Abstract
Objectives:
The purpose of this study was to assess the diagnostic property of intraoral bitewing radiographs (BTW) for early stage interproximal caries, and to compare them with periapical radiographs (PA) at different levels of caries progression.
Methods:
A total of 241 interproximal surfaces of BTW and corresponding PAs were used. Seven teaching faculty consisting of three oral and maxillofacial radiologists, two operative faculty, and two prosthodontists evaluated the images. The observers graded images as either “intact”, “enamel caries <1/2 width”, “enamel caries >1/2 width”, or “caries into dentin”. The gold-standard was established by consensus of two experienced faculty with 35 years and 27 years of experience. Specificity, sensitivity, positive-predictive value, and negative-predictive value were calculated for the different level of caries progression. Furthermore, receiver operating curves) of BTW and PAs of each evaluator were made and the area under the curve of BTW and PAs were compared.
Results:
There was no significant difference in the specificity of BTW and PAs. BTW showed significantly higher sensitivity than PAs in all levels of caries progression (p<0.01). Positive-predictive value and negative-predictive value of BTWs were also significantly higher than PAs. One-way ANOVA and Tukey HSD test showed a significant difference in sensitivity with different levels of caries progression. The average area under the curve was significantly higher for BTWs than PAs (p<0.01).
Conclusions:
BTWs offer a significant advantage over PAs in the diagnoses of early stages of interproximal carious lesions.
Introduction
Although the prevalence of dental caries has been declining in the United States in the last few decades, CDC reports that 53 million people have untreated tooth decay of their permanent teeth and dental caries remains a serious chronic disease.1,2 The new paradigm of operative conservatism, sometimes referred to as “minimally invasive dentistry,” incorporates the dental science of detecting, diagnosing, intercepting and treating dental caries at the microscopic level.3 Expanding the sensitivity of early caries detection would enhance the achievement of the goal of operative conservatism. There are a number of commercially available optical methods for caries detection, including digital imaging under transillumination,4,5 quantitative light-induced fluorescence,6 and laser fluorescence.7–9 Optical diagnostic tools have great potential since they exploit changes in light scattering. However, due to a lack of light penetration, application in the clinical setting, and insufficient volume of bacterial byproducts, none of these systems in their current form are effective enough in the detection of early stage caries. To date, intraoral bitewing radiographs (BTW) are still the primary diagnostic tool used for the detection of interproximal caries despite several disadvantages, including radiation exposure and discomfort. The currently digital radiology systems have significantly reduced radiation dose, save time, allow for image enhancement and simplify image storage, retrieval and transmission.10,11
It was reported that diagnosis of interproximal caries in premolar and molar teeth, observer performance was best when using intraoral BTW.10–13 However, no investigation has been conducted of their use for different levels of caries progression, specifically including carious lesions that are less than half of outer enamel.14 Radiographic images usually underestimate the actual size or depth of a carious lesion.15 By the time a lesion has visibly reached the dentino-enamel junction (DEJ) on a radiograph, the only effective therapy is a Class II restoration, that may potentially subject the tooth to a lifetime of treatment. If carious lesions can be detected when they are limited to just the enamel, especially less than half of enamel, non-invasive treatment, such as fluoride application through prescription toothpaste and varnish, and frequent maintenance visits, can be rendered to stop the progression of the carious lesion.16 Thus, the accurate diagnosis of interproximal caries lesions in their early stage is critical.
Resin-based composite restorations have largely replace dental amalgams for posterior Class II restorations replacing amalgam because of patient’s esthetic demands and less invasive tooth preparation techniques.17–21 However, a higher risk of failure has been reported for posterior resin composite restorations than for amalgam,22–27 and recent literature indicates the importance of adequate follow-up to detect secondary caries which occurred after 3 years.28 Thus, early caries detection at a stage where preventive interventions can still stop further progression is key. For the dental education in the USA, all pre-doctoral students learn how to take and interpret intraoral BTW and diagnose interproximal caries. Most USA dental insurers cover intraoral BTW every 6 months, and BTW are part of the standard of care in the USA. By contrast, some countries, such as Japan bitewing radiography is not the standard of care, and they are rarely covered by dental insurance for the diagnosis of interproximal caries.29
The goal of this study is to enhance the adoption of digital bitewing radiography for the early diagnosis of interproximal caries. The purpose of this study was to confirm the superiority of the diagnostic accuracy of intraoral BTW for early stage interproximal caries, and to compare them to periapical radiographs (PA) for different levels of caries progression.
