Abstract
Liver metastasis from breast cancer is associated with poor prognosis and is a major cause of early morbidity and mortality. When liver resection is not feasible, minimally invasive directed therapies are considered to attempt to prolong survival. Selective internal radiation therapy (SIRT) with yttrium-90 microspheres is a liver-directed therapy that can improve local control of liver metastases from colorectal cancer. We present a case of a patient with a ductal breast adenocarcinoma, who developed liver and bone metastasis despite extensive treatment with systemic chemotherapies. Following SIRT to the liver, after an initial response, the patient ultimately progressed in the liver after 7 months. Liver tumor histology obtained 20 months after the SIRT intervention demonstrated the presence of the resin microspheres in situ. This case report demonstrates the long-term control that may be achieved with SIRT to treat liver metastases from breast cancer that is refractory to previous chemotherapies, and the presence of microspheres in situ long-term.
Keywords: Selective internal radiation therapy, SIRT, Transarterial radioembolization, Liver-directed therapy
Background
Breast cancer is a leading cause of mortality worldwide [1]. It represents a heterogeneous group of subtypes with characteristic molecular features, prognosis, and responses to treatment [2]. The development of liver metastasis from breast cancer heralds a poor prognosis [3]. If it is feasible, hepatic resection is of offered to patients, but unfortunately the vast majority are unresectable at the time of diagnosis of liver metastasis [3]. Other liver-directed therapies have been attempted for liver-only disease with the primary aim of palliating and prolonging survival. These treatments include radiofrequency [4], [5] and microwave ablation, transarterial chemoembolization [6], selective internal radiation therapy (SIRT) [7], and stereotactic body radiotherapy [8], [9].
SIRT was developed as a liver-directed therapy for metastatic colorectal cancer and primary liver cancer [10]. Recently, a randomized multicenter clinical trial has demonstrated an improvement in radiological response rate and hepatic progression-free survival in patients with metastatic colorectal cancer treated with SIRT [11]. In case reports and small case series, SIRT has also been used to treat liver metastases from other primary tumors including breast [12], neuroendocrine [13], and pancreas [14].
SIRT involves the administration of yttrium-90 (90Y) microspheres via the arterial blood supply of liver metastases [15]. The microspheres are typically about 30 µm in diameter. The administered dose is targeted by meticulous angiography of the tumor and its vasculature. Potential adverse events of SIRT therapy include postradioembolization syndrome (PRS) (20%-55% of patients), radioembolization-induced liver disease (REILD), damage to the biliary system including radiation cholecystitis, portal hypertension, radiation pneumonitis, gastroduodenal ulceration, vascular injury, and lymphopenia [10], [16], [17]. PRS typically consists of mild fatigue, gastrointestinal upset, anorexia, fever, and cachexia and it may require symptomatic management. REILD is defined as jaundice and ascites 1 to 2 months after a radioembolization procedure, in the absence of tumor progression or bile duct occlusion [18]. One prospective study evaluated 45 patients without chronic liver disease who underwent radioembolization for the liver in a single institution and 9 (20%) went on to develop REILD in the first 90 days [19]. All these patients had received chemotherapy preprocedure or postprocedure and population analysis strongly suggested that the syndrome associated with the combined effect of radiation and chemotherapy.
There are some reports of tumur pathology from hepatic resection after SIRT, all of which focus on colorectal liver metastases [20], [21], [22]. In colorectal patients, microspheres have been identified in the vascular tumor bed and the portal tract vessels within liver parenchyma following SIRT [20]. These microspheres were typically associated with giant cell reaction or histiocytes. In the tumor bed, the blood vessels and fibroblasts showed changes suggestive of radiation injury. Necrosis, mucin and calcification have also been described. Another study describes a patient with metastatic cholangiocarcinoma, who had a partial response to SIRT and went on to a liver resection and remained disease-free at 9 months [22]. Histology demonstrated residual microspheres with evidence of moderate-to-severe hepatic inflammation and early stage fibrosis, with 50% overall tumor necrosis. In the same study, a patient with metastatic rectal cancer also went on to have a liver resection, and this time histology revealed residual microspheres, moderate-to-severe inflammation with no evidence of fibrosis and 80% tumor necrosis. In addition, the study describes residual microsphere embolization surrounding resected esophageal and cholangiocarcinoma liver metastases with 80% and 45% tumor necrosis, respectively. Another study examined the histology of the nonneoplasic liver in 7 patients 6-23 months following SIRT in metastatic colorectal cancer [3], hepatocellular carcinoma [2], and neuroendocrine tumor [1], [23]. Of the 7 tissue samples, 6 were core biopsies and 1 was a wedge resection. Of the biopsies, 5/6 demonstrated microspheres, typically in the portal tracts, and without significant associated inflammation. Mild portal vein dilatation was seen in 3/6, mild portal fibrosis in 2/6, and in 1 case a particle was demonstrated in an artery with associated thrombus. The wedge resection demonstrated extensive coagulative necrosis of hepatocytes due to portal vein thrombosis. The study suggests that SIRT causes little diagnostic pathologic change in the normal liver surrounding the liver metastasis, and that the microspheres are generally found in the portal tracts with little inflammatory reaction.
