Abstract
Purpose
To establish a new evaluation method to quantify residual ophthalmic viscosurgical device (OVD) volume and corneal endothelium adhesion properties for phacoemulsification surgery.
Methods
We compared the performance of four OVDs (Viscoat®, Healon5®, Healon® and DisCoVisc®) using porcine eyes. First, OVDs were mixed with fluorescent-conjugated dextrans to render them visible under the microscope. A corneal side port was opened, followed by a continuous curvilinear capsulorhexis, and a corneal tunnel incision was made. OVDs were injected, then the lens was removed using one-handed phacoemulsification. After this procedure, the anterior segment of the eye was isolated via an equatorial incision and the tissue was immediately frozen in shimmering liquid nitrogen. Sagittal slices (20 μm) were cut with a Cryostat from limbus to limbus. Every tenth slide was imaged using a fluorescent microscope with a CCD camera. We evaluated the percentage of the corneal endothelium covered by each OVD as the OVD adhesion to corneal endothelium ratio (OAE ratio) and the volume of residual OVD in the anterior chamber.
Results
Viscoat® showed significantly higher endothelium coverage compared with both Healon® and DisCoVisc®. A statistically larger volume of Healon5® remained in the anterior chamber compared with Healon® and DisCoVisc®.
Conclusion
The new evaluation methods used here provide precise quantitative analysis of OAE ratio and residual OVD volume. These results show that Viscoat® and Healon5® have a high potential for coating the corneal endothelium during phacoemulsification and aspiration surgery.
Keywords: Ophthalmology, Surgery
1. Introduction
Phacoemulsification was introduced as a novel cataract removal technique by Kelman in 1967 [1]. In the years following the introduction of this technique, investigators showed that damage to the corneal endothelium could occur because of mechanical contact to the corneal endothelial surface by surgical instruments, dispersed lens fragments, and intraocular lens (IOL) implants [2, 3, 4, 5, 6]. Because of improvements made to ultrasound (US) tips, silicone sleeves, and intraocular lenses, surgeons can now perform more efficient phacoemulsification procedures through small incisions [7, 8]. Moreover, the introduction of ophthalmic viscosurgical devices (OVDs) has successfully decreased the probability of corneal endothelial damage. Thus, OVDs have become indispensable tools to achieve better outcomes following cataract surgery. OVDs not only protect the corneal endothelium and the anterior chamber but also contribute to decreasing the incidence of bullous keratopathy following cataract surgery [9, 10, 11, 12]. Many different OVDs are commercially available, and they typically fall into one of four types based on their physicochemical and rheological properties: cohesive, dispersive, viscoadaptive, and viscous-dispersive [13, 14]. Each type has its own unique advantages and disadvantages. Cohesive high-molecular-weight OVDs, for instance, are known for their ability to help maintain adequate space in the anterior chamber. However, they also are prone to flowing out of the eye during phacoemulsification and have poor corneal endothelium protective properties [15]. Dispersive high-molecular-weight OVDs are less effective for maintaining adequate space in the anterior chamber than cohesive OVDs and are also difficult to remove from the chamber [16]; however, they are more effective for coating and protecting the corneal endothelium against fragment of lens tissues and surgical instruments during phacoemulsification procedures. Viscoadaptive OVDs such as Healon5® work as a viscous, cohesive viscoelastic agent at a low flow rate and as a pseudodispersive viscoelastic agent at a high flow rate [17, 18, 19]. Viscous–dispersive OVDs, such as the DisCoVisc®, are a newly developed type of OVD with an intermediate cohesive/dispersive index, meaning they have the dual advantage of providing good endothelial protection and of maintaining adequate space in the anterior chamber. This allows DisCoVisc® to be used alone to efficiently protect the corneal endothelium [20, 21, 22], in contrast to the current gold standard of combining Viscoat® and a cohesive OVD together to maximize corneal endothelium protection, known as the “soft-shell technique” [13]. The goal of this study was to establish a new histological evaluation method using adult postmortem porcine eyes to detect residual OVDs and to assess their corneal endothelium adhesion properties after phacoemulsification.