Methods and materials
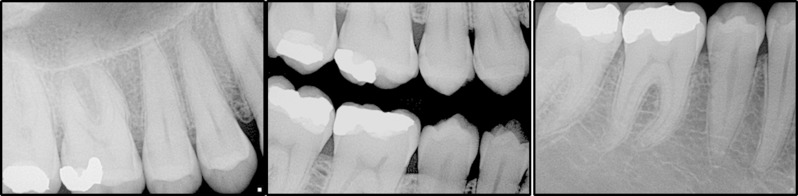
This study was performed in collaboration with Harvard School of Dental Medicine (HSDM) and Iwate Medical University School of Dentistry (IMUSD) in Japan. The study was approved by Institutional Review Board of Harvard Medical School (IRB17-1266) and Iwate Medical University (IMUSDM 002241). Full mouth intraoral radiographs consisting of 14 PA and 4 BTW taken at Harvard School of Dental Center from 2014 to 2017 were randomly exported from the electronic health record system without any personal identifiers. The radiographs were taken using a digital X-ray (Planmeca ProxTM , Renew Digital, Norcross, GA) using Schick 33 sensors (Sirona, USA) operating at 65 kVp and 7 mA at 0.08 s according to manufacturer’s settings. The images were carefully reviewed by the study PI at HSDM. Image sets were included if they contained images of posterior teeth from the distal surface of the first premolar to the mesial surface of the second molar. Images were excluded if they had interproximal restorations, cone cuts, overlapping surfaces, or if they were significantly over/under exposed or distorted. 33 sets of BTWs and the corresponding PAs were selected and saved as lossless TIF files with the original resolution. A total of 234 interproximal surfaces were used for the evaluation (Figure 1).
Figure 1.
Sample images of intraoral bitewing and periapical radiographs used for evaluation.
Seven IMUSD study participants consisting of three oral and maxillofacial radiologists with 20, 15, and 3 years of practice experience respectively, two operative faculty with 30 and 25 years of practice experience, and two prosthodontists with 27 and 10 years of practice experience, evaluated each of the images. Images were displayed on a 15-inch Dell monitor with a 1920×1080 pixel screen resolution on a computer running Windows 7. The evaluators were encouraged to adjust sharpness, contrast, and brightness as needed to aid in their analysis.
The observers examined the interproximal surfaces on the BTW images and graded them as either “intact”, “enamel caries<1/2 width”, “enamel caries>1/2 width”, or “caries into dentin”. Approximately 2 weeks later, the observers examined corresponding periapical images in the same manner.
Statistical analysis
The gold-standard for this study was established by a consensus of eight HSDM experienced faculty with 35 years and 27 years of experience. Each faculty evaluated images separately, and the consensus was obtained by discussion for any readings where there was a disagreement. Agreement between the two HSDM faculty independent grading prior to any discussion was assessed by Cohen’s κ coefficient.
Specificity, sensitivity, positive-predictive value, and negative-predictive value, values of BTW and PAs were calculated in the different level of caries progression (dentin caries, enamel caries with >1/2 of enamel width, and enamel caries with <1/2 of enamel width), and compared by the Student's t-test. The sensitivity and specificity values of three levels of caries progression were compared by one-way ANOVA and Tukey HSD post-hoc test.
Receiver operating curves (ROC) of BTW and PAs for each evaluator were made in four ranking categories with positive (dentin caries and enamel caries >1/2) and negative (enamel caries <1/2 and intact). The area under the curve (AUC) of BTW and PAs were compared.
