Abstract
Subjects with metabolic syndrome (MetS) or obesity have worse arterial stiffness. However, there have been no studies addressing time-sequential changes in pulse wave velocity (PWV) after weight loss and then regaining weight in obese non-diabetic men with MetS.
We prospectively enrolled 40 obese, non-diabetic men with MetS undergoing a 3-month weight reduction program. Another 26 lean and healthy men were recruited for comparisons. Oral glucose tolerance test and brachial ankle (ba) PWV were assessed in study subjects. Eighteen obese non-diabetic MetS and 15 lean control subjects had follow-ups at the 60th month.
The body weight of obese MetS decreased from 94.8 ± 7.6 to 86.1 ± 9.0 (N = 18, P < .001) after a 3-month weight reduction program but regained gradually thereafter to 93.6 ± 11.6 kg at the 60th month (P < .001 versus 3rd month). baPWV decreased after weight loss slightly (P = .240) while weight regain significantly increased the baPWV (from 3rd month, 1358 ± 168 to 60th month 1539 ± 264 cm/sec, P < .001). Systolic and diastolic blood pressure increments correlated with the increment of baPWV after weight regain. At the 60th month, lean controls (N = 15) had increases in body weight while their baPWV increased non-significantly. The increments of baPWV after weight regain in obese MetS were significantly higher than the increment of baPWV in lean controls after weight gain.
In conclusion, regaining body weight after weight reduction worsened arterial stiffness with significant increase of baPWV in obese non-diabetic MetS.
Keywords: arterial stiffness, metabolic syndrome, obesity, pulse wave velocity, weight reduction, weight regain
1. Introduction
Progressive atherosclerotic disease with declined aortic elastin quality and content leads to increased aortic stiffness and, therefore, increased pulse wave velocity (PWV).[1] PWV measures aortic and large vessel distensibility and increases with age, blood pressure, smoking, dyslipidemia and peripheral artery disease.[1–4] Increased arterial stiffness or PWV has been shown to be a useful surrogate marker for future cardiovascular events, cardiovascular death or even total mortality.[5–7] Insulin resistance (IR) either manifested as impaired fasting glucose or impaired glucose tolerance or diabetes mellitus (DM) is associated with worse arterial stiffness.[2,8] Subjects with metabolic syndrome (MetS) or obesity have a higher PWV, too.[3,9]
Ankle-brachial index (ABI) is a useful noninvasive examination for diagnosis of lower extremity arterial disease and a surrogate marker for future cardiovascular events.[10] According to previous studies, an ABI value <0.9 is an independent risk factor for cardiovascular disease and mortality.[10,11]
Few studies have investigated the time sequential change of arterial stiffness in obese non-diabetic men with MetS, who had undergone weight reduction but regained body weight in the long term follow-up.[12,13] Weight loss could induce significant reduction in blood pressure and large arterial stiffness.[12,14] In patients with type 2 DM, weight loss significantly improves the aortic PWV.[15] However, it is unknown whether the reduction in arterial stiffness is due to weight reduction per se or whether it is correlated with changes of adipokines, IR or inflammatory markers accompanying weight loss.[14] Moreover, the effect of regaining body weight after weight reduction on PWV and ABI is not investigated in obese non-diabetic men yet. The aim of the present study was to record and analyze time-sequential changes of PWV, ABI, IR index, adipokines, inflammatory markers, and anthropometric profiles after weight loss and regained weight in obese non-diabetic men with MetS.