Publications of long-term outcomes, including histology, in breast cancer patients with liver metastases who have received SIRT are currently lacking, so it is not known what histological changes are observed in breast cancer metastases. This report describes radiological outcomes and histology in an extensively pretreated breast cancer patient who underwent tumor biopsies 20 months after receiving SIRT. It represents the first report of tumor histology so long after SIRT in a patient with metastatic breast cancer, demonstrating the presence of microspheres distributed in the stroma surrounding viable tumor.
Clinical presentation
Written informed consent was obtained from the patient for publication of this case report, including accompanying images. A 49-year-old woman, with no past medical history of note, presented with right-sided grade 2, node positive, estrogen-receptor (ER) positive breast cancer in 1996. She received 6 cycles of neoadjuvant cyclophosphamide, epirubicin, fluorouracil prior to resection, after which she was commenced on adjuvant cyclophosphamide, methotrexate, 5-fluorouracil . A 3.4 cm grade 2 ER-positive tumor was resected, and 6/8 lymph nodes were positive. She received a course of adjuvant tamoxifen over a period of 4 years, which was then stopped due to menorrhagia. Five years’ post diagnosis, the patient relapsed with a new 1 cm, grade 2, left-sided primary breast tumor, which was ER- and progesterone-receptor (PR) positive, lymph node negative. She received surgery, radiotherapy, and adjuvant letrozole, which was completed in 2006.
There was relapse in 2010 with a solitary bone metastasis in the pubic ramus of the right hemipelvis, but no evidence of soft tissue disease elsewhere. Exemestane was commenced, with a single fraction of 8 Gy palliative radiotherapy to the affected area. The cancer antigen (CA 15-3) biochemical marker was elevated at 121 U/mL at this stage, but fell on this therapy. Two liver metastases developed in November 2011, the largest of which was 17 mm, and at this stage exemestane was replaced with a further course of tamoxifen and alendronic acid was also commenced. The liver metastasis initially remained stable on this treatment. A year later, the serum CA 15-3 marker had demonstrated gradual increases up to the low 300s and the patient was given radiotherapy to right hip. Fulvestrant hormonal therapy was started, which initially reduced the serum CA 15-3. However, liver disease was progressive in 2012 and fulvestrant was replaced with capecitabine. Liver disease continued to progress in 2013, consequently capecitabine was stopped and she was referred for consideration of SIRT, which was recommended by multidisciplinary team meeting discussion.
Treatment
The SIRT procedure to the liver was performed in Oxford, UK in September 2013. The pre-SIRT procedure utilized a right femoral approach to perform coil embolization at the origin of the main gastroduodenal trunk, the first branch of the left hepatic artery and the right gastric artery allowing isolation of the right gastric territory. The SIRT procedure itself involved a further coil embolization to the segment IV vessel to allow more distal delivery of Y90 SIR-spheres in the right hepatic circulation. During the SIRT procedure, 1100 megabecquerel (MBq) was delivered in a split dose, 800 MBq to right hepatic artery, and 300 MBq to left hepatic artery. A single-photon emission computed tomography (CT) scan was performed 18 hours post-SIRT procedure; distribution of radioactivity within and around the liver metastases was demonstrated (Fig. 1).
Fig. 1.
