Abstract
In birds, vocal learning enables the production of sexually selected complex songs, dialects and song copy matching. But stressful conditions during development have been shown to affect song production and complexity, mediated by changes in neural development. However, to date, no studies have tested whether early-life stress affects the neural processes underlying vocal learning, in contrast to song production. Here, we hypothesized that developmental stress alters auditory memory formation and neural processing of song stimuli. We experimentally stressed male nestling zebra finches and, in two separate experiments, tested their neural responses to song playbacks as adults, using either immediate early gene (IEG) expression or electrophysiological response. Once adult, nutritionally stressed males exhibited a reduced response to tutor song playback, as demonstrated by reduced expressions of two IEGs (Arc and ZENK) and reduced neuronal response, in both the caudomedial nidopallium (NCM) and mesopallium (CMM). Furthermore, nutritionally stressed males also showed impaired neuronal memory for novel songs heard in adulthood. These findings demonstrate, for the first time, that developmental conditions affect auditory memories that subserve vocal learning. Although the fitness consequences of such memory impairments remain to be determined, this study highlights the lasting impact early-life experiences can have on cognitive abilities.
Keywords: vocal learning, developmental stress, auditory, IEG, electrophysiology, zebra finch
1. Introduction
The ability to learn affects all aspects of animal behaviour and yet the fitness implications of learning abilities are virtually unknown. Studies are focused on the functional significance [1] or the underlying mechanisms [2] of learning, but we know very little about either the ecological significance or the evolutionary implications of learning ability. Imitative vocal learning occurs in only a few taxonomically diverse groups and is thought to have arisen independently through convergent evolution [3]. In songbirds, vocal learning allows for the production of complex songs that have evolved as a result of sexual selection [4,5]. Under current sexual selection theory, sexual signals are hypothesized to be costly to produce or to maintain [6,7], but the cost of producing complex songs remains unclear [8].
Vocal learning abilities have associated fitness benefits, thought to be mediated through the influence of early-life conditions on neural development [9,10]. For instance, song sparrows (Melospiza melodia) that learn more effective copies of tutor songs attract more female copulation solicitation displays [11]. The ‘developmental stress hypothesis’ [12–14] suggests that early developmental stress may detrimentally affect the development of brain structures controlling song learning and production, and therefore, vocal learning abilities. Empirical studies have confirmed the direct effects of early-life stress on both the songbird brain morphology [14–16] and its functional outputs, in terms of song complexity and copy accuracy [13,17,18], with consequences for female mate choice [11,19,20]. However, it is still not clear from these studies what the causal links are between developmental stress and impaired song production: do males which experience early developmental stress fail to produce such complex songs because they lack the neural mechanisms to produce the song? Or does early developmental stress affect the process of initial song learning, even before motor production?
In songbirds, both vocal learning and production are mediated by a series of interconnected brain nuclei known as the ‘song-control system’ [21–23]. To date, studies investigating the effect of developmental stress on song have almost exclusively focused on changes in neural morphology [14–16], overlooking the functional mechanisms underlying vocal learning per se. Yet, differences in brain anatomy do not necessarily result in differences in song learning abilities. For instance, Gahr et al. [24] showed that individuals with distinct volumes of song control nuclei, such as the nucleus HVC (proper name) and the robust nucleus of the arcopallium (RA), can produce similar songs through differential gene expression patterns within these brain areas. Therefore, while there is good evidence for condition-dependent neural development and vocal learning [13–18], the underlying mechanisms remain untested.
Early in life, males learn their songs through a two-step process of vocal imitation: a ‘sensory’ phase of learning (listening to and memorization of tutor song) and a ‘sensorimotor’ phase (practice vocalizations) [4,21]. In addition to the classical song-control system, essential for song production and sensorimotor learning, there are specific regions in the caudal pallium which are involved in the perception and processing of auditory information, including the caudomedial nidopallium (NCM) and the caudomedial mesopallium (CMM) [21,23]. Both NCM and CMM show stronger neuronal activation in response to conspecific song than to other sounds, as seen in both immediate early gene (IEG) expression studies (e.g. [25]) and electrophysiological activity recordings (e.g. [26]). The evoked auditory responses show stimulus-specific adaptation (SSA; decrements in neural activity and IEG expression) to repeated presentation of conspecific song, which is maintained for days after passive exposure [26,27]. Importantly, neural responses in NCM and CMM adapt more rapidly to playback of novel songs than familiar songs, a property that can be tested to provide a neural index of long-term memory formation [26,28]. In songbirds, although IEG response to song exposure, and therefore memory formation, is known to vary with age, sex and social context [29,30]; to date, there have been no tests of the role of early developmental conditions in auditory memory formation.
In this study, we hypothesized that early developmental stress affects auditory memory formation and neural processing of song stimuli, as a basis for learning throughout adulthood. We evaluated this hypothesis in zebra finches (Taeniopygia guttata) reared under different dietary conditions (stressed and control) in two separate experiments assessing neural responses to song playback in the NCM and CMM: by measuring IEG expression (experiment 1) and electrophysiological response (experiment 2). Specifically, we tested the hypotheses that developmental stress (i) affects the ability of individuals to memorize tutor song, and (ii) may have long-term effects on neural function, causing deficits in the formation of new auditory memories in adulthood.
2. Methods
(a). General husbandry (experiments 1 and 2)
Domesticated zebra finches were housed at Deakin University (experiment 1) and Rutgers University (experiment 2), in breeding cages (118 × 50 × 50 cm) in temperature-controlled rooms. All pairs were allowed a choice of partner before breeding (n = 21 pairs in experiment 1, n = 12 pairs in experiment 2). Photoperiod was held at 14 L : 10 D throughout the experiments. Birds were offered a nest-box and a variety of nesting materials, and cages contained seed ad libitum, automatic drinkers, shell grit, fresh cucumber, boiled egg and two perches. Once egg laying had commenced, breeding pairs were randomly assigned to either a control or stressed diet. Birds allocated to the control diet were allowed seed ad libitum, but no fresh greens or egg. Birds allocated to the stressed diet received a restricted amount of seed daily (scaled to number and age of nestlings) mixed with three times the volume of ground rice husk, which has no nutritional value [15]. The dietary treatments were started at 5 days post-hatch (dph) and every offspring was marked for individual identification and weighed daily from this time. Once birds had fledged, they were then individually colour banded. At 30 dph, offspring were moved to the ad libitum control diet and were physically separated from their parents, although kept within visual and acoustic contact, using mesh cage dividers. Offspring were weighed every 10 days until 60 dph, and then placed in single-sex groups.
