Abstract
Ultraviolet (UV) light, a main cause of photoaging, leads to collapse of skin structure, resulting in wrinkle formation and dehydration. The present study assessed the anti-photoaging and moisturizing effects of Bouea macrophylla extract (BRE). UVB-irradiated hairless mice were orally administered with BME (300 mg/kg/day) for 8 weeks. BME ameliorated wrinkle formation, skin thickening, and inelasticity. BME upregulated COL1A1, COL3A1, COL4A1, and COL7A1 mRNA levels through activation of the transforming growth factor-β (TGF-β)/Smad pathway, thereby recovering the content of collagen reduced by UVB. Further, BME suppressed UVB-induced matrix metalloproteinase (MMP)-3 and MMP-13 expression and inhibited MMP-2 and MMP-9 activity by mediating the mitogen-activated protein kinases (MAPKs)/activator protein-1 (AP-1). BME improved moisture content by stimulating the expression of cornified envelope proteins and filaggrin-processing enzymes. Overall, the results show that BME prevents photoaging and promotes moisturization in UVB-irradiated hairless mice, suggesting its potential as a nutraceutical candidate for anti-photoaging and moisturizing effects.
Keywords: Bouea macrophylla, Matrix metalloproteinase, Collagen, Anti-photoaging, Moisturizing effect
Introduction
Skin aging, which results in roughness, dryness, sagging, and formation of wrinkles, is classified extrinsic or intrinsic aging [1]. Intrinsic aging is a part of the regular aging process, while extrinsic aging is promoted by chronic exposure to ultraviolet B (UVB) radiation. UVB irradiation causes various skin disorders, such as irregular pigmentation, sunburn, photoaging, and skin cancer [2]. UVB stimulates the production of reactive oxygen species (ROS), including hydroxyl radical, peroxides, superoxide, and singlet oxygen. A marked increase in ROS levels leads to collapse of cell structure and accumulation of oxidative stress in cells, resulting in photoaging [3].
The stimulation of ROS formation induces the collapse of the extracellular matrix (ECM) components that provide biochemical support to the cell structure [4]. An increase in ROS levels stimulates mitogen-activated protein kinases (MAPKs), comprising extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 kinase. Stimulation of MAPKs leads to the upregulation of the activator protein-1 (AP-1) complex consisting of c-Jun and c-Fos [5, 6]. Increased AP-1 complex expression promotes matrix metalloproteinases (MMPs) expression, leading to degradation of various ECM components [7]. The AP-1 complex also downregulates procollagen type I synthesis via regulation of the transforming growth factor-β (TGF-β)/Smad signaling pathway [8]. Smad7, stimulated by AP-1 complex, suppresses phosphorylation of Smad2/3, leading to the inactivation of the TGF-β/Smad signaling pathway [9].
Nuclear factor-kappa B (NF-κB) controls cell survival and cytokine production. This protein generally exists in its inactive form with an inhibitory protein, known as inhibitor of nuclear factor kappa B kinase (IκB) subunit alpha. However, excessive production of ROS stimulates the activation of IκB kinase, leading to NF-κB expression [10, 11]. Activated NF-κB induces the expression of proinflammatory cytokines, resulting in further oxidative damage.
MMPs are associated with tissue remodeling processes such as angiogenesis, morphogenesis, tissue repair, and metastasis [12]. Generally, MMPs degrade damaged structural proteins and facilitate the process of wound healing. UVB irradiation induces AP-1 complex expression, leading to excessive expression of MMPs, resulting in digestion of ECM components. Therefore, excessive expression of MMPs stimulates wrinkle formation and is considered a major target in prevention of wrinkle formation.
The stratum corneum (SC) is the outermost part of the epidermis and is composed of corneocytes, which are differentiated keratinocytes [13]. Corneocytes contain natural moisturizing factors (NMFs), which are generated by filaggrin processing and are surrounded by cornified envelope (CE) made up of envoplakin, involucrin, and periplakin [14]. NMFs contribute to skin hydration and formation of the CE, which plays a major role in skin barrier function. Thus, dysfunction of these two factors leads to adverse effects on skin hydration and barrier function.