2. Materials and methods
2.1. Porcine eyes
We purchased porcine eyes from a slaughterhouse at the Osaka city municipal market. The eyes were derived from pigs sacrificed at the age of 6 months. Because the eyes are commercially available, we did not require ethics approval.
2.2. Fluorescein isothiocyanate staining
Each OVD was first extracted from commercial syringes to measure the exact volume required. To visualize OVDs under a microscope, they were stained by mixing with fluorescent materials (solid powder) in a laboratory tube via vortexing to visualize the OVDs in vivo [23]. We first attempted to stain them with fluorescein sodium, but it diffused too quickly out of the tissue to allow for a proper examination (Fig. 1). We then stained with fluorescein isothiocyanate dextran (molecular weight 70,000, Sigma-Aldrich, St. Louis, MO, USA) and did not encounter any diffusion issues (Fig. 2). Therefore, all further analyses were performed using fluorescein isothiocyanate dextran staining.
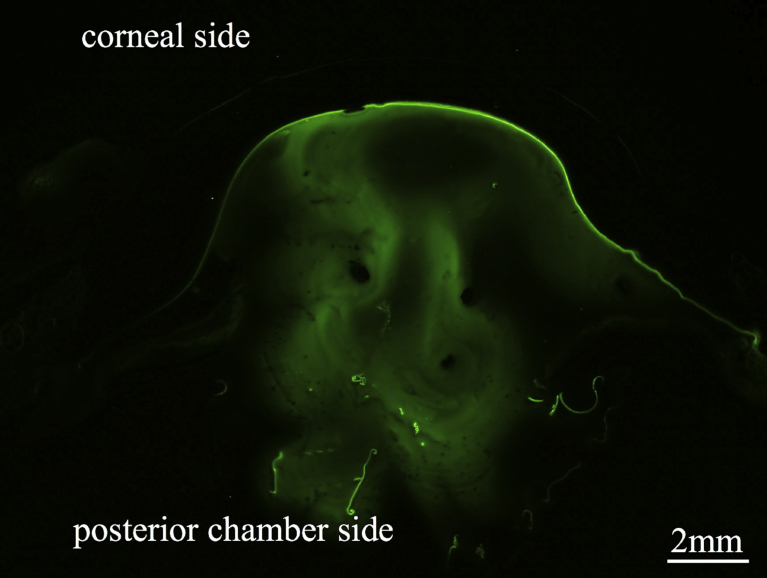
Fig. 1.
Fluorescence microscopy at 1.25× magnification revealed the anterior segment of the porcine eye. (A) Fluorescent material without dextran was found to leak through the tissue and therefore was not able to properly stain the OVDs.
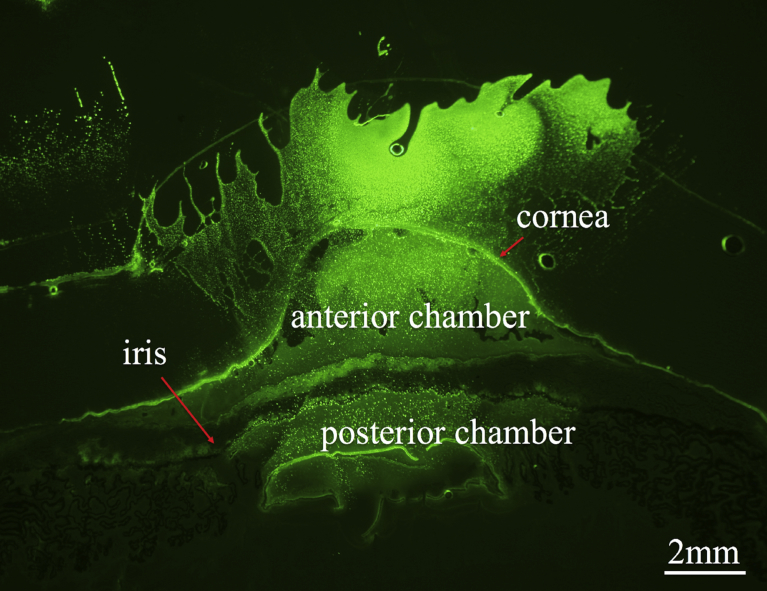
Fig. 2.
Dextran-conjugated fluorescence isothiocyanate allowed us to measure properly stained OVDs by fluorescence microscope at 1.25× magnification.
