Abstract
Most current efforts to advance medical technology proceed along one of two tracks. The first is dedicated to the improvement of clinical tasks through the incremental refinement of medical instruments. The second comprises engineering endeavors to support basic science studies that often only remotely relate to human medicine. Here we survey emerging research approaches that aim to populate the sprawling frontier between these tracks. We focus on interdisciplinary single-live-cell techniques that have overcome limitations of traditional biological methods to successfully address vital questions about medically relevant cellular behavior. Most of the presented case studies are based on the controlled manipulation of nonadherent human immune cells using one or more micropipettes. The included studies have (i) examined one-on-one encounters of immune cells with real or model pathogens, (ii) assessed the physiological role of the expandable surface area of immune cells, and (iii) started to dissect the spatiotemporal organization of signaling processes within these cells. The unique aptitude of such single-live-cell studies to fill conspicuous gaps in our quantitative understanding of medically relevant cause-effect relationships provides a sound basis for new insights that will inform and drive future biomedical innovation.
Keywords: single-cell, immune cell, host-pathogen, neutrophil, micropipette, phagocytosis, chemotaxis, adhesion, signaling, calcium
Introduction
When we think of recent progress in medical technology, we tend to picture imaging devices that improve diagnoses or assist with surgical procedures, robotic instruments that automate laboratory analyses and surgeries, miniaturized point-of-care monitors, and “on-a-chip” devices that enhance the quality of life of patients, etc. The refinement of such familiar technologies continues to advance biomedicine at a steady, incremental pace. Transformative breakthroughs, on the other hand, are rare and unpredictable, although not entirely random. Throughout medical history, almost all such breakthroughs have been linked to fundamental discoveries in science and engineering – discoveries that ultimately provided new mechanistic insight into how a healthy organism functions, and how it may fail in disease. It thus seems inescapable that the acceleration of progress in biomedicine requires a shift in balance from currently prevailing correlative studies to a more future-oriented, quantitative research strategy that aims to uncover the underlying sequences of causes and effects. Enabling new approaches to address unresolved medical questions is, therefore, a foremost challenge of biomedical engineering. It usually starts with the development of new assays in a research-lab setting. Successful assays will provide the fundamental insights that can inform the development of new diagnostics, drugs, and medical procedures. Some research assays may even be suitable for direct adaptation in a clinical setting.
In this paper, we address modern research methodologies to study the behavior of a particularly important functional unit of living organisms: a single cell. Comprehensive quantitative understanding of how individual cells sense, process, and respond to chemical and physical stimuli is key to a future precision medicine that will be truly personalized and predictive. Most of the studies we discuss here use primary human immune cells which, despite their enormous medical importance, remain understudied in comparison to model systems such as animal cells or cell lines. Although it is well known that such model systems can be poor substitutes for human cells [1,2], the conspicuous imbalance between their study and studies of human cells is not surprising. Human immune cells such as neutrophils or monocytes are short-lived and not amenable to many traditional methods of cell and molecular biology. These cells’ hyperexcitability further necessitates the development of alternative research techniques. Almost all existing assays inadvertently pre-activate immune cells, generally to a degree that is poorly defined, which prohibits meaningful quantitative comparisons of different literature reports. The single-cell manipulation techniques discussed in the following sections are a valuable complement to mainstream biological assays and have exposed a wealth of medically relevant insights.
Manipulation of Single, Nonadherent Immune Cells to Examine Biomedically Relevant Cell and Molecular Mechanisms
Microscopic observation of living cells is a cornerstone of biomedical research. By enabling studies of the time-dependent behavior of cells, it has produced an abundance of detailed qualitative information about cellular dynamics that is inaccessible to bulk assays or studies of fixed samples. On the other hand, several limitations make it difficult to gain quantitative insight into cause-effect mechanisms from traditional live-cell observations. The irregular shapes of adherent cells generally prohibit measurements of the cell geometry. Also, due to the lack of positioning control, studies of cell-cell interactions or interactions between cells and other objects such as pathogens must rely on chance encounters between the participants [3,4]. Especially in experiments with immune cells, many variables can affect the cell response to stimuli, such as pre-activation through adhesion [5], interaction with foreign surfaces such as chamber walls, or the presence of chemoattractants. It is often difficult to determine to what extent these variables bias a particular immune-cell response and which features of the response are specific to the used experimental setup.
Many of these limitations are adequately addressed by live-cell experiments that allow the operator to pick up cells and other objects and reposition them at will. The most successful tool enabling such manipulation is a glass micropipette attached to a 3D micromanipulator. Micropipettes of the desired tip diameter and shape are relatively easy to make [6] and are commercially available. They already have found widespread medical use in in vitro fertilization, and they are the core component of the Nobel-Prize-winning patch-clamp technique. Other biophysical studies of live cells and model cells such as lipid vesicles have a long tradition of using micropipettes as well; in fact, most of our current knowledge about membrane mechanics comes from micropipette-aspiration experiments. Yet biophysical studies tend to primarily address fundamental mechanistic or material questions that only remotely relate to the cells’ physiological functions. It is the realization that micropipette-manipulation techniques are ideally suited to examine immune-cell behavior within a biomedical context that has recently led to new types of single-live-cell studies.
In the following sections, we will discuss select case studies that demonstrate the advantages of tightly controlled manipulation of individual immune cells. We will showcase the aptitude of such experiments to provide unparalleled detail about the immune-cell response to pathogens by addressing a variety of cross-disciplinary questions. For instance, why are certain pathogens able to evade short-range chemotactic recognition? For those that are recognized, what is the maximum distance over which an immune cell can detect target particles? Such questions can often be answered directly and unequivocally by using human immune cells as uniquely capable biodetectors of chemoattractants. This approach also allows for the quantitative comparison of immune-cell responses to different species of pathogens including the hierarchical ranking of these responses by strength. Questions that probe the mechanistic underpinnings of immune cell behavior include the following: How sensitive are immune cells to chemoattractants? What limits the number of pathogenic target particles that a single immune cell can phagocytose? How fast and how far do chemical signals spread inside immune cells? By beginning to answer these questions, single-cell research reaffirms its potential to inform and drive biomedical innovation.
