cancer care hospital, to treat all PEs, including segmental and subsegmental PEs, in cancer patients who do not have contraindications to anticoagulation. However, a study by Wiener et al comparing the incidence of PE before and after the introduction of CT angiography found that incidence of PE increased 81% between these time periods while PE mortality and case fatality minimally decreased, leading the authors to conclude that incremental PEs detected at CT angiography were overdiagnosed (1). Similarly, a study by Sheh et al compared outcomes of PEs diagnosed at ventilation-perfusion scintigraphy to those found at CT angiography and found that the fatality rate of PE decreased from 5.7% to 3.3% with increased use of CT angiography (19). This suggests that PEs detected at CT angiography are less lethal than PEs detected at scintigraphy. A study by Pena et al reported that of 18 patients diagnosed with isolated subsegmental PEs at CT angiography and not treated, none experienced recurrent venothromboembolism or death due to PE in a 3-month follow-up period (20). It is entirely possible that the additional small PEs detected on iodine maps in our study are not clinically meaningful. For 11 patients with a new diagnosis of PE based on iodine map findings, four were treated with anticoagulation, either for a pre-existing condition or because the clinical DE CT angiogram review was positive. All four of these treated patients, as well as four untreated patients (total, 72.7% [eight of 11] patients with new PE found on iodine map), died within 6 months of DE CT angiography. The cause of death for these patients was unknown in five cases, and in the remaining cases was not clearly attributable to PE. Of note, studies have reported that death related to PE usually occurs within the first 1–2 weeks of diagnosis (21,22), and only one of these 11 patients (who had been taking anticoagulation medication) died within this time frame in hospice care. Additionally, mortality in patients with cancer and PE is high even if the PE is treated, with median survival after diagnosis of PE in patients with lung cancer reported at 5.6–6.2 months (23).
In evaluating the type of PEs seen on iodine maps, we found that the majority of occlusive PEs (74%) had corresponding wedge-shaped defects on iodine maps, while only a minority (11%) of nonocclusive PEs had matched findings on iodine maps. These findings are similar to prior reports by Pontana et al describing iodine map defects in 14 of 17 occlusive PEs (82%) versus five of 51 nonocclusive PEs (10%), and by Thieme et al finding defects on iodine maps in 42 of 44 (95%) occlusive PEs and only two of 44 (5%) nonocclusive PEs (6,15). Prior studies have found the extent of perfusion defects on iodine maps to correlate with the Qanadli CT angiography obstruction index (24), which has independently been shown to correlate with PE severity as measured by pulmonary artery pressure and oxygen saturation (25). Other studies have correlated perfusion defects on iodine maps with right heart dysfunction (24,26,27). Further study to evaluate the clinical significance of iodine map defects is warranted.
Consensus reviews were performed as DE CT angiography is a relatively recent technology without an established standardized reading pattern. For our study, readers reviewed CT angiograms and noted size and location of PEs, then immediately reviewed iodine map images. CT angiograms were rereviewed to determine whether additional iodine map findings corresponded to findings on the previewed CT angiograms. No attempt was made to interpret iodine maps independently of CT angiograms. The workflow in our study mirrors the workflow of radiologists in our department, as our study aims to determine whether iodine maps help radiologists detect PE in the clinical setting. A senior thoracic radiologist reviewed all DE CT angiograms positive for PE in our study, mitigating potential “overcall” of subsegmental PEs, as one study reported 11% of examinations with an initial diagnosis of subsegmental PE were reinterpreted by a thoracic radiologist to be without any PE (20). The amount of time to interpret iodine maps was not measured as a part of this study, but was estimated at 30–60 seconds by study participants, which would not be burdensome in clinical practice.
We acknowledge several limitations of our study. First, the CT angiograms and iodine maps were not independently reviewed, so we did not try to determine the efficacy of iodine maps alone for detecting PE. Second, no attempt was made to determine whether the senior thoracic radiologist would miss PEs described by the junior radiologist. It is possible that small PEs initially missed on CT angiograms would have been detected by a more experienced radiologist. Our study included interpretations of DE CT angiography examinations by thoracic radiologists who were blinded to clinical DE CT angiography reports. No attempt was made to compare interpretations from this study to clinical DE CT angiography interpretations, which were performed by a larger pool of multiple subspecialty radiologists at our hospital. Evaluation of interobserver agreement was beyond the scope of this study, but a high rate of interobserver agreement on CT angiography for pulmonary embolism has been reported (28). Next, no invasive pulmonary angiography examination was performed for the 33 patients in our study who had DE CT iodine map findings suspicious for PE but no corresponding CT angiogram finding. The clinical significance of these defects is not well understood and was not clearly evident from the available clinical data in these 33 patients. A recent study found the volume of perfusion defects in the absence of thromboembolic clot on CT angiograms to be predictive of all-cause mortality (29). We do not have detailed long-term follow-up of all patients in our study to determine the clinical relevance of matched versus unmatched perfusion defects, although other studies have correlated iodine map defects with embolism severity, as discussed earlier. Finally, our study was conducted at an oncologic referral center in patients with high pretest probability of PE. It is possible that extra scrutiny is given to DE CT angiography in this population compared to the general population.
