Abstract
Non-alcoholic fatty liver disease (NAFLD) is a condition that is characterized by the presence of >5% fat in the liver and affects over one billion people worldwide. If adequate and early precautions are not taken, NAFLD can progress to cirrhosis and death. The current reference standard for detecting hepatic steatosis is a liver biopsy. However, due to the potential morbidity associated with liver biopsies, non-invasive imaging biomarkers have been extensively investigated. Magnetic resonance imaging (MRI)-based methods have proven accuracy in quantifying liver steatosis; however, these techniques are costly and have limited availability. Ultrasound-based quantitative imaging techniques are increasingly utilized because of their widespread availability, ease of use, and relative cost-effectiveness. Several ultrasound-based liver fat quantification techniques have been investigated, including techniques that measure changes in the acoustic properties of the liver due to the presence of fat. In this review, we will focus on quantitative ultrasound approaches and their diagnostic performance in the realm of NAFLD.
Keywords: NAFLD, quantitative, ultrasound, attenuation, backscatter, CAP, speed of sound
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease with an estimated one billion patients globally (Loomba and Sanyal 2013). NAFLD is the most common form of hepatic steatosis and affects approximately 40% of men and 20% of women in the general population (Browning et al. 2004). Its prevalence is estimated to be 13–32% in the general population, and up to 90% in patients with diabetes or obesity as co-morbidities (Chalasani et al. 2018). NAFLD prevalence increases proportionally with obesity and other components of the metabolic syndrome and is expected to be the leading cause of liver transplantation in future (Byrne and Targher 2015; Wong et al. 2015).
NAFLD encompasses a wide spectrum of hepatic abnormalities from simple fat accumulation in greater than 5% of the hepatocytes (non-alcoholic fatty liver: NAFL) to more severe forms with pathological injury (non-alcoholic steatohepatitis: NASH) (Takahashi and Fukusato 2014). In NASH, lobular inflammation, hepatocyte ballooning and progression to fibrosis occur (Bedossa and Patel 2016). Hepatic steatosis can be present in other chronic liver diseases, like chronic hepatitis B and C, in which the rapid onset of the fibrosis process is more common (Yoon and Hu 2006). Although NAFLD does have certain pathognomonic features on a liver biopsy, it is generally considered a diagnosis of exclusion (e.g. steatosis without a known cause and in the absence of associated NASH features). Other causes of hepatic steatosis include metabolic, nutritional, and drug induced (chemotherapy and steroids) (Kneeman et al. 2012). NAFLD is accepted as the hepatic manifestation of metabolic syndrome and is strongly correlated with insulin resistance, atherosclerosis, obesity, dyslipidemia and hypertension (de Alwis and Day 2008; Targher 2007).
Risk stratification in NAFLD is performed by distinguishing NASH patients from simple steatosis patients, as NASH patients are at far higher long-term risk of cirrhosis, liver failure, and hepatocellular carcinoma (Stern and Castera 2017). The current reference standard for diagnosing NASH remains percutaneous biopsy, and biopsies currently play an essential role in staging hepatic steatosis and fibrosis (Chalasani et al. 2018). A common steatosis scoring system is the NASH Clinical Research Network (NASH CRN) scoring system: S0 (<5% hepatocytes), S1 (5%–33% hepatocytes), S2 (34%–66% hepatocytes), and S3 (>66% hepatocytes)(Kleiner et al. 2005). Unless otherwise stated, the steatosis staging system used in this review is the NASH CRN system.
A liver biopsy has well known limitations, including sampling variability and invasiveness (Goldstein et al. 2005; Nalbantoglu and Brunt 2014; Ratziu et al. 2005). Liver biopsies are not suitable for large scale screening due to the relatively high prevalence of NAFLD/NASH in the general population (Chalasani et al. 2012).
Because of the increasing prevalence of NAFLD over the past decade, there is a growing interest in the non-invasive assessment of steatosis with two main approaches: serum biomarkers and imaging biomarkers (Noureddin and Loomba 2012). Accurate quantification of the fat profile of the liver is also critical in liver donor selection, as steatosis may cause complications and is associated with perigraft mortality (Sharma et al. 2013). While there have been numerous articles looking at the NAFLD diagnostic performance of conventional imaging modalities and ultrasound approaches, there is a lack of literature on quantitative ultrasound approaches to hepatic steatosis quantification. The aim of this article is to briefly review the currently used imaging techniques to quantify hepatic fat, specifically focusing on potential advantages of ultrasound-based fat quantitation and summarize the diagnostic performance of quantitative ultrasound approaches to date. We searched the keywords ‘steatosis’, ‘quantification’, ‘ultrasound’ using the Pubmed search platform, and reviewed most recent papers. To understand the principal concepts, we used pioneer studies. To our knowledge, apart from review publications, all the citations below are based on IRB or IACUC approved studies. Area under the receiver operating curve (AUROC) value is extensively used in research to express discriminating power and diagnostic performance (Obuchowski 2004). In this review, we will use AUROC values to express the diagnostic performance of the techniques.
Overview of Conventional Imaging Modalities for Hepatic Fat Quantification
While CT and MRI are known to be able to detect and quantify liver fat, they are unsuitable for widespread use owing to multiple limitations.
Computerized Tomography (CT):
CT is known to detect liver steatosis in moderate to severe stages as reduced hepatic attenuation (Ricci et al. 1997). CT uses ionizing radiation and is therefore unsuitable for widespread use in asymptomatic patients. Furthermore, several factors, including iron, copper, and glycogen deposition, fibrosis and edema, may confound the ability of CT to diagnose hepatic fat (Limanond et al. 2004; Reeder et al. 2011). New CT-based modalities have been proposed to quantify hepatic fat, and these modalities are promising in terms of accuracy (Hyodo et al. 2017). However, given the high incidence of NAFLD and the ionizing radiation of CT imaging, CT is likely to remain unsuitable for widespread use.
Magnetic Resonance Imaging (MRI):
MRI based studies are accurate for quantification of fat, but are expensive and challenging to deploy across the large NAFLD population (Paige et al. 2017). In-phase and opposed-phased MRI and MRI Spectroscopy (MRS) have been extensively utilized for detecting liver fat (Longo et al. 1993; Mitchell et al. 1991; Satkunasingham et al. 2015). A newer MRI technique - proton density fat fraction (MR-PDFF) – offers great potential in quantifying liver fat with a diagnostic performance that may exceed liver biopsy, with the advantage of linear steatosis percentage staging (Le et al. 2012; Noureddin et al. 2013)(Figure1). MR-PDFF is superior to US and CT in diagnosing NAFL, particularly when used in combination with MRI Elastography (Bohte et al. 2011; Yoon et al. 2015). In addition, it has shown high correlation 0.69 (p<0.001) with liver biopsy evaluation (Tang et al.2013) and 0.98 (p<0.001) with MR spectroscopy (Idilman et al. 2016). A study by Idilman et al. also showed high correlation of MRIPDFF and histology-determined steatosis (r=0.743, p<0.001) while MR Spectroscopy showed equally good results(r=0.712, p<0.001) with no difference between them(z=0.19, p=0.849)(2016). As a result, MR-PDFF is also increasingly relied upon as the gold standard for hepatic fat quantification. Despite the high accuracy of MRI in hepatic fat quantification, the biggest limitations of MR-PDFF for the evaluation of NAFL and NASH are the limited availability and high cost of MRI.
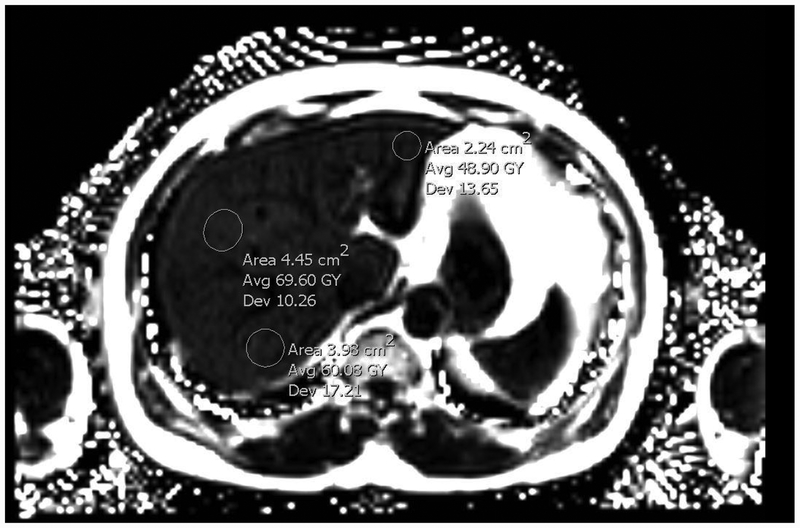
Figure1.
MR-PDFF image with multiple region of interest (ROI) showing 6%, 6.9% and 4.8% fat accumulation (Courtesy of Amirkasra Mojtahed, MD).
Conventional Ultrasound (CUS):
CUS is the most commonly used technique to assess hepatic steatosis because of its accessibility and low cost (Dasarathy et al. 2009). In addition, ultrasound can be performed easily in conscious patients, which is an advantage when imaging pediatric patients or adults unable to tolerate MRI without sedation. Conventional ultrasound uses qualitative sonographic patterns to assess the severity of hepatic fat infiltration, including increased liver echogenicity, portal vein blurring, hepatic vein blurring, posterior beam attenuation, poor diaphragm visualization, and a tightly packed echo pattern (Joseph et al. 1979; Saadeh et al. 2002)(Figure 2). CUS has low accuracy to detect steatosis (Paige et al. 2017), has intra/inter-observer variability, and is often not feasible in patients with high body mass index(BMI) (Bohte et al. 2011; Bohte et al. 2012; Strauss et al. 2007; Williamson et al. 2011).