Highly Controlled Encounters Between Single Cells and Pathogens
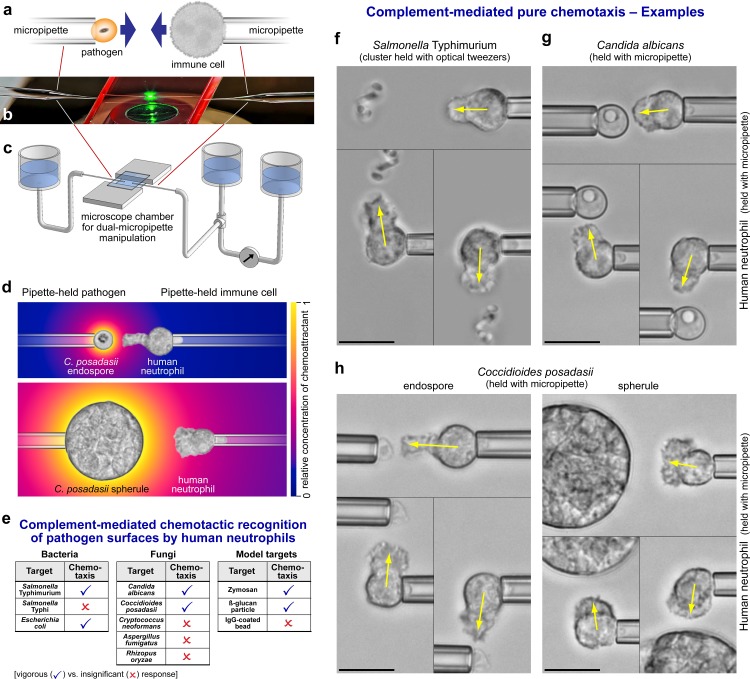
One particularly useful micromanipulation setup consists of two opposing micropipettes – one to hold an immune cell and the other to hold a pathogen or a pathogenic model particle (Figure 1a-c) [7,8]. In a typical experiment, the cell and target particle are lifted above the chamber bottom and first held at a distance from each other to test for a purely chemotactic response, which manifests as a cellular pseudopod extended toward the target (Figure 1d,e). We use the term “pure chemotaxis” to distinguish this behavior from chemotactic migration of adherent cells on a substrate. If pure chemotaxis is observed, the particle is moved to different sides of the cell to verify specificity of the response (Figure 1f-h). Eventually, the particle is brought into soft contact with the cell and released from its pipette. The response of individual immune cells to such contacts provides clear and direct evidence of the ability of the cell’s adhesive receptors and phagocytosis machinery to recognize specific pathogens and model surfaces [9]. (Example videos of such experiments have been compiled into Movie 13.5 of a popular textbook [10] and can be viewed online [11].) Possible variations of this approach include the use of optical tweezers to hold target particles [9,12], or the direct application of jets of chemoattractant from a pipette that had been prefilled with the desired solution and placed opposite the cell [13,14].
Figure 1.
Single-live-cell, single-target pure-chemotaxis assay. a. Sketch of a dual-micropipette experiment to examine interactions between a single immune cell and a single pathogenic particle. b. Photograph of a dual-micropipette setup as used on an inverted microscope. c. Sketch of the microscope chamber including water reservoirs used to control and measure the pipette-aspiration pressure. d. Illustration of pure-chemotaxis experiments to test the response of human neutrophils to two forms of C. posadasii. The underlaid color gradient depicts the local concentration of chemoattractant produced by the host’s complement system at the surface of the differently sized target particles. e. Summary of the results of pure-chemotaxis assays to test the chemotactic response of human neutrophils to 11 different pathogenic and model targets. f-h. Examples of pure-chemotaxis experiments testing human neutrophils against clusters of S. Typhimurium (f), C. albicans cells (g), and endospores and spherules of C. posadasii (h). The positive neutrophil response is triple-checked by positioning the target at three different sides of the neutrophil. All scale bars denote 10 µm.
There are many advantages to using micropipettes in studies of immune-cell interactions with pathogens. Selecting initially quiescent cells and performing the experiments above the chamber bottom minimizes variability in the baseline from which the experiments start and eliminates possible bias from cell-substrate adhesion. Being able to subdivide the cell response into stages such as chemotaxis, adhesion, and phagocytosis facilitates a reductionist approach that is quintessential for mechanistic studies. Because the cells are often roughly axisymmetric when held in a pipette, geometric parameters such as the cells’ surface area can be assessed throughout their interactions with pathogens [14,15]. Axial symmetry also makes the experiments particularly amenable to comparison with computational models [7,16]. On the other hand, experiments using micropipettes can be challenging. They require a low-noise environment and suitable vibration-isolation equipment. Moreover, they can be tedious and often require laborious repetition to accumulate statistically meaningful amounts of data.
The benefits of dual-micropipette experiments are best illustrated with case studies that have brought about new, medically relevant insights. Such experiments have been used, for instance, to examine the chemotactic responses of human neutrophils to 11 different pathogenic targets – three types of bacteria, five fungal pathogens, two fungal model targets, and IgG-opsonized beads (Figure 1) [7,8,12,14,15]. Because the same conditions were maintained throughout these experiments, the neutrophil responses to different pathogens could be compared directly. Based on the chemotactic cell responses, the tested pathogens were categorized into two distinct groups: those that induced vigorous chemotaxis by human neutrophils, and those that were not recognized (Figure 1e). For example, neutrophils reacted strongly to the fungal pathogens Candida albicans and Coccidioides posadasii, and these responses were essentially indistinguishable from the cell behavior during interactions with zymosan and β-glucan particles. This agreement supports the credibility of the latter particles as suitable models of the recognized fungi. However, these results also reveal that assays using zymosan or β-glucan particles are not representative of interactions between neutrophils and the other, unrecognized species of fungal pathogens.
