Abstract
Study objective:
To evaluate the feasibility and safety of vaginal vault drainage after complicated singleport access laparoscopic-assisted vaginal hysterectomy (SPA-LAVH).
Design:
Retrospective cohort study.
Setting:
Ulsan University Hospital (tertiary teaching hospital), South Korea.
Patients:
A total of 359 women underwent SPA-LAVH for the following conditions: benign uterine tumor, preinvasive uterine lesion, and microinvasive cervical cancer.
Interventions:
The participants included 124 women with vault drains and 235 women without drains.
Measurements:
Surgical outcomes, perioperative complications and morbidity, postoperative febrile morbidity.
Results:
There were no differences in background features between drain and no-drain groups. In surgical outcomes, mean uterine weight (364.2 ± 184.9 g vs. 263.7 ± 138.6 g; p < 0.001), operation time (87.4 ± 21.5 min vs. 73.0 ± 17.6 min; p < 0.001), blood loss (225.3 ± 122.2 mL vs. 150.4 ± 95.2 mL; p < 0.001), and hemoglobin decline (1.97 ± 0.96 g/dL vs. 1.42 ± 0.89 g/dL; p < 0.001) were significantly larger for the drain group compared with the no-drain group. However, with regard to postoperative morbidity and complications, there were no group differences in the transfusion rates (6.5% vs. 3.8%; p = 0.300), intraoperative complications (2.4% vs. 1.3%; p = 0.420), perioperative complications (2.4% vs. 0.9%; p = 0.345), and febrile morbidity ≥ 37.5°C (8.9% vs. 11.5%; p = 0.477), although the drain group was more prone to the development of pelvic fluid collection and febrile morbidity than the no-drain group.
Conclusion:
Vaginal vault drainage could be a safe alternative that allows for the management of postoperative morbidity and retains the advantages of minimally invasive surgery after complicated SPA-LAVH.
Keywords: febrile morbidity, hysterectomy, pelvic fluid collection, single-port access laparoscopy, vaginal vault drainage
Introduction
Advances in laparoscopic techniques have resulted in minimally invasive hysterectomy surgery using a single-port access (SPA) system, also referred to as laparoendoscopic single site.1,2,3 Similar to the complications for other hysterectomy procedures, SPA laparoscopic-assisted vaginal hysterectomy (SPA-LAVH) can result in residual pelvic fluid collection, which is a possible cause of febrile morbidity. The reported incidence of pelvic fluid collection ranges between 25% and 98%.4,5
Traditionally, after gynecologic laparoscopy, pelvic drains were used to reduce postoperative morbidity by evacuating pelvic fluid and to allow the evaluation of fluid consistency without the need for more invasive procedures. Additionally, drainage may be beneficial if intraoperative oozing or a pelvic abscess might result after the dissection of a wide area during a complicated laparoscopic hysterectomy. Conservative measures, including systemic antibiotics, may not be successful for preventing or treating large-volume fluid collection, especially if an infection develops.6
The role of a drain in the abdominal approach is well known,7 and several studies have examined the insertion of a drain through an abdominal port site after LAVH.8,9 SPA procedures seem ideally suited for LAVH because the vagina is a natural orifice for transluminal endoscopic surgery (NOTES). The vagina can also serve as a route for hysterectomy using the pouch of Douglas, and a uterine manipulator can be applied through the vagina and used as another grasper.10 Additionally, the vagina can serve as a drainage route to reduce postoperative morbidity without compromising the cosmetic advantage of SPA-LAVH. However, no formal study focusing on the methodology and safety of vaginal vault drainage in SPA-LAVH has been performed.
Therefore, we describe the methodology of closed-suction drainage (Jackson Pratt, or JP, drain) through the vaginal vault for complicated SPA-LAVH to prevent pelvic fluid collection and postoperative febrile morbidity. To show the feasibility and safety of vault drainage after SPA-LAVH, we also compare the operative outcomes and postoperative morbidity of patients with and without drains.