Bouea macrophylla (marian plum) is an edible fruit cultivated widely in Indonesia, the Philippines, and Thailand. An antioxidant effect of Bouea macrophylla was evaluated previously [15]. Additionally, previous studies have reported that this antioxidant property is associated with an anti-photoaging effect [3, 16]. Thus, we hypothesized that Bouea macrophylla has anti-photoaging and moisturizing effects. In this study, we evaluated the effects of Bouea macrophylla ethanol extract (BME) on prevention of photoaging and enhancement of skin barrier function in UVB-irradiated hairless mice.
Materials and methods
Preparation of BME
Dried fruits of Bouea macrophylla obtained from Klong Toey Market (Bangkok, Thailand) were stored in the Department of Biotechnology, Yonsei University (Seoul, Korea). The dried fruits were powdered and soaked in 95% ethanol for 3 h at 37 °C. Bouea macrophylla filtrate was evaporated using a rotary evaporator (Heidolph, Schwabach, Germany) to obtain BME.
Animal experiment
Five-week-old female albino hairless mice (SKH-1) were purchased from Daehan Biolink Ltd. (Eumsung, Korea). The mice were housed in conditions of 55 ± 10% humidity, 12-h day/night cycles, and a temperature of 23 ± 2 °C at Yonsei Laboratory Animal Research Center (Seoul, Korea). All experimental protocols were approved by the Institutional Animal Care and Use Committee of Yonsei University (Permit #: 201509-471-03). The mice were then randomly divided into three groups: control group (normal), UVB-irradiated group (UVB), and UVB-irradiated and 300 mg/kg/day BME-treated group (UVB + BME). The BME-treated group was orally administered BME daily, with exposure to UVB radiation three times a week on the dorsal section of the body for 8 weeks. The experiment was performed as previously described [17]. Dorsal sections of sacrificed mice were promptly soaked in liquid nitrogen and kept at -80 °C in an Ultralow Temperature Freezer (NuAire, Plymouth, MN, USA).
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from dorsal skin tissue using Trizol reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was obtained with Reverse Transcription Premix (Elpis-Biotech., Inc., Daejeon, Korea). The cDNA was amplified with primer pairs (Bioneer, Daejeon, Korea) and HiPi PCR Premix (Elpis-Biotech). The following primers were used: catalase (forward, 5′-GGT GCC CCC AAC TAT TAC CC-3′; reverse, 5′-GAA TGT CCG CAC CTG AGT GA-3′), COL1A1 (forward, 5′-GTC CCC AAT GGT GAG ACG TG-3′; reverse, 5′-GCA CGG AAA CTC CAG CTG AT-3′), COL3A1 (forward, 5′-AGC GGC TGA GTT TTA TGA CG-3′; reverse, 5′-AGC ACA GGA GCA GGT GTA GA-3′), COL4A1 (forward, 5′-GCC AAA GCC AAA CCC ATT CC-3′; reverse 5′-TGG TAC GTG TGG TAA CTT CTC-3′), COL7A1 (forward, 5′-AAG CCG AGA TTA AGG GCT GG-3′; reverse 5′-CAC CAAA TGG AGC ACA GCA G-3′), MMP-3 (forward, 5′-TAG CAG GTT ATC CTA AAA GCA-3′; reverse 5′-CCA GCT ATT GCT CTT CAA T-3′), MMP-13 (forward, 5′-CAT CCA TCC CGT GAC CTT AT-3′; reverse 5′-GCA TGA CTC TCA CAA TGC GA-3′), loricrin (LOR; forward, 5′-TAC CTG GCC GTG CAA GTA AG -3′; reverse 5′-CAA TGG CTT CTT CTG GGG GA-3′), involucrin (INV; forward, 5′-AAG GAC CAA AAA GCC TGG GT-3′; reverse 5′-GCT GTG TCC GGT TCT CCA AT-3′), transglutaminase (TGM; forward, 5′-CCC CCA TTC AAC CTG GTC TT-3′; reverse 5′-TAA GCA CTG CCC TGG TGA AG-3′), filaggrin (FLG; forward, 5′-AGC AAG TGG TCA GGG AGG ATA-3′; reverse 5′-TGC TGA AGA AAG GGC AGA TCC-3′), caspase-14 (CASP14; forward, 5′-CGT GTT TGC CAT AAG TCG GG-3′; reverse 5′-CTT GGA GAC AGA CAA GCC AGG-3′), and β-actin (forward, 5′-CAG CTC AGT AAC AGT CCG CC-3′; reverse 5′-TCA CTA TTG GCA ACG AGC GG-3′). Polymerase chain reaction (PCR) was performed with a Gene Amp PCR System 2700 (Applied Biosystems, Foster City, CA, USA). The products were separated by 1.5% agarose gel electrophoresis and detected with G-BOX EF imaging system (Syngene, Cambridge, UK) using loading star (Dyne Bio, Seoul, Korea). Relative band expression was measured by ImageJ (National Institute of Health, Bethesda, MD, USA).