How does the neutrophil response to pathogens in dual-micropipette experiments compare with clinical findings and results of past in vitro studies? In some cases, cells behaved as expected, while in others, single-cell experiments seemed to be inconsistent with clinical observations. The distinct neutrophil responses to two important bacterial pathogens, Salmonella enterica serovar Typhimurium (S. Typhimurium) and S. Typhi, serve as an example in which single-cell experiments confirmed and expanded existing knowledge. S. Typhimurium is associated with a localized gastroenteritis in humans, whereas S. Typhi causes typhoid fever, a severe systemic infection. The contrasting virulence of these two serovars indicates a marked difference in the ability of the human immune system to cope with them. Micropipette experiments testing for pure chemotaxis of human neutrophils toward these bacteria demonstrated that this difference in immune recognition is indeed mirrored even at the level of individual immune cells. Whereas neutrophils exhibited a vigorous chemotactic response toward S. Typhimurium (Figure 1f), their response to S. Typhi was insignificant [12]. The same study also showed that a capsular polysaccharide protects S. Typhi from this type of microbe-guided, host-serum-dependent chemotactic recognition.
By contrast, the in vitro neutrophil response to C. posadasii, a fungal pathogen that causes Valley fever,seemed incongruent with clinical observations and past experimental reports. In clinical manifestations of coccidioidal infection, patients generally fail to mobilize a significant neutrophil response. However, when pipette-held human neutrophils were exposed to nearby C. posadasii in the presence of autologous serum, the cells responded strongly to both endospores and spherules of the fungus, irrespective of whether the neutrophils were obtained from healthy donors or infected patients (Figure 1h) [15]. Because neutrophils were well able to recognize C. posadasii at close range, the lack of neutrophil mobilization in Valley fever patients was attributed to a suppression of long-range neutrophil recruitment. Furthermore, this study dispelled the long-held misconception that Coccidioides spherules are protected from recognition by human neutrophils [17]. Although the spherules are too large to be completely engulfed by individual neutrophils, the immune cells started to extend chemotactic pseudopods in less than 1 minute after their placement near spherules, and following contact, proceeded to attack the spherules by frustrated phagocytosis.
In all pure-chemotaxis experiments discussed above, the chemotactic neutrophil response required the presence of host serum, suggesting that it was mediated by serum-based complement components. Chemotaxis did not occur in experiments using serum that had been heat-treated at temperatures of 52°C or higher [18], consistent with the temperature-dependent inactivation of the heat-labile complement system. This and other evidence [12,14] confirmed that complement-mediated chemotaxis is the primary mechanism by which human neutrophils detect nearby fungi and live bacteria. In short, complement proteins from the serum assemble into proteases on recognized pathogen surfaces, then cleave other complement proteins to release small peptides known as anaphylatoxins [19]. Anaphylatoxins diffuse rapidly and stimulate chemotactic receptors on immune cells but are also quickly deactivated by serum carboxypeptidases [19]. The most potent of these complement fragments is C5a [20]. Its acute medical importance is underlined by its involvement in sepsis [21] and other diseases [20].
Whereas biochemical aspects of complement-mediated chemotaxis have been explored qualitatively in some depth, many quantitative details remain uncertain, such as: How far from a pathogen can we expect an immune cell to sense it? How much anaphylatoxin must a neutrophil detect before initiating a chemotactic response? Micropipette experiments have started to answer such questions by exploiting the unique capability of neutrophils to act as highly sensitive biodetectors. When used in this capacity, the neutrophil was first placed at a relatively large distance from its target. Then, the separation distance between cell and target was decreased in steps until the neutrophil clearly formed a protrusion in the direction of the target particle [22]. A realistic mathematical model of the evolution of the chemoattractant gradient [23] was used to calculate the concentration of anaphylatoxin at the cell surface for the measured recognition distance. Integrating theory and experiments in this manner provided a quantitative estimate of the neutrophil sensitivity to anaphylatoxins produced at the surface of β-glucan particles [22]. The sensitivity value was then used to predict the recognition distance of pathogens as a function of their size, which established complement-mediated chemotaxis as a short-range mechanism whose physiological role is to facilitate a last-minute course correction of chemotaxing immune cells toward actual pathogen surfaces rather than chemokine-producing host cells [23]. The same study also concluded that small pathogens could be effectively protected from complement-mediated chemotactic recognition simply due to their size.
It is not surprising that a minimum concentration of chemoattractant is required to induce chemotaxis by immune cells. Conversely one might ask if there is a maximum concentration beyond which the cell response exhibits some form of saturation. In the case of the anaphylatoxin C5a, micropipette experiments have clearly answered this question. Chemotaxis of pipette-held human neutrophils stalled or even reversed when the cells were subjected to jets of high concentrations of C5a (≥100 nM; ejected from a pipette facing the cell) [14], in agreement with earlier experiments that exposed chemotaxing neutrophils to high concentrations of the chemoattractant tripeptide fMLP [24]. The impairment of neutrophil chemotaxis at high concentrations of C5a in such experiments bears a striking resemblance to neutrophil paralysis in sepsis [25], which is associated with systemically elevated C5a concentrations [21]. Therefore, experiments of the kind discussed here could open an instructive new window into the behavior of single immune cells during sepsis or other pathological conditions.