Materials and methods
A total of 359 women who underwent SPA-LAVH were included from April 2010 to August 2014. We compared 124 women who received a vaginal vault drain (the drain group) and 235 women who did not receive a drain (the no-drain group) after SPA-LAVH.
All women who were candidates for conventional LAVH underwent the SPA-LAVH procedure. The inclusion criteria were: (1) uterine size below 20 gestational weeks and without definite pelvic adhesions on pelvic examination; (2) a main diagnosis of uterine fibroids, preinvasive cervical lesion, endometrial hyperplasia, or microinvasive cervical cancer; (3) no suspected uterine or adnexal malignancy, previous abdominal surgery for malignancies, or suspected endometriosis; and (4) appropriate medical status for laparoscopic surgery (American Society of Anesthesiologists Physical Status Classification I-II). All of the women were informed that the conventional laparoscopic approach or laparotomy would be performed if unexpected difficulties were encountered during the SPA procedures.
Allocation to the vault drain or no-drain group was based on the surgeon’s decision. The general inclusion criteria for vault drain insertion after SPA-LAVH specified patients with blood coagulation defects, a wide dissection area, intraoperative oozing, intra-operative blood loss, and coexisting pelvic lesions that could increase the possibility of large-volume residual pelvic fluid collection and subsequent pelvic infection. The vault drain was removed within 48 hours after surgery if the drainage volume was < 100 mL/24 hours and/or the pelvic fluid had a hemoserous consistency. When there were infected or large-volume fluid collections or > 100 mL of fresh blood was observed in the drain bulb, the drain was not removed unless the abnormal pelvic fluid completely ceased and/or the volume was < 100 mL/24 hours and the patient was hemodynamically stable with stable hemoglobin (no decrease > 1 g/dL). The vault drain was always removed transvaginally by cutting the fixation suture material. The removal site spontaneously healed in a few days without any intervention.
Operative time was defined as the length of time from the umbilical skin incision to closure, including the time of vaginal vault closure, SPA introduction, and vaginal JP insertion. Blood loss was estimated based on the suction bottle volume and gauze count. Uterine weight was measured immediately after specimen retrieval in the operating room. Postoperative febrile morbidity was defined as a body temperature ≥ 37.5° C, a definition that has been used previously in a number of studies assessing postoperative infectious morbidity.11,12 Temperature was measured every 4 hours in the postoperative ward, excluding the 1st day after surgery. If a body temperature ≥ 37.5° C was noted on any postoperative day, we confirmed febrile status by checking the temperature hourly two times and appropriately controlling the fever. The postoperative hemoglobin level was determined on postoperative Day 1.
All of the women were managed with the standard hospital protocol. The women underwent vaginal preparation on the day of the SPA-LAVH and received cefotetan 2 g intravenously after the induction of general anesthesia. A Foley catheter was maintained for 24 hours for bladder drainage. Postoperative cefotetan 2 g every 12 hours was given to the women until postoperative Day 1 if there was no infectious morbidity.
Operative techniques
Surgical procedures of SPA-LAVH
All SPA procedures were performed using a homemade singleport platform, as previously described.10 After laparoscopic examination using a rigid 0° 5-mm video laparoscope, a uterine manipulator (Acorn uterine manipulator, Richard Wolf GmbH, Knittlingen, Germany) was inserted to facilitate visualization and accessibility in the surgical field. We used articulating instruments such as Realhand (Novare Surgical System, Cupertino, CA, USA) or Roticulator (Covidien, Norwalk, CT, USA) to avoid the clashing of instruments and to allow fine dissection (Figure 1). Each SPA hysterectomy procedure was similar to conventional LAVH. Briefly, after the patient was put into the deep Trendelenburg position, the uterus was deviated to one side with a uterine manipulator. Either the infundibulopelvic ligament or the utero-ovarian ligament was secured and divided following transection of the round ligament. The broad ligament was opened up to the vesicouterine fold, and the bladder was mobilized by blunt and sharp dissection from the anterior vagina. The uterine vessels were skeletonized with partial cutting of the uterosacral ligament. Following the laparoscopic procedures of SPA-LAVH, anterior and posterior colpotomy was performed transvaginally. The uterosacral ligament and uterine vessels were secured with sutures, and the uterus was extracted through the vagina. Vaginal vault closure was performed transvaginally with a single-layer technique, using a running 1-0 polyglactin 910 suture.