2.3. OVDs
We used four types of OVDs. Ex vivo cataract surgery studies were performed by using either Healon® (sodium hyaluronate 1%, Advanced Medical Optics, Inc, Santa Ana, CA, USA), Healon5® (sodium hyaluronate 2.3%, Advanced Medical Optics, Inc, Santa Ana, CA, USA), Viscoat® (a 1:3 mixture of 4% sodium chondroitin sulfate and 3% sodium hyaluronate, Alcon Laboratories, Inc., Fort Worth, TX, USA), or DisCoVisc® (a 1:1 mixture of 4% sodium chondroitin sulfate with 1.65% sodium hyaluronate, Alcon Laboratories, Inc., Fort Worth, TX, USA). Characteristics of each OVD tested in this study are shown in Table 1.
Table 1.
Characteristics of OVDs analyzed in this study.
| OVD name | Characteristic | Manufacturer | Content | Molecular weight × 104 | The number of eyes |
|---|---|---|---|---|---|
| Viscoat | Dispersive | Alcon | SH3% CS4% |
SH(50) CS(2.25) |
5 |
| Healon5 | Viscoadaptive | AMO | SH2.3% | SH(400) | 5 |
| Healon | Cohesive | AMO | SH1% | SH(190–390) | 4 |
| DisCovis | Viscous dispersive | Alcon | SH1.65% CS4% |
SH(160–180) CS(2–2.4) |
4 |
*SH = sodium hyaluronate, CS = sodium chondroitin sulfate.
2.4. Phacoemulsification procedure
All surgeries were performed by a single surgeon (H.M.). First, a 20-gauge corneal side port was made, then a continuous curvilinear capsulorhexis was performed under water irrigation. A corneal tunnel incision was then created with a 3.0 mm angled slit knife (MSL30, MANI, Utsunomiya, Japan), after the anterior capsule was removed. To measure OVD residue without other surgical procedures affecting OVD rheological properties, OVDs were injected just before phacoemulsification. Exactly 1.2 cc of stained OVD was injected into the anterior chamber through a corneal side port. This quantity provided adequate space in the anterior chamber to simulate human cataract surgery. Phacoemulsification was performed using a US handpiece (Universal Ⅱ®, Alcon Laboratories, Inc., Fort Worth, TX, USA) with a fixed flow rate of 25 ml/min and a maximum linear vacuum level of 200 mmHg. A 20-gauge straight phaco needle with a 30-degree angulated tip without a bypass hole was used at fixed 70% continuous energy. The BSS® (Alcon Laboratories, Inc., Fort Worth, TX, USA) bottle was positioned 65 cm above the eye. To avoid unexpected fluid flow, hydrodissection was not performed. The lens was removed using the one-handed phacoemulsification technique. The crystalline lens of porcine eyes is very soft and usually has no nucleus. Therefore, we had to remove the crystalline lenses by aspiration only. However, in routine clinical surgery, ultrasound oscillation is performed to remove the lens nucleus. Therefore, after the lens was completely removed, a 10-second ultrasound oscillation was performed in the anterior chamber to simulate a human eye with a lens nucleus.
2.5. Tissue sectioning and analysis
After the operation, the anterior segment of the eyeball was carefully isolated by performing a circumferential incision 1.5 mm posterior to the corneal limbus. The anterior segment was then immediately frozen by shimmering liquid nitrogen. Sagittal sliced sections (20 μm) were cut from one side of the corneal limbus to the opposite side using a cryostat (Leica CM3050S®, Solms, Germany). From approximately 700 slides per eye, every tenth slide (approximately 70 slides per eye) was selected, placed on a glass slide, and dried at room temperature. These slides were then mounted with Aqua-polymount® water-soluble mounting media (Polysciences, Inc, Warrington, PA, USA), and the anterior chamber and corneal endothelium were immediately imaged under a fluorescent microscope with a CCD camera (BX-51, Olympus, Tokyo, Japan) (Fig. 3). All images were taken with the same pixel size and resolution (12.5 million pixel inch resolution). We then measured two key performance parameters using WinRoof® (Mitani shoji, Tokyo, Japan) software. First, OVD adhesion to the corneal endothelium ratio (OAE ratio) was measured by calculating the percentage of OVDs that were linearly attached to the corneal endothelium in each slice (Fig. 4: red bidirectional arrows × 100%). Second, residual OVD volume in the anterior chamber was quantified by summing the number of stained areas in each slice (Fig. 4: boxed by red color). The anterior chamber area was defined as the area between the corneal endothelium and the iris surface. The total OAE ratio and residual OVD volume were calculated using the following formula:
| Residual OVD volume (ml) = ∑ Each area of fluorescent signal in the anterior chamber × 20 (thickness of slide:μm) × the number of sections × 10 (Selection from every 10th slide) × 10−9 (μm3→ml) |
Fig. 3.