Results
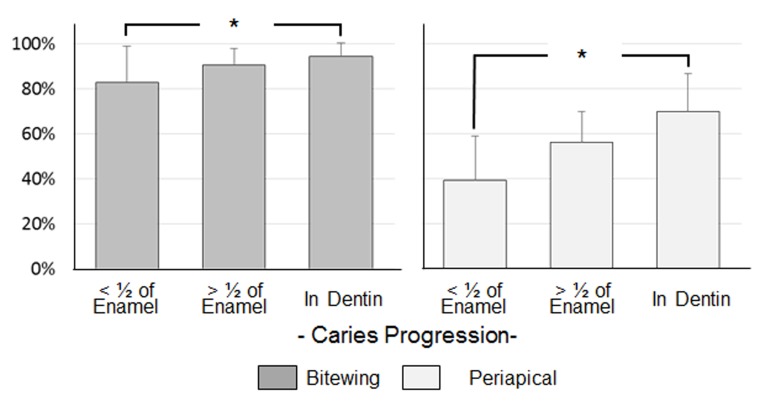
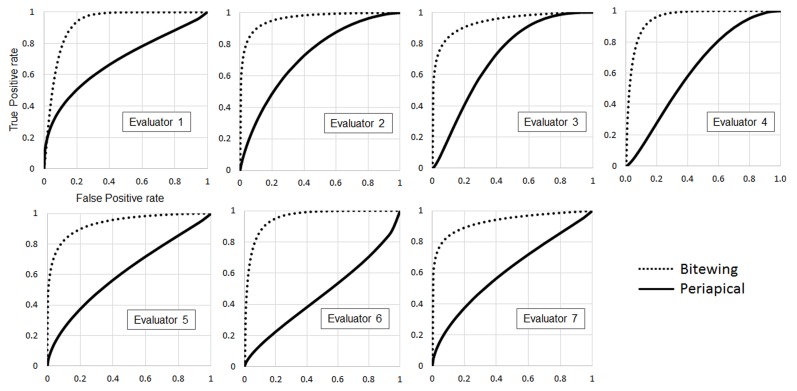
There was excellent agreement between the two HSDM faculty to establish the gold-standard, with an agreement of 90%, and Cohen’s κ coefficient of 0.8710, indicating a near-perfect agreement. There was no significant difference in the specificity of BTW and PAs. BTW showed a significantly better sensitivity than PAs for all levels of caries progression (p<0.01, Table 1). For both BTW and PAs, the positive-predictive- and negative-predictive value of BTWs were also significantly higher than PAs (Table 2). One-way ANOVA and Tukey HSD test showed a significant difference in sensitivity between dentin caries and enamel caries with <1/2 enamel thickness on both BTWs and PAs (p<0.05, Figure 2). ROC curves of BTWs and PAs diagnoses by seven evaluators are shown in Figure 3. The average of AUC (ROC) was significantly higher in BTWs (0.944 ± 0.014) than PAs (0.665 ± 0.112, p<0.01, n=7).
Table 1.
The mean specificity and sensitivity for bitewing and periapical images
| Specificity | Sensitivity | |||
| Dentin caries | Enamel caries (>1/2) | Enamel caries (<1/2) | ||
| Bitewing | 79.2 ± 16.04 | 94.55 ± 5.84 | 90.43 ± 7.42 | 82.73 ± 16.21 |
| Periapical | 68.5 ± 21.69 | 69.72 ± 16.93 | 56.22 ± 13.71 | 39.14 ± 19.82 |
Table 2.
The mean of PPV and NPV on different levels of caries progression
| Caries progression | PPV | NPV | |
| In Dentin | BTW | 0.83 ± 0.11 | 0.944 ± 0.06 |
| PA | 0.65 ± 0.21 | 0.731 ± 0.16 | |
| Enamel caries (>1/2) | BTW | 0.82 ± 0.10 | 0.910 ± 0.06 |
| PA | 0.43 ± 0.23 | 0.697 ± 0.07 | |
| Enamel caries (<1/2) | BTW | 0.62 ± 0.12 | 0.901 ± 0.08 |
| PA | 0.34 ± 0.17 | 0.711 ± 0.05 |
BTW, biteweing radiographs; NPV, negative-predictive value; PA, periapical radiographs; PPV, positive-predictive value;
Figure 2. Fig.2.
Comparison of mean sensitivity of BTW and PA in different levels of caries progression. BTW, biteweing radiographs; PA, periapical radiographs.
Figure 3.
ROCs of bitewing and periapical radiographs for seven evaluators. ROCs, receiver operating characteristics.
