ABSTRACT
Four phototropin genes (PHOTA1, PHOTA2, PHOTB1, PHOTB2) have been isolated in the moss Physcomitrella patens. These genes encode phototropins that mediate blue-light–induced chloroplast movement. However, the individual functions of these phototropins, including the function of mediating blue-light–induced phototropism, remain unclear. To elucidate the individual functions of P. patens phototropins, each of these phototropin genes was expressed in a phototropin-deficient mutant of Arabidopsis (phot1-5 phot2-1). In addition, fluorescence of GFP fused to these phototropins was examined to determine the subcellular localization of each phototropin. Our results demonstrate that all four P. patens phototropins mediate blue-light–induced phototropism and are associated with the plasma membrane in Arabidopsis.
Abbreviations GFP: green fluorescent protein; Pp_phot: Physcomitrella patens phototropin
KEYWORDS: Phototropism, phototropin, moss, Physcomitrella, blue light, red light, plasma membrane
Introduction
Plants perceive environmental light signals and respond with a variety of physiological changes. Among the most well-known blue-light responses in plants is phototropism, which was described in 1881 as a ‘movement’ of plants by Charles Darwin and his son.1 Over a century later, the phototropin gene has been identified as a blue-light receptor for phototropism from studies of phototropism-deficient mutants of Arabidopsis.2 This gene encodes a polypeptide composed of a Ser/Thr protein kinase domain and tandemly repeated LOV (Light, Oxygen, Voltage) domains to which a flavin mononucleotide attaches as a chromophore.3 Two phototropin genes (PHOT1 and PHOT2) are present in the Arabidopsis genome and subsequent studies have further demonstrated that phototropins are associated with the plasma membrane4,5 and regulate not only phototropism but also blue-light–dependent chloroplast movement.6 These phototropin-mediated responses are thought to improve light absorption by the plant.7
Four phototropin genes (PHOTA1, PHOTA2, PHOTB1, PHOTB2) were isolated in the moss P. patens based on sequence similarity with Arabidopsis phototropin genes.8 So far, at least 7 mRNA sequences of phototropin, including these 4 phototropin genes, have been deposited in public databases, and another 4 phototropin-like gene loci have been annotated on the P. patens genome. Studies of combinations of PHOT gene-targeted disruption indicate that the 4 earliest identified phototropins are involved in blue-light–induced chloroplast movement.8 Phototropic response in P. patens is well documented.9,10 Phototropism of P. patens is clearly induced by red light and is mediated by the red/far-red light receptor phytochrome.11,12 Although phototropin genes have been found in this moss, the blue-light–dependent phototropic response is somewhat complicated. In the protonemal stage of P. patens, two distinct cell types (chloronemata and caulonemata) are present. Jenkins and Cove have noted that primary chloronemata growing from germinated spores have sensitivities for blue-light–dependent phototropic responses;10 however, some lines of evidence have indicated that blue light does not induce any tropic response in dark-adapted caulonemata.13,14 Recently, Bao et al. demonstrated a very weak phototropic bending toward incident blue light in dark-adapted etiolated gametophytic shoots.14 This curvature was very small and required irradiation at a high fluence rate for up to 48 h. Accordingly, blue-light–dependent phototropism has been reported in certain types of cells and organs of P. patens; however, it is still unknown whether phototropins of P. patens function as blue-light receptors mediating this tropic response. Since phototropins are encoded by a multigene family in P. patens, it is not easy to identify each phototropin gene function independently by approaching loss-of-function of these genes. In this report, we transferred individual phototropin genes from P. patens (PHOTA1, PHOTA2, PHOTB1, PHOTB2) into the phototropin-deficient mutant of Arabidopsis (phot1-5 phot2-1) to examine the phototropic function of each gene. The subcellular localization site of each phototropin in transgenic Arabidopsis was also analyzed using fluorescence from phototropin-GFP fusions. Our results provide insight into the relationship between blue-light–dependent phototropism and phototropins in P. patens, which remains unclear in cryptogams and imply that there is evolutionary conservation in the signaling mechanism for phototropism in plants.