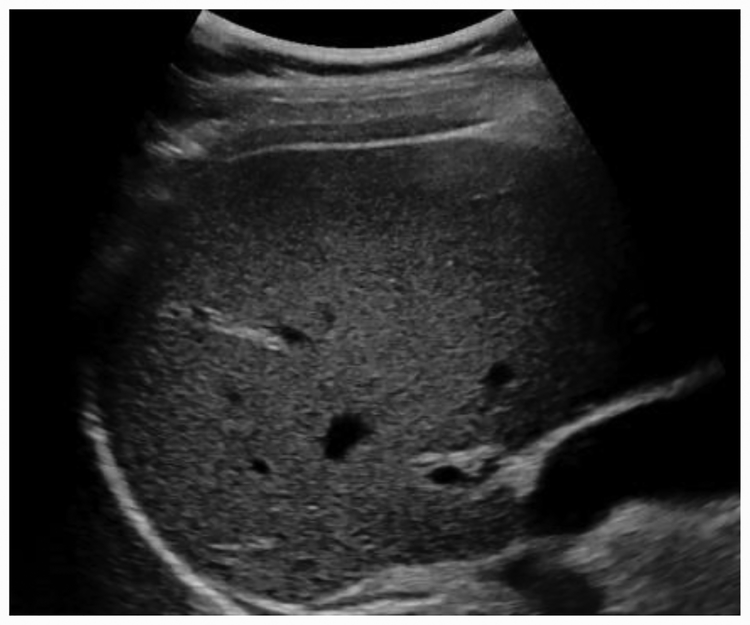
Figure2.
Difference between healthy liver and fatty liver. Increased echogenicity and poor beam attenuation are visible in figure on the right.
Quantitative Ultrasound (QUS) Approaches
Due to the limited utility of conventional ultrasound in quantifying hepatic steatosis, there has been a significant effort to develop quantitative ultrasound approaches for liver fat quantification.
Most QUS techniques use different approaches to identify tissue microstructure by measuring essential acoustic parameters (Ghoshal et al. 2012). Phantom studies have shown that quantitative US parameters are accurate, reproducible, and not operator dependent (Nam et al. 2012; Yao et al. 1990).
QUS techniques can essentially be divided into two groups: (1) image based analysis (sometimes referred to as envelope statistics and/or characterization) and (2) analysis based on the raw RF data acquired directly from the scanner. Development of the latter techniques has accelerated recently with the availability of research scanners given their ability to capture raw data including those from individual transducer elements. B-mode based techniques have been analyzed in many studies in the last few decades (Edens et al. 2009; Gaitini et al. 2004; Thijssen et al. 2008) and non-image based spectral techniques, including speed of sound, US backscatter and attenuation coefficients, have been studied for decades (Bamber et al. 1981; Chen et al. 1987; Goss et al. 1978; O’Brien Jr. et al. 1988). Summary of the studies using quantitative techniques, is presented in Table1. However, their acceptance into routine practice and into commercial scanners has been slow. The rapid advancement of computation in the last decade has improved some of the data analysis approaches. The aim of this article is to review these approaches, especially speed of sound estimation, backscatter coefficient and attenuation parameters, with a comparison of their diagnostic performance for hepatic fat quantification.
Table1.
Technical summary of the research studies using various fat quantification methods. SoS-Speed of Sound, AC-Attenuation Coefficient, BSC-Backscatter Coefficient, SWD-Shear wave dispersion
| Study | Assessed Parameter | Liver type | Ultrasound system | Probe | Frequency 1 |
|---|---|---|---|---|---|
| Imbault et al. 2017 | SoS | Phantom, healthy and CLD subjects | Supersonic imagine | XC 6–1 Curved probe | |
| Paige et al. 2017 | AC and BSC | NAFLD subjects | Siemens ACUSON S3000 | 4C1 Curved probe | 1–4.5 |
| Jaeger et al. 2015 | SoS | Phantom | Verasonics V1–64 | HDI L7–4 linear probe | 5 MHz |
| Barry et al. 2015 | SWD | Rat liver | GE Logiq E9 | L8–18i-D linear probe | 150 Hz |
| Lin et al. 2015 | BSC | Healthy and NAFLD subjects | Siemens ACUSON S3000 | 4C1 Curved probe | 1–4.5 |
| Barry et al. 2014 | SWD | Phantoms and rat liver | Ultrasonix | L40–8/12 | 200–360 Hz |
| Mahmoud et al. 2014 | SoS | Rat Liver | Ultrasonix SonixTOUCH | L14–5/38 linear Probe | 5–14MHz |
| Kumagai et al. 2014 | SoS | Rat Liver | Experimental setup | Panasonic linear Probe | 7 MHz |
| Barry et al. 2012 | SWD | Phantoms and rat liver | GE Logiq E9 | M12L Linear Probe | 100–400 Hz |
| Ghoshal et al. 2012 | SoS, AC, BSC | Rabbit liver | Experimental setup | Olympus probe | 20 MHz |
| Nam et al. 2012 | AC and BSC | Phantom | Ultrasonix RP | L14–5/38 | 6MHz |
| Thijjsen et al. 2008 | AC | Cow liver | Toshiba Power Vision 6000 | Curved probe | 4.2MHz |
| Gaitini et al. 2004 | AC and BSC | CLD subjects | Philips HDI5000 | 2–5 MHz | |
| Fujii et al. 2002 | AC | Phantom and healthy subj ects | Aloka SSD-5500 | 3.75MHz | |
| Lu et al. 1999 | AC and BSC | Phantom, healthy and CLD subjects | Siemens SL-1 | 3.5, 5MHz | |
| Zagzebski et al. 1993 | AC and BSC | Healthy subjects | Siemens Sonoline SL-1 | 3.5, 5, 7.5MHz | |
| Boote et al. 1992 | BSC | Phantom | Experimental setup | 3–5MHz | |
| Yao et al. 1990 | BSC | Phantom | ATL Mark III | 2.5–5.5 | |
| Kuni et al. 1989 | AC | Healthy and CLD subjects | 3.5MHz | ||
| Itoh et al. 1988 | AC | Phantom, healthy and CLD subjects | Aloka SSD-270 | Curved and linear probes | 3.5MHz |
| Parker et al. 1988 | AC | Healthy and CLD subjects | Ausonics Octoson | 2.5MHz | |
| O’Brien Jr. et al. 1988 | SoS and AC | Rat Liver | Experimental setup | 100 MHz | |
| Lin et al. 1987 | SoS | Excised human liver | Experimental device | 5 MHz | |
| Chen et al. 1987 | SoS | Normal and CLD | UI Octoson | 3 MHz | |
| Taylor et al. 1986 | AC | Phantom, healthy and CLD subjects | GE Datason | 3 MHz | |
| Bamber et al. 1981 | SoS, AC, BSC | Excised human liver | 1, 2.5, 4MHz |
Speed of Sound
The measurement of the speed of sound in tissue has been of major interest not only for the potential of being able to diagnose disease, but also to improve ultrasound image quality. The majority of scanners today assume a constant speed of sound of 1540 m/s throughout the entire imaging domain. This assumption is demonstrably invalid in the average human but nonetheless its use in image formation results in reasonable image quality. New methods for the estimation of longitudinal wave speed (LWS) of sound in tissue and to establish the ability of such methods to stage the progression of steatosis and fatty infiltration are increasingly being studied. Both shear wave speed (current paradigm) and the longitudinal wave speed have similar mathematical relationships to the elastic properties of tissue. Various approaches to derive these have been studied on phantoms, animal models or excised human liver samples (Bamber et al. 1981; Chen et al. 1987; Ghoshal et al. 2012; Imbault et al. 2017; Lin et al. 1987)
With most image-based methods of speed of sound estimation, characteristics of the B-mode image are analyzed for brightness or sharpness (high frequency content) near the focal zone. The technical origins of this approach are based on the work of Burckhard et al and Wagner et al., who recognized the similarity between laser and ultrasound speckle, and were able to demonstrate analytically increased brightness and reduced speckle size in the focal region (Burckhardt 1978; Wagner et al. 1983). These insights have been taken advantage of in several settings involving speed of sound, for example, image quality improvement (Napolitano et al. 2006), aberration correction (Nock and Trahey 1985), as well as several efforts in liver steatosis estimation (Kumagai et al. 2014) or in general speed of sound measurements (Gyöngy and Kollár 2015). In addition to speckle based analysis, other researchers have used seismology based methods (Anderson et al. 2000), triangulation or biprisms (Chen et al. 1987; Robinson et al. 1991), and tomographically determined phase shifts within the imaged area from different viewing angles (Jaeger et al. 2015).
Speed of sound in normal healthy liver and fatty liver has been reported as a range of 1538–1588 m/s for normal liver, and from 1423–1567 m/s for fatty liver. There is a high negative correlation between speed of sound and the fat content of the liver, with increases in the fat content of the liver resulting in a proportional reduction in the speed of sound (Bamber et al. 1981; Chen et al. 1987; O’Brien Jr. et al. 1988). As a confounding factor, the effect of temperature has been assessed by research groups. The speed of sound in normal liver increases with temperature and shows a maximum value at around 50°C. For fatty liver, a negative correlation occurs (Bamber and Hill 1979). Mahmoud et al, introduced an ultrasound technique called ultrasound induced thermal strain imaging (US-TSI) to assess fat in ex-vivo mice liver by using Bamber and Hill’s (1979) proposed speed-temperature theory(2014). US-TSI identifies fatty liver (20% fat) with 70% sensitivity, 90% specificity and an AUROC of 0.775 (Mahmoud et al. 2014). Most of the above reported studies used multiple acoustic windows with multiple transducers, but in one study, the same negative correlation between speed of sound and hepatic fat content was proposed with a single transducer (Kumagai et al. 2014).