Quantitative Characterization of the Expandable Surface Area of Immune Cells
The simple geometric principle that a sphere is the shape of minimum surface area of an object with given volume has important consequences for the behavior of immune cells. Quiescent, nonadherent white blood cells are generally round, which means that their resting shape is the shape of minimum cell surface area (bearing in mind that the cell volume is expected to remain constant as long as the osmotic conditions do not change). Consequently, the surface area of white blood cells invariably increases during cell deformations that accompany physiological functions such as chemotaxis and phagocytosis. By contrast, red blood cells are non-spherical under physiological conditions and can readily change their shape without changing their surface area. In fact, it is well known that smooth lipid membranes—such as the membranes of red cells and liposomes—cannot expand their area by more than 2 to 5 percent before they lyse [26].
On the other hand, a cursory inspection of common deformations of white blood cells shows that these cells can increase their apparent surface area by much more than 5 percent. This has been demonstrated for spreading neutrophils [27], macrophages [28], and lymphoblasts [29], as well as during osmotic swelling of neutrophils [30], and in phagocytosis experiments with neutrophils [7,15,31] and macrophages [32]. Yet the amount of membrane material that a cell can expend is not unlimited, which gives rise to the hypothesis that the capacity of immune cells to expand their surface area is a limiting factor in chemotactic deformations and for the maximum number of particles a cell can phagocytose. It is not surprising that questions about the sizes and types of surface area reservoirs of immune cells have attracted considerable attention [33-36].
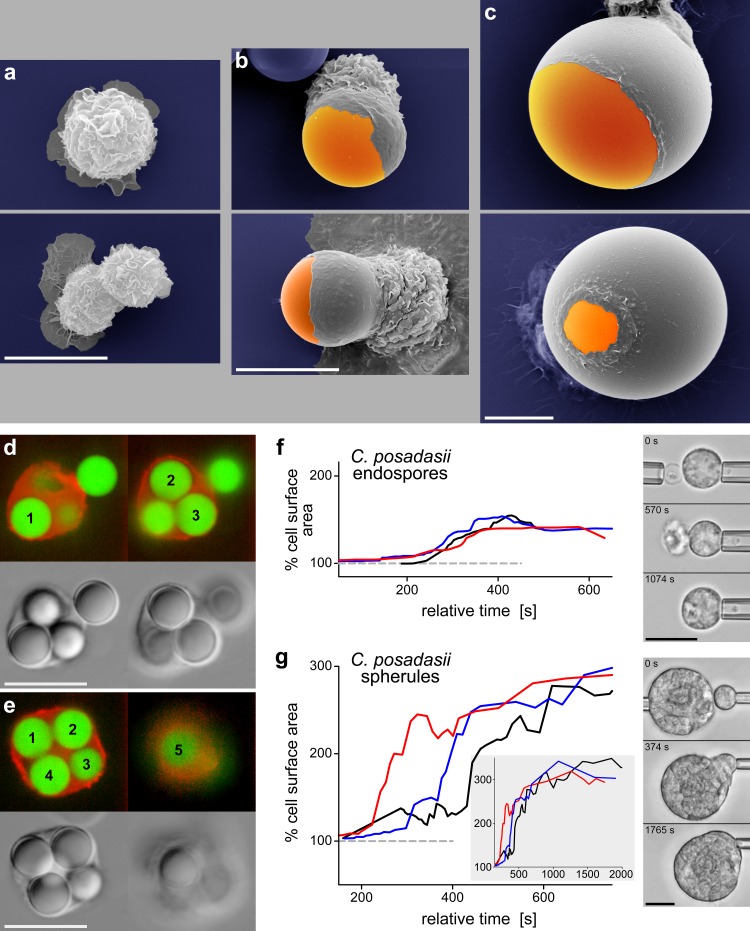
So far, we have used the term “surface area” to denote the apparent cell surface area, i.e., the area that can be inferred from a smooth curve circumscribing an image of the cell taken on an optical microscope. This apparent cell surface area is different from the microscopic area of the cell’s plasma membrane. Scanning electron micrographs of immune cells convey a clear picture of the large reserves of membrane area stored in wrinkles such as microvilli, ridges, pits, nanotubes, etc. (Figure 2a-c). It is difficult to reliably determine the actual size of the microscopic membrane area from such images. On the other hand, the apparent surface area of a pipette-aspirated cell is often relatively easy to measure. A suitable strategy to also obtain an estimate of an immune cell’s membrane reservoirs is to subject the cell to deformations that “iron out” [34] its wrinkles, in which case the growing apparent surface area approaches the value of the microscopic area (Figure 2). This strategy has a caveat though – it is difficult to distinguish between ironed-out wrinkles and membrane addition through fusion of internal vesicles with the plasma membrane; therefore, such estimates generally report the combined size of both types of membrane reservoir.
Figure 2.
Expansion of the surface area of immune cells during phagocytosis. a-c. Scanning electron micrographs of J774 macrophages attached to a substrate (a) and during phagocytosis of 10-µm (b) and 30-µm (c) IgG-coated beads. These snapshots of fixed cells demonstrate how cell-surface wrinkles are progressively “ironed out” as the cells increase their apparent surface area. Note that the images in c are shown at a reduced scale compared to a and b. d,e. Fluorescence and transmitted-light images of human neutrophils (actin labeled red) that were fixed during phagocytosis of several 5-µm IgG-coated beads (labeled green). Each panel includes two different confocal slices, allowing us to determine the correct count of internalized beads. f,g. Graphs of the time course of the apparent surface area of human neutrophils during phagocytosis of C. posadasii endospores (f) and frustrated phagocytosis of C. posadasii spherules (g) (reproduced from [15]). Each plot includes the results from 3 different experiments. The inset in g shows the surface area over an extended time period. Included are videomicrographs recorded during representative dual-micropipette, single-live-cell experiments. All scale bars denote 10 µm.
