CASE
A previously healthy, fully immunized 10-year-old girl from Minnesota presented in July with a 1-week history of headaches, dizziness, myalgias, pharyngitis, and fever and a 1-day history of double vision, unsteady gait, and slurred speech. Her symptoms began approximately 4 days after returning from a family vacation to northern Wisconsin, where she had spent significant time outdoors and had exposure to mosquitoes.
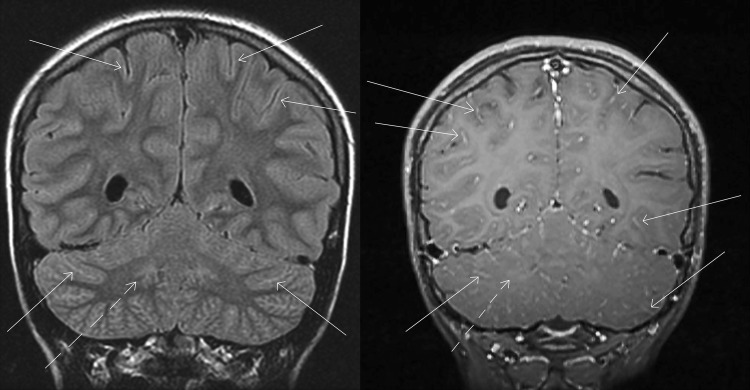
Upon evaluation in the emergency department, she was afebrile with normal vital signs. She was oriented but drowsy, demonstrated dysarthric (i.e., slurred) speech and dysmetria (i.e., lack of coordination of movement), and had trouble walking. A complete blood count showed a hemoglobin of 13.3 g/dl (normal range, 12.0 to 14.5 g/dl), white blood cell count of 9.1 × 109/liter (3.4 × 109 to 10.8 × 109/liter), and platelet count of 124 × 109/liter (150 × 109 to 450 × 109/liter). Reactive lymphocytes (i.e., large, antigen-stimulated cells) were observed on a peripheral blood smear. A basic metabolic panel and liver function tests were within normal limits, and a urine drug screen was negative. A cranial computed tomography (CT) scan was normal, while magnetic resonance imaging (MRI) of the brain with intravenous contrast showed subtle signs of inflammation (leptomeningeal enhancement concentrated in the brainstem, cortical, limbic, and deep gray structures), which is a pattern that can be seen with viral meningoencephalitis (Fig. 1). An electroencephalogram was performed, which showed no lateralized periodic discharges or other abnormalities.
FIG 1.
MRI findings suggestive of infectious meningoencephalitis. Coronal FLAIR (left) and postcontrast T1 (right) magnetic resonance imaging show subtle signs of inflammation, including hyperintense signal along multiple cerebral gyri and contrast enhancement throughout the arachnoid space, outlining multiple cerebellar folia and scattered cerebral sulci (examples shown by solid arrows). The dentate nuclei of the cerebellum also have abnormal signal (dashed arrows).
Laboratory studies included serologic testing for herpes simplex virus 1 and 2 (HSV1/2), Bartonella henselae, B. quintana, and Lyme disease, all of which were negative. A tick-borne disease real-time PCR panel targeting Anaplasma phagocytophilum, Ehrlichia species, Babesia species, and Borrelia miyamotoi, performed on whole blood, was negative. A lumbar puncture was performed, and cerebrospinal fluid (CSF) showed a total nucleated cell count of 219/μl, with a differential of 44% lymphocytes, 44% neutrophils, and 12% monocytes. In addition, the CSF showed a normal glucose level of 53 mg/dl (normal range, 40 to 70 mg/dl) and an elevated protein level of 95 mg/dl (12 to 60 mg/dl). Gram stain of the CSF showed white blood cells but no organisms. Bacterial culture of the CSF grew one colony of Corynebacterium species and Lactobacillus gasseri (1+ growth), both of which were interpreted as contaminants. Further testing on CSF included real-time PCR for cytomegalovirus, enterovirus, Epstein-Barr virus, HSV1/2, West Nile virus (WNV), varicella-zoster virus (VZV), and free-living amoebae (Acanthamoeba species, Balamuthia mandrillaris, and Naegleria fowleri), all of which were negative. CSF testing was also negative by serology for neuroinvasive Lyme disease.