Figure 1.
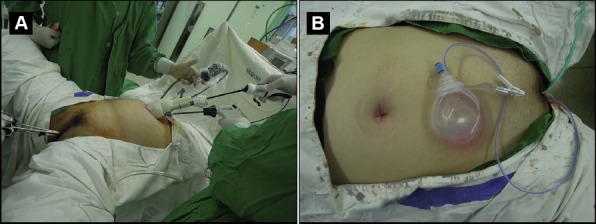
Operative view of single-port access laparoscopic-assisted vaginal hysterectomy (SPA-LAVH). (A) Home-made single-port platform made of a wound retractor, a glove and three trocars (12 mm, 5 mm, and 5 mm): a uterine manipulator is inserted to facilitate visualization of the pelvis. (B) Immediate postoperative view following SPA-LAVH with vault drain.
Surgical technique of closed suction drain (JP) insertion through the vaginal vault after complicated SPA-LAVH
Once the vault was closed, a laparoscope was used to check the pelvis for hemostasis and any abnormal lesion. If we found the patients with the risk of large volume residual pelvic fluid collection, and subsequent pelvic infection in the surgical field, we decided on the insertion of vault drainage. A JP drain was inserted through the vault, and intraperitoneal placement of the drain in the pouch of Douglas was performed under laparoscopic visualization. First, the tip of the JP drain was sutured using 2-0 polyglactin 910, and the suture material was grasped with a Fascial suture instrument (B. BraunMelsungen AG, Melsungen, Germany). After the vault was downwardly grasped with Allis forceps, the fascial suture instrument with the sutured JP drain was inserted through the apex of the vault into the peritoneal cavity under the guidance of laparoscopy. Then, the delivered suture material was pulled intra-corporeally using flexible grasping forceps. After the tip of the JP drain was identified, it was moved upward into the pouch of Douglas. The JP drain was fixed to the posterior vaginal vault to prevent it from falling out of the vagina (Figure 2). The suture material of the JP drain was delivered extracorporeally through the SPA system, and removed from the drain.
Figure 2.

JP drain insertion through the vaginal vault after single-port access laparoscopic-assisted vaginal hysterectomy (SPA-LAVH). (A) Insertion of sutured drain through the apex of the vault. (B) Placement of drain in peritoneal cavity. (C) Fixation of drain in posterior vaginal vault.
Statistical analysis
The statistical analysis was performed using SPSS version 21.0 (SPSS, Chicago, IL, USA) and the R package, version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Student t test was used to compare normally distributed continuous variables. Categorical variables were analyzed using Fisher’s exact test. All of the tests were two-tailed, and a p value < 0.05 was considered statistically significant for all comparisons.
Results
The background features of the 359 women are detailed in Table 1. There was no difference in general demographics between the two groups. The primary indication for hysterectomy was uterine fibroids in 77.2% of the women.
Table 1.
Background features.a
| Preoperative variables | Vaginal JP drainage (n = 124) | No drainage (n = 235) | p |
|---|---|---|---|
| Age (y) | 47.9 ± 6.0 | 47.1 ± 6.3 | 0.237 |
| BMI (kg/m2) | 24.0 ± 3.4 | 23.7 ± 2.6 | 0.375 |
| Parity (n) | 2.0 ± 0.6 | 2.1 ± 0.8 | 0.701 |
| History of previous abdominal surgery (n) | 38 (30.6) | 59 (25.1) | 0.264 |
| Numbers of previous abdominal surgeries (n) | 0.6 ± 0.9 | 0.4 ± 0.8 | 0.144 |
| Indications for hysterectomy (n) | 0.917 | ||
| Uterine fibroids | 97 (78.2) | 180 (76.6) | |
| Preinvasive cervical lesion | 19 (15.3) | 38 (16.2) | |
| Uterine lesion with adnexal tumor | 4 (3.2) | 5 (2.1) | |
| Endometrial hyperplasia | 3 (2.4) | 9 (3.8) | |
| Microinvasive cervical cancer | 1 (0.8) | 3 (1.3) |
BMI = body mass index.
a Data are presented as mean ± standard deviation or number (%).