Fluorescence microscopy at 1.25× magnification revealed the anterior segment of the porcine eye at the level of the midsection.
Fig. 4.
Illustration of the two measurement methods. Residual OVD in the anterior chamber was calculated as the area outlined in red. The OVD adhesion to the corneal endothelium ratio (OAE ratio) was defined as the area of the cornea covered by bidirectional arrows (red color) × 100 %.
Using the above equations, the total OAE ratio and residual OVD volume were evaluated as three dimensional form by accumulating each 2D image of certain planes and were statistically compared using Fisher's least significant difference (LSD) test for parametric data using the StatView software package (version 5.0, Abacus Concepts Inc., Berkeley, USA). Group differences were considered statistically significant if p < 0.05.
3. Results
Each OVD was tested in 4–5 porcine eyes, and each eye tested was assessed for both OAE ratio and residual OVD volume. OAE ratios for each OVD type are shown in Fig. 5. The OAE ratio was 99 (±0.28) % for Viscoat® (n = 5), 83 (±2.59) % for Healon5® (n = 4), 60 (±10.81) % for Healon® (n = 4), and 66 (±12.92) % for DisCoVisc® (n = 4). Statistical comparisons among each OVD group revealed that Viscoat® coated a greater percentage of the corneal endothelium than did Healon® or DisCoVisc® (p = 0.0037 and p = 0.0095, respectively). Residual OVD volume characteristics are shown in Fig. 6. Mean residual OVD volumes were 0.56 ± 0.02 ml for Viscoat®, 0.86 ± 0.20 ml for Healon5®, 0.18 ± 0.15 ml for Healon®, and 0.21 ± 0.16 ml for DisCoVisc®. The residual OVD volume in the anterior chamber was significantly larger for Healon5® than for Healon® and DisCoVisc® (p = 0.0039 and p = 0.0053, Fisher's LSD).
Fig. 5.
OAE ratio of each OVD type after phacoemulsification. The mean OAE ratios (±figure represents standard deviation) for Viscoat®, Healon5®, Healon®, and DisCoVisc® were 99 ± 0.28%, 83 ± 2.59%, 60 ± 10.81%, and 66 ± 12.92%, respectively. Significant differences were found between Viscoat® and Healon® and between Viscoat® and DisCoVisc® (p = 0.0037 and p = 0.0095, respectively, Fisher's LSD).
Fig. 6.
Residual OVD volume after phacoemulsification. The mean residual OVD volumes (±figure represents standard deviation) for Viscoat®, Healon5®, Healon®, and DisCoVisc® were 0.56 ± 0.02 ml, 0.86 ± 0.20 ml, 0.18 ± 0.15 ml, and 0.21 ± 0.16 ml, respectively. Significant differences were found between Healon5® and Healon® and between Healon5® and DisCoVisc® (p = 0.0039 and p = 0.0053, respectively, Fisher's LSD).