(b). Experimental designs (experiments 1 and 2)
The two experiments differed in their design. In experiment 1, parents were allowed to breed twice, with the dietary treatment being imposed in a balanced manner across breeding attempts. Siblings, therefore, acted as their own controls, across breeding attempts. In experiment 2, nestlings were cross-fostered at 5 dph. Cross-fostering was completed by moving half of each brood into the nest of another family, with similar age and number of offspring as their own, but of the opposite experimental condition. This allowed for control of heredity and genetic influences.
In both experiments, when subjects reached adulthood (≈90 dph), we assessed their neural responses to song playback. In experiment 1, we quantified ZENK and Arc expression in the NCM and CMM in response to tutor and novel song playbacks to assess the degree to which tutor song was memorized in adult males. In experiment 2, we measured the neuronal responses to relevant song stimuli (i.e. tutor, familiar and novel songs) through electrophysiological recordings in NCM and CMM in order to assess the memory for (i) tutor song and (ii) passively familiar trained stimuli.
(c). Brain collection, histology and immunohistochemistry (experiment 1)
Before brain collection, male offspring from each nest (n(stressed) = 15, n(control) = 16) were randomly assigned to playback treatment groups for playback of either a novel male song or their tutor song. Where brothers were available, one was allocated to each of the different playback treatments and any additional males were then randomly assigned. The brains of all male offspring aged 80–99 (mean 87) dph were collected following song playback in the dark (see the electronic supplementary material). Briefly, after 3 h of dark acclimatization, the male was then played 1 h of song playback. The playback consisted of 16 s repeats of stimulus song followed by 16 s of silence, played in a loop. All playback stimuli were in the .wav format (sampling rate: 44.1 kHz, standardized amplitude: 80–90 dB). Thirty minutes after the end of the playback, males were euthanized, and the brains were collected, flash frozen, weighed and then stored at −80°C. One-half (left side) of each brain was sectioned into 30 µm parasagittal sections. Each section was mounted onto Superfrost Plus microscope slides (Menzel Glaser, Braunschweig, Germany). In situ hybridization of these slides detected ZENK and Arc mRNA expression in the brain sections using cRNA probes labelled with 35S-CTP. This technique followed a previously described protocol for the androgen receptor mRNA [31]. The cloning and characterization of the zebra finch ZENK and Arc genes have been described previously (see [32,33]; GenBank accession numbers: ZENK: AF026084; Arc: AY792623). These slides then underwent 35S film autoradiography and developed in a Kodak cassette for 14 days. Developed and fixed film then underwent densitometry calculations to estimate the intensity of gene expression from optical density (ImageJ software; see the electronic supplementary material). Overall, 20 and 24 male offspring brains were successfully imaged for Arc and ZENK expression, respectively.
(d). Electrophysiological recordings (experiment 2)
When males reached 90 dph, 13 subjects (n(stressed) = 6, n(control) = 7) underwent surgical preparation for the neuronal activity recordings; surgical and electrophysiological recording procedures are described in detail in the electronic supplementary material. One day later, the birds were passively exposed to playback of the songs of eight zebra finches unknown to the subject (200 repetitions, blocked, 8 s inter-stimulus interval (ISI)) which would then serve as ‘familiar’ stimuli for subsequent neural recordings. In brief, 20 h after presentation, the awake bird was comfortably restrained in a plastic tube, and the head pin clamped to a stereotaxic apparatus located in a soundproof booth. To record neuronal activity, a multi-electrode microdrive (Eckhorn, Thomas Recording, Giessen, Germany) was used to lower 16 tungsten micro-electrodes (Type ESI2ec, impedance: 2–4 MΩ, Thomas Recording) bilaterally into the brain, targeting areas NCM and CMM (16 electrodes total, four in each area in each hemisphere). Experimental sets of song stimuli were played through a speaker placed 30 cm in front of the subjects while recording multi-unit neural responses (amplified: ×19 000, band-pass filtered: 0.5–5 kHz; Spike2 software, CED, Cambridge, UK). Stimulus sets included the ‘familiar’ songs, eight completely ‘novel’ songs, and the subject's ‘tutor’ songs (equated for loudness: 75 dB average; sampling rate: 44.4 kHz). Song stimuli were presented for 25 repetitions each, in a shuffled order (8 s ISI).
Analyses of electrophysiological data were conducted on multi-unit responses collected in NCM and CMM (see the electronic supplementary material for details). To quantify the strength of the response for a particular song (tutor or familiar) compared to novel song (i.e. response selectivity), relative response strength (RRS) was calculated at each recording site by subtracting the absolute response magnitude (ARM) to each of the relevant stimuli (tutor or familiar) from the ARM to novel stimuli, and dividing by the average of both ARMs to normalize for the response of the site [34]. In addition, we calculated the adaptation rate of neural responses to familiar song stimuli at each site, using the slope of decline in neuronal responses between trial 6 and 25, and dividing this rate by the ARM to normalize for the level of response of the site [28]. Stimuli that are novel show a higher rate of adaptation than stimuli that are familiar (i.e. remembered; [26,28]). Thus, to quantify the strength of the ‘20 h memory’, we calculated a familiarity index (FI) at each site as the ratio of the adaptation rate for novel song to that for familiar song [28]. RRS and FI values from recording sites within the same auditory brain area were pooled for statistical analyses (average RRS and FI in NCM or CMM).