The quantitative analysis of the cell geometry during physiologically relevant deformations simplifies greatly when the observed cell shapes can be approximated by axisymmetric 3D objects or composites of such objects. In some cases, the pipette-held cell can simply be assumed to consist of spheroidal and cylindrical parts. A more advanced method describes the visible outline of the cell in terms of a suitable mathematical function, and then reconstructs the 3D cell body assuming that this outline represents a 2D cross-section of the cell and contains its rotational symmetry axis [14]. Such approaches have enabled detailed analyses of the time course of the cell surface area of pipette-held immune cells during pure chemotaxis [14] and phagocytosis [15,37] (Figure 2f,g). Human neutrophils have been found to expand their apparent surface area to ~300 percent of their resting area [7,15] and the analogous capacity of macrophages has been estimated at an astounding ~600 percent [32]. Based on these values, it is straightforward to predict how many spherical particles of a certain size an immune cell can engulf. For example, an average human neutrophil is expected to be able to phagocytose up to six spheres with a diameter of 4.5 µm, or 13 spherical particles with a diameter of 3 µm. The enormous membrane reserves of a macrophage should allow it to completely surround a bead that is close to twice its own resting size. Cases that confirmed these quantitative predictions have indeed been observed in phagocytosis experiments in our lab (Figure 2).
The great expandability of the apparent surface area of leukocytes is also crucial for other physiological functions, such as the firm arrest, spreading, and migration of cells on the inflamed endothelium, as well as their extravasation into the surrounding tissue. For example, human T-lymphoblasts have been found to increase their apparent surface area up to ~2.5-fold during extravasation and cell spreading [29] – an increase that seems to be realized once more by a combination of membrane unwrinkling and vesicle fusion.
Autonomous deformations during which immune cells actively increase their surface area require the cells to do mechanical work. The cortex of the cells is under perpetual tension, as established by early micropipette-aspiration experiments [38]. By its very nature, the cortical tension acts to minimize the cell surface area and thus opposes deformations that require an increase of the area. To create cellular protrusions, the interior cytoskeleton must push against the cortex strongly enough to overcome the resistance of this tension. The required mechanical effort becomes greater as the cell surface area increases, because the cortical tension itself increases with the area. This dependence has been analyzed in detail for human neutrophils [37], including its mechanistic consequences during phagocytosis [9]. Most pertinent to the present discussion is the result that the relationship between the tension and surface area of human neutrophils has a biphasic character. A certain amount of cortical slack allows passive neutrophils to increase their surface area by 25 to 30 percent with relatively little mechanical effort. Larger deformations are accompanied by a much steeper rise of the cortical tension, making it harder and harder for the cell to increase its surface area further.
These fundamental insights into the mechanical behavior of the cell cortex have interesting implications. For example, neutrophils that actively formed protrusive pseudopods during pure chemotaxis were unable to expand their apparent surface area by more than 25 to 30 percent – an amount that coincides with the cortical slack of passive neutrophils [14]. Because the cortical tension remained within normal bounds for the observed deformations, this limited surface-area expansion was attributed to a limited ability of neutrophils to generate protrusive force in this situation. When compared to the 8-fold larger capacity of neutrophils to expand their apparent surface area during phagocytosis and cell spreading, this result suggests the existence of two qualitatively distinct mechanisms of actomyosin-based protrusive force generation, and it implies that adhesion to a target particle or substrate is an essential prerequisite for the generation of large protrusive forces [14].
Spatiotemporal Organization of Signaling in Immune Cells
In view of the vast expanse of biological signaling research, the amount of medically relevant mechanistic knowledge about cell signaling that life scientists have accumulated to date seems small. A key challenge of future-oriented signaling research is to shift its focus from traditional qualitative explorations of the molecular participants in signaling paths to mechanistic cause-effect studies that aim to quantify the kinetics of the signaling biochemistry and account for the spatiotemporal organization of the participating reactants within a cell. Tackling this challenge in live-cell studies of fully differentiated human immune cells is particularly difficult, but some progress has been made. For example, immune cells can be preloaded with a fluorescent calcium (Ca2+) indicator to monitor the cytosolic Ca2+ concentration during vital cell functions. The Ca2+ concentration in neutrophils and other immune cells is known to exhibit sudden cell-wide bursts during which it increases rapidly to several-fold of the base level [39-44]. Unlike in electrically excitable cells, these bursts can be induced biochemically [41,45-48] or mechanically [49-52]. Yet although such Ca2+ bursts are among the most conspicuous signaling events known and can transmit a signal throughout the entire cell in record time, their exact physiological role in immune cells remains uncertain.
The prominence of these Ca2+ bursts, and the fact that divalent cations like Ca2+ participate in the regulation of numerous cellular functions, assigns a very high priority to the systematic dissection of the underlying cause-effect sequences. A promising strategy combines fluorescence imaging with single-live-cell micromanipulation experiments similar to those discussed in the previous sections. The ability to separate cell-substrate adhesion—which has been implicated as an important factor in Ca2+ bursts [49-51]—from other immune-cell functions is a key advantage of this strategy. Pipette-held human neutrophils were found to reliably exhibit Ca2+ bursts shortly after beginning to phagocytose IL-8-coated beads [53,54], IgG-coated beads, zymosan, and β-glucan particles [14]. The detailed information about the time-dependent cell morphology, cell-target contact area, cell surface area, and cortical tension that such phagocytosis experiments provide is an excellent starting point to examine possible correlations with the dynamics of Ca2+ bursts.
Of course, adhesion—in this case to a target particle—is still a necessary part of phagocytosis experiments. Surprisingly, the extension of purely chemotactic pseudopods by nonadherent neutrophils toward nearby zymosan and β-glucan particles did not trigger Ca2+ bursts [14]. Consistent with a small number of previous studies [24,55], these experiments provided clear evidence that pure, complement-mediated chemotaxis neither causes nor requires Ca2+ bursts in human neutrophils, calling for a careful reevaluation of the widespread view that such bursts mediate rearrangement of the actin cytoskeleton in response to the activation of G protein-coupled receptors [47,48,56,57].