4. Discussion
The ideal OVD allows for better retention, protection, and removal during phacoemulsification surgery. An increasing number of OVDs with different compositions and characteristics are now commercially available, and several studies have reported on the characteristics of the various OVD types in clinical and experimental studies [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22]. However, it is essential that we adequately evaluate OVD performance to help cataract surgeons in selecting the best OVD for cataract surgery. In this study, we visualized OVDs by staining with fluorescent dyes, as in a previous study [23]. Oshika et al. measured the time to remove OVDs completely from the anterior chamber using a phaco needle in order to evaluate the approximate volume of OVDs in the anterior chamber [21]. However, what Oshika et al. observed in their experiment was not accurate, as they did not evaluate the OVD volume directly. In this study, we evaluated a quantitative method to measure residual OVD volume by simulating 3D volume as separate rectangular blocks and the ability of OVDs to cover the corneal endothelium. Fluorescent material bound to dextran enabled quantitative measurement of OVDs by blocking fluorescein dye from flowing out of the anterior chamber. In previous studies, the injection of OVDs was performed before making a continuous curvilinear capsulorhexis or a corneal tunnel [21, 22, 24], a method that is very similar to what we usually do in clinical surgery settings. However, to measure OVD residue more accurately when using phacoemulsification, it is best to inject the OVDs just prior to performing phacoemulsification. After phacoemulsification, we reconstructed a three-dimensional image by accumulating each two-dimensional image of certain planes with regards to the OAE ratio and residual OVD volume. The three-dimensional evaluation allowed us to overcome the limitations of previous reports. By doing so, we succeeded in quantitatively measuring residual OVD volume and OAE ratios.
The first performance parameter we assessed was the OAE ratio after phacoemulsification. It was impossible to evaluate corneal endothelial damage accurately because post-mortem porcine eyes were used, and thus corneal endothelial cell death had occurred. We considered using rodent eyes but decided this to be inappropriate because the endothelial cells of rodents undergo regeneration. Thus, corneal endothelial damage/protection could not be assessed directly in this study, as was also the case in previous reports [20, 24]. For the above reasons, in this study, we investigated the OVD corneal endothelium protection and coating properties by staining the OVD with fluorescein isothiocyanate dextran and subsequently calculating the OAE ratio. Using rabbit eyes, Petroll et al revealed that Viscoat® and DisCoVisc® had better endothelium coating properties than Provisc®, Healon®, Healon5®, and Amvisc Plus® using in vivo confocal microscopy through-focusing [20]. Peck et al showed that Viscoat® and Healon-D® remained on the corneal endothelium during phacoemulsification in rabbit eyes [24]. Thus, previous studies have shown that dispersive OVDs, including DisCoVisc®, covered the endothelial surface more extensively than cohesive OVDs [20, 24]. In this study, there was a significant difference in corneal endothelium coverage between Viscoat® and Healon® and between Viscoat® and DisCoVisc®. We also observed high corneal endothelium coverage from dispersive OVDs, with the exception of DisCoVisc®.
The second OVD performance parameter we measured was residual OVD volume, or the volume of OVD remaining in the anterior chamber after phacoemulsification. A previous clinical report has shown that during phacoemulsification, Healon5® remains in the anterior chamber to a greater extent than Viscoat® [19]. Oshika reported that a significantly higher volume of DisCoVisc® and Viscoat® remained in the anterior chamber after phacoemulsification than Healon® and ProVisc® [21]. The data from the present study show that a higher volume of Healon5 remained in the anterior chamber than Healon® and DisCoVisc®. In contrast to the present findings, previous experimental studies have shown that DisCoVisc®, which has the properties of both cohesive and dispersive OVDs, demonstrated more effective retention and protection properties than cohesive OVDs after phacoemulsification [20, 21, 22]. Conversely, Bissen-Miyajima revealed that DisCoVisc® had a significantly shorter removal time compared with Healon5, and acted similarly to cohesive OVDs during aspiration [22]. In this study, DisCoVisc® was found to perform similarly to Healon® in terms of OAE ratio and residual OVD volume in the anterior chamber.
The present study had some limitations. Firstly, we tested the performance of each OVD individually, whereas many surgeons perform cataract surgeries using a soft shell technique by mixing different OVDs. Moreover, porcine eyes are larger than human eyes and they require a greater volume of OVD compared to human eyes. Future research projects should compare various OVD combinations to determine the optimal mixtures to maximize protection and anterior chamber space in clinical surgery settings. Secondly, our model did not accurately represent human phacoemulsification surgery given the particular texture of the 6 month old porcine lens. In this study, lens was removed by aspiration only with phacoemulsification power being applied for 10 seconds without occlusion at the end of the operation. Further factors that impact how much/how fast OVD is aspirated might be phaco tip positioning in human surgery.