In a recent study investigating a NAFLD population ranging from simple steatosis to cirrhosis, Imbault et al. used a complex technique including aberration correction, optimization of the spatial coherence of backscattered speckle noise for different speeds of sound thereby correcting for bias caused by variations in the intercostal layer thickness, with a reference standard of MR-PDFF and biopsy (2017). The speed of sound assessment technique proposed in this study indicated AUROC values of 0.942 and 0.952 (p<0.0001 for both) in a 16-patient study, when compared with reference standards of MR-PDFF and biopsy, respectively. They defined the speed of sound cutoff value between healthy and fatty liver as 1.555mm.μs−1 (Imbault et al. 2017).
These results are promising, and further clinical validation is needed for establishing speed of sound to quantify fat in the liver, especially for the diagnostic performance with accepting biopsy, MR-PDFF or MRS as reference. Furthermore, there is a need to ascertain clinical and biological confounders that may affect speed of sound estimation in the underlying tissue. Some of the factors that need to be accounted for include the subcutaneous fat layer overlying the liver and other pathophysiological processes that occur in the liver along with steatosis, such as fibrosis and inflammation.
Attenuation Coefficient
Ultrasound attenuation, the energy loss when a sound wave passes through a medium, depends on the frequency of the ultrasound wave and the nature of the tissue. Biochemical components of the tissue, including fat, glycogen and fibrotic tissue, directly affect the attenuation of the acoustic wave (Parker et al. 1988; Tuthill et al. 1989). The attenuation coefficient is a quantitative measure of the loss of ultrasound energy due to transmission within a medium (Lin et al. 2015). Attenuation has been studied by many research groups, and many different calculations and analyses have been proposed including spectral shift-zero crossing and sound field correction. The common point of these studies was the positive correlation between the attenuation coefficient and steatosis severity (Fujii et al. 2002; Itoh et al. 1988; Kuni et al. 1989; Taylor et al. 1986). Attenuation coefficient can be measured using various other techniques as well. For example, the spectral shift technique assumes a Gaussian spectral shape of the propagating pulse and echo and estimates the attenuation coefficient slope by measuring the downshift in the center frequency with respect to depth. A drawback of this method is that it does not account for diffraction effects. In order to remedy this, methods that use reference phantoms have been developed. One of these is the spectral difference method, which measures the decay of the power spectrum frequency components with respect to depth to estimate the attenuation coefficient as a function of frequency. Another reference-based technique is the spectral log difference method, which assumes that the attenuation has a linear frequency dependence and obtains an estimate of the attenuation coefficient slope by calculating the slope of the straight line that fits the log ratio (difference between log spectra) of the two power spectra from the proximal and the distal segments of the region of interest (ROI) (Labyed and Bigelow 2011). Additionally, the hybrid method is a recently developed technique that estimates the attenuation coefficient slope by measuring the downshift in the center frequency of the spectra that results from multiplying the power spectra of windowed segments at various depths of the ROI by a Gaussian filter (Labyed and Bigelow 2011). Some of these techniques have shown promising results with AUROC values of 0.793 (95%CI, 0.676–0.911) to detect steatosis grade S1 vs ≥ S2 and 0.804 (95%CI, 0.696–0.913) to detect steatosis grade ≤S2 vs S3(Paige et al. 2017). It has also shown high correlation values ρ=0.69 (0.53–0.81) and ρ=0.55 (0.34–0.71), with reference standard steatosis diagnostic measurements obtained via MRI-PDFF and liver biopsy, respectively (Paige et al. 2017).
That being said, however, the use of attenuation coefficients to diagnose NAFLD/NASH does have its drawbacks. Attenuation is generally measured along the path of propagation, without providing the requisite spatial information. In the case of NAFLD and NASH, a bulk attenuation coefficient might prove impractical in identifying cirrhotic or inflamed regions of the liver (Labyed and Bigelow 2010). Additionally, the estimation of attenuation coefficients from backscattered echoes is greatly affected by the presence of aberrating layers (subcutaneous fat in obese patients) and diffraction effects within the insonified region. These effects modify the spectrum of the backscattered RF signals, leading to errors in the estimated values; these errors get progressively worse with increasing depths (Labyed and Bigelow 2010). In some instances (such as when using the Spectral Fit Algorithm), explicit knowledge of the scatterers within the insonified region (density, size, distribution etc.) is required when estimating the attenuation coefficients and might prove impractical for diagnosing such a widespread condition (Labyed and Bigelow 2010)
Controlled Attenuation Parameter
The most widely clinically studied attenuation assessment method is the controlled-attenuation parameter (CAP) implemented by FibroScan (Paris, France). With CAP, total attenuation at the central frequency of the regular or M (mono-element) probe of the Fibroscan (3.5 MHz) is calculated in units of dB/m and used to estimate steatosis severity (Sasso et al. 2010). The algorithm itself is proprietary, and details of it are restricted to Fibroscan. CAP has been shown to be useful in both adult and pediatric patients with NAFLD or alcoholic liver disease (Ahn et al. 2016; Desai et al. 2016; Sasso et al. 2010; Thiele et al. 2018). The XL probe, designed for obese patients, can be used when the conventional M probe is insufficient (Chan et al. 2017). CAP measurements with both M and XL probe, can succesfully differentiate steatosis stages, at frequency value 3.5MHz (Sasso et al. 2016).
In a recent meta-analysis study of 19 CAP studies with histologic features as reference, CAP had overall AUROC values of 0.823 (95%CI, 0.809–0.837) to detect S0 vs. S1–S3, 0.865 (95%CI,0.850–0.880) to detect S0–S1 vs. S2–S3 and 0.882(95%CI, 0.858–0.906) to detect S0–2 vs. S3 level steatosis (Karlas et al. 2017). These results are concordant with other meta-analyses (Shi et al. 2014; Wang et al. 2015). A summary of some CAP studies is presented in Table 2. Other studies have shown that CAP is successful in the assessment of steatosis caused by multiple liver disease etiologies including viral, alcoholic and non-alcoholic (Ahn et al. 2016; Ferraioli et al. 2014b; Mi et al. 2014; Thiele et al. 2018). Kumar et al. also reported no difference between CAP acquisitions in HBV infected, HCV infected and NAFLD patients (p=0.592 for S0, p=0.964 for S1, p=0.062 for S2 and p=0.728 for S3) (2013).
Table2.
Summary of the research studies using CAP in patients with NAFLD. *, N/A, AUROC, LCI and UCI, denote; median values, Not available, Area under the curve, lower confidence interval and upper confidence interval respectively. ≥ S1, ≥ S2 and ≥ S3 represents fat accumulation in 5%–33%, 33%–66%, and >66% of hepatocytes, respectively.
| Study ID | N | Etiology | Reference method | Probe type | Steatosis Level | Mean/Median CAP value (dB/m) | Optimal cut-off value (dB/m) | AUROC | AUROC LCI | AUROC UCI | Sensitivity % | Specificity % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Friedrich-Rust et al. 2012 | 46 | NAFLD | Biopsy | M | ≥ S1 | 241 | N/A | N/A | N/A | N/A | N/A | N/A |