(e). Statistical analyses
Statistical analyses were conducted in R v. 3.3.3 [35], using the package ‘lme4’ for the main statistical mixed-effects models. We tested the effect of dietary treatment on nestling growth rates (experiments 1 and 2) using mixed-effects models, with ‘body mass’ (repeated measures) as the dependent variable, and ‘treatment’ (stressed or control), ‘sex’ (male or female), ‘age’ (in days) and its quadratic term ‘age2’ and two-way interactions involving treatment as fixed effects. We included a random intercept and a random slope effects for individual identity (ID) in the models, and given the specific design of each experiment, also included brood ID nested within pair ID (experiment 1) or both natal and foster nest IDs (experiment 2) as random factors (see the electronic supplementary material). We then tested the effects of developmental stress on IEGs expression in response to song playbacks in adult males (experiment 1) using mixed-effects models. Models included ‘Arc or ZENK expression’ as the dependent variable, experimental ‘treatment group’ (four-level factor: ‘control diet/tutor song’, ‘control diet/novel song’, ‘stressed diet/tutor song’, ‘stressed diet/novel song’), brain ‘area’ (NCM or CMM) and their interaction as fixed effects, and individual ID nested within brood ID as random factors. When needed, post-hoc comparisons of least square means with adjusted p-values (Bonferroni correction) were performed. Finally, we tested whether developmental stress affected the strength of the neuronal response to tutor song and the ability of birds to memorize recently heard songs (‘20 h memory’) in adulthood (experiment 2). We used mixed-effects models with mean ‘RRS’ or ‘FI’ as the dependent variable, developmental ‘treatment’ (stressed or control), ‘area’ (NCM or CMM) and their interaction as fixed effects and individual ID as a random factor. All models were fitted using restricted maximum-likelihood (REML) estimation and degrees of freedom (d.f.) were estimated according to the Kenward–Roger approximation.
3. Results
(a). Effects of experimental diet manipulation on nestling growth
In both experiments, nestlings showed a nonlinear rate of mass gain (figure 1a,b), and importantly, their growth patterns were affected by the dietary treatment (significant effects of ‘treatment × age’ and ‘treatment × age2’ interactions; table 1a,b). Specifically, at the start of the treatment (5 dph), there was no difference in body mass between control and stressed nestlings (mixed-effects models at age 5, ‘treatment’ effect: F1,55.82 = 2.66, p = 0.109 and F1,8.78 = 0.32, p = 0.587, in experiment 1 and experiment 2, respectively). During early-life dietary restriction (i.e. before fledging), stressed nestlings exhibited a slower growth than control nestlings, particularly between 5 and 13–14 dph (figure 1a,b). As a result, stressed nestlings were significantly lighter than controls from 10 to 17 dph in experiment 1 (models run for each day separately, ‘treatment’ effect: all p < 0.042 at ages 10–17; figure 1a) and from 9 to 29 dph in experiment 2 (all p < 0.041 at ages 9–29; figure 1b). However, although dietary restriction initially reduced the growth of stressed nestlings, they compensated. Consequently, by 18 (experiment 1) and 30 dph (experiment 2), nestlings in the stressed group had caught up, reaching similar body masses to those of controls, (p > 0.05, figure 1a,b) and treatment groups showed similar weights throughout adulthood (all p > 0.220 at ages 40, 50 and 60 dph in both experiments).
Figure 1.
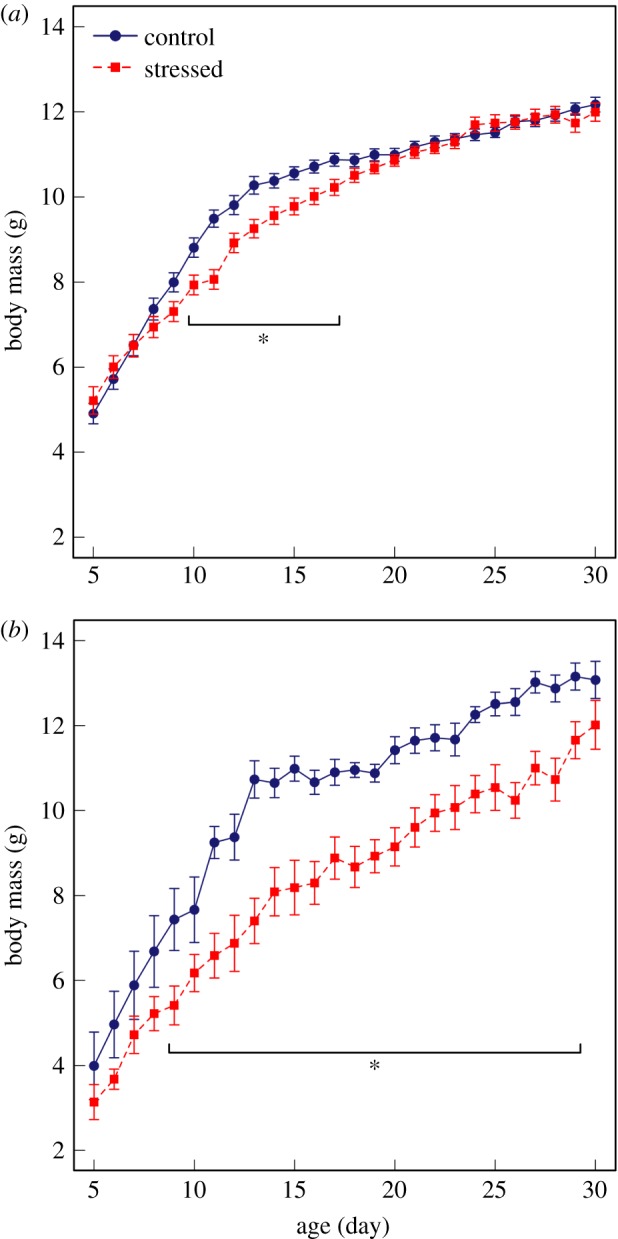
Relationship between body mass (mean ± s.e.) and age for nestling zebra finches reared on a control (ad libitum) or stressed (restricted) diet, in experiment 1 (a) and experiment 2 (b). Control and stressed subjects began and ended the nutritional stress paradigm at similar weights. However, during early-life deprivation, stressed nestlings weighed significantly less than controls (table 1 and text for details). An asterisk * indicates days when stressed nestlings were significantly lighter than controls. (Online version in colour).
Table 1.