Discussion
In all levels of caries progression, BTWs had significantly higher sensitivity compared to the PAs. The sensitivity of dentin caries, which is typically considered the threshold in which a restorative procedure is required for therapy, is near perfect with the BTWs. However, for PAs, a mean sensitivity of approximately 70% indicates that nearly a third of lesions are being underdiagnosed, and therefore undertreated. By the time these lesions are detected radiographically by PAs, the decay would continue to extend. This could impact the treatment required to treat the lesions, ranging from requiring a larger restoration, a restoration including cuspal coverage such as a crown or onlay, or even greater decay extension into the pulp. Even enamel lesions have a mean sensitivity of 83–90% with BTWs, indicating that almost all lesions can be detected in the early stages such that noninvasive treatment, such as fluoride varnish and improving oral hygiene, can be utilized to arrest the progression of caries and possibly allow for the remineralization of the enamel.
Although the specificity is higher for BTWs than PAs, the specificity was lower in this study than in prior studies.29 However, the lower specificity can be misleading, because this study differentiates between different levels of decay, rather than simply decay or intact. In addition, the evaluators were not accustomed to using BTWs for diagnosing caries, nor for enamel caries in general, as they normally use PAs for caries diagnosis. In this study, the sensitivity for the diagnosis of enamel caries is poor. Because of this, a couple of evaluators were much more likely to overdiagnose artifacts or possible radiolucencies of healthy teeth as enamel lesions, which lowered the mean specificity. Evaluators in this study, as well as in the real world, would likely caution on diagnosing enamel caries if there is any suspicion of decay. Since noninvasive interventions would be very low cost and offer very little downside it would be better to over diagnose initial lesions. Rarely were healthy teeth misdiagnosed as dentinal lesions, which would be much more clinically significant and potentially detrimental if treated unnecessarily. This is reflected in the ROC curves, and as well as the high AUC for BTWs which offer a more comprehensive view of the effectiveness of BTWs.
Although the histological analysis is the best method for the diagnosis of caries, it is impractical and unethical to do so clinically, especially for early stage caries where invasive intervention is not necessary. Instead, the consensus of two experienced faculty of HSDM was used as the standard for comparison. The use of the calibrated faculty as a gold-standard has been previously utilized.11 With an agreement of 90% and Cohen’s κ coefficient of 0.8710 indicating a near-perfect agreement, the use of the consensus of the two faculty is a valid methodology. Artificially created lesions on extracted teeth in an ex vivo setting and mounted to mimic a clinical scenario is another method that could be used to compare BTWs and PAs, which would allow for histologic analysis and could be interesting to assess in the future and compare the results found in this study.
Another limitation of this study was a discrepancy of observer’s clinical experience and actual experience with reading BTW. BTW are not the standard diagnostic tool for diagnosing interproximal caries in Japan. IMUSDM is one of the few schools that are beginning to use them. Although there was an overall remarkably high sensitivity and specificity and large AUC with BTWs, especially for evaluators new to using BTWs, inter-rater agreement for each surface was relatively low. It would be important to calibrate the evaluators towards a diagnostic standard using BTWs, and assess the inter-rater agreement for BTWs compared to PAs in the future.
Many optical devices have been introduced for diagnosing interproximal lesions, and the recent report utilizing the near-infrared transillumination method showed a high concordance with BTW, especially for dentin caries with Cohen’s κ coefficient of 0.9, but only 0.24 for enamel caries.5 The authors also tested Near Infrared Fluorescence Imaging for interproximal enamel caries diagnosis with significantly higher sensitivity than bitewings.30 However, BTW provide a wealth of information in addition to interproximal caries, such as bone levels, restorative margins, shape and proximity of the pulp, detailed anatomy, and much more, thus BTW remain a standard tool for interproximal caries diagnosis. Using an optical method in addition to the radiograph may be a clinical application that would also be useful in acting as an additional standard for comparison in a similar study such as this, in lieu of using a histologic analysis.
Conclusion
BTW offer significant advantages to PAs in diagnosing interproximal carious lesions, even in a population where PAs are the standard diagnostic tool. BTWs have significantly better sensitivity and specificity and AUC in ROC curves compared to PAs, especially in diagnosing enamel lesions that have not reached the DEJ. In the current model of minimally invasive dentistry, it is essential that the use of BTW is extended to other countries so that early carious can be detected and treated in a cost effective and noninvasive manner.