Calcium bursts can flood the entire cell with secondary messenger in a matter of seconds. This type of signaling mechanism is poorly suited to selectively trigger localized cell functions or control targeted intracellular communication. How then can we assess other, more refined mechanisms of signal transduction in a cell? The basic idea is still straightforward: (i) apply a well-defined stimulus to the cell, and (ii) monitor the response in terms of a quantity that is more discriminatory than the global Ca2+ concentration.
Advanced micromanipulation techniques have implemented the first part of this idea by using functionalized “test probes” to administer stimuli to individual pipette-held immune cells. Suitable test probes include micrometer-sized beads or red blood cells whose surfaces have been decorated with the desired composition of specific ligands. During experiments, a test probe is brought into contact with an immune cell using a second micropipette. The experimental protocol determines how many surface receptors of the immune cell are engaged during such contacts. For example, to deliver a relatively large dose of the immobilized chemokine IL-8 to human neutrophils, the cells were allowed to completely phagocytose IL-8-coated beads [53,58]. Alternatively, a test probe can be touched to the cell surface briefly and repeatedly. In this case, automated pipette manipulation can provide accurate control over the duration and repetition rate of such touches. This type of protocol was used to co-stimulate T cells from mice with a mixture of pMHC and ICAM-1, and to expose human platelets successively to vWF-A1 and then fibronectin [59].
Implementing the second part of the above idea—i.e., the spatiotemporal analysis of downstream consequences of such stimuli—is more challenging. An elegant method detects local inside-out signaling events based on changes in the molecular adhesion mechanics of a cell [60]. By bringing yet another pipette-held object (a “force probe”) into repeated contact with the cell, the response to various stimuli can be monitored in terms of the dynamic strength of adhesive interactions between this force probe and the cell surface. In short, small deformations of the cell and/or force probe during probe retraction reveal whether adhesive bonds have formed during a contact. Simply counting how many touches produce an adhesion event provides the adhesion frequency. A sudden change in adhesion frequency signifies the arrival of a signal at the probed location. Because this approach assesses only a small number of localized adhesion receptors, it can characterize the cell response with superior spatiotemporal resolution and without the use of labels. Force probes decorated with immobilized ICAM-1 molecules have successfully detected changes in the adhesive strength between ICAM-1 and its receptors, the β2-integrins LFA-1 and Mac-1, expressed on the surfaces of human neutrophils [53,58] and murine T cells [59]. The experiments established that it takes several minutes to transduce a signal from the original stimulus to a local cell response, which in this case takes the form of integrin activation and/or upregulation. Moreover, variation of the type of stimulus and the use of inhibitors or genetically modified mice provided valuable insight into the underlying signaling paths.
Outlook
The studies reviewed in this paper demonstrate how micromanipulation experiments with individual immune cells can successfully address medically relevant questions that are inaccessible to traditional biological methods. In some cases, the results of these experiments have provided direct and quantitative evidence in support of concepts of cellular behavior that previously had been based solely on correlative reasoning and cumulative circumstantial evidence. Other single-cell studies have cast doubt on broadly accepted biomedical notions or even overturned them.
We have outlined in the Introduction how such single-cell research can inform and drive biomedical innovation. Rather than let advances in technology define the primary directions of future biomedical research, we believe that unresolved vital questions of human medicine should more often become the driving force of purposeful technological advancement. A top priority of future-oriented technology development should be the enablement of research that strives to fill gaps in our mechanistic understanding of the cause-effect relationships that govern health and disease – starting at the cell and molecular level.
It is not difficult to envision translational paths to clinical applications of some of the single-cell assays featured in the above discussion. Routine diagnostics are bound to benefit greatly from an increased number of parameters measured in standard clinical blood tests – provided the interpretation of the parameter values is based on a sound understanding of the underlying mechanisms. For example, calcium bursts in immune cells are relatively easy to detect. Patient-specific variations in the thresholds of different stimuli that trigger such bursts could potentially serve as indicators of immune deficiencies. The analysis of such variations might even inform the design of personalized therapies. Other parameters include the sensitivity of white blood cells to various chemoattractants, the cells’ mechanical deformability, or the dynamics of prominent signaling paths. Microfabrication of high-throughput devices that enable the measurement of such parameters is currently an active area of technological research and development [61-63]. The long-term success of these devices depends critically on the degree of fundamental understanding of single-cell behavior that informs the original device design.
Acknowledgments
Some of the experiments included in the figures were conducted by Cheng-Yuk Lee and Jonathan Lam. We are grateful to Soichiro Yamada for his help with the confocal images of Figure 2d,e.
Glossary
- Ca2+
calcium
- C5a
complement component 5a
- fMLP
N-formyl-met-leu-phe
- ICAM-1
intercellular adhesion molecule 1
- IgG
immunoglobulin G
- IL-8
interleukin 8
- LFA-1
lymphocyte function-associated antigen 1
- Mac-1
macrophage-1 antigen
- pMHC
peptide-bound major histocompatibility complex
- vWF-A1
von Willebrand factor A1 domain
Author Contributions
Both authors contributed equally to this review. This work was supported by the NIH (grant R01 GM098060).