Radiological imaging obtained from the patient treated with SIRT in 2013. Top: Fluoroscopy performed during (left) and immediately following injection of yttrium-90 resin microspheres (right), the later image showing blush of radioopacity predominantly overlying the right side of liver. Bottom left: Fat saturation MRI in axial section obtained 9 days prior to SIRT, with the white arrow demonstrating the location of the tumor subsequently biopsied in an early phase cancer study in 2015. Bottom right: Post-SIRT SPECT-CT scan at a similar axial section demonstrating high doses of radioactivity in segments V and VI of the liver coregistering the tumor distribution.
Outcome and follow-up
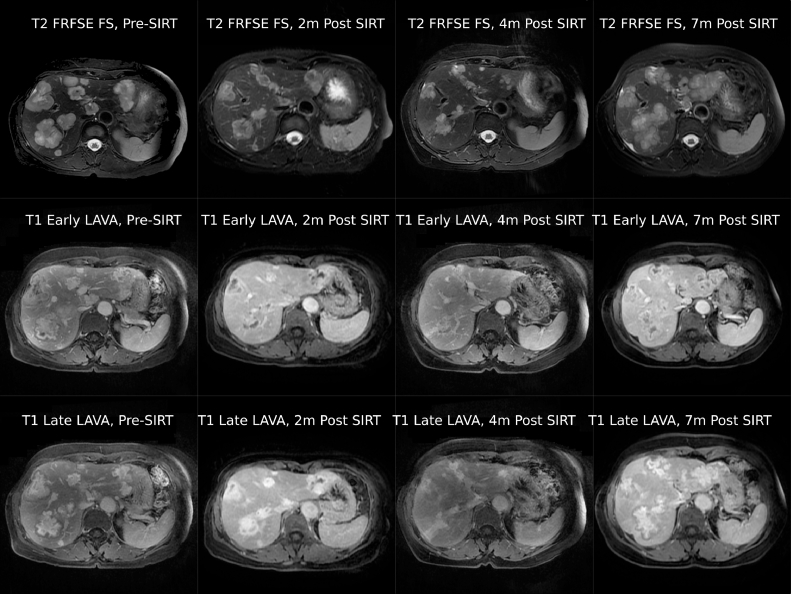
Follow-up magnetic resonance imaging (MRI) 11 weeks after SIRT revealed a partial response of liver metastases, and MRI analysis using specialist 3D volumetric software (Advantage Workstation Volume Share v2) showed that the liver volume was 1184 cm3 of which 39 cm3 was metastatic tumor burden (3.3% hepatic replacement). This was compared to a preSIRT liver volume of 1401 mL on baseline MRI, of which 208 mL was metastatic tumor (14.8% hepatic replacement). The follow-up MRI also demonstrated new vertebral bony metastasis in the cervical, thoracic, and lumbar spine and denosumab and megace were started. Further radiological follow-up of liver metastases with MRI demonstrated stable disease at 4 months, after which there was disease progression at 7 months (Fig. 2).
Fig. 2.
Top row: Axial T2 fast relaxation fast spin-echo fat-saturated (T2 FRFSE FS) MRI before (far left), 2 months (middle left), 4 months (middle right), and 7 months (far right) following the SIRT procedure. Middle row: Early phase T1 LAVA MRI of the same axial sections for the same time points. Bottom row: Late phase T1 LAVA MRI of the same. The liver tumors initially respond well to therapy at 2 months, are stable at 4 months, before subsequent progression at 7 months.
Megace was discontinued in April 2014 due to disease progression and oral vinorelbine commenced. The patient continued to progress on subsequent scans, demonstrating aggressive liver disease with a markedly elevated CA 15-3 tumor marker at this stage, and consequently the patient was referred for early-phase clinical studies. Twenty months following the SIRT procedure, 3, 18-guage core biopsies of a liver metastasis within the segments previously treated with SIRT were obtained under ultrasound-guidance as part of an ethically approved clinical study in targeted drug delivery [24], [25]. Sections of these biopsies were formalin-fixed, paraffin-embedded for subsequent microscopic analysis. Routine hematoxylin and eosin staining was performed and demonstrated tumor, consistent with breast cancer, set in fibrous stroma (Fig. 3). One of 3 available biopsy specimens revealed the presence of 6 resin microspheres, which were not associated with a surrounding macrophage response. All biopsies were taken from the core of the target tumor and no control biopsies were taken, thus it was not possible to assess the tumor bed or surrounding normal liver architecture for radiation damage or other background liver disease such as steatohepatitis.