Contributor Information
Cliff Lee, Email: cliff.lee.dmd@gmail.com.
John Daren Da Silva, Email: john_dasilva@hsdm.harvard.edu.
Hiroe Ohyama, Email: hiroe_ohyama@hsdm.harvard.edu.
Wataru Hatakeyama, Email: wataru_hatakeyama@hsdm.harvard.edu.
Shigemi Ishikawa-Nagai, Email: shigemi_nagai@hsdm.harvard.edu.
REFERENCES
- 1. Brown LJ, Wall TP, Lazar V. Trends in total caries experience: permanent and primary teeth. J Am Dent Assoc 2000; 131: 223–31. doi: 10.14219/jada.archive.2000.0151 [DOI] [PubMed] [Google Scholar]
- 2. Prevention, C.f.D.C.a.. preventing cavities, gum disease, and tooth loss : D.o.O.H. , US; 2009. http://www.cdc.gov/nccdphp/publications/aag/doh.htm. [Google Scholar]
- 3. Murdoch-Kinch CA, McLean ME. Minimally invasive dentistry. J Am Dent Assoc 2003; 134: 87–95. doi: 10.14219/jada.archive.2003.0021 [DOI] [PubMed] [Google Scholar]
- 4. Schneiderman A, Elbaum M, Shultz T, Keem S, Greenebaum M, Driller J, et al. Assessment of dental caries with Digital Imaging Fiber-Optic TransIllumination (DIFOTI): in vitro study. Caries Res 1997; 31: 103–10. doi: 10.1159/000262384 [DOI] [PubMed] [Google Scholar]
- 5. Lara-Capi C, Cagetti MG, Lingström P, Lai G, Cocco F, Simark-Mattsson C, et al. Digital transillumination in caries detection versus radiographic and clinical methods: an in-vivo study. Dentomaxillofac Radiol 2017; 46: 20160417. doi: 10.1259/dmfr.20160417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heinrich-Weltzien R, Kühnisch J, van der Veen M, de Josselin de Jong E, Stösser L. Quantitative light-induced fluorescence (QLF)--a potential method for the dental practitioner. Quintessence Int 2003; 34: 181–8. [PubMed] [Google Scholar]
- 7. Shi XQ, Welander U, Angmar-Månsson B. Occlusal caries detection with KaVo DIAGNOdent and radiography: an in vitro comparison. Caries Res 2000; 34: 151–8. doi: 10.1159/000016583 [DOI] [PubMed] [Google Scholar]
- 8. Menem R, Barngkgei I, Beiruti N, Al Haffar I, Joury E. The diagnostic accuracy of a laser fluorescence device and digital radiography in detecting approximal caries lesions in posterior permanent teeth: an in vivo study. Lasers Med Sci 2017; 32: 621–8. doi: 10.1007/s10103-017-2157-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bizhang M, Wollenweber N, Singh-Hüsgen P, Danesh G, Zimmer S. Pen-type laser fluorescence device versus bitewing radiographs for caries detection on approximal surfaces. Head Face Med 2016; 12: 30. doi: 10.1186/s13005-016-0126-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamburoglu K, Kolsuz E, Murat S, Yüksel S, Ozen T. Proximal caries detection accuracy using intraoral bitewing radiography, extraoral bitewing radiography and panoramic radiography. Dentomaxillofac Radiol 2012; 41: 450–9. doi: 10.1259/dmfr/30526171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terry GL, Noujeim M, Langlais RP, Moore WS, Prihoda TJ. A clinical comparison of extraoral panoramic and intraoral radiographic modalities for detecting proximal caries and visualizing open posterior interproximal contacts. Dentomaxillofac Radiol 2016; 45: 20150159. doi: 10.1259/dmfr.20150159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akarslan ZZ, Akdevelioğlu M, Güngör K, Erten H. A comparison of the diagnostic accuracy of bitewing, periapical, unfiltered and filtered digital panoramic images for approximal caries detection in posterior teeth. Dentomaxillofac Radiol 2008; 37: 458–63. doi: 10.1259/dmfr/84698143 [DOI] [PubMed] [Google Scholar]