Western blot analysis
Total homogenized dorsal skin tissues were analyzed by western blot according to previously described method [17]. The primary antibodies against MMP-3, MMP-13, catalase, NF-κB, p-ERK, t-ERK, p-JNK, t-JNK, p-p38, t-p38, p-c-Jun, t-c-Jun, c-Fos, TGF-β, Smad2/3, Smad7, LOR, INV, TGM, FLG, prostasin (PRSS), matriptase (MTSP), CASP14 (1:1000 dilution; Santa Cruz Biotechnology Inc.), and α-tubulin (1:1000 dilution, Cell Signaling, Beverly, MA, USA) were used for detection. Horseradish peroxidase-linked secondary antibodies (Bethyl Laboratories, Montgomery, TX, USA) were used for visualizing the blotted membrane and detected using ECL detection solution (GE Healthcare, Uppsala, Sweden) and the G:BOX Image Analysis System (Syngene).
Gelatin zymography assay
Activity of MMP-2 and MMP-9 was estimated by gelatin zymography assay. Gelatin zymography was carried out as previously described [18]. After staining, the gel was destained with 10% acetic acid and 30% methanol. MMP-2 (72 kDa) and MMP-9 (92 kDa) were detected by the G:BOX Image Analysis system (Syngene).
Hydroxyproline analysis
Hydroxyproline content in dorsal skin tissues was estimated by the chemical colorimetric method using a commercial hydroxyproline assay kit (Quickenzyme Bioscience, Leiden, Netherlands) according to the manufacturer’s instructions.
Morphological and histopathological observation
Histological changes in skin were observed with Visioline VL650 (CK Electronics GmbH, Cologne, Germany) and Replica (Epigem, Seoul, Korea). Before sacrifice, a caliper (Ozaki MFG Co., Ltd., Tokyo, Japan) was used for measuring skinfold thickness. Elasticity of the skin was determined using a Cutometer® MPA580 (CK Electronics GmbH). Fixed dorsal skins were stained with hematoxylin and eosin, Masson’s trichrome, and Verhoeff’s stain. The stained sections were visualized using an Eclipse TE2000U Inverted Microscope with twin CCD cameras (Nikon, Tokyo, Japan).
Evaluation of skin hydration and transepidermal water loss (TEWL)
Skin hydration was measured with a Corneometer® 825 (Khazaka Electronic, Köln, Germany), and TEWL as a marker of epidermal skin barrier function was measured by Tewameter® TM300 (Khazaka Electronic), which was mounted on a Multi Probe Adapter® MPA5 (CK Electronics GmbH).
Statistical analysis
Each experiment was performed in triplicate. Data are represented as the mean ± standard deviation (SD). Differences between groups were assessed by analysis of variance (ANOVA) followed by a Scheff’s test or Student’s t test using IBM SPSS Statistics 23 software (SPSS, Chicago, IL, USA). ## p < 0.01, *p < 0.05, and **p < 0.01 were considered statistically significant.
Results and discussion
The effects of BME on wrinkle formation, skinfold thickness, and elasticity
UVB-irradiated skin is characterized by symptoms such as wrinkle formation, erythema, skin dehydration, skin thickening, and elastosis [19]. Wrinkle formation, which is considered a noticeable feature of photoaging, results from the destruction of ECM components such as fibronectin, collagen, and gelatin [20]. This study evaluated whether BME protected against UVB-induced photoaging by assaying wrinkle formation, skinfold thickness, and elasticity in hairless mice. UVB irradiation induced coarse and deep wrinkle formation in the dorsal skin of hairless mice. In contrast, BME significantly downregulated UVB-induced wrinkle parameters, which showed decreases in wrinkle area, depth, length, and number by 59.6, 37.8, 35.5, and 28.2%, respectively, compared to the values obtained for the UVB-irradiated group [Fig. 1(A)]. UVB irradiation increases skinfold thickness by stimulating excessive proliferation of keratinocytes; this is known as hyperplasia and is a crucial factor in inducing wrinkle formation [21]. Elastosis (abnormal structure of elastic fibers) decreases elasticity of the skin and is closely related to the early processes of wrinkle formation [22]. In this study, compared to the UVB-irradiated group, the BME-treated group not only showed effective suppression of skin thickening, but also showed inhibition of accumulation of abnormal elastic fibers, thereby showing inhibition of a decrease in skin elasticity [Fig. 1(B), (C)]. Thus, these data demonstrate that BME suppresses the early process of wrinkle formation through inhibition of skin thickening and the abnormal structure of elastic fibers, indicating that BME has a preventive effect against photoaging symptoms.