Fig. 3.
Histology images obtained using slide scanner at 20× magnification. Top: (a) Hematoxylin and eosin stain of liver breast metastasis biopsy core demonstrating presence of the characteristic spherical yttrium-90 microspheres within tumor set in abundant fibrous stroma. Bottom: (b) A further tumor fragment from the same biopsy core demonstrates further tumor set in a smaller amount of fibrous stroma and this time, absence of microspheres. Neither sample demonstrates tumor necrosis.
Discussion
Several retrospective studies have explored the use of SIRT in chemorefractory metastatic breast cancer to the liver. In 2007, Bangash et al demonstrated a complete or partial response at 90 days in 9/27 women with progressive breast cancer who had failed standard of care chemotherapy, whilst 2/27 progressed and the remainder were stable [26]. The same year Coldwell et al published series of 44 heavily pretreated women with breast liver metastasis, showing a 47% partial response rate by CT at 12 weeks, and higher response rate by PET [27]. In 2008, Jakob et al published a study which demonstrated partial response in 61% in 23 women with at a median follow-up of 4.2 months with a median overall survival of 11.7 months, 23.6 months in the responding group vs 5.7 months in nonresponding group [28]. In 2012, Haug et al demonstrated a similar median overall survival of 47 weeks in 58 women receiving SIRT for breast liver metastasis [12]. Further following 2-[18F]-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography analysis, SUVmax correlated significantly with survival, and indeed the change in its value 3 months’ post-SIRT was the only independent predictor of survival. At around the same time, Cianni et al demonstrated similar outcomes with a median overall survival of 11.5 with a 56% partial response in 52 breast cancer patients receiving liver-directed SIRT [29]. In the largest study of SIRT in breast cancer patients to date, Gordon et al showed a partial response of 35.3% and stable disease in 63.2%, with a median overall survival of 6.6 months in 75 patients [30].
One systematic review included 6 retrospective cohort studies in which SIRT was used to treat breast cancer liver metastases, including 198 patients overall [31]. The study concluded that SIRT is well tolerated and effective for this patient cohort with end-stage disease and a high tumor burden. Gastric ulceration was seen in 5% of patients and treatment-related mortality in 2% thought due to radiation-induced liver disease. In these retrospective cohorts, disease control (complete response, partial response or stable disease) was seen in 78%-96% at 2-4 months. Prospective data in this patient cohort are currently lacking.
There are some reports of tumor histopathology from hepatic resection after SIRT, all of which focus on colorectal liver metastases [20], [21], [22]. In colorectal patients, microspheres have been identified in the vascular tumor bed and the portal tract vessels within liver parenchyma following SIRT [20]. These microspheres were typically associated with giant cell reaction or macrophages, a feature not seen in the case of the breast tumor histology presented here. In the tumor bed, the blood vessels and fibroblasts showed changes suggestive of radiation injury. Necrosis, mucin, and calcification have also been described. Another study describes a patient with metastatic cholangiocarcinoma who had a partial response to SIRT and went on to a liver resection and remained disease-free at 9 months [22]. Histology demonstrated residual microspheres with evidence of moderate-to-severe hepatic inflammation and early stage fibrosis, with 50% overall tumor necrosis. In the same study, a patient with metastatic rectal cancer also went on to have a liver resection, and this time histology revealed residual microspheres, moderate-to-severe inflammation with no evidence of fibrosis and 80% tumor necrosis. In addition, the study describes residual microsphere embolization surrounding resected esophageal and cholangiocarcinoma liver metastases with 80% and 45% tumor necrosis respectively.
Another study examined the histology of the nonneoplasic liver parenchyma in 7 patients 6-23 months following SIRT in metastatic colorectal cancer.[3], hepatocellular carcinoma [2], and neuroendocrine tumor [1], [23]. Of the 7 tissue samples, 6 were core biopsies and 1was a wedge resection. Of the biopsies, 5/6 demonstrated microspheres, typically in the portal tracts and without significant associated inflammation. Mild portal vein dilatation was seen in 3/6, mild portal fibrosis in 2/6, and in 1 case a particle was demonstrated in an artery with associated thrombus. The wedge resection demonstrated extensive coagulative necrosis of hepatocytes due to portal vein thrombosis. The study suggests that SIRT causes little diagnostic pathologic change in the normal liver surrounding the liver metastasis, and that the microspheres are generally found in the portal tracts with little inflammatory reaction.