| ≥ S2 | 298 | 245 | 0.78 | 0.58 | 0.99 | 97 | 67 | |||||
| ≥ S3 | 319 | 301 | 0.72 | 0.57 | 0.86 | 76 | 68 | |||||
| Myers et al. 2012 | 153 | NAFLD | Biopsy | M | ≥ S1 | 299* | 289 | 0.79 | 0.71 | 0.87 | 68 | 88 |
| ≥ S2 | 319* | 288 | 0.76 | 0.69 | 0.84 | 85 | 62 | |||||
| ≥ S3 | 320* | 283 | 0.7 | 0.6 | 0.81 | 94 | 47 | |||||
| Kumar et al. 2013 | 63 | NAFLD | Biopsy | N/A | ≥ S1 | 213* | N/A | N/A | N/A | N/A | N/A | N/A |
| ≥ S2 | 284* | 258 | 0.79 | N/A | N/A | 78.4 | 73.1 | |||||
| ≥ S3 | 291* | 283 | 0.76 | N/A | N/A | 71.4 | 67.9 | |||||
| Chan et al. 2014 | 161 | NAFLD | Biopsy | M | ≥ S1 | 305* | 263 | 0.97 | N/A | N/A | 91.8 | 93.7 |
| ≥ S2 | 320* | 263 | 0.86 | N/A | N/A | 96.9 | 67.7 | |||||
| ≥ S3 | 324* | 281 | 0.75 | N/A | N/A | 100 | 53.1 | |||||
| Karlas et al. 2014 | 50 | NAFLD | Biopsy | M | ≥ S1 | 253 | 233.5 | 0.93 | 0.86 | 1 | 93 | 87 |
| ≥ S2 | 321 | 268.5 | 0.94 | 0.88 | 0.99 | 97 | 81 | |||||
| ≥ S3 | 335 | 301.2 | 0.82 | 0.7 | 0.93 | 82 | 76 | |||||
| Imajo et al. 2016 | 142 | NAFLD | Biopsy | M | ≥ S1 | 262.9 | 236 | 0.88 | 0.8 | 0.95 | 82.3 | 91 |
| ≥ S2 | 289.6 | 270 | 0.73 | 0.64 | 0.81 | 64.3 | 73.6 | |||||
| ≥ S3 | 304.9 | 302 | 0.7 | 0.58 | 0.83 | 64.3 | 73.6 | |||||
| Sasso et al. 2016 | 59 | NAFLD | MRI fat fraction | M | S≥ 2% | 275 | 251 | 0.83 | 0.71 | 0.95 | 78 | 78 |
| S≥ 8% | 276 | 267 | 0.87 | 0.78 | 0.97 | 80 | 79 | |||||
| S≥ 16% | 268 | 299 | 0.92 | 0.85 | 0.99 | 92 | 88 | |||||
| XL | S≥ 2% | 279 | 254 | 0.84 | 0.73 | 0.95 | 83 | 78 | ||||
| S≥ 8% | 272 | 270 | 0.90 | 0.82 | 0.99 | 88 | 79 | |||||
| S≥ 16% | 275 | 301 | 0.91 | 0.83 | 0.99 | 92 | 81 | |||||
| de Ledinghen 2016 | 261 | NAFLD | Biopsy | M | ≥ S1 | 264 | N/A | N/A | N/A | N/A | N/A | N/A |
| ≥ S2 | 331 | 310 | 0.8 | 0.73 | 0.86 | 79 | 71 | |||||
| ≥ S3 | 336 | 311 | 0.66 | 0.59 | 0.72 | 87 | 47 | |||||
| Lee et al. 2016 | 183 | NAFLD | Biopsy | N/A | ≥ S1 | 265* | 247 | 0.95 | 0.92 | 0.98 | 88.2 | 100 |
| ≥ S2 | 313* | 280 | 0.85 | 0.79 | 0.91 | 84.7 | 80 | |||||
| ≥ S3 | 322* | 300 | 0.72 | 0.64 | 0.80 | 72.7 | 60.7 | |||||
| Chan et al. 2017 | 57 | NAFLD | Biopsy | M | ≥ S1 | 324* | 266 | 0.94 | 0.86 | 0.98 | 91.1 | 87 |
| ≥ S2 | 321* | 266 | 0.80 | 0.69 | 0.88 | 91.1 | 87 | |||||
| ≥ S3 | 330* | 267 | 0.69 | 0.57 | 0.79 | 100 | 40.6 | |||||
| XL | ≥ S1 | 339* | 271 | 0.97 | 0.90 | 0.99 | 94.6 | 91.3 | ||||
| ≥ S2 | 345* | 271 | 0.81 | 0.71 | 0.89 | 95.3 | 61.1 | |||||
| ≥ S3 | 345* | 304 | 0.67 | 0.56 | 0.77 | 80 | 54.7 | |||||
| Park et al. 2017 | 104 | NAFLD | Biopsy | M and XL | ≥ S1 | N/A | 261 | 0.85 | 0.75 | 0.96 | 71.8 | 85.7 |
| ≥ S2 | N/A | 305 | 0.70 | 0.58 | 0.82 | 63.3 | 68.8 | |||||
| ≥ S3 | N/A | 312 | 0.73 | 0.58 | 0.89 | 63.6 | 70.1 | |||||
| Siddiqui et al. 2018 | 393 | NAFLD | Biopsy | M and XL | ≥ S1 | 306* | 285 | 0.76 | 0.64 | 0.89 | 80 | 77 |
| ≥ S2 | 340* | 311 | 0.70 | 0.64 | 0.75 | 77 | 57 | |||||
| ≥ S3 | 340* | 306 | 0.58 | 0.51 | 0.64 | 80 | 40 | |||||
| Garg et al. 2018 | 76 | NAFLD | Biopsy | XL | ≥ S1 | 320* | 323 | 0.75 | 0.61 | 0.89 | 58.6 | 83.3 |
| ≥ S2 | 354* | 336 | 0.74 | 0.62 | 0.86 | 73.9 | 75.5 | |||||
| ≥ S3 | 362* | 357 | 0.82 | 0.73 | 0.91 | 100 | 77.8 | |||||
| Caussy et al. 2018 | 119 | NAFLD | MRI-PDFF | M and XL | ≥5% | 315 | 288 | 0.80 | 0.70 | 0.90 | 75 | 77.1 |
| ≥10% | N/A | 306 | 0.87 | 0.80 | 0.94 | 78.6 | 82.5 | |||||
| Chan et al. 2018 | NAFLD | Biopsy | M | ≥ S1 | 293* | 253 | 0.84 | 0.78 | 0.89 | 92.6 | 70.6 | |
| ≥ S2 | 327* | 294 | 0.76 | 0.69 | 0.82 | 84.8 | 58.8 | |||||
| ≥ S3 | 330* | 294 | 0.61 | 0.54 | 0.69 | 87.9 | 36.1 | |||||
| XL | ≥ S1 | 302* | 279 | 0.91 | 0.85 | 0.94 | 82.8 | 88.2 | ||||
| ≥ S2 | 342* | 303 | 0.78 | 0.71 | 0.84 | 79.5 | 64.7 | |||||
| ≥ S3 | 351* | 325 | 0.65 | 0.58 | 0.72 | 75.8 | 54.4 | |||||
| Runge et al. 2018 | 55 | NAFLD | Biopsy | M | ≥ S1 | N/A | 260 | 0.77 | 0.63 | 0.87 | 90 | 60 |
| ≥ S2 | N/A | 296 | 0.78 | 0.65 | 0.88 | 92.3 | 55.2 | |||||
| ≥ S3 | N/A | 334 | 0.78 | 0.65 | 0.88 | 77.8 | 76.1 |
Steatosis assessment is critical in the living-donor liver transplantation setting as hepatic steatosis is associated with graft loss (Stern and Castera 2017). A combination of CAP and elastographic liver fibrosis assessment with the transient elastography function of FibroScan(Figure 3) can be used preoperatively to comprehensively assess the graft liver condition (Mancia et al. 2015). Elastography attempts to estimate the speed of propagation of shear waves within a medium to estimate its stiffness (elasticity), which is often indicative of disease progression (Ozturk et al. 2018). CAP is a useful technique to exclude potential liver donor candidates with a steatosis level >33% (Hong et al. 2017).
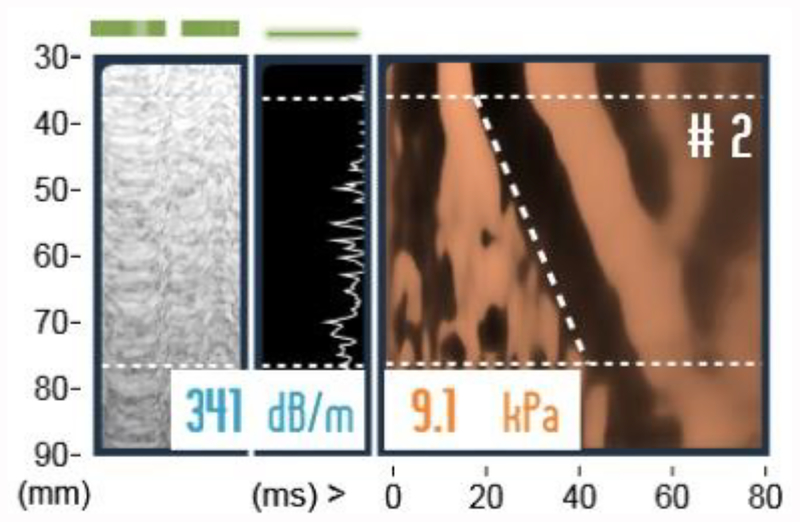
Figure3.
Time-motion(TM) mode, Amplitude(A) mode and shear wave propagation map, respectively. TM and A modes are used to locate ideal liver part for reliable acquisitions. Shear wave propagation image.y-axis is distance from skin, x-axis is time. Slope of the dashed line is shear wave speed(Vs). Median CAP shows 289dB/m, and median stiffness value shows 7.1kPa.
CAP has been compared with other imaging modalities in steatosis assessment. Comparing CAP and conventional ultrasound (CUS) based Hamaguchi steatosis scoring system, Carvalhana et al. reported a correlation of ρ=0.75(P<0.001) between ultrasound and CAP (2014). Several research groups compared the diagnostic accuracy rates of CAP and MR based methods (Imajo et al. 2016; Karlas et al. 2014; Park et al. 2017). Park et al. showed that MR-PDFF had diagnostic AUROC values of 0.90 (95% CI, 0.82–0.97) for diagnosing S2–S3 vs S0–1 and 0.92 (95% CI, 0.84–0.99) for diagnosing S3 vs S0–2, while CAP AUROC values were 0.70 (95% CI, 0.58–0.82) and 0.73 (95% CI, 0.58–0.89), respectively (2017). Direct comparison of these two techniques showed that MRI-PDFF is significantly superior at each steatosis grade (p=0.0091 for S1–3 vs S0, p=0.0017 for S2–3 vs S0–1, p=0.0238 for S3 vs S0–2) (Park et al. 2017).
CAP also correlates with several other biomarkers of hepatic fat: BMI (ρ=0.55), waist circumference (ρ=0.62), fatty liver index (ρ =0.69), Steatotest (ρ=0.308), triglyceride levels (ρ=0.49), HOMA-IR (ρ =0.26), cholesterol levels (ρ=0.19) and fasting C-peptide (ρ=0.402) levels (Carvalhana et al. 2014; Chon et al. 2016; de Ledinghen et al. 2012).
Although CAP is an accurate and feasible technique to quantify steatosis, it has some limitations that needs to be considered. At the end stage of NAFLD fibrosis, cirrhosis, normal attenuation values may be observed, implying that attenuation may not be a reliable measure of steatosis in severe diffuse liver disease (Maklad et al. 1984). Other confounders for attenuation measurements as a measure of hepatic fat include: (1) attenuation coefficient changes with temperature, with 40 °C being a turning point (Bamber and Hill 1979), (2) Prolonged fatty diet (6 weeks) may increase the attenuation coefficient by 90% (Ghoshal et al. 2012), (3) High skin to capsule distance (SCD) may cause overestimation of steatosis level, although there is no significant difference between AUROC values in patient groups SCD < 25mm and SCD > 25mm (Shen et al. 2015) (4) CAP measurements are affected by the intake of meals prior to examination and as such, a minimum fast of 150 minutes is suggested prior to examinations (Ratchatasettakul et al. 2017). (5) CAP value improvements occur after short term low-calorie diet and vitamin D correction (Arslanow et al. 2016; Papapostoli et al. 2016).