- 13. Abu El-Ela WH, Farid MM, Mostafa MS. Intraoral versus extraoral bitewing radiography in detection of enamel proximal caries: an ex vivo study. Dentomaxillofac Radiol 2016; 45: 20150326. doi: 10.1259/dmfr.20150326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Senneby A, Elfvin M, Stebring-Franzon C, Rohlin M. A novel classification system for assessment of approximal caries lesion progression in bitewing radiographs. Dentomaxillofac Radiol 2016; 45: 20160039. doi: 10.1259/dmfr.20160039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White SC, Pharoah MJ. Oral Radiology-E-Book: Principles and Interpretation In: Elsevier Health Sciences; 2014. [Google Scholar]
- 16. Fejerskov O, Kidd E. Dental caries: the disease and its clinical management. 3rd Edition: The British Institute of Radiology.; 2009. [Google Scholar]
- 17. Lynch CD, Opdam NJ, Hickel R, Brunton PA, Gurgan S, Kakaboura A, et al. Guidance on posterior resin composites: Academy of Operative Dentistry - European Section. J Dent 2014; 42: 377–83. doi: 10.1016/j.jdent.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 18. Mitchell RJ, Koike M, Okabe T. Posterior amalgam restorations--usage, regulation, and longevity. Dent Clin North Am 2007; 51: 573–89. doi: 10.1016/j.cden.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 19. Jackson RD. Class II composite resin restorations: faster, easier, predictable. Br Dent J 2016; 221: 623–31. doi: 10.1038/sj.bdj.2016.856 [DOI] [PubMed] [Google Scholar]
- 20. Christensen GJ. Should resin-based composite dominate restorative dentistry today? J Am Dent Assoc 2010; 141: 1490–3. doi: 10.14219/jada.archive.2010.0112 [DOI] [PubMed] [Google Scholar]
- 21. Opdam NJ, van de Sande FH, Bronkhorst E, Cenci MS, Bottenberg P, Pallesen U, et al. Longevity of posterior composite restorations: a systematic review and meta-analysis. J Dent Res 2014; 93: 943–9. doi: 10.1177/0022034514544217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forss H, Widström E. Reasons for restorative therapy and the longevity of restorations in adults. Acta Odontol Scand 2004; 62: 82–6. doi: 10.1080/00016350310008733 [DOI] [PubMed] [Google Scholar]
- 23. Sunnegårdh-Grönberg K, van Dijken JW, Funegård U, Lindberg A, Nilsson M. Selection of dental materials and longevity of replaced restorations in Public Dental Health clinics in northern Sweden. J Dent 2009; 37: 673–8. doi: 10.1016/j.jdent.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 24. Kopperud SE, Tveit AB, Gaarden T, Sandvik L, Espelid I. Longevity of posterior dental restorations and reasons for failure. Eur J Oral Sci 2012; 120: 539–48. doi: 10.1111/eos.12004 [DOI] [PubMed] [Google Scholar]
- 25. Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: findings from the New England children’s amalgam trial. J Am Dent Assoc 2007; 138: 763–72. [DOI] [PubMed] [Google Scholar]
- 26. Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitão J, et al. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc 2007; 138: 775–83. doi: 10.14219/jada.archive.2007.0265 [DOI] [PubMed] [Google Scholar]
- 27. Ástvaldsdóttir Á, Dagerhamn J, van Dijken JW, Naimi-Akbar A, Sandborgh-Englund G, Tranæus S, et al. Longevity of posterior resin composite restorations in adults – a systematic review. J Dent 2015; 43: 934–54. doi: 10.1016/j.jdent.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 28. Rasines Alcaraz MG, et al. Direct composite resin fillings versus amalgam fillings for permanent or adult posterior teeth. Cochrane Database Syst Rev 2014; 3: Cd005620. [DOI] [PubMed] [Google Scholar]
- 29. Dove SB. Radiographic diagnosis of dental caries. J Dent Educ 2001; 65: 985–90. [PubMed] [Google Scholar]
- 30. Da Silva J GM, Nagai M, Chen G, Sun J, Stock S, Ishikawa-Nagai S. Near infrared fluorescence imaging for early caries detection. Sci J Res Dentistry 2017; 1: 027–32. [Google Scholar]