2. Materials and methods
2.1. Study subjects
We prospectively enrolled 40 men in total, who were diagnosed with MetS using the ATP-III criteria, from outpatient clinics at Taichung Veterans General Hospital (Taichung, Taiwan) in April to September 2008. Parts of the results have been published previously.[16–19] In brief, the study enrollment criteria included central obesity, which was defined as waist circumference ≥ 90 cm, in addition to at least 2 other ATP-III MetS criteria.[20] Patients with DM requiring oral anti-diabetic agents or insulin shots were excluded from the study. The patients undertook a 3-month weight reduction program. The dietitian instructed the study subjects to consume a 1200 Kcal diet per day comprising 55% carbohydrate, 30% fat, and 15% protein. Patients were educated how to estimate the energy and nutritional components per unit amount of food.[18,21,22] They all received an instruction brochure, which stated all the nutritional facts and energy per unit amount of food for instant check. The patients were asked to keep a very detailed record of each meal. A dietitian would estimate the energy based on the record in order to keep the daily requirement of 1200Kcal per day. In addition, the dietitian closely followed them with phone-call consultations. We encouraged peer competition for weight loss and showed weight and waist circumference changes in bar graphs at each gathering, which was held once every week in the first month, then biweekly in the 2nd and 3rd month. The patients were encouraged to have at least 2 to 3 light physical exercise periods per week. We provided a 60-minute fitness program with a physical trainer at the biweekly gatherings. The study subjects underwent anthropometric data check, blood sampling and arterial stiffness examinations again at the 3rd and 60th month after the beginning of weight loss program if they were compliant with our recalls. Among the study participants, 18 obese non-diabetic MetS and 15 lean control subjects had follow-ups at the 60th month. All subjects provided written informed consent. The authors confirm that all ongoing and related trials for this study/intervention are registered. The study protocol was approved by the Human Research Ethics Review Committee of Taichung Veterans General Hospital (Taichung, Taiwan). The protocol and case enrollment were conducted after getting the ethics committee approval; however, the registration at www.clinicaltrials.gov (Multi-faceted Evaluations Following Weight Reduction in Subjects with MetS NCT 01065753) was delayed till Feb 8, 2010 because clinical trial registration was not compulsory in our country back then.
2.2. Biochemical analysis, inflammatory markers, adipokines, and oral glucose tolerance tests (OGTTs)
All obese MetS subjects underwent blood tests and OGTT after an overnight fast to exclude those with unknown diabetes. After a fasting blood sample was collected, glucose load of 75 g was ingested over 5 minutes. Blood samples were collected at 30 minutes, 60 minutes, 90 minutes, and 120 minutes after the test load. Blood glucose and insulin concentrations were measured in each sample. Serum insulin was determined by a commercially available assay kit (IMMULITE, I-2000, EURO/Diagnostic Products Corporation, Gwynedd, UK). The inter- and intra-assay coefficients of variation for insulin (range 10.7 to 439 μU/mL) were 4.3% and 5.4%, respectively. Insulin-resistance was estimated using the homeostasis model assessment of IR (HOMA-IR), defined as fasting glucose mg/dl x fasting insulin μU/mL/405.[23,24] Serum high-sensitivity C- reactive protein (hs-CRP) was determined by particle-enhanced immunoturbidimetry (Latex microparticles sensitized with duck anti-CRP IgY kit, provided by Good Biotech Corp., Taichung, Taiwan). The intra- and inter-assay coefficients of variance were 1.4% and 1.42%, respectively.[25] Serum monocyte chemotactic protein-1 (MCP-1) was determined by enzyme-linked immunosorbent assays (R&D Systems, Inc., Minneapolis, MN).[26,27] The intra- and inter-assay coefficients of variation for MCP-1 were 5.80% and 5.70%, respectively, with a minimum detectable concentration of <5.0 pg/mL. Serum adiponectin and leptin were determined by enzyme-linked immunosorbent assays (R&D Systems, Inc., Minneapolis, MN). The intra- and inter-assay coefficients of variation for adiponectin were 3.53% and 6.50%, respectively, with a minimum detectable concentration of 0.079 to 0.891 ng/mL. The intra- and inter-assay coefficients of variation for leptin were 3.17% and 4.37%, respectively, with a minimum detectable concentration of <7.8 pg/mL.
2.3. Measurement of ankle-brachial index (ABI) and brachial ankle (ba) PWV
After seating and resting for 5 minutes, the study subjects’ brachial and ankle blood pressure, PWV and ABI were measured by VP-2000 Vascular Profiling System (OMRON Healthcare Co., LTD, Kyoto, Japan), using simultaneous blood pressure and waveform measurements on all 4 limbs with electrocardiogram tracings. Brachial-ankle pulse wave velocity (baPWV) was calculated as the distance in centimeters between arterial sites of interest over time (in seconds) that the pressure waveforms travel from the heart to the respective arterial sites. Distance between peripheral sites was calculated using a height based algorithm as required by the Colin waveform analyzer.[28] Time was calculated using the foot-to-foot velocity method of waveforms measured at various sites.[29] The average of 2 runs was used in this analysis.