CAP has been shown to be more cost-effective but inferior to liver biopsy in the diagnosis of steatosis (Thavorn and Coyle 2015). CAP is available on the same platform that can perform transient elastography. Synchronous assessment of liver steatosis and fibrosis from the same region of the liver may be helpful when performing risk stratification in NAFLD.
Backscatter Coefficient (BSC)
Backscatter coefficient is the quantitative value of the ultrasound signals (echoes) returned from the tissue under examination (Lin et al. 2015). It is defined as the differential scattering cross-section per unit volume in the 180° direction (Sigelmann and Reid 1973). Generally, the higher the echogenicity observed, the higher the BSC values (Lin et al. 2015). In vivo and in vitro studies have shown positive correlation between backscatter and steatosis (Bamber et al. 1981; Boote et al. 1992; Zagzebski et al. 1993). Several techniques derived from backscatter coefficient including effective scatterer diameter (ESD) and effective acoustic concentration (EAC) have shown similar correlations (Ghoshal et al. 2012).
The diagnostic performance of BSC to detect steatosis grade S1 vs ≥S2 was shown to have an AUROC value of 0.854 (95%CI, 0.748–0.961). The AUROC value for detecting steatosis grade ≤S2 vs S3 was 0.830 (95%CI, 0.719–0.942) (Paige et al. 2017). BSC correlates with steatosis measured by MRI-PDFF and liver biopsy with Spearman correlation values ρ=0.72 (0.57–0.83) and ρ=0.67 (0.49–0.79) (Paige et al. 2017). In a study comparing healthy volunteers and patients with diffuse liver disease, Lu et al. reported 14 times higher BSC values for fatty liver than healthy liver (6.8×10−3cm−1sr−1 vs 0.5×10−3cm−1sr−1, p<0.0001) (1999).(Lu et al. 1999)
Shear Wave Dispersion
Dispersion is defined as frequency dependence of shear wave speed and shear wave attenuation(Barry et al. 2012). By evaluating the crawling wave pattern at different frequencies, shear wave velocity and attenuation can be monitored (Barry et al. 2012). Using this technique, researchers found that hepatic fat accumulation is associated with increased dispersion (Barry et al. 2012). The accepted unit for dispersion is ‘m/s per Hz’. Current knowledge of shear wave dispersion is mainly from animal models, with limited clinical data available. Barry et al. showed increased dispersion with increasing steatosis in mice (2014). This finding suggests that dispersion measurement techniques may have a role in staging hepatic steatosis. However, a clinical study of 135 NAFLD patients demonstrated poor performance of dispersion for predicting steatosis, with an AUROC of 0.47 (Nightingale et al. 2015). Further studies are needed to validate this technique in clinical settings. A possible confounding factor in dispersion measurement is increased abdominal fat thickness (Barry et al. 2012). The accuracy of this technique in early hepatic steatosis as well as steatosis in the presence of concomitant fibrosis still require validation (Barry et al. 2014; Barry et al. 2015).
Conclusions/Discussion
Hepatic steatosis evaluation is important in the assessment of chronic liver disease, particularly for the NAFLD spectrum of disease (NAFL or NASH), metabolic syndrome, and alcoholic liver disease. The increasing incidence of NAFLD disease worldwide, and the realization that steatosis alone (NAFL) can progress to clinically significant fibrosis (McPherson et al. 2015; Sharma et al. 2015), make hepatic steatosis an important public health concern, with a risk of future cirrhosis requiring liver transplantation (Farrell and Larter 2006). Percutaneous biopsy is not practical for large scale steatosis screening due to its invasive nature, high cost, and diagnostic limitations (Nalbantoglu and Brunt 2014). Several imaging biomarkers have been explored to detect and quantify hepatic steatosis, including ultrasound, CT and MRI based techniques. The non-invasive nature of these techniques is ideally suited to serial imaging assessment of steatosis and potentially provides a non-invasive endpoint of therapeutic efficacy. Conventional US is cost effective and easy to implement but lacks quantitative assessment (Lee and Park 2014). CT based modalities are accurate and sensitive but involve ionizing radiation exposure. MR based modalities like standard MRI, MR spectroscopy and MR-PDFF are the most accurate modalities; however, high cost and limited availability limit their broad use. Consequently, lower cost, easier to perform bedside tests such as serum biomarkers or ultrasound techniques are the biomarkers of choice for serial assessment of hepatic steatosis.
While imaging based ultrasound quantification methods hold promise, quantification methods using underlying ultrasound data acquisition are a more robust approach. Among these, CAP has been the most widely studied. This has resulted in the generation of clinical data regarding accuracy of the technique in hundreds of patients. Though some of these studies have shown good performance (AUROC value of 0.97) (Chan et al. 2014), others have not shown such good results (AUROC value of 0.65) (Kumar et al. 2013).
There is limited data for the performance of attenuation coefficient, backscatter coefficient and speed of sound techniques in the clinical setting; however, these quantitative ultrasound techniques are highly dependent on tissue structure and have shown high correlation with an increase in the histology-determined steatosis stage (Imbault et al. 2017; Lin et al. 2015; Paige et al. 2017). QUS techniques have the potential to be reliable techniques with minor inter-operator variability. CAP is also an operator friendly technique with a high inter-rater reliability concordance correlation coefficient (CCC) value of 0.82 (95%CI, 0.78–0.85) (Ferraioli et al. 2014a). Reliability of attenuation coefficient and backscatter coefficient have been studied with phantoms and in clinical settings (Han et al. 2017; Paige et al. 2017). Attenuation coefficient has high inter-rater reliability with 90% (79.5–96.2) agreement value between operators (p<0.0001) (Paige et al. 2017). Backscatter coefficient has a relatively high inter-rater reliability with 71.7% (58.6–82.5) agreement value between operators (p=0.0012) (Paige et al. 2017).
Differentiation of NASH from simple steatosis is also critical. Speed of sound, attenuation, CAP and BSC are promising in terms of sensitivity and feasibility to detect steatosis, but current studies have focused on NAFLD patients without attempting to discriminate NASH patients from simple steatosis. Inflammation in the liver may influence quantitative techniques (Matsuhashi et al. 1996), so NASH discrimination may be a fruitful area for future research. In summary, quantification techniques using ultrasound based approaches hold significant promise. Larger clinical trials are needed to see if these approaches can be used in combination for achieving a robust hepatic fat quantification technique that provides an alternative to MR based approaches.
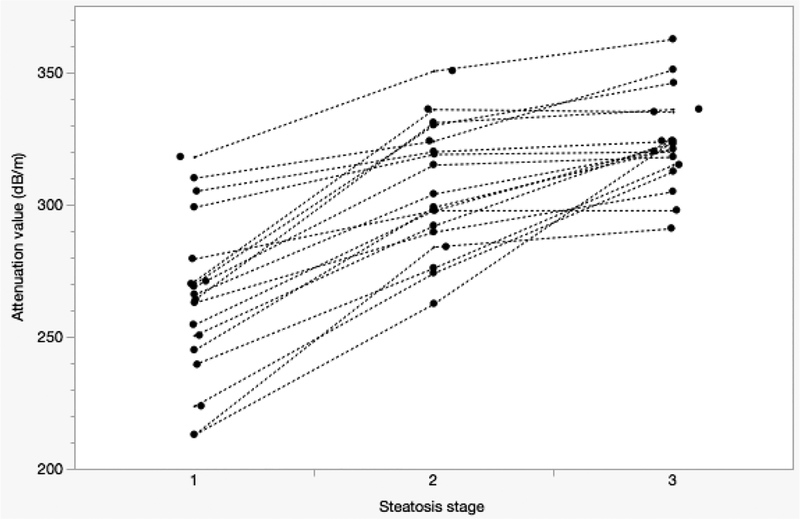
Figure4.
Spagetti plot of mean/median of attenuation values from multiple research groups using controlled attenuation parameter. X axis represents steatosis stage (S1–3). Y axis represents controlled attenuation parameter in dB/m units. Attenuation value increases at higher steatosis stages (Sasso et al. 2010, Myers et al. 2012, Kumar et al. 2013, Wang et al. 2014, Ferraioli et al. 2014, Chan et al. 2014, Shen et al. 2014, Chon et al. 2014, Mi et al. 2014, Karlas et al. 2014, Lupsor-Platon et al. 2015, Shen et al. 2015, Imajo et al. 2016, de Ledinghen et al. 2016, Ahn et al. 2016, Kwok et al. 2016, Andrade et al. 2017, Thiele et al. 2018).