Fig. 1.
Effect of BME on UVB-induced photoaging symptoms. (A) Replica photographs indicating wrinkle formation and wrinkle parameters were determined by skin replica analysis. (B) Measurement of skinfold thickness and hematoxylin and eosin staining of dorsal skin tissue section. (C) Gross elasticity (Ua/Uf) of skin and Verhoeff’s staining of elastic fiber in dermis. Data are represented as the mean ± SD (n = 5). ## p < 0.01 (Normal group vs. UVB-irradiated group); **p < 0.01 (UVB-irradiated group vs. BME-treated group)
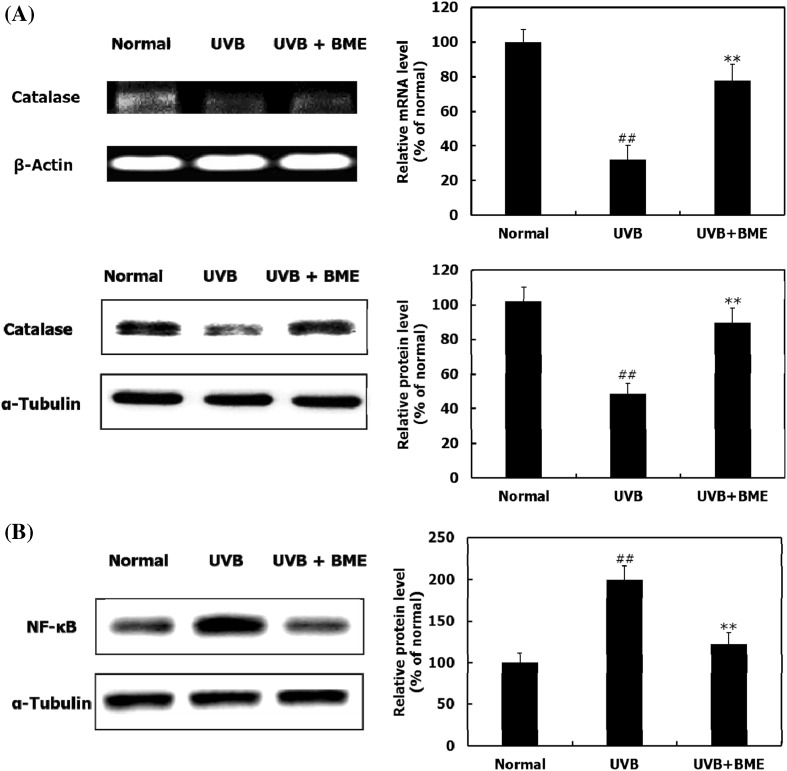
The effects of BME on catalase and NF-κB expression
Inflammation and oxidative stress are involved in UVB-induced skin photodamage [23]. Increased oxidative stress by UVB plays a central role in photoaging through activation of MAPKs and NF-κB, a main protein for inflammation [5, 6, 23]. Increasing the expression or activation of catalase, an anti-oxidant enzyme, is a major strategy to protect skin against photo-oxidative stress [22]. Activated NF-κB stimulates release of proinflammatory cytokines, including interleukin-6 and interleukin-8, which induce MMPs expression [24]. The mRNA and protein levels of catalase increased in the BME-treated group compared to those in the UVB-irradiated group, indicating that BME may contribute to reduction of oxidative stress [Fig. 2(A)]. BME treatment effectively reduced the expression of NF-κB in UVB-irradiated hairless mice [Fig. 2(B)]. Therefore, these results demonstrate that BME upregulates catalase and downregulates NF-κB expression, suggesting that BME suppresses wrinkle formation and skin thickening.