In conclusion, our cataract surgery simulation study using porcine eyes histologically demonstrated that Healon5® possesses excellent anterior chamber retention properties and that Viscoat® has a consistently high OAE ratio during phacoemulsification surgery. The novel evaluation methods used here provide precise quantitative measurements of OVD volume retention and corneal endothelium adhesion properties.
Declarations
Author contribution statement
Hidetsugu Mori: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Haruhiko Yamada, Keiko Toyama: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kanji Takahashi: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kelman C.D. Phaco-emulsification and aspiration: a new techniques of cataract removal. Am. J. Ophthalmol. 1967;64:23–35. PMID: 6028631. [PubMed] [Google Scholar]
- 2.McCarey B.E., Polack F.M., Marshall W. The phacoemulsification procedure. I. The effect of intraocular irrigating solutions on the corneal endothelium. Investig. Ophthalmol. 1976;15:449–457. PMID: 931689. [PubMed] [Google Scholar]
- 3.Polack F.M., Sugar A. The phacoemulsification procedure. II. Corneal endothelial changes. Investig. Ophthalmol. 1976;15:458–469. PMID: 931690. [PubMed] [Google Scholar]
- 4.Binder P.S., Sternberg H., Wickman M.G., Worthen D.M. Corneal endothelial damage with phacoemulsification. Am. J. Ophthalmol. 1976;82:48–54. doi: 10.1016/0002-9394(76)90663-2. PMID: 937457. [DOI] [PubMed] [Google Scholar]
- 5.Irvine A.R., Kratz R.P., O’Donnel J.J. Endothelial damage with phacoemulsification and intraocular lens implantation. Arch. Ophthalmol. 1978;96:1023–1026. doi: 10.1001/archopht.1978.03910050547011. PMID: 655939. [DOI] [PubMed] [Google Scholar]
- 6.Waltman S.R., Cozean C.H., Jr. The effect of phacoemulsification on the corneal endothelium. Ophthalmic Surg. 1979;10:31–33. PMID: 424170. [PubMed] [Google Scholar]
- 7.Tsuneoka H., Shiba T., Takahashi Y. Ultrasonic phacoemulsification using a 1.4 mm incision: clinical results. J. Cataract Refract. Surg. 2002;28:81–86. doi: 10.1016/s0886-3350(01)01235-4. PMID: 11777714. [DOI] [PubMed] [Google Scholar]
- 8.Rainer G., Kiss B., Dallinger S., Menapace R., Findl O., Schmetterer K., Georgopoulos M., Schmetterer L. Effect of small incision cataract surgery on ocular blood flow in cataract patients. J. Cataract Refract. Surg. 1999;25:964–968. doi: 10.1016/s0886-3350(99)00077-2. PMID: 10404373. [DOI] [PubMed] [Google Scholar]
- 9.Miyata K., Maruoka S., Nakahara M., Otani S., Nejima R., Samejima T., Amano S. Corneal endothelial cell protection during phacoemulsification: low- versus high-molecular-weight sodium hyaluronate. J. Cataract Refract. Surg. 2002;28:1557–1560. doi: 10.1016/s0886-3350(02)01540-7. PMID: 12231310. [DOI] [PubMed] [Google Scholar]
- 10.Rainer G., Menapace R., Findl O., Kiss B., Petternel V., Georgopoulos M., Schneider B. Intraocular pressure after small incision cataract surgery with Healon5 and Viscoat. J. Cataract Refract. Surg. 2000;26:271–276. doi: 10.1016/s0886-3350(99)00367-3. PMID: 11159474. [DOI] [PubMed] [Google Scholar]
- 11.Arshinoff S.A., Jafari M. New classification of ophthalmic viscosurgical devices— 2005. J. Cataract Refract. Surg. 2005;31:2167–2171. doi: 10.1016/j.jcrs.2005.08.056. PMID: 16412934. [DOI] [PubMed] [Google Scholar]