Results of mixed-effects models testing the effect of dietary treatment on nestling mass gain in experiment 1 (a) and experiment 2 (b). (Full models included treatment (stressed versus control), age (centered at day 5), age2, sex and treatment × age and treatment × age2 interactions as fixed effects and individual ID as random factor. Model (a) also included brood ID nested within pair ID as random factors. Model (b) also included natal and foster nest IDs as random factors. Since the effect of sex was not significant in experiment 1 (F1,78.16 = 0.00, p = 0.951), sex was removed from model (a).)
| fixed effects | estimate | s.e. | d.f. | F | p |
|---|---|---|---|---|---|
| (a) experiment 1 (n = 1685 observations on 65 nestlings) | |||||
| intercept | 5.665 | 0.232 | — | — | — |
| age | 0.617 | 0.014 | 1, 336.11 | 2720.08 | <0.001 |
| age2 | −0.015 | 0.0004 | 1, 1553.25 | 1777.53 | <0.001 |
| treatment[stressed] | −0.185 | 0.352 | 1, 63.69 | 0.27 | 0.608 |
| treatment[stressed] × age | −0.077 | 0.022 | 1, 336.11 | 12.07 | <0.001 |
| treatment[stressed] × age 2 | 0.004 | 0.0006 | 1, 1553.25 | 38.84 | <0.001 |
| (b) experiment 2 (n = 410 observations on 19 nestlings) | |||||
| intercept | 4.646 | 1.049 | — | — | — |
| sex[male] | 1.079 | 0.277 | 1, 8.09 | 11.41 | 0.010 |
| age | 0.673 | 0.046 | 1, 108.38 | 409.07 | <0.001 |
| age2 | −0.015 | 0.0013 | 1, 377.16 | 192.66 | <0.001 |
| treatment[stressed] | −1.516 | 1.298 | 1, 16.50 | 1.36 | 0.260 |
| treatment[stressed] × age | −0.175 | 0.058 | 1, 108.39 | 9.19 | 0.003 |
| treatment[stressed] × age2 | 0.007 | 0.0017 | 1, 377.15 | 17.07 | <0.001 |
(b). Effects of developmental stress on tutor song neuronal memory
(i). As measured by song-induced immediate early gene expression (experiment 1)
Adult body mass and brain mass did not differ between treatment groups (mixed-effects models, body mass: F3,18.27 = 0.93, p = 0.445, brain mass: F3,19.77 = 1.50, p = 0.245). However, there were significant effects of treatment group on IEG expression. Specifically, Arc expression levels were affected by both treatment group (F3,12.80 = 6.40, p = 0.007) and brain area (F1,19.00 = 63.06, p < 0.001). Overall, Arc expression levels were higher in CMM than in NCM (figure 2a). However, there was no effect of the interaction between brain area and treatment group (‘treatment group × area’ interaction: F3,16.00 = 2.06, p = 0.146), indicating that the effects of treatment group on Arc expression did not depend on the brain area: across the NCM and CMM, post-hoc comparisons between treatment groups revealed that in control birds, tutor song playback elicited a significant increase in Arc expression relative to novel song playback (t11.84 = 3.77, p.adj = 0.013; figure 2a). In addition, Arc expression levels were significantly greater in control birds experiencing tutor song than in stressed subjects hearing either tutor (t15.97 = 3.22, p.adj = 0.025) or novel (t15.97 = 3.80, p.adj = 0.008) songs (figure 2a). All other comparisons were not significant (all p.adj ≥ 0.875; figure 2a). ZENK expression levels were only affected by treatment group (mixed-effects models, F3,14.80 = 5.72, p = 0.008), and post-hoc comparisons between treatment groups showed that ZENK expression followed similar patterns as for Arc expression, with highest expression levels in the control birds that experienced tutor song playback (figure 2b; post-hoc tests: t11.60 = 3.73, p.adj = 0.014 relative to control birds/novel song; t19.27 = 3.02, p.adj = 0.033 relative to stressed birds/tutor song; t18.43 = 3.20, p.adj = 0.023 relative to stressed birds/novel song; all other comparisons, p.adj ≥ 0.910). Furthermore, there was no effect of brain area (F3,23.00 = 0.04, p = 0.844) or the ‘treatment group × area’ interaction (F3,20.00 = 0.38, p = 0.771) on ZENK expression, indicating that levels were similar overall in CMM and NCM and that the observed differences in ZENK expression between treatment groups did not depend on the brain area (figure 2b).
Figure 2.
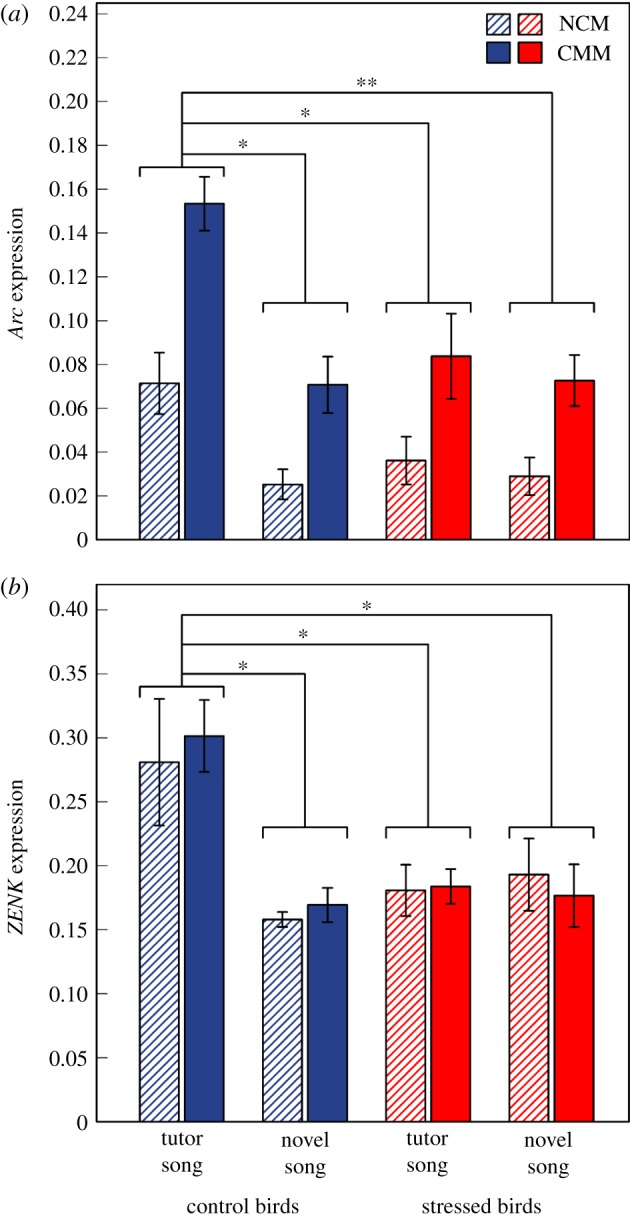
IEG expression levels (mean ± s.e.) for Arc (a) and ZENK (b) in the NCM and CMM in response to playback of tutor or novel songs in adult males raised under either control or stressed conditions. (a) n = 5 for all treatment groups, (b) n = 6 for control/tutor song, n = 5 for control/novel song, n = 5 for stressed/tutor song and n = 8 for stressed/novel song. Significant differences between treatment groups are symbolized: *p < 0.05, **p < 0.01. IEG expression after tutor song playback was higher in controls than stressed subjects (see text for details). (Online version in colour).