Fig. 2.
Effects of BME on catalase and NF-κB expression. (A) RT-PCR and western blot analysis indicating the expression of catalase at mRNA and protein levels. (B) Western blot analysis indicating the expression of NF-κB at protein level. Data are represented as the mean ± SD (n = 5). ## p < 0.01 (Normal group vs. UVB-irradiated group); **p < 0.01 (UVB-irradiated group vs. BME-treated group)
The effects of BME on collagen synthesis and the TGF-β/Smad signaling pathway
Collagen is a major constituent of dermis and plays as important role in wrinkle formation [25]. Type I collagen and type III collagen are located in the ECM and connective tissue and are the most abundant types of collagen. Type IV collagen is only found in basement membranes, and together with type VII collagen, plays a major role in dermal-epidermal adhesion [26]. In the present study, the mRNA expression levels of COL1A1, COL3A1, COL4A1, and COL7A1 were decreased in UVB-irradiated mice, while BME remarkably elevated the expression levels of these collagen synthesis genes in UVB-irradiated mice [Fig. 3(A)]. The upregulation of collagen synthesis gene expression leads to an increase in hydroxyproline content, which is considered an indicator of collagen fiber [27]. This study showed that hydroxyproline content was markedly increased by BME treatment [Fig. 3(B)].
Fig. 3.
Effects of BME on collagen content and synthesis. (A) RT-PCR analysis indicating the expression of COL1A1, COL3A1, COL4A1, and COL7A1 mRNA. (B) Amount of hydroxyproline and Masson’s trichrome stain of collagen fibers. (C) Western blot analysis indicating the expression of TGF-β/Smad signaling pathway at protein level. Data are represented as the mean ± SD (n = 5). ## p < 0.01 (Normal group vs. UVB-irradiated group); **p < 0.01 (UVB-irradiated group vs. BME-treated group)
TGF-β binds to its receptor, leading to activation of Smad2/3 and thereby stimulating procollagen type I synthesis. Smad7 acts as an antagonist of the TGF-β/Smad signaling pathway by inhibiting activation of Smad2/3 [8]. In this study, the protein expression levels of TGF-β and Smad2/3 were increased by BME treatment, while Smad7 was suppressed in dorsal skin tissue of BME-treated hairless mice [Fig. 3(C)]. These results suggest that the anti-oxidant effect of BME effectively increases UVB-induced downregulation of collagen synthesis genes and activates TGF-β/Smad signaling pathway, subsequently increasing collagen contents. However, whether BME directly mediates the interaction of TGF-β/Smad signaling pathway and collagen synthesis gene expression remains to be elucidated.
The effects of BME on MMPs expression and the MAPK/AP-1 signaling pathway
MMPs accelerate wrinkle formation in response to degradation of collagen, gelatin, and other ECM components [28]. In this study, UVB irradiation increased the activity of MMP-2 and MMP-9 in dorsal skin tissue of hairless mice, while BME treatment effectively decreased the activity of these MMPs, compared to that in the UVB-irradiated group [Fig. 4(A)]. Gelatinases (MMP-2 and MMP-9) digest type IV collagen (which plays an important role in epidermal-dermal adhesion) and gelatin [29]. Furthermore, MMP-3 digests a large number of ECM components and promotes other pro-MMPs activation [30]. In this study, BME dramatically reduced the mRNA and protein expression levels of MMP-3 and MMP-13 in dorsal skin tissue of UVB-irradiated hairless mice [Fig. 4(B), (C)], These results suggest that BME prevents destruction of skin structure through regulation of MMPs expression.
Fig. 4.