In conclusion, we have described the type and location of PE detected on iodine maps and have found that iodine maps helped to newly diagnose only small (segmental or subsegmental) PEs in 1% of our patients. The added diagnostic value of DE CT angiography iodine maps for pulmonary embolism is beyond what is currently available with routine CT angiography. The clinical importance of these additional newly detected, often subsegmental, pulmonary emboli remains unknown.
Summary
Iodine maps helped detect additional occlusive segmental or subsegmental pulmonary emboli.
Implications for Patient Care
■ Iodine maps helped to detect segmental or subsegmental pulmonary emboli.
■ The majority of small pulmonary emboli detected with the addition of iodine maps were occlusive.
Acknowledgments
Acknowledgments
The authors would like to thank Joanne Chin, MFA, ELS, and Sumar Hayan, BS, for assisting with the preparation of the manuscript.
Supported by the National Institutes of Health and National Cancer Institute Cancer Center Support Grant (P30 CA008748).
Disclosures of Conflicts of Interest: E.K.W. disclosed no relevant relationships. A.J.P. disclosed no relevant relationships. D.F.H. disclosed no relevant relationships. S.A.H. disclosed no relevant relationships. R.P.J. disclosed no relevant relationships. J.Z. disclosed no relevant relationships. C.M. disclosed no relevant relationships. M.S.G. disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- DE
- dual energy
- PE
- pulmonary embolism
References
- 1.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med 2011;171(9):831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonneau G, Sors H, Charbonnier B, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. The THESEE Study Group. Tinzaparine ou Heparine Standard: Evaluations dans l’Embolie Pulmonaire. N Engl J Med 1997;337(10):663–669. [DOI] [PubMed] [Google Scholar]
- 3.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999;353(9162):1386–1389. [DOI] [PubMed] [Google Scholar]
- 4.Baile EM, King GG, Müller NL, et al. Spiral computed tomography is comparable to angiography for the diagnosis of pulmonary embolism. Am J Respir Crit Care Med 2000;161(3 Pt 1):1010–1015. [DOI] [PubMed] [Google Scholar]
- 5.Hess S, Frary EC, Gerke O, Madsen PH. State-of-the-art imaging in pulmonary embolism: ventilation/perfusion single-photon emission computed tomography versus computed tomography angiography - controversies, results, and recommendations from a systematic review. Semin Thromb Hemost 2016;42(8):833–845. [DOI] [PubMed] [Google Scholar]
- 6.Pontana F, Faivre JB, Remy-Jardin M, et al. Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol 2008;15(12):1494–1504. [DOI] [PubMed] [Google Scholar]
- 7.Fink C, Johnson TR, Michaely HJ, et al. Dual-energy CT angiography of the lung in patients with suspected pulmonary embolism: initial results. Rofo 2008;180(10):879–883. [DOI] [PubMed] [Google Scholar]
- 8.Geyer LL, Scherr M, Körner M, et al. Imaging of acute pulmonary embolism using a dual energy CT system with rapid kVp switching: initial results. Eur J Radiol 2012;81(12):3711–3718. [DOI] [PubMed] [Google Scholar]
- 9.Sadigh G, Kelly AM, Cronin P. Challenges, controversies, and hot topics in pulmonary embolism imaging. AJR Am J Roentgenol 2011;196(3):497–515. [DOI] [PubMed] [Google Scholar]
- 10.Zhang LJ, Zhao YE, Wu SY, et al. Pulmonary embolism detection with dual-energy CT: experimental study of dual-source CT in rabbits. Radiology 2009;252(1):61–70. [DOI] [PubMed] [Google Scholar]
- 11.Thieme SF, Becker CR, Hacker M, Nikolaou K, Reiser MF, Johnson TR. Dual energy CT for the assessment of lung perfusion--correlation to scintigraphy. Eur J Radiol 2008;68(3):369–374. [DOI] [PubMed] [Google Scholar]
- 12.Thieme SF, Graute V, Nikolaou K, et al. Dual energy CT lung perfusion imaging–correlation with SPECT/CT. Eur J Radiol 2012;81(2):360–365. [DOI] [PubMed] [Google Scholar]