(ii). As measured by electrophysiological response to songs (experiment 2)
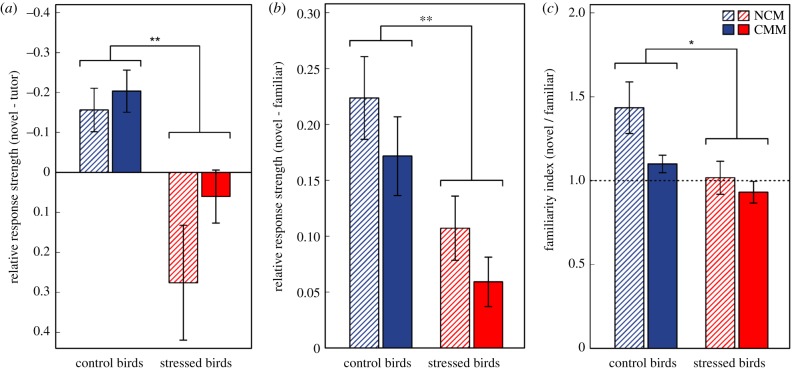
Control and stressed birds significantly differed in the strength of their neuronal response to tutor song, as measured through electrophysiological recordings in NCM and CMM (tutor RRS: mixed-effects model, treatment effect: F1,11 = 12.42, p = 0.005). Specifically, control males showed stronger neuronal responses (i.e. increased electrophysiological activity) to tutor song than novel song, resulting in mean RRS values (novel–tutor) lower than 0, while males that had experienced early-life nutritional stress did not (figure 3a). Furthermore, there was no effect of brain ‘area’ (F1,12 = 3.52, p = 0.090) or the ‘treatment × area’ interaction (F1,11 = 1.68, p = 0.222) on tutor RRS, indicating that the observed differences in tutor song selectivity between control and stressed birds did not differ across NCM and CMM (figure 3a).
Figure 3.
Mean (±s.e.) tutor RRS (a), familiar RRS (b) and familiar FI (c) in the NCM and CMM of adult males raised under either control or stressed conditions. Significant difference between control and stressed birds are symbolized: *p < 0.05, **p < 0.01. Male subjects showed impaired neuronal memory for tutor song (a) and recently heard songs (b,c). (a) Tutor RRS scores were significantly lower in control than stressed subjects (scale reversed, mixed-effects model: p = 0.005), and only control birds had RRS < 0 (indicating response selectivity for tutor song). (b,c) Familiar RRS and FI indices were significantly higher in control than stressed subjects (RRS: p = 0.006, FI: p = 0.022) and only control birds had FI > 1 (indicating memory of the recently heard song). (Online version in colour).
(c). Effects of developmental stress on 20 h memory in adulthood (experiment 2)
In adulthood, neuronal responses in NCM and CMM are weaker and adapt more slowly to playback of recently heard (≈2 days) familiar songs than to novel songs [26]. Therefore, for recently heard songs that are remembered, RRS is expected to be greater than 0, and FI to be greater than 1. The measured RRS and FI values for familiar songs showed that nutritionally stressed males had impaired 20 h neuronal memory (figure 3b,c). Specifically, there was a significant main effect of developmental condition on mean familiar RRS (mixed-effects model, ‘treatment’ effect: F1,11 = 11.77, p = 0.006), with control birds showing higher familiar RRS values than stressed birds (figure 3b). There was, however, no effect of brain ‘area’ (F1,12 = 2.81, p = 0.120) or the ‘treatment × area’ interaction (F1,11 = 0.004, p = 0.950) on familiar RRS. The mean FI for familiar song was affected by both developmental condition (F1,11 = 7.08, p = 0.022) and brain area (F1,12 = 4.97, p = 0.046). Specifically, familiar FI indices were overall higher in the NCM than in the CMM (figure 3c). However, there was no effect of the interaction between brain area and treatment (‘treatment × area’ interaction: F1,11 = 1.65, p = 0.225), indicating that the effects of developmental condition on 20 h memory did not depend on the brain area: across the NCM and CMM, familiar FI indices were higher in control than stressed birds (figure 3c), indicating that memory for recently heard familiar songs was stronger in controls than in individuals that were subjected to early-life nutritional stress.