Effects of BME on UVB-induced MMPs activity and expression. (A) Gelatin zymography analysis indicating the activity of MMP-2 and MMP-9. (B) RT-PCR analysis indicating the mRNA expression levels of MMP-3 and MMP-13. (C) Western blot analysis indicating the protein expression of MMP-3 and MMP-13. Data are represented as the mean ± SD (n = 5). ## p < 0.01 (Normal group vs. UVB-irradiated group); **p < 0.01 (UVB-irradiated group vs. BME-treated group)
MMPs expression and secretion are regulated through mediation of the MAPKs/AP-1 complex signaling pathway [28]. Excessive oxidative stress induces activation of MAPKs signaling components, which leads to phosphorylation of JNK. The activated JNK stimulates protein expression of c-Fos and phosphorylation of c-Jun, leading to the formation of the AP-1 complex [31]. In the present study, UVB-induced phosphorylation of MAPK components such as ERK, JNK, and p-38 was inhibited by BME treatment in dorsal skin tissue of hairless mice [Fig. 5(A)]. BME also downregulated protein level of the AP-1 complex by suppressing JNK phosphorylation [Fig. 5(B)]. Thus, the anti-oxidant effect of BME effectively downregulates UVB-induced MMPs expression via inactivating the MAPKs/AP-1 signaling pathway. Further study is needed to elucidate whether BME directly disturbs the interaction of MAPK/AP-1 and MMP expression.
Fig. 5.
Effect of BME on UVB-induced MAPKs/AP-1 complex signaling pathway. Western blot analysis indicating the expression of (A) MAPKs and (B) AP-1 complex at protein levels. Data are represented as the mean ± SD (n = 5). ## p < 0.01 (Normal group vs. UVB-irradiated group); **p < 0.01 (UVB-irradiated group vs. BME-treated group)
The effect of BME on CE formation
Terminally-differentiated keratinocytes are known as corneocytes, which are responsible for CE formation and NMF production [13]. The CE is formed by the cross-linkage of various proteins, such as loricrin and involucrin. Loricrin is the main component of the CE (80% of the protein mass) and involucrin plays a role early in the formation of the CE [32]. In this study, BME treatment significantly increased the expression levels of loricrin and involucrin in UVB-irradiated hairless mice [Fig. 6(A)]. Transglutaminase is involved in CE formation through stimulation of cross-linkage between loricrin and involucrin [33]. This study showed that the protein expression level of transglutaminase was downregulated in the UVB-irradiated group, while BME upregulated the expression of this protein [Fig. 6(A)]. The TEWL value (which indicates water loss in epidermis) decreased by 29.0% through stimulation of the CE formation [Fig. 6(B)]. Thus, these data suggest that BME is effective in alleviating skin dehydration in response to enhancement of CE formation.
Fig. 6.
Effects of BME on CE formation and filaggrin processing. (A) The mRNA and protein expression of loricrin, involucrin, and transglutaminase. (B) TEWL as a marker of skin dehydration in UVB-irradiated dorsal skin. (C) The mRNA and protein expression of filaggrin and caspase-14. (D) The protein levels of matriptase and prostasin. (E) Graph indicating skin hydration in UVB-irradiated dorsal skin. Data are represented as the mean ± SD (n = 5). ## p < 0.01 (Normal group vs. UVB-irradiated group); *p < 0.05, **p < 0.01 (UVB-irradiated group vs. BME-treated group)
The effects of BME on filaggrin formation and degradation
The decomposition of filaggrin by caspase-14 produces NMF, a crucial factor for moisturization [34]. In the present study, BME treatment stimulated the expression of filaggrin and caspase-14 in UVB-irradiated hairless mice [Fig. 6(C)]. Profilaggrin is degraded into filaggrin by profilaggrin degradation enzymes such as matriptase and prostasin [35]. The protein expression levels of matriptase and prostasin were enhanced by BME treatment in UVB-induced hairless mice [Fig. 6(D)]. In addition, the chronically UVB-irradiated dorsal skin of hairless mice showed a 60% decrease in skin hydration level compared to that of the normal group, while BME increased skin hydration level by 30.5%, compared to that in the UVB-irradiated group [Fig. 6(E)]. These data demonstrate that BME stimulates filaggrin formation by upregulating profilaggrin degradation enzymes and enhances NMF by stimulating caspase-14, suggesting that BME enhances the water retention of the skin. Previous studies have revealed that the activation of Smad2/3 or the inhibition of ERK and JNK, members of MAPKs, stimulated keratinocyte differentiation required for CE formation and skin hydration [36, 37]. The activated TGF-β/Smad signaling, inactivated MAPK/AP-1 signaling, or the combination of both signaling pathways were also involved in the moisturizing effect of BME in UVB-induced skin in mice.