Due to the patient's travel history and mosquito exposure, additional arbovirus testing was performed (Table 1). A serum sample was sent to the Minnesota Department of Health (MDH) (St. Paul, MN) and yielded atypical staining (i.e., the observed fluorescence was not consistent with patterns typically produced by positive samples) by immunofluorescence assays (IFAs) for IgM to St. Louis encephalitis (SLE) virus, eastern equine encephalitis (EEE) virus, western equine encephalitis (WEE) virus, and California group encephalitis viruses. The atypical IFA findings were followed up at MDH using an IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA) for SLE, EEE, and WEE, all of which were negative, suggesting nonspecific staining by IFA. In addition, an IgM enzyme immunoassay (EIA) for Powassan virus was negative; however, the IgM EIA for Jamestown Canyon virus (JCV) was reported as preliminary positive, prompting submission of serum and CSF to the Centers for Disease Control and Prevention (CDC) for additional arbovirus testing. This included a JCV IgM EIA performed on serum, which was classified as equivocal. Plaque reduction neutralization testing (PRNT) for snowshoe hare virus (SSHV) and La Crosse virus (LACV) demonstrated an endpoint titer of 1:20. Notably, PRNT for JCV yielded an endpoint titer of 1:2,560 (reference value, <1:10) in serum and 1:16 (reference value, <1:2) in the CSF (Table 1).
TABLE 1.
Test results for California serogroup viruses and other arboviruses in cerebrospinal fluid and acute- and convalescent-phase serum samplese
| Test name and virus | Testing location | Acute-phase serum resulta (titer) | Acute-phase CSF resulta (titer) | Convalescent-phase serum resultb (titer) |
|---|---|---|---|---|
| Jamestown Canyon virus | ||||
| IgM EIA | MDH | Preliminary positive | TNP | Preliminary positive |
| IgM EIA | CDC | Equivocal | TNP | TNP |
| PRNT | CDC | Positive (1:2,560)c | Positive (1:16)d | Positive (1:2,560)c |
| Snowshoe hare virus | ||||
| IgM EIA | CDC | Negative | TNP | TNP |
| PRNT | CDC | Positive (1:20)c | TNP | Positive (1:20)c |
| La Crosse virus | ||||
| IgM IFA | Mayo | TNP | Negative | TNP |
| IgG IFA | Mayo | TNP | Negative | TNP |
| IgM IFA | MDH | Atypical | TNP | TNP |
| IgM EIA | MDH | Negative | TNP | TNP |
| IgM EIA | CDC | Equivocal | TNP | TNP |
| PRNT | CDC | Positive (1:20)c | Negative | Positive (1:20)c |
| West Nile virus | ||||
| IgM EIA | MDH | Negative | TNP | Negative |
| IgM EIA | Mayo | Negative | Negative | TNP |
| IgG EIA | Mayo | Negative | Negative | TNP |
| Real-time PCR | MDH | Negative | TNP | Negative |
| Real-time PCR | Mayo | TNP | Negative | TNP |
| Powassan virus | ||||
| IgM EIA | MDH | Negative | TNP | Negative |
| Saint Louis encephalitis virus | ||||
| IgM IFA | MDH | Atypical | TNP | Negative |
| IgM EIA | MDH | Negative | TNP | TNP |
| IgM IFA | Mayo | TNP | Negative | TNP |
| IgG IFA | Mayo | TNP | Negative | TNP |
| Eastern equine encephalitis virus | ||||
| IgM IFA | MDH | Atypical | TNP | Negative |
| IgM EIA | MDH | Negative | TNP | TNP |
| IgM IFA | Mayo | TNP | Negative | TNP |
| IgG IFA | Mayo | TNP | Negative | TNP |
| Western equine encephalitis virus | ||||
| IgM IFA | MDH | Atypical | TNP | Negative |
| IgM IFA | Mayo | TNP | Negative | TNP |
| IgG IFA | Mayo | TNP | Negative | TNP |
| California group encephalitis viruses | ||||
| IgM IFA | MDH | Atypical | TNP | Negative |
Sample collected approximately 1 week following symptom onset.