Results
To study P. patens phototropin (Pp_phot) function, we isolated transgenic Arabidopsis lines transformed with gene transfer vectors carrying 4 phototropin genes (PHOTA1, PHOTA2, PHOTB1, PHOTB2). Each Pp_phot gene was translationally fused to the GFP gene at their 3ʹ end to form Pp_phot:GFP (Figure 1A). To confirm whether the transgenic lines expressed the Pp_phot:GFP polypeptides, crude protein extracts were prepared from 3-day-old dark-grown seedlings of T2 lines. The predicted molecular mass of Pp_photA1:GFP, Pp_photA2:GFP, Pp_photB1:GFP and Pp_photB2:GFP were 135 kDa, 138 kDa, 141 kDa and 146 kDa, respectively. Immunoblot analysis using the anti-GFP monoclonal antibody clearly showed that each transgenic line contained the Pp_phot:GFP as an expected polypeptide size (Figure 1B). Among these polypeptides, Pp_photA1:GFP and Pp_photB2:GFP exhibited electrophoretic mobility retardation depending on the light irradiation (Supplemental Figure 1).
Figure 1.
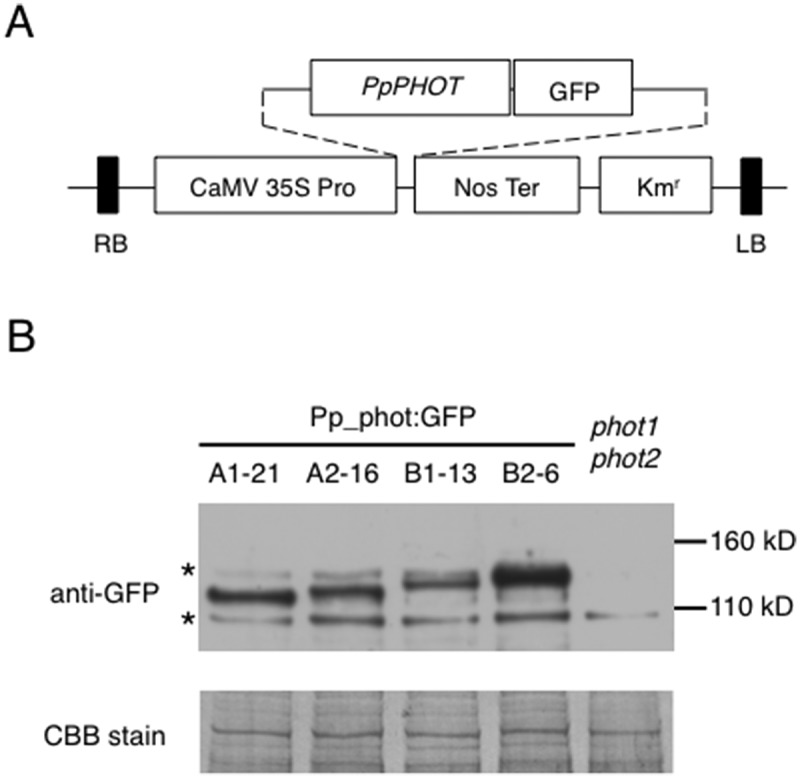
Expression of Pp_phot:GFP in transgenic Arabidopsis (a) Schematic illustration of the binary vector construct used to make transgenic Arabidopsis expressing Pp_phot:GFP fusion protein. CaMV 35S Pro: cauliflower mosaic virus 35S promoter; Nos Ter: nopaline synthase terminator; Kmr: kanamycin resistant gene cassette(b) Immunoblot analysis of Pp_phot:GFP protein. Crude protein extract (30 μg) prepared from dark-grown transgenic Arabidopsis seedlings expressing Pp_phot:GFP (photA1:GFP-21, photA2:GFP-16, photB1:GFP-13, or photB2:GFP-6) was probed with the anti-GFP antibody. An asterisk on the left indicates a nonspecific band that had cross-reacted with GFP antibody. After chemiluminescence detection, the polyvinylidene difluoride (PVDF) membrane was stained with Coomassie brilliant blue (CBB) to visualize the amount of protein loaded in each lane (CBB stain).
To examine the blue-light–dependent phototropic response of the transgenic seedlings, the 3-day-old dark-grown etiolated seedlings were laterally irradiated with 5 μmol m−2 s−1 blue light for 16 hr. Figure 2 shows seedling images and percentage distribution of hypocotyl curvature degrees measured after blue-light irradiation. In contrast to the phot1phot2 transgenic background, a proportion of T2 seedlings in all 4 Pp_phot:GFP expressing lines clearly showed a positive phototropic response to blue light (Figure 2). As these T2 lines were heterozygous for the transferred gene, a clear segregation of bending seedlings was observed. On the other hand, no obvious phototropic response was detected in any of the tested lines (including 4 Pp_phot:GFP transgenic plants) when they were laterally irradiated with 5 μmol m−2 s−1 red light (Supplemental Figure 2).