4. Discussion
Our study demonstrates, for the first time to our knowledge, that the auditory neural response to a learned sound stimulus is detrimentally affected by developmental stress. These results suggest that early-life stress, through impoverished nutrition, leads to impaired sensory learning of tutor's song, as well as impaired memory for new songs heard in adulthood in a songbird species. Consistent with this interpretation, Schmidt et al. [36] recently showed that early-life stress alters the neural response to song in females, such that control females showed distinct differences in their response to conspecific and heterospecific songs while stressed females did not. Similar to our study, their results suggest that developmental stress can reduce the ability of the brain to discriminate between different auditory signals [36]. In our study, importantly, the results, in terms of both growth trajectories and neuronal activation by song, were remarkably consistent between our two separate experiments. In accordance with other studies on zebra finches and other bird species (e.g. see [37,38]), early-life nutritional restriction resulted in delayed growth in the stressed nestlings of both experiments. We further demonstrated the effect of developmental stress on tutor song memory by testing the expression of two different IEGs (Arc and ZENK) in forebrain auditory areas (experiment 1). Previous studies have established that IEG expression in the NCM in response to tutor song correlates with song learning performance in the adult zebra finch, suggesting that the forebrain auditory areas are implicated in tutor song memory [28,39,40]. The results of our experiment showed that males with developmental stress experience exhibited weaker responses (i.e. reduced IEG expression) to playback of their tutor's song than controls. This suggests that the process of memorization of the tutor song template [21,28] has not occurred as effectively in individuals challenged by limited food resources in early life. Further, recordings of electrophysiological activity of neurons in NCM and CMM (experiment 2) replicated these effects, in addition to testing the effect of early-life developmental stress on auditory memory in adulthood, in a 20 h memory paradigm. Overall, the results of electrophysiological recordings of NCM and CMM responses to tutor song playback supported the IEG data, indicating impairments in tutor song memory after early-life nutritional stress. Furthermore, stressed birds showed poorer memories for passively familiar stimuli in adulthood than controls, suggesting that nutritional stress during early development may cause organizational or functional effects that impair auditory learning far beyond the time of the stress, perhaps even throughout a subject's entire lifespan.
Here, the sensitive period for song learning (neural development and ‘sensory’ phase of song memorization in particular; [29]) started during the period of nutritional restriction. Zebra finches are ‘close-ended’ learners, which means that their song learning process only occurs during a limited developmental period [4]. Therefore, although they may be able to compensate later in life for certain detrimental effects of developmental stress, e.g. on their morphology, they may not show the same resilience in terms of vocal learning. Our findings provide strong support for the developmental stress hypothesis [12–14], which suggests that the effects of early-life stress on song occur through detrimental effects on brain development and song learning, which cannot be compensated for later in life. Previous studies have documented the effects of developmental stress on adult brain morphology, song output and song copying accuracy (e.g. [13–18] see also [41] for a review), as well as male song attractiveness and mate choice decisions [19,20], but our study provides an experimental demonstration that the mechanisms underlying vocal learning per se, and auditory memory formation in particular, are condition-dependent. Some species, referred to as ‘open-ended’ learners, can learn new songs in adulthood [4], and might, consequently, have a greater ability to compensate for the effects of developmental stress on song learning. Future studies should, therefore, usefully assess how developmental conditions affect auditory memory in these species, to improve our understanding of the evolution of birdsong, and possibly to provide a model system for exploring compensatory neural mechanisms.
The proximate causes of the memory impairments (for either tutor or familiar trained songs) observed in birds that were nutritionally restricted during development remain to be determined. Two hypotheses could indeed explain our findings. First, males with developmental stress experience could have not formed a memory of the song because they initially failed to perceive the auditory stimulus (e.g. through hearing or attentional deficits). Alternatively, developmental stress could have affected brain development and the neural processes underlying auditory memory formation and/or storage (e.g. altered synaptic plasticity or other processes). In our study, the first hypothesis seems unlikely, as neuronal responses to playbacks of pure tones (i.e. synthetic stimuli) measured through electrophysiological recordings in NCM and CMM (experiment 2) were similar between stressed and control birds (see the electronic supplementary material). This suggests that hearing abilities were not impaired in nutritionally stressed males, and thus, that differences in neural responses to learned acoustic stimuli between the two treatment groups were most likely owing to effects on auditory memory processes. However, we cannot exclude the possibility that early-life stress may have impacted birds' attention during the period of memorization of the tutor song, reducing input, and therefore tutor song memory. Further investigations would therefore be useful to fully disentangle the two hypotheses.
By affecting their imitative vocal learning abilities [14,18], the observed impairments in tutor song memory after early-life nutritional stress could have important implications for developing birds (e.g. affecting their ability to attract a mate, hold a territory, integrate into the population [4,5,42]). However, the implications of such variation in auditory memory formation for individual social interactions, dialect recognition and larger population processes remain to be tested. Furthermore, our results not only provide evidence that developmental stress affects tutor song memory, and thus probably song learning abilities, but also alters auditory learning long after the stress has abated. As adults, these birds may be unable to remember identity information which may be essential for territory acquisition or local dialect copying. For instance, individuals with early-life stress experience may find it more difficult to settle into a territory, respond appropriately to known neighbours [4,5] or recognize conspecific songs [36], affecting individual resource defence, social integration and ultimately lowering fitness.
Although the exact mechanisms through which early-life nutritional stress affects auditory memory formation and/or storage (e.g. trade-offs in resource allocation, action of glucocorticoids), and the fitness consequences of such memory impairments require further investigations, our data provide new evidence demonstrating the effects of developmental stress on cognitive abilities [43]. Our study indeed reports the effects of developmental conditions on memory formation in a vocal learning context. Similarly, evidence for condition dependency of other learning abilities have also been described [10]. For instance, early-life stress has been shown to detrimentally affect the hippocampus and associated cognitive functions, such as spatial learning and memory, in birds [44,45], but also in mammals [46–48]. Altogether, these findings emphasize that the neural processes underlying the ability to learn, particularly those implicated in memory formation (whether it is used for vocal, spatial learning or individual recognition), probably have commonalities, which may be impacted by early-life conditions to produce long-term impacts on general cognitive abilities. However, it remains unclear whether developmental stress detrimentally affects general learning mechanisms per se or selectively impacts specific brain areas, and thus specific cognitive functions (e.g. see adaptive priorities in brain development hypothesis [49,50]). In this context, future studies investigating how early-life stress affects simultaneously different brain areas and neural mechanisms involved in different learning contexts (e.g. spatial and vocal learning) in the same species would provide crucial new insights.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Dr Amanda Moehring and two anonymous referees for helpful comments on the manuscript. We acknowledge the support of Rod Collins with animal husbandry for the first experiment. In addition, Ashley Conway and Marina Sharobeam assisted in animal care and data gathering, whilst David Clayton commented on an earlier draft.
Ethics
All applicable institutional and national guidelines for the care and use of animals were followed, and all experimental procedures were approved by Deakin University Animal Ethics Committee (A63-2009) and Animal Care and Use Committee of Rutgers University.
Data accessibility
Available as the electronic supplementary material.