Acknowledgements
Dr. Samir’s contribution was supported by the NIBIB of the National Institutes of Health under award number K23 EB020710. Dr. Samir is solely responsible for the content and the work does not represent the official views of the National Institutes of Health. Dr. Anthony’s contribution was supported in part by GE Global Research through the Medical Electronic Device Realization Center (MEDRC) at MIT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn JM, Paik YH, Min SY, Cho JY, Sohn W, Sinn DH, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Relationship between controlled attenuation parameter and hepatic steatosis as assessed by ultrasound in alcoholic or nonalcoholic fatty liver disease. Gut Liver 2016;10:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME, McKeag MS, Trahey GE. The impact of sound speed errors on medical ultrasound imaging. J Acoust Soc Am 2000;107:3540–3548. [DOI] [PubMed] [Google Scholar]
- Andrade P, Rodrigues S, Rodrigues-Pinto E, Gaspar R, Lopes J, Lopes S, Macedo G. Diagnostic Accuracy of Controlled Attenuation Parameter for Detecting Hepatic Steatosis in Patients with Chronic Liver Disease. GE Port J Gastroenterol 2017;24:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslanow A, Teutsch M, Walle H, Grünhage F, Lammert F, Stokes CS. Short-Term Hypocaloric High-Fiber and High-Protein Diet Improves Hepatic Steatosis Assessed by Controlled Attenuation Parameter. Clin Transl Gastroenterol 2016;7:e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamber J, Hill C. Ultrasonic attenuation and propagation speed in mammalian tissues as a function of temperature. Ultrasound Med Biol 1979;5:149–157. [DOI] [PubMed] [Google Scholar]
- Bamber JC, Hill CR, King JA. Acoustic properties of normal and cancerous human liver-II Dependence on tissue structure. Ultrasound Med Biol 1981;7:135–144. [DOI] [PubMed] [Google Scholar]
- Barry C, Mills B, Hah Z, Mooney R. Shear wave dispersion measures liver steatosis. Ultrasound Med … 2012;38:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CT, Hah Z, Partin A, Mooney RA, Chuang KH, Augustine A, Almudevar A, Cao W, Rubens DJ, Parker KJ. Mouse liver dispersion for the diagnosis of early-stage fatty liver disease: A 70-sample study. Ultrasound Med Biol 2014;40:704–713. [DOI] [PubMed] [Google Scholar]
- Barry CT, Hazard C, Hah Z, Cheng G, Partin A, Mooney RA, Chuang KH, Cao W, Rubens DJ, Parker KJ. Shear wave dispersion in lean versus steatotic rat livers. J Ultrasound Med 2015;34:1123–1129. [DOI] [PubMed] [Google Scholar]
- Bedossa P, Patel K. Biopsy and Noninvasive Methods to Assess Progression of Nonalcoholic Fatty Liver Disease. Gastroenterology Elsevier, Inc, 2016;150:1811–1822. [DOI] [PubMed] [Google Scholar]
- Bohte AE, Koot BGP, Van Der Baan-Slootweg OH, Rijcken THP, Van Werven JR, Bipat S, Nederveen AJ, Jansen PLM, Benninga MA, Stoker J. US Cannot Be Used to Predict the Presence or Severity of Hepatic Steatosis in Severely Obese Adolescents. Radiology 2012;262:327–334. [DOI] [PubMed] [Google Scholar]
- Bohte AE, Van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: A meta-analysis. Eur Radiol 2011;21:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boote E, Zagzebski J, Madsen E. Backscatter coefficient imaging using a clinical scanner. Med Phys 1992;19:1145–1152. [DOI] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004;40:1387–1395. [DOI] [PubMed] [Google Scholar]
- Burckhardt CB. Speckle in Ultrasound B-Mode Scans. IEEE Trans Sonics Ultrason 1978;1–6. [Google Scholar]
- Byrne CD, Targher G. NAFLD: A multisystem disease. J Hepatol European Association for the Study of the Liver, 2015;62:S47–S64. [DOI] [PubMed] [Google Scholar]
- Carvalhana S, Leitão J, Alves AC, Bourbon M, Cortez-Pinto H. How good is controlled attenuation parameter and fatty liver index for assessing liver steatosis in general population: Correlation with ultrasound. Liver Int 2014;34:111–117. [DOI] [PubMed] [Google Scholar]
- Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, Ajmera V, Bettencourt R, Collier S, Hooker J, Sy E, Rizo E, Richards L, Sirlin CB, Loomba R. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018;67:1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–2023. [DOI] [PubMed] [Google Scholar]
- Chan WK, Mustapha NRN, Mahadeva S, Wong VWS, Cheng JYK, Wong GLH. Can the same controlled attenuation parameter cut-offs be used for M and XL probes for diagnosing hepatic steatosis? J Gastroenterol Hepatol 2018;1–8. [DOI] [PubMed] [Google Scholar]
- Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol 2014;29:1470–1476. [DOI] [PubMed] [Google Scholar]
- Chan WK, Nik Mustapha NR, Wong GLH, Wong VWS, Mahadeva S. Controlled attenuation parameter using the FibroScan® XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United Eur Gastroenterol J 2017;5:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Robinson D, Wilson L, Griffiths K, Manoharan A, Doust B. Clinical sound speed measurement in liver and spleen in vivo. Ultrason Imaging 1987;9:221–235. [DOI] [PubMed] [Google Scholar]
- Chon YE, Jung KS, Kim SU, Park JY, Park YN, Kim DY, Ahn SH, Chon CY, Lee HW, Park Y, Han KH. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: A prospective study of a native Korean population. Liver Int 2014;34:102–109. [DOI] [PubMed] [Google Scholar]
- Chon YE, Kim KJ, Jung KS, Kim SU, Park JY, Kim DY, Ahn SH, Chon CY, Chung JB, Park KH, Bae JC, Han KH. The relationship between type 2 diabetes mellitus and non-alcoholic fatty liver disease measured by controlled attenuation parameter. Yonsei Med J 2016;57:885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, Mccullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis : A prospective study q. J Hepatol European Association for the Study of the Liver, 2009;51:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alwis N, Day C. Non-alcoholic fatty liver disease : The mist gradually clears. J Hepatol 2008;48:S104–S112. [DOI] [PubMed] [Google Scholar]
- de Ledinghen V, Vergniol J, Foucher J, Merrouche W, Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int 2012;32:911–918. [DOI] [PubMed] [Google Scholar]
- de Lédinghen V, Wong GLH, Vergniol J, Chan HLY, Hiriart JB, Chan AWH, Chermak F, Choi PCL, Foucher J, Chan CKM, Merrouche W, Chim AML, Le Bail B, Wong VWS. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2016;31:848–855. [DOI] [PubMed] [Google Scholar]
- Desai NK, Harney S, Raza R, Al-ibraheemi A, Shillingford N, Mitchell PD, Jonas MM. Comparison of Controlled Attenuation Parameter and Liver Biopsy to Assess Hepatic Steatosis in Pediatric Patients. J Pediatr Elsevier, 2016;173:160–164.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens MA, van Ooijen PMA, Post WJ, Haagmans MJF, Kristanto W, Sijens PE, van der Jagt EJ, Stolk RP. Ultrasonography to Quantify Hepatic Fat Content: Validation by 1H Magnetic Resonance Spectroscopy . Obesity Nature Publishing Group, 2009;17:2239–2244. [DOI] [PubMed] [Google Scholar]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006;43:99–112. [DOI] [PubMed] [Google Scholar]
- Ferraioli G, Tinelli C, Lissandrin R. Interobserver reproducibility of the controlled attenuation parameter (CAP) for quantifying liver steatosis. Hepatol Int 2014a;8:576–581. [DOI] [PubMed] [Google Scholar]
- Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Bello BD, Filice G, Ferraioli G, Lissandrin R, Zicchetti M, Bello BD. Controlled attenuation parameter for evaluating liver steatosis in chronic viral hepatitis. 2014b;20:6626–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich-Rust M, Romen D, Vermehren J, Kriener S, Sadet D, Herrmann E, Zeuzem S, Bojunga J. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol Elsevier Ireland Ltd, 2012;81:e325–e331. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Tanguchi N, Itoh K, Shigeta K, Wang Y, Tsao JW, Kumasaki K, Itoh T. A New Method for Attenuation Coefficient Measurement in the Liver. J Ultrasound Med 21 783–788, 2002. 2002;783–788. [DOI] [PubMed] [Google Scholar]
- Gaitini D, Baruch Y, Ghersin E, Veitsman E, Kerner H, Shalem B, Yaniv G, Sarfaty C, Azhari H. Feasibility study of ultrasonic fatty liver biopsy: Texture vs. attenuation and backscatter. Ultrasound Med Biol 2004;30:1321–1327. [DOI] [PubMed] [Google Scholar]
- Garg H, Aggarwal S, Shalimar, Yadav R, Datta Gupta S, Agarwal L, Agarwal S. Utility of transient elastography (fibroscan) and impact of bariatric surgery on nonalcoholic fatty liver disease (NAFLD) in morbidly obese patients. Surg Obes Relat Dis Elsevier Inc., 2018;14:81–91. [DOI] [PubMed] [Google Scholar]
- Ghoshal G, Lavarello RJ, Kemmerer JP, Miller RJ, Oelze ML. Ex vivo Study of Quantitative Ultrasound Parameters in Fatty Rabbit Livers. Ultrasound Med Biol 2012;38:2238–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein NS, Hastah F, Galan MV., Gordon SC. Fibrosis heterogeneity in nonalcoholic steatohepatitis and hepatitis C virus needle core biopsy specimens. Am J Clin Pathol 2005;123:382–387. [DOI] [PubMed] [Google Scholar]
- Goss SA, Johnston RL, Dunn F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J Acoust Soc Am 1978;64:423–457. [DOI] [PubMed] [Google Scholar]
- Gyöngy M, Kollár S. Variation of ultrasound image lateral spectrum with assumed speed of sound and true scatterer density. Ultrasonics 2015;56:370–380. [DOI] [PubMed] [Google Scholar]