In the present case report, we present the long-term radiological and histological outcomes post-SIRT in a single patient with liver metastasis from breast cancer refractory to standard systemic chemotherapies. Our histological findings are consistent with previous histology reports and demonstrate the persistence of the resin microspheres in situ. In this particular isolated case, no surrounding inflammatory response was demonstrated.
Summary
SIRT may have a role for liver-directed therapy in selected patients with advanced metastatic liver disease, were other treatment options are limited. We have presented the radiological outcomes and histopathological findings of long-term retention of microspheres in a breast cancer patient with extensive liver metastasis treated with SIRT.
Learning points
In the treatment of unresectable, chemorefractory metastatic liver tumors:
-
1.
The safety of SIRT has been demonstrated in both early phase and later phase studies, typically in the setting of colorectal cancer metastasis.
-
2.
SIRT is a potential treatment modality in the treatment of liver metastases from breast cancer, where it has been shown to be well-tolerated in retrospective series.
-
3.
Postradioembolization syndrome (fatigue, nausea, gastrointestinal upset, fever, and cachexia) is commonly seen post-SIRT and is generally mild and self-limiting; symptomatic management may be required.
-
4.
Radioembolisation-induced liver disease (jaundice and ascites 1-2 months’ postprocedure) is 1 potential complication post-SIRT.
-
5.
Reports of post-SIRT tumor histology are limited but typically residual microspheres are demonstrated in the portal tracts of surrounding liver with little inflammatory change.
-
6.
MRI, CT, or 2-[18F]-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography is typically used to evaluate radiological response at 1-2 months post-SIRT.
-
7.
Several studies have demonstrated that SIRT can significantly improve hepatic progression-free survival in patients with liver metastasis from colorectal cancer.
-
8.
Evaluation of SIRT in Phase 3 studies is on-going, particularly in liver-only or liver-dominant disease.
Acknowledgments
The authors acknowledge the patient and SIRTeX Medical who provided SIR-Spheres and funding from Cancer Research UK grant A8971 CRUK/07/030. The early phase trial (NCT02181075) leading to acquisition of the tumor tissue was funded by the UK's National Institute for Health Research, Oxford Biomedical Research Centre, and the research was supported by the Oxford Centre for Drug Delivery Devices under a programme grant (EP/L024012/1) from the Engineering and Physical Sciences Research Council.
Contributor Information
Paul C. Lyon, Email: paul.lyon@nhs.net.
Helen Winter, Email: helen.winter2@gtc.ox.ac.uk.
Karin Herbschleb, Email: Karin.Herbschleb@radboudumc.nl.
Leticia Campo, Email: leticia.campo@oncology.ox.ac.uk.
Robert Carlisle, Email: robert.carlisle@eng.ox.ac.uk.
Feng Wu, Email: feng.wu@nds.ox.ac.uk.
Robert Goldin, Email: r.goldin@imperial.ac.uk.
Constantin C. Coussios, Email: constantin.coussios@eng.ox.ac.uk.
Mark R. Middleton, Email: Mark.Middleton@ouh.nhs.uk.
Fergus V. Gleeson, Email: Fergus.Gleeson@ouh.nhs.uk.
Philip Boardman, Email: phil.boardman@ouh.nhs.uk.
Ricky A. Sharma, Email: ricky.sharma@ucl.ac.uk.