- 13.Bauer RW, Kramer S, Renker M, et al. Dose and image quality at CT pulmonary angiography-comparison of first and second generation dual-energy CT and 64-slice CT. Eur Radiol 2011;21(10):2139–2147. [DOI] [PubMed] [Google Scholar]
- 14.Schenzle JC, Sommer WH, Neumaier K, et al. Dual energy CT of the chest: how about the dose? Invest Radiol 2010;45(6):347–353. [DOI] [PubMed] [Google Scholar]
- 15.Thieme SF, Johnson TR, Lee C, et al. Dual-energy CT for the assessment of contrast material distribution in the pulmonary parenchyma. AJR Am J Roentgenol 2009;193(1):144–149. [DOI] [PubMed] [Google Scholar]
- 16.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008;248(1):254–263. [DOI] [PubMed] [Google Scholar]
- 17.Shinagare AB, Guo M, Hatabu H, et al. Incidence of pulmonary embolism in oncologic outpatients at a tertiary cancer center. Cancer 2011;117(16):3860–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang CX, Zhang LJ, Han ZH, et al. Dual-energy CT based vascular iodine analysis improves sensitivity for peripheral pulmonary artery thrombus detection: an experimental study in canines. Eur J Radiol 2013;82(12):2270–2278. [DOI] [PubMed] [Google Scholar]
- 19.Sheh SH, Bellin E, Freeman KD, Haramati LB. Pulmonary embolism diagnosis and mortality with pulmonary CT angiography versus ventilation-perfusion scintigraphy: evidence of overdiagnosis with CT? AJR Am J Roentgenol 2012;198(6):1340–1345. [DOI] [PubMed] [Google Scholar]
- 20.Pena E, Kimpton M, Dennie C, Peterson R, LE Gal G, Carrier M. Difference in interpretation of computed tomography pulmonary angiography diagnosis of subsegmental thrombosis in patients with suspected pulmonary embolism. J Thromb Haemost 2012;10(3):496–498. [DOI] [PubMed] [Google Scholar]
- 21.Nijkeuter M, Söhne M, Tick LW, et al. The natural course of hemodynamically stable pulmonary embolism: clinical outcome and risk factors in a large prospective cohort study. Chest 2007;131(2):517–523. [DOI] [PubMed] [Google Scholar]
- 22.Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med 1992;326(19):1240–1245. [DOI] [PubMed] [Google Scholar]
- 23.Shinagare AB, Okajima Y, Oxnard GR, et al. Unsuspected pulmonary embolism in lung cancer patients: comparison of clinical characteristics and outcome with suspected pulmonary embolism. Lung Cancer 2012;78(2):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chae EJ, Seo JB, Jang YM, et al. Dual-energy CT for assessment of the severity of acute pulmonary embolism: pulmonary perfusion defect score compared with CT angiographic obstruction score and right ventricular/left ventricular diameter ratio. AJR Am J Roentgenol 2010;194(3):604–610. [DOI] [PubMed] [Google Scholar]
- 25.Yu T, Yuan M, Zhang Q, Shi H, Wang D. Evaluation of computed tomography obstruction index in guiding therapeutic decisions and monitoring percutanous catheter fragmentation in massive pulmonary embolism. J Biomed Res 2011;25(6):431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer RW, Frellesen C, Renker M, et al. Dual energy CT pulmonary blood volume assessment in acute pulmonary embolism - correlation with D-dimer level, right heart strain and clinical outcome. Eur Radiol 2011;21(9):1914–1921. [DOI] [PubMed] [Google Scholar]
- 27.Apfaltrer P, Bachmann V, Meyer M, et al. Prognostic value of perfusion defect volume at dual energy CTA in patients with pulmonary embolism: correlation with CTA obstruction scores, CT parameters of right ventricular dysfunction and adverse clinical outcome. Eur J Radiol 2012;81(11):3592–3597. [DOI] [PubMed] [Google Scholar]
- 28.Domingo ML, Martí-Bonmatí L, Dosdá R, Pallardó Y. Interobserver agreement in the diagnosis of pulmonary embolism with helical CT. Eur J Radiol 2000;34(2):136–140. [DOI] [PubMed] [Google Scholar]
- 29.Takx RAP, Henzler T, Schoepf UJ, et al. Predictive value of perfusion defects on dual energy CTA in the absence of thromboembolic clots. J Cardiovasc Comput Tomogr 2017;11(3):183–187. [DOI] [PubMed] [Google Scholar]