Authors' contributions
K.L.B., S.L., D.S.V. and M.L.P. designed the study, B.A.B. and J.K.E. carried out experimental procedures and collected the data, K.L.B., B.A.B. and A.M. performed statistical analyses, and K.L.B., B.A.B., M.L.P. and A.M. wrote the manuscript (with input from D.S.V., S.L. and J.K.E.). All authors approved the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
This project was supported with funding from Deakin University and Australian Research Council grant no. FT140100131.
References
- 1.Shettleworth SJ. 2010. Cognition, evolution, and behavior. New York, NY: Oxford University Press. [Google Scholar]
- 2.Brainard MS, Doupe AJ. 2000. Auditory feedback in learning and maintenance of vocal behaviour. Nat. Rev. Neurosci. 1, 31–40. ( 10.1038/35036205) [DOI] [PubMed] [Google Scholar]
- 3.Jarvis ED. 2004. Learned birdsong and the neurobiology of human language. Ann. NY Acad. Sci. 1016, 749–777. ( 10.1196/annals.1298.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catchpole CK, Slater PJ. 2008. Bird song: biological themes and variations. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Marler P, Slabbekoorn H. 2004. Nature's music: the science of birdsong. San Diego, CA: Elsevier Academic Press. [Google Scholar]
- 6.Zahavi A. 1975. Mate selection: a selection for a handicap. J. Theor. Biol. 53, 205–214. ( 10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 7.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 8.Gil D, Gahr M. 2002. The honesty of bird song: multiple constraints for multiple traits. Trends Ecol. Evol. 17, 133–141. ( 10.1016/S0169-5347(02)02410-2) [DOI] [Google Scholar]
- 9.Spencer KA, MacDougall-Shackleton SA. 2011. Indicators of development as sexually selected traits: the developmental stress hypothesis in context. Behav. Ecol. 22, 1–9. ( 10.1093/beheco/arq068) [DOI] [Google Scholar]
- 10.Buchanan KL, Grindstaff JL, Pravosudov VV. 2013. Condition dependence, developmental plasticity, and cognition: implications for ecology and evolution. Trends Ecol. Evol. 28, 290–296. ( 10.1016/j.tree.2013.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowicki S, Searcy WA, Peters S. 2002. Quality of song learning affects female response to male bird song. Proc. R. Soc. Lond. B 269, 1949–1954. ( 10.1098/rspb.2002.2124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowicki S, Peters S, Podos J. 1998. Song learning, early nutrition and sexual selection in songbirds. Am. Zool. 38, 179–190. ( 10.1093/icb/38.1.179) [DOI] [Google Scholar]
- 13.Buchanan KL, Spencer KA, Goldsmith AR, Catchpole CK. 2003. Song as an honest signal of past developmental stress in the European starling (Sturnus vulgaris). Proc. R. Soc. Lond. B 270, 1149–1156. ( 10.1098/rspb.2003.2330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowicki S, Searcy WA, Peters S. 2002. Brain development, song learning and mate choice in birds: a review and experimental test of the ‘nutritional stress hypothesis’. J. Comp. Physiol. A 188, 1003–1014. ( 10.1007/s00359-002-0361-3) [DOI] [PubMed] [Google Scholar]
- 15.Buchanan KL, Leitner S, Spencer KA, Goldsmith AR, Catchpole CK. 2004. Developmental stress selectively affects the song control nucleus HVC in the zebra finch. Proc. R. Soc. B 271, 2381–2386. ( 10.1098/rspb.2004.2874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald IF, Kempster B, Zanette L, MacDougall-Shackleton SA. 2006. Early nutritional stress impairs development of a song-control brain region in both male and female juvenile song sparrows (Melospiza melodia) at the onset of song learning. Proc. R. Soc. B 273, 2559–2564. ( 10.1098/rspb.2006.3547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer KA, Buchanan KL, Goldsmith AR, Catchpole CK. 2003. Song as an honest signal of developmental stress in the zebra finch (Taeniopygia guttata). Horm. Behav. 44, 132–139. ( 10.1016/S0018-506X(03)00124-7) [DOI] [PubMed] [Google Scholar]
- 18.Holveck M-J, Vieira de Castro AC, Lachlan RF, ten Cate C, Riebel K. 2008. Accuracy of song syntax learning and singing consistency signal early condition in zebra finches. Behav. Ecol. 19, 1267–1281. ( 10.1093/beheco/arn078) [DOI] [Google Scholar]
- 19.Spencer KA, Wimpenny JH, Buchanan KL, Lovell PG, Goldsmith AR, Catchpole CK. 2005. Developmental stress affects the attractiveness of male song and female choice in the zebra finch (Taeniopygia guttata). Behav. Ecol. Sociobiol. 58, 423–428. ( 10.1007/s00265-005-0927-5) [DOI] [Google Scholar]
- 20.Holveck M-J, Riebel K. 2010. Low-quality females prefer low-quality males when choosing a mate. Proc. R. Soc. B 277, 153–160. ( 10.1098/rspb.2009.1222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brainard MS, Doupe AJ. 2002. What songbirds teach us about learning. Nature 417, 351–358. ( 10.1038/417351a) [DOI] [PubMed] [Google Scholar]
- 22.Nottebohm F. 2005. The neural basis of birdsong. PLoS Biol. 3, e164 ( 10.1371/journal.pbio.0030164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolhuis JJ, Gahr M. 2006. Neural mechanisms of birdsong memory. Nat. Rev. Neurosci. 7, 347–357. ( 10.1038/nrn1904) [DOI] [PubMed] [Google Scholar]