The mean surgical data for the 359 women are summarized in Table 2. The uterine weight was significantly greater in the drain group than in the no-drain group (364.2 ± 184.9 g vs. 263.7 ± 138.6 g; p < 0.001). Moreover, the drain group had more cases with uterine weight ≥ 500 g than the no-drain group (n = 28, 22.6% vs. n = 19, 8.1%; p < 0.001). The two groups were comparable for operative procedures and main pathologic features. However, the drain group had greater blood loss (225.3 ± 122.2 mL vs. 150.4 ± 95.2 mL; p < 0.001), a greater hemoglobin drop (1.97 ± 0.96 g/dL vs. 1.42 ± 0.89 g/dL; p < 0.001), and longer operation times (87.4 ± 21.5 vs. 73.0 ± 17.6 minute; p < 0.001) than the no-drain group.
Table 2.
Surgical outcomes.a
| Surgical variables | Vaginal JP drainage (n = 124) | No drainage (n = 235) | p |
|---|---|---|---|
| Uterus weight (g) | 364.2 ± 184.9 | 263.7 ± 138.6 | < 0.001 |
| Uterus weight ≥ 500 g (n) | 28 (22.6) | 19 (8.1) | < 0.001 |
| Operative procedures (n) | 0.490 | ||
| Hysterectomy only | 94 (75.8) | 177 (75.3) | |
| Hysterectomy + BSO | 18 (14.5) | 42 (17.9) | |
| Hysterectomy + USO | 6 (4.8) | 11 (4.7) | |
| Hysterectomy + BOC | 1 (0.8) | 0 (0.0) | |
| Hysterectomy + other procedures | 5 (4.0) b | 5 (2.0) c | |
| Main pathology of uterus (n) | 0.935 | ||
| Leiomyoma | 72 (59.8) | 139 (59.0) | |
| Adenomyosis | 29 (21.4) | 46 (18.4) | |
| CIS of cervix | 18 (15.2) | 38 (17.5) | |
| Endometrial hyperplasia | 4 (2.7) | 9 (3.8) | |
| Microinvasive cervical cancer | 1 (0.9) | 3 (1.4) | |
| Estimated blood loss (mL) | 225.3 ± 122.2 | 150.4 ± 95.2 | < 0.001 |
| Hemoglobin decline (g/dL) | 1.97 ± 0.96 | 1.42 ± 0.89 | < 0.001 |
| Operation time from umbilical skin incision to closure (min) | 87.4 ± 21.5 | 73.0 ± 17.6 | < 0.001 |
BOC = bilateral ovarian cystectomy; BSO = bilateral salpingoophorectomy; USO = unilateral salpingoophorectomy.
a Data are presented as or mean ± standard deviation or number (%).
b In the drain group, other procedures include one case of ureteral stent insertion to the left ureter, two cases of primary repair of the bladder through the vagina, two cases of staging operation (borderline ovarian tumor-LAVH BSO, pelvic and para-aortic lymph node sampling, ometum and both paracolic gutter biopsy, appendectomy; cervical cancer-LAVH BSO, pelvic and para-arotic lymph node sampling).
c In the no-drain group, other procedures include three cases of primary repair of bladder wall through the vagina, and two cases of colporrhaphy.
The postoperative outcomes for the 324 women are summarized in Table 3. A total of 17 (4.7%) patients required a blood transfusion during the postoperative period; eight (6.5%) were in the drain group and nine (3.8%) were in the no-drain group. The drain group had a longer mean hospital stay (3.1 ± 1.6 days vs. 2.5 ± 1.3 days; p = 0.002) than the no-drain group. No significant operative morbidity occurred, and there were no group differences in terms of intraoperative and perioperative complications.