- Han A, Andre MP, Erdman JW, Loomba R, Sirlin CB, O’Brien WD. Repeatability and Reproducibility of a Clinically Based QUS Phantom Study and Methodologies. IEEE Trans Ultrason Ferroelectr Freq Control 2017;64:218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YM, Yoon KT, Cho M, Chu CW, Rhu JH, Yang KH, Lee JW. Clinical usefulness of controlled attenuation parameter to screen hepatic steatosis for potential donor of living donor liver transplant. Eur J Gastroenterol Hepatol 2017;29:805–810. [DOI] [PubMed] [Google Scholar]
- Hyodo T, Hori M, Lamb P, Sasaki K, Wakayama T, Chiba Y, Mochizuki T, Murakami T. Multimaterial Decomposition Algorithm for the Quantification of Liver Fat Content by Using Fast-Kilovolt-Peak Switching Dual-Energy CT: Experimental Validation. Radiology 2017;282:381–389. [DOI] [PubMed] [Google Scholar]
- Idilman IS, Keskin O, Celik A, Savas B, Elhan AH, Idilman R, Karcaaltincaba M. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta radiol 2016;57:271–278. [DOI] [PubMed] [Google Scholar]
- Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H, Sumida Y, Ono M, Eguchi Y, Inoue T, Yamanaka T, Wada K, Saito S, Nakajima A. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology Elsevier, Inc, 2016;150:626–637e7. [DOI] [PubMed] [Google Scholar]
- Imbault M, Faccinetto A, Osmanski B-F, Tissier A, Deffieux T, Gennisson J-L, Vilgrain V, Tanter M. Robust sound speed estimation for ultrasound-based hepatic steatosis assessment. Phys Med Biol 2017;62:3582–3598. [DOI] [PubMed] [Google Scholar]
- Itoh K, Yasuda Y, Suzuki O, Itoh H, Itoh T, Jing-Wen T, Konishi T, Koyano A. Studies on frequency-dependent attenuation in the normal liver and spleen and in liver diseases, using the spectral-shift zero-crossing method. J Clin Ultrasound 1988;16:553–562. [DOI] [PubMed] [Google Scholar]
- Jaeger M, Held G, Peeters S, Preisser S, Grünig M, Frenz M. Computed Ultrasound Tomography in Echo Mode for Imaging Speed of Sound Using Pulse-Echo Sonography: Proof of Principle. Ultrasound Med Biol 2015;41:235–250. [DOI] [PubMed] [Google Scholar]
- Joseph A, Dewbury K, McGuire P. Ultrasound in the detection of chronic liver disease (the ‘bright liver’). Br J Radiol 1979;52:184–188. [DOI] [PubMed] [Google Scholar]
- Karlas T, Petroff D, Garnov N, Böhm S, Tenckhoff H, Wittekind C, Wiese M, Schiefke I, Linder N, Schaudinn A, Busse H, Kahn T, Mössner J, Berg T, Tröltzsch M, Keim V, Wiegand J. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS One 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VWS, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GLH, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol European Association for the Study of the Liver, 2017;66:1022–1030. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- Kneeman JM, Misdraji J, Corey KE. Secondary causes of nonalcoholic fatty liver disease. Therap Adv Gastroenterol 2012;5:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai H, Yokoyama K, Katsuyama K, Hara S, Yamamoto H, Yamagata T, Taniguchi N, Hirota N, Itoh K. A New Method for Measuring the Speed of Sound in Rat Liver ex Vivo Using an Ultrasound System: Correlation of Sound Speed with Fat Deposition. Ultrasound Med Biol 2014;40:2499–2507. [DOI] [PubMed] [Google Scholar]
- Kumar M, Rastogi A, Singh T, Behari C, Gupta E, Garg H, Kumar R, Bhatia V, Sarin SK. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: Does etiology affect performance? J Gastroenterol Hepatol 2013;28:1194–1201. [DOI] [PubMed] [Google Scholar]
- Kuni CC, Johnson TK, Crass JR, Snover DC. Correlation of Fourier spectral shift-determined hepatic acoustic attenuation coefficients with liver biopsy findings. J Ultrasound Med 1989;8:631–634. [DOI] [PubMed] [Google Scholar]
- Kwok R, Choi KC, Wong GLH, Zhang Y, Chan HLY, Luk AOY, Shu SST, Chan AWH, Yeung MW, Chan JCN, Kong APS, Wong VWS. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016;65:1359–1368. [DOI] [PubMed] [Google Scholar]
- Labyed Y, Bigelow TA. Estimating the total ultrasound attenuation along the propagation path by applying multiple filters to backscattered echoes from a single spherically focused source. IEEE Trans Ultrason Ferroelectr Freq Control 2010;57:900–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labyed Y, Bigelow TA. A theoretical comparison of attenuation measurement techniques from backscattered ultrasound echoes. J Acoust Soc Am 2011;129:2316–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, Cohen BL, Brenner D, Sirlin C, Loomba R. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: A randomized controlled trial. Hepatology 2012;56:922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Park SY, Kim SU, Jang JY, Park H, Kim JK, Lee CK, Chon YE, Han KH. Discrimination of Nonalcoholic Steatohepatitis Using Transient Elastography in Patients with Nonalcoholic Fatty Liver Disease. PLoS One 2016;11:e0157358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol 2014;20:7392–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, Saab S, Lu DS. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology 2004;230:276–280. [DOI] [PubMed] [Google Scholar]
- Lin SC, Heba E, Wolfson T, Ang B, Gamst A, Han A, Erdman JW, O’Brien WD, Andre MP, Sirlin CB, Loomba R. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin Gastroenterol Hepatol Elsevier, 2015;13:1337–1345.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Ophir J, Potter G. Correlations of sound speed with tissue constituents in normal and diffuse liver disease. Ultrason Imaging 1987;9:29–40. [DOI] [PubMed] [Google Scholar]
- Longo R, Ricci C, Masutti F, Vidimari R, Crocé LS, Bercich L, Tiribelli C, Dalla Palma L. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest. Radiol 1993. pp. 297–302. [PubMed] [Google Scholar]
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol Nature Publishing Group, 2013;10:686–690. [DOI] [PubMed] [Google Scholar]
- Lu ZF, Zagzebski JA, Lee FT. Ultrasound backscatter and attenuation in human liver with diffuse disease. Ultrasound Med Biol 1999;25:1047–1054. [DOI] [PubMed] [Google Scholar]
- Lupsor-Platon M, Feier D, Stef??nescu H, Tamas A, Botan E, Sparchez Z, Maniu A, Badea R. Diagnostic accuracy of controlled attenuation parameter measured by transient elastography for the non-invasive assessment of liver steatosis: A prospective study. J Gastrointest Liver Dis 2015;24:35–42. [DOI] [PubMed] [Google Scholar]
- Mahmoud AM, Ding X, Dutta D, Singh VP, Kim K. Detecting hepatic steatosis using ultrasound-induced thermal strain imaging: An ex vivo animal study. Phys Med Biol 2014;59:881–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad N, Ophir J, Balsara V. Attenuation of ultrasound in normal liver and diffuse liver disease in vivo. 1984;6:117–125. [DOI] [PubMed] [Google Scholar]
- Mancia C, Loustaud-Ratti V, Carrier P, Naudet F, Bellissant E, Labrousse F, Pichon N. Controlled attenuation parameter and liver stiffness measurements for steatosis assessment in the liver transplant of brain dead donors. Transplantation 2015;99:1619–1624. [DOI] [PubMed] [Google Scholar]
- Matsuhashi T, Yamada N, Shinzawa H, Takahashi T. An evaluation of hepatic ultrasound speed in injury models in rats: Correlation with tissue constituents. J Ultrasound Med 1996;15:563–570. [DOI] [PubMed] [Google Scholar]
- McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J Hepatol European Association for the Study of the Liver, 2015;62:1148–1155. [DOI] [PubMed] [Google Scholar]
- Mi YQ, Shi QY, Xu L, Shi RF, Liu YG, Li P, Shen F, Lu W, Fan JG. Controlled Attenuation Parameter for Noninvasive Assessment of Hepatic Steatosis Using Fibroscan®: Validation in Chronic Hepatitis B. Dig Dis Sci 2014;60:243–251. [DOI] [PubMed] [Google Scholar]
- Mitchell D, K I, Chang T, Vinitski S, Consigny M, Saponaro S, Ehrlich S, Rifkin M, Rubin R. Fatty liver Chemical shift phase difference and suppression magnetic resonance imaging techniques IN Animals, Phantoms and Humans. Invest Radiol 1991;26:1041–1052. [PubMed] [Google Scholar]
- Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, Duarte-Rojo A, Wong D, Crotty P, Elkashab M. Controlled Attenuation Parameter (CAP): A noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int 2012;32:902–910. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu I, Brunt EM. Role of liver biopsy in nonalcoholic fatty liver disease. World J Gastroenterol 2014;20:9026–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam K, Rosado-Mendez IM, Wirtzfeld LA, Ghoshal G, Pawlicki AD, Madsen EL, Lavarello RJ, Oelze ML, Zagzebski JA, O’Brien WD, Hall TJ. Comparison of ultrasound attenuation and backscatter estimates in layered tissue-mimicking phantoms among three clinical scanners. Ultrason Imaging 2012;34:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano D, Chou CH, McLaughlin G, Ji TL, Mo L, DeBusschere D, Steins R. Sound speed correction in ultrasound imaging. Ultrasonics 2006;44:44–47. [DOI] [PubMed] [Google Scholar]
- Nightingale K, Rouze N, Rosenzweig S, Wang M, Abdelmalek M, Guy C, Palmeri M. Derivation and analysis of viscoelastic properties in human liver: Impact of frequency on fibrosis and steatosis staging. IEEE Trans Ultrason Ferroelectr Freq Control 2015;62:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock L, Trahey G. Phase aberration correction in medical ultrasound using speckle brightness as a quality factor. J Acoust Soc Am 1985;85:1819–1833. [DOI] [PubMed] [Google Scholar]
- Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, Bettencourt R, Changchien C, Brenner DA, Sirlin C, Loomba R. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013;58:1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noureddin M, Loomba R. Nonalcoholic fatty liver disease: Indications for liver biopsy and noninvasive biomarkers. Clin Liver Dis 2012;1:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WD Jr., Erdman JW Jr., Hebner TB. Ultrasonic propagation properties (@ 100 MHz) in excessively fatty rat liver. JAcoustSocAm 1988;83:1159–1166. [DOI] [PubMed] [Google Scholar]