- 12.Behndig A. Phacoemulsification in spherophakia with corneal touch. J. Cataract Refract. Surg. 2002;28:189–191. doi: 10.1016/s0886-3350(01)00904-x. PMID: 11777730. [DOI] [PubMed] [Google Scholar]
- 13.Arshinoff S.A. Dispersive-cohesive viscoelastic soft shell technique. J. Cataract Refract. Surg. 1999;2:167–173. doi: 10.1016/s0886-3350(99)80121-7. PMID: 9951659. [DOI] [PubMed] [Google Scholar]
- 14.Arshinoff S.A., Wong E. Understanding, retaining, and removing dispersive and pseudodispersive ophthalmic viscosurgical devices. J. Cataract Refract. Surg. 2003;29:2318–2323. doi: 10.1016/j.jcrs.2003.09.045. PMID: 14709292. [DOI] [PubMed] [Google Scholar]
- 15.Pandey S., Thankur J., Werner L., Izak A., Apple D. Update on ophthalmic viscosurgcal devices. In: Agarwal A., editor. Phacoemulsification. third ed. Taylor and Francis; Boca Raton, FL: 2004. pp. 179–195. [Google Scholar]
- 16.Bollinger K., Smith S. Ophthalmic viscosurgical device. In: Henderson B., editor. Essentials of Cataract Surgery. Slank Inc; Thorofare, NJ: 2007. pp. 63–68. [Google Scholar]
- 17.Schwenn O., Dick H.B., Krummenauer F., Christmann S., Vogel A., Pfeiffer N. Healon5 versus Viscoat during cataract surgery: intraocular pressure, laser flare and corneal changes. Graefes Arch. Clin. Exp. Ophthalmol. 2000;238:861–867. doi: 10.1007/s004170000192. PMID: 11127574. [DOI] [PubMed] [Google Scholar]
- 18.Dick H.B., Krummenauer F., Augustin A.J., Pakula T., Pfeiffer N. Healon5 viscoadaptive formulation: comparison to Healon and Healon GV. Cataract Refract. Surg. 2001;27:320–326. doi: 10.1016/s0886-3350(00)00482-x. PMID: 11226801. [DOI] [PubMed] [Google Scholar]
- 19.Tetz M.R., Holzer M.P., Lundberg K., Auffarth G.U., Burk R.O., Kruse F.E. Clinical results of phacoemulsification with the use of Healon5 or Viscoat. J. Cataract Refract. Surg. 2001;27:416–420. doi: 10.1016/s0886-3350(00)00569-1. PMID: 11255054. [DOI] [PubMed] [Google Scholar]
- 20.Petroll W.M., Jafari M., Lane S.S., Jester J.V., Cavanagh H.D. Quantitative assessment of ophthalmic viscosurgical device retention using in vivo confocal microscopy. J. Cataract Refract. Surg. 2005;31:2363–2368. doi: 10.1016/j.jcrs.2005.05.032. PMID: 16473232. [DOI] [PubMed] [Google Scholar]
- 21.Oshika T., Okamoto F., Kaji Y., Hiraoka T., Kiuchi T., Sato M., Kawana K. Retention and removal of a new viscous dispersive ophthalmic viscosurgical device during cataract surgery in animal eyes. Br. J. Ophthalmol. 2006;90:485–487. doi: 10.1136/bjo.2005.085969. PMID: 16547332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bissen-Miyajima H. In vitro behavior of ophthalmic viscosurgical devices during phacoemulsification. J. Cataract Refract. Surg. 2006;32:1026–1031. doi: 10.1016/j.jcrs.2006.02.039. PMID: 16814065. [DOI] [PubMed] [Google Scholar]
- 23.Assia El, Apple D.J., Lim E.S., Morgan R.C., Tsai J.C. Removal of viscoelastic materials after experimental cataract surgery in vitro. J. Cataract Refract. Surg. 1992;18:3–6. doi: 10.1016/s0886-3350(13)80376-8. PMID 1531233. [DOI] [PubMed] [Google Scholar]
- 24.Peck C.M., Joos Z.P., Zaugg B.E., Abdel-Aziz S., Stringham J.D., Werner L., Mamalis N., Olson R.J. Comparison of the corneal endothelial protective effects of Healon-D and Viscoat. Clin. Exp. Ophthalmol. 2009;37:397–401. doi: 10.1111/j.1442-9071.2009.02034.x. PMID: 19594567. [DOI] [PubMed] [Google Scholar]