- 24.Gahr M, Metzdorf R, Schmidl D, Wickler W. 2008. Bi-directional sexual dimorphisms of the song control nucleus HVC in a songbird with unison song. PLoS ONE 3, e3073 ( 10.1371/journal.pone.0003073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mello CV, Vicario DS, Clayton DF. 1992. Song presentation induces gene expression in the songbird forebrain. Proc. Natl Acad. Sci. USA 89, 6818–6822. ( 10.1073/pnas.89.15.6818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. 1995. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc. Natl Acad. Sci. USA 92, 3406–3410. ( 10.1073/pnas.92.8.3406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mello C, Nottebohm F, Clayton D. 1995. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene's response to that song in zebra finch telencephalon. J. Neurosci. 15, 6919–6925. ( 10.1523/JNEUROSCI.15-10-06919.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan ML, Pytte CL, Vicario DS. 2006. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc. Natl Acad. Sci. USA 103, 1088–1093. ( 10.1073/pnas.0510136103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin H, Clayton DF. 1997. Localized changes in immediate-early gene regulation during sensory and motor learning in zebra finches. Neuron 19, 1049–1059. ( 10.1016/S0896-6273(00)80396-7) [DOI] [PubMed] [Google Scholar]
- 30.Vignal C, Andru J, Mathevon N. 2005. Social context modulates behavioural and brain immediate early gene responses to sound in male songbird. Eur. J. Neurosci. 22, 949–955. ( 10.1111/j.1460-9568.2005.04254.x) [DOI] [PubMed] [Google Scholar]
- 31.Metzdorf R, Gahr M, Fusani L. 1999. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J. Comp. Neurol. 407, 115–129. ( 10.1002/(SICI)1096-9861(19990428)407:1%3C115::AID-CNE9%3E3.0.CO;2-W) [DOI] [PubMed] [Google Scholar]
- 32.Long KD, Salbaum JM. 1998. Evolutionary conservation of the immediate-early gene ZENK. Mol. Biol. Evol. 15, 284–292. ( 10.1093/oxfordjournals.molbev.a025925) [DOI] [PubMed] [Google Scholar]
- 33.Leitner S, Voigt C, Metzdorf R, Catchpole CK. 2005. Immediate early gene (ZENK, Arc) expression in the auditory forebrain of female canaries varies in response to male song quality. Dev. Neurobiol. 64, 275–284. ( 10.1002/neu.20135) [DOI] [PubMed] [Google Scholar]
- 34.Tsoi SC, Aiya UV, Wasner KD, Phan ML, Pytte CL, Vicario DS. 2014. Hemispheric asymmetry in new neurons in adulthood is associated with vocal learning and auditory memory. PLoS ONE 9, e108929 ( 10.1371/journal.pone.0108929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 36.Schmidt KL, McCallum ES, MacDougall-Shackleton EA, MacDougall-Shackleton SA. 2013. Early-life stress affects the behavioural and neural response of female song sparrows to conspecific song. Anim. Behav. 85, 825–837. ( 10.1016/j.anbehav.2013.01.029) [DOI] [Google Scholar]
- 37.Krause ET, Naguib M. 2011. Compensatory growth affects exploratory behaviour in zebra finches, Taeniopygia guttata. Anim. Behav. 81, 1295–1300. ( 10.1016/j.anbehav.2011.03.021) [DOI] [Google Scholar]
- 38.Schmidt KL, MacDougall-Shackleton EA, MacDougall-Shackleton SA. 2012. Developmental stress has sex-specific effects on nestling growth and adult metabolic rates but no effect on adult body size or body composition in song sparrows. J. Exp. Biol. 215, 3207–3217. ( 10.1242/jeb.068965) [DOI] [PubMed] [Google Scholar]
- 39.Bolhuis JJ, Hetebrij E, Boer-Visser D, Ardie M, De Groot JH, Zijlstra GG. 2001. Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. Eur. J. Neurosci. 13, 2165–2170. ( 10.1046/j.0953-816x.2001.01588.x) [DOI] [PubMed] [Google Scholar]
- 40.Terpstra NJ, Bolhuis JJ, den Boer-Visser AM. 2004. An analysis of the neural representation of birdsong memory. J. Neurosci. 24, 4971–4977. ( 10.1523/JNEUROSCI.0570-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacDougall-Shackleton SA, Spencer KA. 2012. Developmental stress and birdsong: current evidence and future directions. J. Ornithol. 153, 105–117. ( 10.1007/s10336-011-0807-x) [DOI] [Google Scholar]
- 42.Nowicki S, Searcy WA. 2005. Song and mate choice in birds: how the development of behavior helps us understand function. The Auk 122, 1–14. ( 10.1642/0004-8038(2005)122%5B0001:SAMCIB%5D2.0.CO;2) [DOI] [Google Scholar]
- 43.Peters S, Searcy WA, Nowicki S. 2014. Developmental stress, song-learning, and cognition. Integr. Comp. Biol. 54, 555–567. ( 10.1093/icb/icu020) [DOI] [PubMed] [Google Scholar]
- 44.Pravosudov VV, Lavenex P, Omanska A. 2005. Nutritional deficits during early development affect hippocampal structure and spatial memory later in life. Behav. Neurosci. 119, 1368–1374. ( 10.1037/0735-7044.119.5.1368) [DOI] [PubMed] [Google Scholar]
- 45.Kriengwatana B, Farrell TM, Aitken SD, Garcia L, MacDougall-Shackleton SA. 2015. Early-life nutritional stress affects associative learning and spatial memory but not performance on a novel object test. Behaviour 152, 195–218. ( 10.1163/1568539X-00003239) [DOI] [Google Scholar]
- 46.Bedi KS. 2003. Nutritional effects on neuron numbers. Nutr. Neurosci. 6, 141–152. ( 10.1080/1028415031000098549) [DOI] [PubMed] [Google Scholar]
- 47.Fumagalli F, Molteni R, Racagni G, Riva MA. 2007. Stress during development: impact on neuroplasticity and relevance to psychopathology. Prog. Neurobiol. 81, 197–217. ( 10.1016/j.pneurobio.2007.01.002) [DOI] [PubMed] [Google Scholar]
- 48.Kosten TA, Kim JJ, Lee HJ. 2012. Early life manipulations alter learning and memory in rats. Neurosci. Biobehav. Rev. 36, 1985–2006. ( 10.1016/j.neubiorev.2012.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nowicki S, Searcy WA. 2005. Adaptive priorities in brain development: theoretical comment on Pravosudov et al. (2005). Behav. Neurosci. 119, 1415–1418. ( 10.1037/0735-7044.119.5.1415) [DOI] [PubMed] [Google Scholar]
- 50.Pravosudov VV. 2009. Development of spatial memory and the hippocampus under nutritional stress: adaptive priorities or developmental constraints in brain development? In Cognitive ecology II (eds Dukas R, Ratcliffe JM), pp. 88–110. Chicago, IL: University of Chicago Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available as the electronic supplementary material.