Taken together, this study is the first to demonstrate that BME prevents wrinkle formation in UVB-irradiated hairless mice by downregulating the MAPKs/AP-1 complex signaling pathway, which regulates MMPs expression. BME increased the mRNA expression of collagen and stimulated the TGF-β/Smad signaling pathway in response to regulation of the AP-1 complex, leading to an increase in collagen content in dermis. Moreover, BME moisturizes skin by stimulating CE formation and filaggrin processing. Thus, Bouea macrophylla may be a potential anti-photoaging and moisturizing ingredient in skin functional foods. Further clinical research is required to determine whether BME is clinically effective as a natural anti-photoaging and moisturizing supplement.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, Ahmad H, Uitto J. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Experi. Dermatol. 11: 398–405 (2002). [DOI] [PubMed]
- 2.Kuhn A, Zahn S, Patsinakidis N, Landmann A, Graef M, Sauerland C, Surber C, Wenzel J. Resistance to water and abrasion of a broad-spectrum sunscreen: a prospective, open-label study. Experi. Dermatol. 2016;25:151–152. doi: 10.1111/exd.12869. [DOI] [PubMed] [Google Scholar]
- 3.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 4.Passeron T, Ortonne J. Skin ageing and its prevention. Press. Medica. 2003;32:1474–1482. [PubMed] [Google Scholar]
- 5.Heng MC. Signaling pathways targeted by curcumin in acute and chronic injury: burns and photo-damaged skin. Int. J. Dermatol. 2013;52:531–543. doi: 10.1111/j.1365-4632.2012.05703.x. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Guo JH, Tu XL, Zhang C, Zhao M, Zhang QW, Gao FH. Tiron inhibits UVB-Induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PloS ONE. 2016;11:e0159998. doi: 10.1371/journal.pone.0159998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson RE, Gibbs NK, Griffiths CE, Sherratt MJ. Damage to skin extracellular matrix induced by UV exposure. Antioxid. Redox. Signal. 2014;21:1063–1077. doi: 10.1089/ars.2013.5653. [DOI] [PubMed] [Google Scholar]
- 8.Chen B, Li R, Yan N, Chen G, Qian W, Jiang HL, Ji C, Bi ZG. Astragaloside IV controls collagen reduction in photoaging skin by improving transforming growth factor-β/Smad signaling suppression and inhibiting matrix metalloproteinase-1. Mol. Med. Rep. 2015;11:3344–3348. doi: 10.3892/mmr.2015.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-β type II receptor/Smad signaling. Am. J. Pathol. 2004;165:741–751. doi: 10.1016/S0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 11.Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh Y, Lim H-W, Kim K, Lim C-J. Ginsenoside Re improves skin barrier function in HaCaT keratinocytes under normal growth conditions. Biosci. Biotehcnol. Biochem. 2016;80:2165–2167. doi: 10.1080/09168451.2016.1206808. [DOI] [PubMed] [Google Scholar]
- 14.Rinnerthaler M, Duschl J, Steinbacher P, Salzmann M, Bischof J, Schuller M, Wimmer H, Peer T, Bauer JW, Richter K. Age-related changes in the composition of the cornified envelope in human skin. Experi. Dermatol. 2013;22:329–335. doi: 10.1111/exd.12135. [DOI] [PubMed] [Google Scholar]
- 15.Rajan NS, Bhat R. Antioxidant compounds and antioxidant activities in unripe and ripe kundang fruits (Bouea macrophylla Griffith) Fruits. 2016;71:41–47. doi: 10.1051/fruits/2015046. [DOI] [Google Scholar]
- 16.Heck DE, Vetrano AM, Mariano TM, Laskin JD. UVB light stimulates production of reactive oxygen species unexpected role for catalase. J. Biol. Chem. 2003;278:22432–22436. doi: 10.1074/jbc.C300048200. [DOI] [PubMed] [Google Scholar]
- 17.Park JE, Pyun HB, Woo SW, Jeong JH, Hwang JK. The protective effect of Kaempferia parviflora extract on UVB-induced skin photoaging in hairless mice. Photodermatol. Photoimmunol Photomed. 2014;30:237–245. doi: 10.1111/phpp.12097. [DOI] [PubMed] [Google Scholar]