Figure 2.
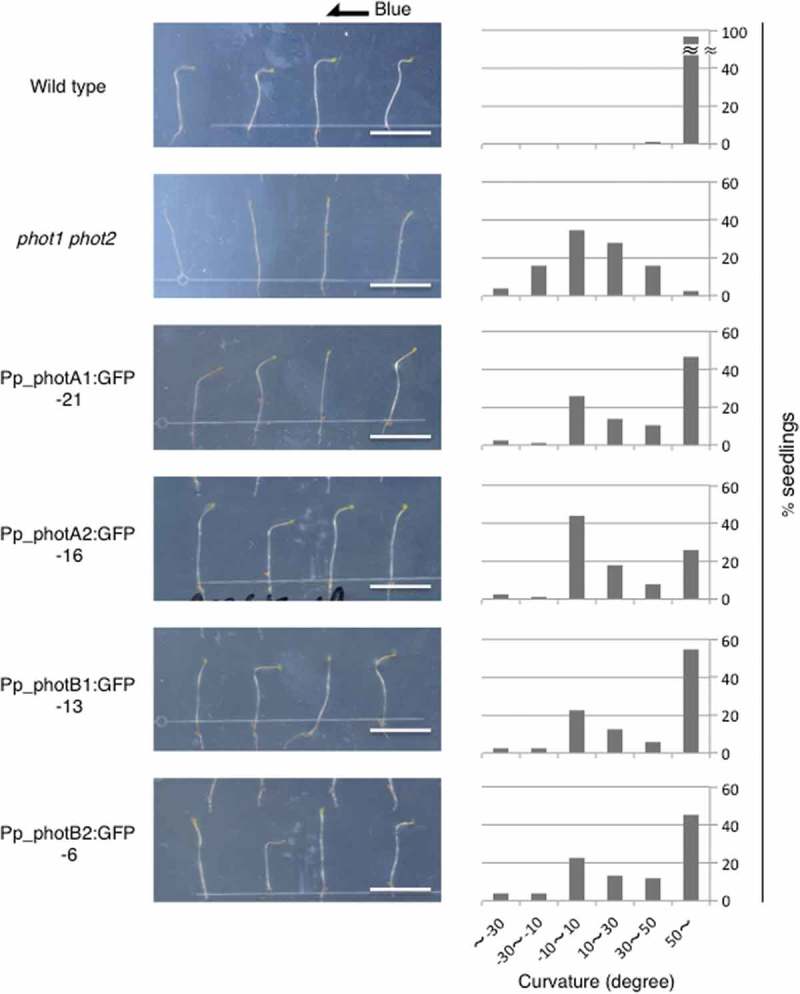
Phototropic responses of transgenic Arabidopsis seedlings Wild-type, phot1-5 phot2-1, and transgenic Arabidopsis lines expressing Pp_phot:GFP were grown in the dark for 3 days, and irradiated by unilateral blue light (5 μmol m−2 s−1) for 16 hr before imaging. All of the transgenic lines were heterozygous for the transferred gene. Scale bars, 1 cm. (left). The percentage of seedlings with hypocotyl angles relative to vertical is shown in 6 classes: less than −30, −30 ~ −10, −10 ~ 10, 10 ~ 30, 30 ~ 50, and more than 50°. A positive value indicates the degree of curvature which shows bending toward the light source. Hypocotyl curvatures were measured in at least 74 seedlings (right).
It has been demonstrated that Arabidopsis phot1 and phot2 are associated with the plasma membrane under dark conditions.4,5 This membrane association is thought to have a close correlation with blue-light–induced hypocotyl phototropism. To localize P. patens phototropins in transgenic Arabidopsis, fluorescence derived from GFP tagged to Pp_phot was observed in 3-day-old dark-grown seedlings. Figure 3 clearly shows that GFP fluorescence was detected on the periphery of epidermal cells of all 4 Pp_phot:GFP transgenic lines. The merged images demonstrated the close correspondence between the distribution of GFP and FM 4–64 fluorescence, indicating that P. patens phototropins were associated with the plasma membrane of Arabidopsis cells. In the microsomal membrane fraction of these transgenic lines, Pp_phot:GFP polypeptide was clearly detected by western blot analysis (Supplemental Figure 3). This result further supports the membrane association of Pp_phot.