Table 3.
Postoperative outcomes.a
| Postoperative variables | Vaginal JP drainage (n = 124) | No drainage (n = 235) | p |
|---|---|---|---|
| Transfusion (n) | 8 (6.5) | 9 (3.8) | 0.300 |
| Postoperative hospital stay (d) | 3.1 ± 1.6 | 2.5 ± 1.3 | 0.002 |
| Total volume of postoperative drain (mL) | 230.5 ± 191.5 | Not available | |
| Intraoperative complication (n) | 3 (2.4) | 3 (1.3) | 0.420 |
| Bladder wall injury | 2 | 3 | |
| Ureter injury | 1 | – | |
| Perioperative complication within 30 d (n) | 3 (2.4) | 2 (0.9) | 0.345 |
| Umbilical wound infection | 2 | 1 | |
| Paralytic ileus | 1 | – | |
| Vaginal stump dehiscence | – | 1 | |
| Postoperative febrile morbidity | |||
| ≥ 37.5°C (n) | 11 (8.9) | 27 (11.5) | 0.477 |
| ≥ 38.0°C (n) | 5 (4.0) | 7 (3.0) | 0.759 |
a Data are presented as or mean ± standard deviation or number (%).
There were six intraoperative complications (three in the drain group and three in the no-drain group), which included five cases of bladder-wall injury and one case of ureteral injury during the SPA-LAVH. All cases were successfully managed without an additional trocar or conversion to laparotomy. In five women with bladder wall injury, primary repair of the bladder wall was performed through the vagina. In one woman with a ureter injury, a minor electrical burn of the left distal ureter was suspected after SPA-LAVH, and a prophylactic double J stent was inserted in the ureter during surgery. All women recovered unremarkably.
During the perioperative period (within 30 days after surgery), five complications were noted. Three women had umbilical wound infections that resolved with antibiotic therapy. One woman in the drain group was readmitted for paralytic ileus and was conservatively managed. In the no-drain group, one woman presented with vault dehiscence on postoperative Day 24; it was managed with resuturing, and there were no long-term consequences.
The bottom line of Table 3 shows the febrile morbidity results for the two groups. A total of 38 (10.6%) women with SPA-LAVH had a temperature ≥ 37.5°C during the postoperative period: 11 (8.9%) were in the drain group and 27 (11.5%) were in the no-drain group (p = 0.477). There were 12 cases with relatively large-volume drainage (≥ 500 mL) in the drain group, but none of these women suffered from febrile morbidity. We also performed a subgroup analysis for women with a temperature ≥ 38.0°C, and there was no difference in the number of women (p = 0.759) in the two groups.
Discussion
There were some controversies in the use of drainage to reduce postoperative morbidity by eliminating pelvic fluid collection. Some studies have reported the advantage of drain usage, but others have not confirmed. However, in real clinical practice, drainage procedures have been used for preventing abnormal pelvic fluid collection in the complicated surgical case for a long time. Thus, we suggested that vaginal vault (NOTES) could be a useful alternative to control postoperative morbidity in selective situations based on surgeon decision without compromising the advantage of SPA laparoscopic surgery. In this study, the indications for vaginal vault drainage included the wide extent of dissection, large volume of operative blood loss, intraoperative oozing, blood coagulation defects, and coexisting pelvic lesions that could increase the possibility of large-volume residual pelvic fluid collection, subsequent pelvic infection, and febrile morbidity after complicated SPA-LAVH.