References
- 1.Rick A, Cammie B, Burke A, Gansler T, Gapstur S, Gaudet M. Breast cancer facts & figures 2013-2014. Am Cancer Soc. 2014;38 Internet Available from http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-040951.pdf. [Google Scholar]
- 2.Engstrom MJ, Opdahl S, Hagen AI, Romundstad PR, Akslen LA, Haugen OA. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res Treat. 2013;140(3):463–473. doi: 10.1007/s10549-013-2647-2. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/23901018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: long-term survival after curative resection. Surgery. 2000;127(4):383–389. doi: 10.1067/msy.2000.103883. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/10776428. [DOI] [PubMed] [Google Scholar]
- 4.Lawes D, Chopada A, Gillams A, Lees W, Taylor I. Radiofrequency ablation (RFA) as a cytoreductive strategy for hepatic metastasis from breast cancer. Ann R Coll Surg Engl. 2006;88(7):639–642. doi: 10.1308/003588406X149129. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/17132311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covey AM, Sofocleous CT. Radiofrequency ablation as a treatment strategy for liver metastases from breast cancer. Semin Interv Radiol. 2008;25(4):406–412. doi: 10.1055/s-0028-1102996. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/21326582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho SW, Kitisin K, Buck D, Steel J, Brufsky A, Gillespie R. Transcatheter arterial chemoembolization is a feasible palliative locoregional therapy for breast cancer liver metastases. Int J Surg Oncol. 2010;2010 doi: 10.1155/2010/251621. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/22312487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cianni R, Pelle G. Evidence-based integration of selective internal radiation therapy into the management of breast cancer liver metastases. Futur Oncol. 2014;10(15 Suppl):93–95. doi: 10.2217/fon.14.233. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/25478777. [DOI] [PubMed] [Google Scholar]
- 8.Goodman K.A., Wiegner E.A., Maturen K.E., Zhang Z., Mo Q., Yang G., Koong A.C. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78(2):486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Kirichenko A., Gayou O., Parda D., Kudithipudi V., Tom K., Khan A., Thai N. Stereotactic body radiotherapy (SBRT) with or without surgery for primary and metastatic liver tumors. HPB (Oxford) 2016;18(1):88–97. doi: 10.1016/j.hpb.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma RA, Van Hazel GA, Morgan B, Berry DP, Blanshard K, Price D. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25(9):1099–1106. doi: 10.1200/JCO.2006.08.7916. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/17369573. [DOI] [PubMed] [Google Scholar]
- 11.Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18(9):1159–1171. doi: 10.1016/S1470-2045(17)30457-6. Available from http://www.sciencedirect.com/science/article/pii/S1470204517304576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haug AR, Tiega Donfack BP, Trumm C, Zech CJ, Michl M, Laubender RP. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med. 2012;53(3):371–377. doi: 10.2967/jnumed.111.096230. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/22331219. [DOI] [PubMed] [Google Scholar]
- 13.Devcic Z, Rosenberg J, Braat AJA, Techasith T, Banerjee A, Sze DY. The efficacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med. 2014;55(9):1404–1410. doi: 10.2967/jnumed.113.135855. Internet Available from http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.113.135855%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/25012459. [DOI] [PubMed] [Google Scholar]
- 14.Michl M, Haug AR, Jakobs TF, Paprottka P, Hoffmann RT, Bartenstein P. Radioembolization with yttrium-90 microspheres (SIRT) in pancreatic cancer patients with liver metastases:efficacy, safety and prognostic factors. Oncology. 2014;86(1):24–32. doi: 10.1159/000355821. [DOI] [PubMed] [Google Scholar]
- 15.Morgan B, Kennedy AS, Lewington V, Jones B, Sharma RA. Intra-arterial brachytherapy of hepatic malignancies: watch the flow. Nat Rev Clin Oncol. 2011;8(2):115–120. doi: 10.1038/nrclinonc.2010.153. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/20924355. [DOI] [PubMed] [Google Scholar]