Figure 3.

Subcellular localization of the Pp_phot:GFP in transgenic Arabidopsis seedlings A single optical section of Pp_phot:GFP expressing transgenic Arabidopsis, which was stained with the FM 4–64, was captured by confocal imaging. Upper hypocotyl epidermal cells of the 3-day-old dark-grown seedlings were observed. Scale bars, 20 µm.
The region corresponding to the membrane association of phototropin has been studied in Arabidopsis phototropins. It has been reported that the short amino-acid stretches in the C-terminus of Arabidopsis phototropins (phot1 and phot2) are important for their membrane association.15 Figure 4 shows the alignment of multiple amino-acid sequences in the C-terminal region of phototropins. The amino-acid residues within the corresponding regions of the Arabidopsis phototropin stretches are highly conserved among all phototropins, including those of the moss P. patens and the liverwort Marchantia polymorpha.
Figure 4.

Multiple alignment of the amino-acid sequences of the C-terminal region in phototropins Residues conserved in all sequences are marked with an asterisk and highly or moderately similar residues are marked with dots or a dot, respectively. The short amino-acid stretches that were suggested to be important for phototropin’s membrane association in Arabidopsis are enclosed in red boxes. Within the stretches region, conserved or similar amino acid residues are highlighted with blue or green background, respectively. Pp (Physcomitrella patens); Mp (Marchantia polymorpha); At (Arabidopsis thaliana).
Discussion
Phototropin genes exist in a broad range of plants from algae to seed plants.16 We have demonstrated here that these 4 Pp_phots induce blue-light–dependent hypocotyl bending when expressed heterologously in Arabidopsis phot1phot2 seedlings. A light-dependent electrophoretic mobility shift of Pp_photA1: GFP and Pp_photB2: GFP was observed, suggesting the possibility that these two Pp_phot may be autophosphorylated by light irradiation like Arabidopsis phototropins. NONPHOTOTROPIC HYPOCOTYL 3 (NPH3) is one of the early signaling components in phototropism, and molecular phylogenetic analysis indicates several Physcomitrella proteins exist in NPH3 clade.17 These findings imply that the basic mechanism of phototropin action is highly conserved in land plants. On the other hand, phototropin of the green alga Chlamydomonas reinhardtii rescued the phototropin-mediated blue light responses such as phototropism, chloroplast photorelocation movement, and stomatal opening in Arabidopsis phot1 phot2 mutant.18 Phototropin from the marine picoalga Ostreococcus tauri (Otphot) also restored chloroplast-accumulation response and stomatal opening when expressed in the phot1 phot2 mutant of Arabidopsis, however, it did not rescue phototropism and chloroplast-avoidance response.19 This loss of the ability of Otphot to restore phototropism may correlate with the inability of Otphot to complex with Arabidopsis NPH3.19 Thus, these results imply that the mode of phototropin action should be closely related to interaction with NPH3.
These Pp_phots were associated with the plasma membrane in the transgenic Arabidopsis cells, and this intracellular localization pattern was quite similar to that of the Arabidopsis phototropins. The short amino-acid stretches in the C-terminal region of phototropins, which are thought to be important for membrane association of Arabidopsis phototropins,15 are retained in P. patens phototropins. This primary sequence structure suggests that Pp_phots could be anchored to the Arabidopsis plasma membrane using the membrane association system for Arabidopsis phototropins. Transient expression of CFP:Pp_phot in P. patens has shown that Pp_phots are associated with the plasma membrane in filament cells.13 A similar amino-acid stretch is found in the C-terminal region of the liverwort M. polymorpha phototropin, which is localized in the plasma membrane.20 These findings suggest that the membrane-association system of phototropins is widely conserved among evolutionarily divergent plant species.
Taken together, these results indicate that the Pp_phots were able to transmit blue-light signals into the signal transduction pathway for phototropism in Arabidopsis and hence imply that each of these 4 Pp_phots may play a role as a photoreceptor mediating blue-light–dependent phototropic responses.