References
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110(9):3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–8. [DOI] [PubMed] [Google Scholar]
- Gilbert AS, Seoane PI, Sephton-Clark P, Bojarczuk A, Hotham R, Giurisato E, et al. Vomocytosis of live pathogens from macrophages is regulated by the atypical MAP kinase ERK5. Sci Adv. 2017;3(8):e1700898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnsen J, Narang P, Hasenberg M, Gunzer F, Bilitewski U, Klippel N, et al. Environmental dimensionality controls the interaction of phagocytes with the pathogenic fungi Aspergillus fumigatus and Candida albicans. PLoS Pathog. 2007;3(2):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan CF. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987;80(6):1550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich V, Rawicz W. Automated, high-resolution micropipet aspiration reveals new insight into the physical properties of fluid membranes. Langmuir. 2005;21(5):1962–71. [DOI] [PubMed] [Google Scholar]
- Herant M, Heinrich V, Dembo M. Mechanics of neutrophil phagocytosis: experiments and quantitative models. J Cell Sci. 2006;119(Pt 9):1903–13. [DOI] [PubMed] [Google Scholar]
- Lee CY, Herant M, Heinrich V. Target-specific mechanics of phagocytosis: protrusive neutrophil response to zymosan differs from the uptake of antibody-tagged pathogens. J Cell Sci. 2011;124(Pt 7):1106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich V. Controlled one-on-one encounters between immune cells and microbes reveal mechanisms of phagocytosis. Biophys J. 2015;109(3):469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, et al. Molecular Biology of the Cell. 6 ed. New York: Garland Science; 2014. 1-1342 p. [Google Scholar]
- Heinrich V. Available from: https://www.youtube.com/user/HeinrichLab .
- Wangdi T, Lee CY, Spees AM, Yu C, Kingsbury DD, Winter SE, et al. The Vi Capsular Polysaccharide Enables Salmonella enterica Serovar Typhi to Evade Microbe-Guided Neutrophil Chemotaxis. PLoS Pathog. 2014;10(8):e1004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelev DV, Alteraifi AM, Chodniewicz D. Controlled pseudopod extension of human neutrophils stimulated with different chemoattractants. Biophys J. 2004;87(1):688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis EA, Heinrich V. Extension of chemotactic pseudopods by nonadherent human neutrophils does not require or cause calcium bursts. Sci Signal. 2018;11(521):eaal4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Thompson GR, 3rd, Hastey CJ, Hodge GC, Lunetta JM, Pappagianis D, et al. Coccidioides Endospores and Spherules Draw Strong Chemotactic, Adhesive, and Phagocytic Responses by Individual Human Neutrophils. PLoS One. 2015;10(6):e0129522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herant M, Lee CY, Dembo M, Heinrich V. Protrusive push versus enveloping embrace: computational model of phagocytosis predicts key regulatory role of cytoskeletal membrane anchors. PLOS Comput Biol. 2011;7(1):e1001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey CL, Drutz DJ. Influence of fungal surface components on the interaction of Coccidioides immitis with polymorphonuclear neutrophils. J Infect Dis. 1986;153(5):933–43. [DOI] [PubMed] [Google Scholar]
- Mankovich AR, Lee CY, Heinrich V. Differential effects of serum heat treatment on chemotaxis and phagocytosis by human neutrophils. PLoS One. 2013;8(1):e54735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I - molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–52. [DOI] [PubMed] [Google Scholar]
- Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4(2):133–42. [DOI] [PubMed] [Google Scholar]
- Francis EA, Heinrich V. Quantifying the sensitivity of human immune cells to chemoattractant. Biophys J. 2017;112(5):834–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich V, Simpson WD, Francis EA. Analytical Prediction of the Spatiotemporal Distribution of Chemoattractants around Their Source: Theory and Application to Complement-Mediated Chemotaxis. Front Immunol. 2017;8(578). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffafian I, Hallett MB. Does cytosolic free Ca2+ signal neutrophil chemotaxis in response to formylated chemotactic peptide? J Cell Sci. 1995;108(Pt 10):3199–205. [DOI] [PubMed] [Google Scholar]
- Huber-Lang MS, Younkin EM, Sarma JV, McGuire SR, Lu KT, Guo RF, et al. Complement-induced impairment of innate immunity during sepsis. J Immunol. 2002;169(6):3223–31. [DOI] [PubMed] [Google Scholar]
- Evans E, Heinrich V, Ludwig F, Rawicz W. Dynamic tension spectroscopy and strength of biomembranes. Biophys J. 2003;85(4):2342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S, Francis RJ, Hallett MB. Ca(2)(+) and calpain control membrane expansion during the rapid cell spreading of neutrophils. J Cell Sci. 2013;126(Pt 20):4627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovari DT, Wei W, Chang P, Toro JS, Beach RF, Chambers D, et al. Frustrated Phagocytic Spreading of J774A-1 Macrophages Ends in Myosin II-Dependent Contraction. Biophys J. 2016;111(12):2698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou L, Babataheri A, Saitakis M, Bohineust A, Dogniaux S, Hivroz C, et al. T-lymphocyte passive deformation is controlled by unfolding of membrane surface reservoirs. Mol Biol Cell. 2016;27(22):3574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting-Beall HP, Needham D, Hochmuth RM. Volume and osmotic properties of human neutrophils. Blood. 1993;81(10):2774–80. [PubMed] [Google Scholar]
- Dewitt S, Hallett MB. Cytosolic free Ca(2+) changes and calpain activation are required for beta integrin-accelerated phagocytosis by human neutrophils. J Cell Biol. 2002;159(1):181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Herant M, Dembo M, Heinrich V. Baseline mechanical characterization of J774 macrophages. Biophys J. 2009;96(1):248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S, Hallett M. Leukocyte membrane “expansion”: a central mechanism for leukocyte extravasation. J Leukoc Biol. 2007;81(5):1160–4. [DOI] [PubMed] [Google Scholar]