Sample collected approximately 4 weeks following symptom onset.
PRNT serum titer of <1:10 is negative.
PRNT CSF titer of <1:2 is negative.
CSF, cerebrospinal fluid; EIA, enzyme immunoassay; MDH, Minnesota Department of Health; CDC, Centers for Disease Control and Prevention; TNP, test not performed; PRNT, plaque reduction neutralization test; IFA, immunofluorescence antibody assay; IgM, immunoglobulin M; IgG, immunoglobulin G.
She was treated empirically with meropenem, vancomycin, and acyclovir. These were discontinued when CSF cultures and HSV testing were negative. Her symptoms improved, and she was discharged after 4 days of hospitalization. She was seen in follow-up 2.5 weeks later and was doing well.
DISCUSSION
JCV is a mosquito-borne RNA virus of the genus Orthobunyavirus. It was first identified in Jamestown, Colorado, in 1961, and it belongs to the California serogroup of arboviruses, which also includes LACV, California group encephalitis viruses, and SSHV (1). JCV is believed to be endemic to most regions of the continental United States, circulating between deer and multiple mosquitoes species (e.g., Aedes species, Anopheles species, Coquilettidia species, etc.). It became a reportable disease in 2004, but reports of human disease are rare (2, 3), with an average of three cases of neuroinvasive disease reported annually between 2006 and 2015 (4). An analysis of 31 confirmed cases of JCV in the United States between 2000 and 2013 showed cases in 13 states, with the majority (42%) reported in Wisconsin. More than 90% of cases occurred between April and September, the typical mosquito season in much of the country. Clinical symptoms vary from a nonspecific febrile illness (e.g., fever with possible malaise, fatigue, headaches, muscle aches, and pharyngitis) to meningitis or meningoencephalitis, and approximately half of patients require hospitalization. Interestingly, neuroinvasive disease is present in ∼54% of reported cases of JCV, but unlike disease caused by WNV, acute flaccid paralysis associated with JCV has not been observed (3). No deaths associated with JCV have been reported. JCV predominantly affects adults, although cases in children have been reported (3). Patients with JCV are managed with supportive care, as no specific therapy or vaccination is currently available.
JCV infection is likely underdiagnosed due to lack of awareness of this virus and limited diagnostic testing options. The laboratory diagnosis of JCV is accomplished using serology, while other methods, such as nucleic acid amplification tests (e.g., real-time PCR) and viral culture, are not routinely performed. In 2013, JCV IgM antibody testing was implemented at the CDC; however, confirmatory testing for JCV must typically be requested by a state health department based on preliminary results and/or exposure history. Confirmatory testing includes anti-JCV IgM antibody screening, with a reflex to PRNT if reactive (3). Seroprevalence studies have demonstrated the presence of neutralizing antibodies to JCV at various rates (3.9 to 17.6% in two studies [5, 6]), which suggests that mild or asymptomatic infections occur.
Serologic testing for JCV is complicated by significant cross-reactivity with other viruses in the California serogroup, as was observed in our patient during testing at both MDH and initial EIA testing at the CDC (Table 1). Previous studies using the JCV IgM EIA demonstrated that ∼46% of patients with JCV infection test positive for LACV IgM, while 15% have inconclusive results (3). Therefore, testing by PRNT is required to establish a definitive diagnosis of JCV and LACV. The CDC performs PRNT for multiple closely related viruses and compares the endpoint neutralizing antibody titers between them. The infectious virus is typically identified as the one with at least a 4-fold or higher endpoint titer compared to the other tested viruses. In our case, the JCV serum PRNT endpoint titer was high at 1:2,560 (reference range, <1:10), although PRNT titers of 1:20 were observed for both SSHV and LACV. Interestingly, PRNT on our patient's CSF specimen was positive for JCV (endpoint titer, 1:16) but negative for LACV. As highlighted in this case, extensive serologic testing is often required to establish a diagnosis of JCV infection. Our patient required serologic testing on serum and CSF, using multiple methods (e.g., EIA, IFA, and PRNT) performed at three laboratories (Mayo Clinic, MDH, and CDC) to confirm the diagnosis and rule out other arbovirus infections.