The pelvic fluid collection and/or hematomas presumably formed as a result of residual fluid, oozing, or bleeding at the end of the hysterectomy procedures. At the end of vaginal hysterectomy procedures, 48% of patients still have pelvic bleeding points; the bleeding comes predominantly from the vaginal vault, but 20% of patients also have bleeding from the uterine artery or the vaginal angle.13 Although some studies reported that the presence of sonographically diagnosed pelvic fluid collection was associated with febrile morbidity following hysterectomy,4,14,15 other studies failed to confirm this claim.4,5 Individual studies did not show a significant relationship between pelvic fluid collection and febrile morbidity, but each study showed an incremental trend toward febrile morbidity in patients with pelvic fluid collection. In this study, we did not simply compare drain group with no-drain group, but focused on the availability and safety of vault drainage after complicated SPA-LAVH. With regard to the operative complication and postoperative morbidity rates, we found that the drain group was comparable to the no-drain group; however, the drain group had a significantly longer operation time and more frequent wide dissection as a result of larger uterine sizes and weights, which made this group more prone to pelvic fluid collection and febrile morbidity. Moreover, there was no complication specifically related to the insertion of the closed-suction drain of the vaginal vault into the peritoneal cavity such as vaginal stump dehiscence, bowel injury, and infection of the stump site.
Generally, a pelvic drain is placed to reduce postoperative morbidity by facilitating the removal of gas and pelvic fluid collection, to assess fluid characteristics, to check for any unsuspected hemorrhages in the surgical field, and to promote tissue apposition and wound healing without the need for further invasive procedures. A randomized study by Dua et al12 suggested that the routine use of vault drainage during vaginal hysterectomy did not influence febrile morbidity, but there were no adverse outcomes noted with the use of the drain. The volume of fluid that may be drained even after extensive pelvic dissection using laparoscopy is unpredictable, and the decision to use postoperative drainage was based on the surgeon’s experience and the patient’s clinical condition.16 With conventional LAVH, drains are still a necessary part of gynecologic laparoscopy for selected women, such as those with persistent oozing from raw surfaces or pelvic abscesses.8 Complex extirpative pelvic surgery via the SPA system has become possible because of recent advances in surgical techniques, but no studies have evaluated the management of postoperative morbidity after complicated SPA hysterectomy. Because the use of prophylactic antibiotics has been clearly shown to have a significant role in reducing infection and is now a standard practice, the efficacy of vault drainage after complicated SPA-LAVH should be reevaluated.
In the current study, the overall complication rate was 3.1%, and the rate of febrile morbidity (at least 38.0°C) was 3.3%. Although this study was the first to evaluate vault drainage after SPA-LAVH, the results were comparable to those of previous studies on laparoscopic hysterectomy.11
The strength of this study was that it was the first preliminary report on the safety of vault drainage after complicated SPA-LAVH in terms of complications and postoperative morbidity. However, there were some limitations. First, this study was a retrospective one and the cohort of women was not unselected cases; in addition, all procedures were performed by one surgeon (H.J Roh). Thus, our data may not be generalizable to all gynecologic surgeons using different SPA devices and drainage procedures. Second, there was a lack of definitive imaging measurements of the pelvic fluid collection with which to compare the postoperative morbidity of the drain and no-drain groups. Thus, our results do not definitively support the use of routine vault drainage for reducing pelvic fluid collection and febrile morbidity in cases of uncomplicated SPA-LAVH; rather, they only evaluate the safety and effectiveness of vault drains following complicated SPA-LAVH.
In conclusion, in selected women following complicated SPA-LAVH, vaginal vault drainage could be a feasible and safe method for managing postoperative morbidity and completing surgery while retaining the advantages of minimally invasive surgery. However, this study did not show that routine use of prophylactic vault drainage may be necessary in elective, uncomplicated SPA-LAVH. The role of vault drainage in the management of postoperative morbidity following SPA-LAVH should be further examined.
Footnotes
Conflicts of interest: The authors have no conflicts of interest relevant to this article.