A red-light–dependent phototropic response mediated by phytochromes is clearly observed in P. patens.9-12 In lines with disrupted phototropin genes, the degrees of red-light–induced curvature are significantly smaller than that in the wild type.13 This result indicates that Pp_phots are partially involved in red-light–dependent phototropism in P. patens. In addition, it has also been shown that Pp_phots physically interact with P. patens phytochrome 4 both in yeast cells and in P. patens filament cells.13 This evidence suggests that P. patens phototropins could possibly integrate the red-light signal received by phytochromes into the phototropin-mediated signaling pathway of the phototropic response. Since red-light–dependent phototropic response is not observed in Arabidopsis seedlings, we investigated whether Pp_phot-expressing Arabidopsis would show hypocotyl bending toward laterally irradiated red light or not. None of the tested transgenic lines, which exhibited blue-light–dependent phototropism, showed red-light–induced hypocotyl curvature. This result suggests that, unlike the case of P. patens, Pp_phots could not transmit red-light signal perceived by Arabidopsis phytochromes into the endogenous signaling pathway for phototropism. Red-light–dependent phototropism is also known in some ferns. In the fern Adiantum capillus-veneris, this red-light response is mediated by an atypical photoreceptor phytochrome 3 (phy3)/neochrome, which is composed of a full-length phototropin with the photosensory domain of phytochromes.21,22 In contrast to Pp_phots, phy3 confers a red-light–induced phototropic response to Arabidopsis phot1phot2 seedlings,23,24 suggesting that Physcomitrella and Adiantum control this red-light response in different ways. Thus, phototropin-mediated red-light–dependent phototropism may have been established during the evolution of Physcomitrella.
As previously mentioned, etiolated gametophytic shoots of P. patens show weak but noticeable blue-light–dependent phototropic responses.14 If we assume that these shoots are structurally comparable to Arabidopsis hypocotyls, it is probable that blue-light–dependent phototropism in P. patens gametophores is controlled by a similar mechanism of phototropism as in Arabidopsis. By contrast, the blue-light–dependent phototropic response was hardly observed in dark-adapted protonemal cells. These filamentous cells form one cylindrical organ, thus their tropic response might be regulated through a mechanism different from that of multicellular organs such as gametophores. In the filamentous growth of this moss, blue light induces side branch formation. Subsequently, filamentous organs show spatial spreading of colonized areas and possibly expand the light-receivable portion of the colonies. Pp_phots are involved in this response and contribute to the determination of branch position.25
In this report, we demonstrated that P. patens phototropins act as a blue-light receptor for phototropism in Arabidopsis, suggesting the possible involvement of Pp_phots in blue-light-dependent phototropism in P. patens. Pp_phots are also involved in chloroplast photorelocation movement and side branch formation. Because these responses are thought to improve the efficiency of light capture for photosynthesis, we speculate that these unique phototropin-mediated photomorphogenic responses may have influenced the evolutionary adaptions of P. patens to its environmental light conditions.
Materials and methods
Plant materials
The Arabidopsis thaliana phot1-5 phot2-1 double mutant line26 was used as a transformation host plant.
Plasmid vector construction and plant transformation
smRS-GFP27 carrying the Ala206Lys mutation28 was used as the GFP. To express the Pp_phot:GFP fusion protein in Arabidopsis, the full-length Pp_phot cDNA sequence fused to the GFP gene was inserted between the cauliflower mosaic virus (CaMV) 35S promoter and the nopaline synthase (Nos) terminator of pPZP211 binary vector.29 Transformation of Arabidopsis was performed as previously described,24 using Agrobacterium tumefaciens GV3101 (pMP90)30 carrying the plasmid described above. Transgenic plants were selected based on T-DNA-linked drug resistance on 1⁄2 Murashige-Skoog medium containing 50 μg ml−1 kanamycin. The experiments were performed using heterozygous T2 lines and the results from the Pp_photA1:GFP-21, Pp_photA2:GFP-16, Pp_photB1:GFP-13 and Pp_photB2:GFP-6 lines were considered to be representative.
Protein preparation and immunoblot analysis
Protein extraction and immunoblot analysis of the transgenic Arabidopsis seedlings were performed as previously described.24 Microsomal membrane fraction was prepared as described previously,21 except that the resuspension buffer contained 0.5% (v/v) protease inhibitor cocktail (P9599, Sigma-Aldrich). The primary antibody used to probe the blotted membrane was either the anti-GFP monoclonal antibody (632375, Clontech) or the anti-H+-ATPase polyclonal antibody (AS07260, Agrisera), and the enhanced-chemiluminescence (ECL) method was performed using ECL Select Western Blotting Detection Reagents (Amersham).