- Obuchowski N Fundamentals of Clinical Research for Radiologists ROC Analysis. AJR Am J Roentgenol 2004;184:364–372. [DOI] [PubMed] [Google Scholar]
- Ozturk A, Grajo JR, Dhyani M, Anthony BW, Samir AE. Principles of ultrasound elastography. Abdom Radiol 2018;43:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige JS, Bernstein GS, Heba E, Costa EAC, Fereirra M, Wolfson T, Gamst AC, Valasek MA, Lin GY, Han A, Erdman JW, O’Brien WD, Andre MP, Loomba R, Sirlin CB. A pilot comparative study of quantitative ultrasound, conventional ultrasound, and MRI for predicting histology-determined steatosis grade in adult nonalcoholic fatty liver disease. Am J Roentgenol 2017;208:W168–W177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapostoli I, Lammert F, Stokes CS. Effect of short-term vitamin D correction on hepatic steatosis as quantified by controlled attenuation parameter (CAP). J Gastrointest Liver Dis 2016;25:175–181. [DOI] [PubMed] [Google Scholar]
- Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH, Valasek MA, Rizo E, Richards L, Brenner D, Sirlin CB, Loomba R. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology Elsevier, Inc, 2017;152:598–607.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Asztely MS, Lerner RM, Schenk EA, Waag RC. In-vivo measurements of ultrasound attenuation in normal or diseased liver. Ultrasound Med Biol 1988;14:127–136. [DOI] [PubMed] [Google Scholar]
- Ratchatasettakul K, Rattanasiri S, Promson K, Sringam P, Sobhonslidsuk A. The inverse effect of meal intake on controlled attenuation parameter and liver stiffness as assessed by transient elastography. BMC Gastroenterol BMC Gastroenterology, 2017;17:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898–1906. [DOI] [PubMed] [Google Scholar]
- Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J Magn Reson Imaging 2011;749:729–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci C, Longo R, Giolius E, Bosco M, Pollesello P, Masutti F, Croce LS, Paoletti S, de Bernard B, Tiribelli C, Dalla Palma L. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol 1997;27:108–113. [DOI] [PubMed] [Google Scholar]
- Robinson DE, Ophir J, Wilson LS, Chen CF. Pulse-echo ultrasound speed measurements: Progress and prospects. Ultrasound Med Biol 1991;17:633–646. [DOI] [PubMed] [Google Scholar]
- Runge JH, Smits LP, Verheij J, Depla A, Kuiken SD, Baak BC, Nederveen AJ, Beuers U, Stoker J. MR Spectroscopy–derived Proton Density Fat Fraction Is Superior to Controlled Attenuation Parameter for Detecting and Grading Hepatic Steatosis. Radiology 2018;286:162931. [DOI] [PubMed] [Google Scholar]
- Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745–750. [DOI] [PubMed] [Google Scholar]
- Sasso M, Audière S, Kemgang A, Gaouar F, Corpechot C, Chazouillères O, Fournier C, Golsztejn O, Prince S, Menu Y, Sandrin L, Miette V. Liver Steatosis Assessed by Controlled Attenuation Parameter (CAP) Measured with the XL Probe of the FibroScan: A Pilot Study Assessing Diagnostic Accuracy. Ultrasound Med Biol 2016;42:92–103. [DOI] [PubMed] [Google Scholar]
- Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): A novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 2010;36:1825–1835. [DOI] [PubMed] [Google Scholar]
- Satkunasingham J, Besa C, Bane O, Shah A, De Oliveira A, Gilson WD, Kannengiesser S, Taouli B. Liver fat quantification: Comparison of dual-echo and triple-echo chemical shift MRI to MR spectroscopy. Eur J Radiol Elsevier Ireland Ltd, 2015;84:1452–1458. [DOI] [PubMed] [Google Scholar]
- Sharma A, Ashworth A, Behnke M, Cotterell A, Posner M, Fisher RA. Donor selection for adult-to-adult living donor liver transplantation: Well begun is half done. Transplantation 2013;95:501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Mitnala S, Vishnubhotla RK, Mukherjee R, Reddy DN, Rao PN. The Riddle of Nonalcoholic Fatty Liver Disease: Progression From Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis. J Clin Exp Hepatol Elsevier Ltd, 2015;5:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Zheng RD, Mi YQ, Wang XY, Pan Q, Chen GY, Cao HX, Chen ML, Xu L, Chen JN, Cao Y, Zhang RN, Xu LM, Fan JG. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis in Chinese patients. World J Gastroenterol 2014;20:4702–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Zheng RD, Shi JP, Mi YQ, Chen GF, Hu X, Liu YG, Wang XY, Pan Q, Chen GY, Chen JN, Xu L, Zhang RN, Xu LM, Fan JG. Impact of skin capsular distance on the performance of controlled attenuation parameter in patients with chronic liver disease. Liver Int 2015;35:2392–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi KQ, Tang JZ, Zhu XL, Ying L, Li DW, Gao J, Fang YX, Li GL, Song YJ, Deng ZJ, Wu JM, Tang KF. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: A meta-analysis of diagnostic accuracy. J Gastroenterol Hepatol 2014;29:1149–1158. [DOI] [PubMed] [Google Scholar]
- Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV., Abdelmalek M, Neuschwander-Tetri BA, Loomba R, Dasarathy S, Brandman D, Doo E, Tonascia JA, Kleiner DE, Chalasani N, Sanyal AJ, NASH Clinical Research Network. Vibration-controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol The American Gastroenterological Association, 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigelmann RA, Reid JM. Analysis and measurement of ultrasound backscattering from an ensemble of scatterers excited by sine-wave bursts. J Acoust Soc Am 1973;53:1351–1355. [Google Scholar]
- Stern C, Castera L. Non-invasive diagnosis of hepatic steatosis. Hepatol Int Springer India, 2017;11:70–78. [DOI] [PubMed] [Google Scholar]
- Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. Am J Roentgenol 2007;189:1449. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Fukusato T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2014;20:15539–15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic Fatty Liver Disease: MR Imaging of Liver Proton Density Fat Fraction to Assess Hepatic Steatosis. Radiology 2013;267:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targher G Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med 2007;24:1–6. [DOI] [PubMed] [Google Scholar]
- Taylor KJW, Riely C a, Hammers L, Flax S, Weltin G, Garciatsao G, Conn HO, Kuc R, Barwick KW. Quantitative US Attenuation in Normal Liver and in Patients with Diffuse Liver-Disease - Importance of Fat. Radiology 1986;160:65–71. [DOI] [PubMed] [Google Scholar]
- Thavorn K, Coyle D. Transient elastography and controlled attenuation parameter for diagnosing liver fibrosis and steatosis in ontario: An economic analysis. Ont Health Technol Assess Ser 2015;15:1–58. [PMC free article] [PubMed] [Google Scholar]
- Thiele M, Rausch V, Fluhr G, Kjærgaard M, Piecha F, Mueller J, Straub BK, Lupșor-Platon M, De-Ledinghen V, Seitz HK, Detlefsen S, Madsen B, Krag A, Mueller S. Controlled attenuation parameter and alcoholic hepatic steatosis: Diagnostic accuracy and role of alcohol detoxification. J Hepatol 2018;68:1025–1032. [DOI] [PubMed] [Google Scholar]
- Thijssen JM, Starke A, Weijers G, Haudum A, Herzog K, Wohlsein P, Rehage J, De Korte CL. Computer-aided B-mode ultrasound diagnosis of hepatic steatosis: a feasibility study. IEEE Trans Ultrason Ferroelectr Freq Control 2008;55:1343–1354. [DOI] [PubMed] [Google Scholar]
- Tuthill TA, Baggs RB, Parker KJ. Liver glycogen and water storage: Effect on ultrasound attenuation. Ultrasound Med Biol 1989;15:621–627. [DOI] [PubMed] [Google Scholar]
- Wagner RF, Smith SW, Sandrik JM, Lopez H. Statistics of Speckle in Ultrasound B-Scans. IEEE Trans Sonics Ultrason 1983;30:156–163. [Google Scholar]
- Wang CY, Lu W, Hu DS, Wang GD, Cheng XJ. Diagnostic value of controlled attenuation parameter for liver steatosis in patients with chronic hepatitis B. World J Gastroenterol 2014;20:10585–10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fan Q, Wang T, Wen J, Wang H, Zhang T. Controlled attenuation parameter for assessment of hepatic steatosis grades: A diagnostic meta-analysis. Int J Clin Exp Med 2015;8:17654–17663. [PMC free article] [PubMed] [Google Scholar]
- Williamson RM, Perry E, Glancy S, Marshall I, Gray C, Nee LD, Hayes PC, Forbes S, Frier BM, Johnston GI, Lee AJ, Reynolds RM, Price JF, Strachan MWJ, Type E, Study D. The use of ultrasound to diagnose hepatic steatosis in type 2 diabetes : Intra- and interobserver variability and comparison with magnetic resonance spectroscopy. Clin Radiol The Royal College of Radiologists, 2011;66:434–439. [DOI] [PubMed] [Google Scholar]
- Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology Elsevier, Inc, 2015;148:547–555. [DOI] [PubMed] [Google Scholar]
- Yao LX, Zagzebski JA, Madsen EL. Backscatter Coefficient Measurements Using a Reference Phantom to extract depth-dependent instrumentation factors. Ultrason Imaging 1990;12:58–70. [DOI] [PubMed] [Google Scholar]
- Yoon EJ, Hu KQ. Hepatitis C virus (HCV) infection and hepatic steatosis. Int J Med Sci 2006;3:53–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Lee JM, Suh K-S, Lee K-W, Yi N-J, Lee KB, Han JK, Choi BI. Combined Use of MR Fat Quantification and MR Elastography in Living Liver Donors: Can It Reduce the Need for Preoperative Liver Biopsy? Radiology 2015;276:453–64. [DOI] [PubMed] [Google Scholar]
- Zagzebski JA, Lu ZF, Yao LX. Quantitative Ultrasound Imaging: In Vivo Results in Normal Liver. Ultrason. Imaging 1993. pp. 335–351. [DOI] [PubMed] [Google Scholar]