- 16.Riaz A, Lewandowski RJ, Kulik LM, Mulcahy MF, Sato KT, Ryu RK. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Intervent Radiol. 2009;20:1121–1130. doi: 10.1016/j.jvir.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol. 2014;4(July):198. doi: 10.3389/fonc.2014.00198. Internet Available from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4114299&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gil-Alzugaray B, Chopitea A, Iñarrairaegui M, Bilbao JI, Rodriguez-Fraile M, Rodriguez J. Prognostic factors and prevention of radioembolization-induced liver disease [Internet] Hepatology. 2013;57:1078–1087. doi: 10.1002/hep.26191. Available from . [DOI] [PubMed] [Google Scholar]
- 19.Sangro B, Gil-Alzugaray B, Rodriguez J, Sola I, Martinez-Cuesta A, Viudez A. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer. 2008;112:1538–1546. doi: 10.1002/cncr.23339. Available from . [DOI] [PubMed] [Google Scholar]
- 20.Wang LM, Jani AR, Hill EJ, Sharma RA. Anatomical basis and histopathological changes resulting from selective internal radiotherapy for liver metastases. J Clin Pathol. 2013;66(3):205–211. doi: 10.1136/jclinpath-2012-201231. Available from http://www.ncbi.nlm.nih.gov/pubmed/23162108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadaki M, Praseedom R, Brais R, See TC, Balan K, Wilson CB. Selective internal radiation therapy with 90Y-SIR-Spheres microspheres for non-resectable colorectal metastases in the liver. BMJ Case Rep. 2011 doi: 10.1136/bcr.01.2011.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitney R, Tatum C, Hahl M, Ellis S, Scoggins CR, McMasters K. Safety of hepatic resection in metastatic disease to the liver after yttrium-90 therapy. J Surg Res. 2011;166(2):236–240. doi: 10.1016/j.jss.2009.05.021. Available from http://www.ncbi.nlm.nih.gov/pubmed/19691985. [DOI] [PubMed] [Google Scholar]
- 23.Chiaffarano J, Marcus A, Nosher J, Hudacko R, Fyfe B, Robert R. Histopathologic evaluation of non-neoplastic liver following selective internal radiation therapy (SIRT) Am J Clin Pathol. 2014;142:A251. Internet Available from http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L72166924%5Cnhttp://ajcp.oxfordjournals.org/search/volume:142issue:suppl+1?facettoc-section-id0=SurgicalPathology%5Cnhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=00029173&id=do. [Google Scholar]
- 24.Lyon PC, Griffiths LF, Lee J, Chung D, Carlisle R, Wu F. Clinical trial protocol for TARDOX: a phase I study to investigate the feasibility of targeted release of lyso-thermosensitive liposomal doxorubicin (ThermoDox®) using focused ultrasound in patients with liver tumours. J Ther Ultrasound. 2017;5(1) doi: 10.1186/s40349-017-0104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyon PC, Gray MD, Mannaris C, Folkes LK, Stratford M, Campo L. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): a single-centre, open-label, phase 1 trial. Lancet Oncol. 2018 Aug 1;19(8):1027–1039. doi: 10.1016/S1470-2045(18)30332-2. Internet Available from . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bangash AK, Atassi B, Kaklamani V, Rhee TK, Yu M, Lewandowski RJ. 90Y radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol. 2007;18(5):621–628. doi: 10.1016/j.jvir.2007.02.019. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/17494843. [DOI] [PubMed] [Google Scholar]
- 27.Coldwell DM, Kennedy AS, Nutting CW. Use of yttrium-90 microspheres in the treatment of unresectable hepatic metastases from breast cancer. Int J Radiat Oncol Biol Phys. 2007;69(3):800–804. doi: 10.1016/j.ijrobp.2007.03.056. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/17524567. [DOI] [PubMed] [Google Scholar]
- 28.Jakobs TF, Hoffmann RT, Fischer T, Stemmler HJ, Tatsch K, La Fougere C. Radioembolization in patients with hepatic metastases from breast cancer. J Vasc Interv Radiol. 2008;19(5):683–690. doi: 10.1016/j.jvir.2008.01.009. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/18440456. [DOI] [PubMed] [Google Scholar]
- 29.Cianni R, Pelle G, Notarianni E, Saltarelli A, Rabuffi P, Bagni O. Radioembolisation with 90Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer. Eur Radiol. 2013;23(1):182–189. doi: 10.1007/s00330-012-2556-5. [DOI] [PubMed] [Google Scholar]
- 30.Gordon AC, Gradishar WJ, Kaklamani VG, Thuluvath AJ, Ryu RK, Sato KT. Yttrium-90 radioembolization stops progression of targeted breast cancer liver metastases after failed chemotherapy. J Vasc Interv Radiol. 2014;25(10):1523–1532. doi: 10.1016/j.jvir.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smits MLJ, Prince JF, Rosenbaum CENM, Van Den Hoven AF, Nijsen JFW, Zonnenberg BA. Intra-arterial radioembolization of breast cancer liver metastases: a structured review. Euro J Pharmacol. 2013;709:37–42. doi: 10.1016/j.ejphar.2012.11.067. [DOI] [PubMed] [Google Scholar]