Measurement of phototropic response
Hypocotyl curvature was measured as previously described21.
Microscopic observation
Transgenic Arabidopsis seedlings grown in darkness for 3 days were observed using a LSM710 (Carl Zeiss) confocal laser scanning microscope. FM 4–64 dye (Molecular Probes) was applied to stain the plasma membrane at a final concentration of 32.9 μM. The fluorescence emission ranges used for the image acquisition were 493–598 nm for GFP and 647–721 nm for FM4-64. Each single optical section was scanned an average of 4 times and acquired images were analyzed using ImageJ (https://imagej.nih.gov/ij).
Computational analysis
The ClustalW alignment method31 was used for the alignment of multiple amino-acid sequences. The accession numbers of the sequences used in the alignment were as follows: Pp_photA1, P. patens photA1 (AB163420); Pp_photA2, P. patens photA2 (AB163421); Pp_photB1, P. patens photB1 (AB163422); Pp_photB2, P. patens photB2 (AB163423); At_phot1, Arabidopsis thaliana phot1 (AF030864); At_phot2, A. thaliana phot2 (AF053941); Mp_phot, Marchantia polymorpha phot (AB938188).
Acknowledgments
We thank A. Kadota for helpful discussions and technical advice for the microscopic observations, and R.D. Vierstra and Arabidopsis Biological Resource Center for the psmRS-GFP plasmid.
Disclosure of interest
The authors report no conflicts of interest.
Supplementary materials
Supplemental data for this Article can be acessed here
References
- 1.Darwin C, Darwin F.. The power of movement in plants. New York: D. Appleton; 1881. [Google Scholar]
- 2.Huala E, Teller PW, Liscum E, Han I-S, Larsen E, Briggs WR.. Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science. 1997;278(5346):2120–2123. [DOI] [PubMed] [Google Scholar]
- 3.Christie JM, Salomon M, Nozue K, Wada M, Briggs WR. LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc Natl Acad Sci USA. 1999;96(15):8779–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakamoto K, Briggs WR. Cellular and subcellular localization of phototropin 1. Plant Cell. 2002;14(8):1723–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong S-G, Suzuki T, Tamura K, Mochizuki N, Hara-Nishimura I, Nagatani A. Blue light-induced association of phototropin 2 with the Golgi apparatus. Plant J. 2006;45(6):994–1005. doi: 10.1111/j.1365-313X.2006.02667.x. [DOI] [PubMed] [Google Scholar]
- 6.Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA. 2001;98(12):6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takemiya A, Inoue S, Doi M, Kinoshita T, Shimazaki K. Phototropins promote plant growth in response to blue light in low light environments. Plant Cell. 2005;17(4):1120–1127. doi: 10.1105/tpc.104.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasahara M, Kagawa T, Sato Y, Kiyosue T, Wada M. Phototropins mediate blue and red light-induced chloroplast movements in Physcomitrella patens. Plant Physiol. 2004;135(3):1388–1397. doi: 10.1104/pp.104.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cove DJ, Schild A, Ashton NW, Hartmann E. Genetic and physiological studies of the effect of light on the development of the moss, Physcomitrella patens. Photochem Photobiol. 1978;27:249–254. doi: 10.1111/php.1978.27.issue-2. [DOI] [Google Scholar]
- 10.Jenkins GI, Cove DJ. Phototropism and polarotropism of primary chloronemata of the moss Physcomitrella patens: responses of the wild-type. Planta. 1983;158:357–364. doi: 10.1007/BF00397338. [DOI] [PubMed] [Google Scholar]
- 11.Mittmann F, Brücker G, Zeidler M, Repp A, Abts T, Hartmann E, Hughes J. Targeted knockout in Physcomitrella reveals direct actions of phytochrome in the cytoplasm. Proc Natl Acad Sci USA. 2004;101(38):13939–13944. doi: 10.1073/pnas.0403140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins GI, Cove DJ. Phototropism and polarotropism of primary chloronemata of the moss Physcomitrella patens: responses of mutant strains. Planta. 1983;159:432–438. doi: 10.1007/BF00392079. [DOI] [PubMed] [Google Scholar]