In summary, we present a case of a 10-year-old girl with meningoencephalitis caused by JCV. She recovered from her illness without long-term sequelae. JCV is likely an underdiagnosed viral infection that health care providers and clinical laboratory professionals should consider. Specifically, infection with JCV should be included in the differential diagnosis of patients with mosquito exposure who present with a central nervous system infection or flu-like illness in the spring to early fall. It is important to be aware that infection with JCV may result in serologic cross-reactivity with other more common arboviruses (e.g., LACV), and atypical serology results (e.g., atypical staining by IFA, weak positivity by multiple arboviral screening tests) may prompt specific testing for JCV. Currently, laboratory testing for JCV may be facilitated through select state health departments and the CDC.
SELF-ASSESSMENT QUESTIONS
- Infection with JCV may result in serologic cross-reactivity with which of the following viruses?
- La Crosse virus
- Cytomegalovirus
- Enterovirus
- Powassan virus
- Which of the following statements about JCV is true?
- Infections most commonly occur between October and March in the United States
- Transmission has been documented for several different species of mosquito vectors
- Laboratory diagnosis of JCV is most commonly made by real-time PCR, with confirmation requiring growth of the virus in cell culture
- Vaccination for JCV is recommended in the United States for all adults 18 to 65 years of age
- Which of the following methods is used to confirm a diagnosis of JCV infection?
- IgM enzyme immunoassay
- Plaque reduction neutralization test
- IgM immunofluorescence assay
- NS1 antigen
For answers to the self-assessment questions and take-home points, see https://doi.org/10.1128/JCM.00255-18 in this issue.
ACKNOWLEDGMENTS
We thank our colleagues at the Minnesota Department of Health and Centers for Disease Control and Prevention for their assistance with this case. We also thank Mai Lan Ho for her help with selection and description of the MRI images.
We have no conflicts of interest to declare.
REFERENCES
- 1.Hughes HR, Lanciotti RS, Blair CD, Lambert AJ. 2017. Full genomic characterization of California serogroup viruses, genus Orthobunyavirus, family Peribunyaviridae including phylogenetic relationships. Virology 512:201–210. doi: 10.1016/j.virol.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). 2011. Human Jamestown canyon virus infection–Montana, 2009. MMWR Morb Mortal Wkly Rep 60:652–655. [PubMed] [Google Scholar]
- 3.Pastula DM, Joang Johnson DK, White JL, Dupuis AP, Fischer M, Staples JE. 2015. Jamestown Canyon virus disease in the United States-2000-2013. Am J Trop Med Hyg 93:384–389. doi: 10.4269/ajtmh.15-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams DA, Thomas KR, Jajosky RA, Foster L, Baroi G, Sharp P, Onweh DH, Schley AW, Anderson WJ, Nationally Notifiable Infectious Conditions Group. 2017. Summary of notifiable infectious diseases and conditions–United States, 2015. MMWR Morb Mortal Wkly Rep 64:1–143. doi: 10.15585/mmwr.mm6453a1. [DOI] [PubMed] [Google Scholar]
- 5.Mayo D, Karabatsos N, Scarano FJ, Brennan T, Buck D, Fiorentino T, Mennone J, Tran S. 2001. Jamestown Canyon virus: seroprevalence in Connecticut. Emerg Infect Dis 7:911–912. doi: 10.3201/eid0705.017529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walters LL, Tirrell SJ, Shope RE. 1999. Seroepidemiology of California and Bunyamwera serogroup (Bunyaviridae) virus infections in native populations of Alaska. Am J Trop Med Hyg 60:806–821. doi: 10.4269/ajtmh.1999.60.806. [DOI] [PubMed] [Google Scholar]