References
- 1.Hong MK, Wang JH, Chu TY, Ding DC. Laparoendoscopic single-site hysterectomy with Ligasure is better than conventional laparoscopic assisted vaginal hysterectomy. Gynecol Minim Invasive Ther. 2014;3:78–81. [Google Scholar]
- 2.Chen YJ, Wang PH, Ocampo EJ, Twu NF, Yen MS, Chao KC. Single-port compared with conventional laparoscopic-assisted vaginal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2011;117:906–912. doi: 10.1097/AOG.0b013e31820c666a. [DOI] [PubMed] [Google Scholar]
- 3.Song T, Lee Y, Kim ML, et al. Single-port access total laparoscopic hysterectomy for large uterus. Gynecol Obstet Invest. 2013;75:16–20. doi: 10.1159/000341141. [DOI] [PubMed] [Google Scholar]
- 4.Antonelli E, Morales MA, Dumps P, Boulvain M, Weil A. Sonographic detection of fluid collections and postoperative morbidity following Cesarean section and hysterectomy. Ultrasound Obstet Gynecol. 2004;23:388–392. doi: 10.1002/uog.1023. [DOI] [PubMed] [Google Scholar]
- 5.Thomson AJ, Sproston AR, Farquharson RG. Ultrasound detection of vault haematoma following vaginal hysterectomy. Br J Obstet Gynaecol. 1998;105:211–215. doi: 10.1111/j.1471-0528.1998.tb10055.x. [DOI] [PubMed] [Google Scholar]
- 6.Segev Y, Auslender R, Lissak A, Lavie O, Abramov Y. Symptomatic pelvic hematoma following transvaginal reconstructive pelvic surgery: incidence, clinical presentation, risk factors, and outcome. Eur J Obstet Gynecol Reprod Biol. 2010;153:211–214. doi: 10.1016/j.ejogrb.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Rosen DM, Cario GM. Vault haematoma following laparoscopic hysterectomy. Aust N Z J Obstet Gynaecol. 1997;37:220–222. doi: 10.1111/j.1479-828x.1997.tb02258.x. [DOI] [PubMed] [Google Scholar]
- 8.Shen CC, Huang FJ, Hsu TY, Weng HH, Chang HW, Chang SY. A prospective, randomized study of closed-suction drainage after laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 2002;9:346–352. doi: 10.1016/s1074-3804(05)60415-x. [DOI] [PubMed] [Google Scholar]
- 9.Shen CC, Wu MP, Lu CH, et al. Effects of closed suction drainage in reducing pain after laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 2003;10:210–214. doi: 10.1016/s1074-3804(05)60301-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim TJ, Lee YY, Cha HH, et al. Single-port-access laparoscopic-assisted vaginal hysterectomy versus conventional laparoscopic-assisted vaginal hysterectomy: a comparison of perioperative outcomes. Surg Endosc. 2010;24:2248–2252. doi: 10.1007/s00464-010-0944-y. [DOI] [PubMed] [Google Scholar]
- 11.McPherson K, Metcalfe MA, Herbert A, et al. Severe complications of hysterectomy: the VALUE study. BJOG. 2004;111:688–694. doi: 10.1111/j.1471-0528.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 12.Dua A, Galimberti A, Subramaniam M, Popli G, Radley S. The effects of vault drainage on postoperative morbidity after vaginal hysterectomy for benign gynaecological disease: a randomised controlled trial. BJOG. 2012;119:348–353. doi: 10.1111/j.1471-0528.2011.03170.x. [DOI] [PubMed] [Google Scholar]
- 13.Wood C, Maher P, Hill D. Bleeding associated with vaginal hysterectomy. Aust N Z J Obstet Gynaecol. 1997;37:457–461. doi: 10.1111/j.1479-828x.1997.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 14.Toglia MR, Pearlman MD. Pelvic fluid collections following hysterectomy and their relation to febrile morbidity. Obstet Gynecol. 1994;83:766–770. [PubMed] [Google Scholar]
- 15.Haines CJ, Shan YO, Hung TW, Chung TK, Leung DH. Sonographic assessment of the vaginal vault following hysterectomy. Acta Obstet Gynecol Scand. 1995;74:220–223. doi: 10.3109/00016349509008943. [DOI] [PubMed] [Google Scholar]
- 16.Chan K, Welsh A, Abbott J. Residual pelvic fluid using two types of drains at laparoscopy: a randomized controlled trial. Obstet Gynecol. 2008;111:1293–1297. doi: 10.1097/AOG.0b013e31817589cd. [DOI] [PubMed] [Google Scholar]