- 18.Anggakusuma Yanti. Hwang JK. Effects of macelignan isolated from Myristica fragrans Houtt. on UVB-induced matrix metalloproteinase-9 and cyclooxygenase-2 in HaCaT cells. J. Dermatol. Sci. 2010;57:114–122. doi: 10.1016/j.jdermsci.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Draelos ZD. The latest cosmeceutical approaches for anti-aging. J. Cosmet. Dermatol. 2007;6:2–6. doi: 10.1111/j.1473-2165.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim YJ, Kim HN, Shin MS, Choi BT. Thread embedding acupuncture inhibits ultraviolet B irradiation-induced skin photoaging in hairless mice. Evid. Based Complement. Alternat. Med. 2015 (2015). [DOI] [PMC free article] [PubMed]
- 21.Shirakata Y. Regulation of epidermal keratinocytes by growth factors. J. Dermatol. Sci. 2010;59:73–80. doi: 10.1016/j.jdermsci.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Kim HK. Garlic supplementation ameliorates UV-induced photoaging in hairless mice by regulating antioxidative activity and MMPs expression. Molecules. 2016;21:70. doi: 10.3390/molecules21010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan JYX, Wang XF, Liu YH, Zhang ZB, Wang L, Chen JN, Huang S, Zeng HF, Lai XP. Andrographolide sodium bisulfate prevents UV-induced skin photoaging through inhibiting oxidative stress and inflammation. Mediators Inflamm. 2016;2016:1–12. doi: 10.1155/2016/3271451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation–a review. Int. J. Cosmet. Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka YT, Tanaka K, Kojima H, Hamada T, Masutani T, Tsuboi M, Akao Y. Cynaropicrin from Cynara scolymus L. suppresses photoaging of skin by inhibiting the transcription activity of nuclear factor-kappa B. Bioorg. Med. Chem. Lett. 2013;23:518–523. doi: 10.1016/j.bmcl.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 26.Amano S, Ogura Y, Akutsu N, Matsunaga Y, Kadoya K, Adachi E, Nishiyama T. Protective effect of matrix metalloproteinase inhibitors against epidermal basement membrane damage: skin equivalents partially mimic photoageing process. Br. J. Dermatol. 2005;153:37–46. doi: 10.1111/j.1365-2133.2005.06968.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen T, Hou H, Lu J, Zhang K, Li B. Protective effect of gelatin and gelatin hydrolysate from salmon skin on UV irradiation-induced photoaging of mice skin. J. Ocean U. China. 2016;15:1–8. doi: 10.1007/s11802-016-2722-5. [DOI] [Google Scholar]
- 28.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016;17:868. doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MS, Oh GH, Kim MJ, Hwang JK. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 2013;89:911–918. doi: 10.1111/php.12061. [DOI] [PubMed] [Google Scholar]
- 32.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 33.Yamane M, Sugimura K, Kawasaki H, Tatsukawa H, Hitomi K. Analysis on transglutaminase 1 and its substrates using specific substrate peptide in cultured keratinocytes. Biochem. Biophys. Res. Commun. 2016;478:343–348. doi: 10.1016/j.bbrc.2016.07.051. [DOI] [PubMed] [Google Scholar]
- 34.Kim H, Lim YJ, Park JH, Cho Y. Dietary silk protein, sericin, improves epidermal hydration with increased levels of filaggrins and free amino acids in NC/Nga mice. Br. J. Nutr. 2012;108:1726–1735. doi: 10.1017/S0007114511007306. [DOI] [PubMed] [Google Scholar]
- 35.Lai CH, Chang SC, Chen YJ, Wang YJ, Lai YJ, Chang HD, Berens EB, Johnson MD, Wang JK, Lin CY. Matriptase and prostasin are expressed in human skin in an inverse trend over the course of differentiation and are targeted to different regions of the plasma membrane. Biol. Open. 2016;5:1380–1387. doi: 10.1242/bio.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Descargues P, Sil AK, Sano Y, Korchynskyi O, Han G, Owens P, Wang XJ, Karin M. IKKα is a critical coregulator of a Smad4-independent TGFβ-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proc. Natl. Acad. Sci. 2008;105:2487–2492. doi: 10.1073/pnas.0712044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu N, Sulpice E, Obeid P, Benzina S, Kermarrec F, Combe S, Gidrol X. The miR-17 family links p63 protein to MAPK signaling to promote the onset of human keratinocyte differentiation. PloS ONE. 2012;7:e45761. doi: 10.1371/journal.pone.0045761. [DOI] [PMC free article] [PubMed] [Google Scholar]