- 13.Jaedicke K, Lichtenthäler AL, Meyberg R, Zeidler M, Hughes J. A phytochrome-phototropin light signaling complex at the plasma membrane. Proc Natl Acad Sci USA. 2012;109(30):12231–12236. doi: 10.1073/pnas.1120203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao L, Yamamoto KT, Fujita T. Phototropism in gametophytic shoots of the moss Physcomitrella patens. Plant Signal Behav. 2015;10(3):e1010900–7. doi: 10.1080/15592324.2015.1010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong S-G, Kagawa T, Wada M, Nagatani A. C-terminal membrane association domain of photoropin 2 is necessary for chloroplast movement. Plant Cell Physiol. 2013;54(1):57–68. doi: 10.1093/pcp/pcs132. [DOI] [PubMed] [Google Scholar]
- 16.Li F-W, Villarreal JC, Kelly S, Rothfels CJ, Melkonian M, Frangedakis E, Ruhsam M, Sigel EM, Der JP, Pittermann J et al. Horizontal transfer of an adaptive chimeric photoreceptor from bryophytes to ferns. Proc Natl Acad Sci USA. 2014;111(18):6672–6677. doi: 10.1073/pnas.1319929111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suetsugu N, Takemiya A, Kong S-G, Higa T, Komatsu A, Shimazaki K, Kohchi T, Wada M. RPT2/NCH1 subfamily of NPH3-like proteins is essential for the chloroplast accumulation response in land plants. Proc Natl Acad Sci USA. 2016;113(37):10424–10429. doi: 10.1073/pnas.1602151113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onodera A, Kong S-G, Doi M, Shimazaki K, Christie J, Mochizuki N, Nagatani A. Phototropin from Chlamydomonas reinhardtii is functional in Arabidopsis thaliana. Plant Cell Physiol. 2005;46(2):367–374. doi: 10.1093/pcp/pci037. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan S, Petersen J, Blackwood L, Papanatsiou M, Christie JM. Functional characterization of Ostreococcus tauri phototropin. New Phytol. 2016;209(2):612–623. doi: 10.1111/nph.13640. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu A, Terai M, Ishizaki K, Suetsugu N, Tsuboi H, Nishihama R, Yamato KT, Wada M, Kohchi T. Phototropin encoded by a single-copy gene mediates chloroplast photorelocation movements in the liverwort Marchantia polymorpha. Plant Physiol. 2014;166(1):411–427. doi: 10.1104/pp.114.245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nozue K, Kanegae T, Imaizumi T, Fukuda S, Okamoto H, Yeh K-C, Lagarias JC, Wada M. A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proc Natl Acad Sci USA. 1998;95(26):15826–15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai H, Kanegae T, Christensen S, Kiyosue T, Sato Y, Imaizumi T, Kadota A, Wada M. Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature. 2003;421(6920):287–290. doi: 10.1038/nature01310. [DOI] [PubMed] [Google Scholar]
- 23.Kanegae T, Hayashida E, Kuramoto C, Wada M. A single chromoprotein with triple chromophores acts as both a phytochrome and a phototropin. Proc Natl Acad Sci USA. 2006;103(47):17997–18001. doi: 10.1073/pnas.0603569103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanegae T, Kimura I. A phytochrome/phototropin chimeric photoreceptor of fern functions as a blue/far-red light-dependent photoreceptor for phototropism in Arabidopsis. Plant J. 2015;83(3):480–488. doi: 10.1111/tpj.12903. [DOI] [PubMed] [Google Scholar]
- 25.Uenaka H, Wada M, Kadota A. Four distinct photoreceptors contribute to light-induced side branch formation in the moss Physcomitrella patens. Planta. 2005;222:3623–3631. doi: 10.1007/s00425-005-0009-y. [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 2001;414(6864):656–660. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- 27.Davis SJ, Vierstra RD. Soluble, highly fluorescent variants of green fluorescent protein (GFP) for use in higher plants. Plant Mol Biol. 1998;36(4):521–528. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Campbell RE, Ting AY, Tsien RY Creating new fluorescent probes for cell biology. Nat Rev Mol Cell Biol. 2002;3:906–918. doi: 10.1038/nrm976. [DOI] [PubMed] [Google Scholar]
- 29.Hajdukiewicz P, Svab Z, Maliga P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol. 1994;25(6):989–994. [DOI] [PubMed] [Google Scholar]
- 30.Koncz C, Schell J. The promoter TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204(3):383–396. doi: 10.1007/BF00331014. [DOI] [Google Scholar]
- 31.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