- Hallett MB, Dewitt S. Ironing out the wrinkles of neutrophil phagocytosis. Trends Cell Biol. 2007;17(5):209–14. [DOI] [PubMed] [Google Scholar]
- Touret N, Paroutis P, Terebiznik M, Harrison RE, Trombetta S, Pypaert M, et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell. 2005;123(1):157–70. [DOI] [PubMed] [Google Scholar]
- Jumaa MA, Dewitt S, Hallett MB. Topographical interrogation of the living cell surface reveals its role in rapid cell shape changes during phagocytosis and spreading. Sci Rep. 2017;7(1):9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herant M, Heinrich V, Dembo M. Mechanics of neutrophil phagocytosis: behavior of the cortical tension. J Cell Sci. 2005;118(Pt 9):1789–97. [DOI] [PubMed] [Google Scholar]
- Evans E, Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989;56(1):151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JR, Naccache PH, Molski TF, Borgeat P, Sha’afi RI. Direct demonstration of increased intracellular concentration of free calcium in rabbit and human neutrophils following stimulation by chemotactic factor. Biochem Biophys Res Commun. 1983;113(1):44–50. [DOI] [PubMed] [Google Scholar]
- Marks PW, Maxfield FR. Local and global changes in cytosolic free calcium in neutrophils during chemotaxis and phagocytosis. Cell Calcium. 1990;11(2-3):181–90. [DOI] [PubMed] [Google Scholar]
- Lew DP. Receptor signalling and intracellular calcium in neutrophil activation. Eur J Clin Invest. 1989;19(4):338–46. [DOI] [PubMed] [Google Scholar]
- Clemens RA, Lowell CA. Store-operated calcium signaling in neutrophils. J Leukoc Biol. 2015;98(4):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N, Nunes P. The role of STIM and ORAI proteins in phagocytic immune cells. Am J Physiol Cell Physiol. 2016;310(7):C496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol. 2009;10(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim FB, Pang SJ, Melendez AJ. Anaphylatoxin signaling in human neutrophils. A key role for sphingosine kinase. J Biol Chem. 2004;279(43):44802–11. [DOI] [PubMed] [Google Scholar]
- Norgauer J, Dobos G, Kownatzki E, Dahinden C, Burger R, Kupper R, et al. Complement fragment C3a stimulates Ca2+ influx in neutrophils via a pertussis-toxin-sensitive G protein. Eur J Biochem. 1993;217(1):289–94. [DOI] [PubMed] [Google Scholar]
- Schorr W, Swandulla D, Zeilhofer HU. Mechanisms of IL-8-induced Ca2+ signaling in human neutrophil granulocytes. Eur J Immunol. 1999;29(3):897–904. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S, Iribarren P, Moreno-Garcia ME, Gao JL, Murphy PM, Oppenheimer N, et al. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J Immunol. 2004;172(3):1896–906. [DOI] [PubMed] [Google Scholar]
- Schaff UY, Yamayoshi I, Tse T, Griffin D, Kibathi L, Simon SI. Calcium flux in neutrophils synchronizes beta2 integrin adhesive and signaling events that guide inflammatory recruitment. Ann Biomed Eng. 2008;36(4):632–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaff UY, Dixit N, Procyk E, Yamayoshi I, Tse T, Simon SI. Orai1 regulates intracellular calcium, arrest, and shape polarization during neutrophil recruitment in shear flow. Blood. 2010;115(3):657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit N, Yamayoshi I, Nazarian A, Simon SI. Migrational guidance of neutrophils is mechanotransduced via high-affinity LFA-1 and calcium flux. J Immunol. 2011;187(1):472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Ling Y, Lin J, Du X, Fang Y, Wu J. Force-dependent calcium signaling and its pathway of human neutrophils on P-selectin in flow. Protein Cell. 2017;8(2):103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakina EB, Waugh RE. Signaling and Dynamics of Activation of LFA-1 and Mac-1 by Immobilized IL-8. Cell Mol Bioeng. 2010;3(2):106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste MT, Lomakina EB, Hammer DA, Waugh RE. Immobilized IL-8 triggers phagocytosis and dynamic changes in membrane microtopology in human neutrophils. Ann Biomed Eng. 2015;43(9):2207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshulam T, Proto P, Diamond RD, Melnick DA. Calcium modulation and chemotactic response: divergent stimulation of neutrophil chemotaxis and cytosolic calcium response by the chemotactic peptide receptor. J Immunol. 1986;137(6):1954–60. [PubMed] [Google Scholar]
- Sogkas G, Vogtle T, Rau E, Gewecke B, Stegner D, Schmidt RE, et al. Orai1 controls C5a-induced neutrophil recruitment in inflammation. Eur J Immunol. 2015;45(7):2143–53. [DOI] [PubMed] [Google Scholar]
- Elsner J, Kaever V, Emmendorffer A, Breidenbach T, Lohmann-Matthes ML, Roesler J. Heterogeneity in the mobilization of cytoplasmic calcium by human polymorphonuclear leukocytes in response to fMLP, C5a and IL-8/NAP-1. J Leukoc Biol. 1992;51(1):77–83. [DOI] [PubMed] [Google Scholar]
- Lomakina EB, Waugh RE. Dynamics of increased neutrophil adhesion to ICAM-1 after contacting immobilized IL-8. Ann Biomed Eng. 2006;34(10):1553–63. [DOI] [PubMed] [Google Scholar]
- Ju L, Chen Y, Li K, Yuan Z, Liu B, Jackson SP, et al. Dual Biomembrane Force Probe enables single-cell mechanical analysis of signal crosstalk between multiple molecular species. Sci Rep. 2017;7(1):14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakina EB, Waugh RE. Micromechanical tests of adhesion dynamics between neutrophils and immobilized ICAM-1. Biophys J. 2004;86(2):1223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Mao W, Byler R, Patel K, Henegar C, Alexeev A, et al. Stiffness dependent separation of cells in a microfluidic device. PLoS One. 2013;8(10):e75901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto O, Rosendahl P, Mietke A, Golfier S, Herold C, Klaue D, et al. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat Methods. 2015;12(3):199-202, 4 p following [DOI] [PubMed] [Google Scholar]
- Islam M, Brink H, Blanche S, DiPrete C, Bongiorno T, Stone N, et al. Microfluidic Sorting of Cells by Viability Based on Differences in Cell Stiffness. Sci Rep. 2017;7(1):1997. [DOI] [PMC free article] [PubMed] [Google Scholar]