2.4. Statistical analysis
Continuous variables are expressed as mean ± SD and categorical data as percentages. Differences in continuous variables between obese MetS and lean controls were measured by the independent Student t test. Differences in continuous variables at baseline and 60th month follow-up in lean controls were measured by the paired Student t test. Differences in continuous variables among different visits (baseline, the 3rd month and the 60th month) in obese MetS were measured by the repeated measure analysis of variance (ANOVA) test. Univariate correlation analysis between changes of body weight, blood pressure, IR indices, inflammatory marker and circulating adipokines versus increments of PWV in obese MetS after weight regain were analyzed using Pearson correlation analysis. The SPSS 17.0 statistical software package (SPSS, Inc., Chicago, IL) was used for all calculations. A 2-tailed P value less than .05 was considered statistically significant.
3. results
3.1. Baseline clinical, biochemical and vascular stiffness data in obese non-diabetic men with MetS and lean controls
Parts of the results of the comparisons of obese MetS versus lean controls have been published previously.[16–19] Obese non-diabetic men with MetS had a larger body weight, body mass index, worse IR profiles, and higher triglyceride and lower high-density lipoprotein cholesterol. Obese non-diabetic men with MetS had a higher baPWV (1365 ± 217 cm/sec, N = 40) than the lean and healthy controls (1283 ± 119 cm/sec, N = 26) but the difference did not reach statistical significance (P = .083). The ages (46 ± 10 versus 40 ± 13, P = .140) were similar between obese non-diabetic MetS (N = 18) and the lean healthy controls (N = 15) that complied with call-backs at 60th month.
3.2. Comparing anthropometric, biochemical and vascular stiffness data in lean healthy controls between baseline and 60th month (N = 15)
Among the lean and healthy controls, 15 of them complied with call-back at 60th month. Average body weight increased from 67.1 ± 6.5 to 69.9 ± 9.7 (N = 15, P = .032). Diastolic blood pressure and total cholesterol increased significantly after weight gain at 60th month (Table 1). Among the IR index, OGTT 2h glucose and insulin increased significantly after weight gain (Table 1). In terms of vascular stiffness indices, ABI values were similar and baPWV increased but the difference did not reach statistical significance (Table 1).
Table 1.
Comparing anthropometric, biochemical and vascular stiffness data in lean controls between baseline and 60th month (N = 15) and obese non-diabetic men with metabolic syndrome after 3-month weight reduction program and regained weight at 60th month (N = 18).
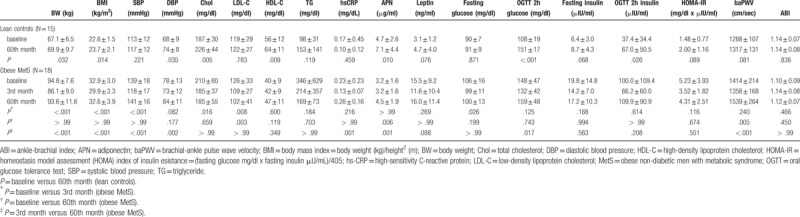
3.3. Anthropometric, biochemical and vascular stiffness data in obese non-diabetic men after 3-month weight reduction program and weight regain at 60th month (N = 18)
Among the obese non-diabetic men with MetS, 18 of them had follow-ups after weight loss at 3rd month and weight regain at 60th month. Average body weight decreased from 94.8 ± 7.6 to 86.1 ± 9.0 (N = 18, P < .001) after a 3-month diet and exercise weight reduction program. Weight regained gradually thereafter and increased to 93.6 ± 11.6 kg at 60th month (P < .001 versus 3rd month (after just finishing weight reduction program)) (Table 1). Systolic and diastolic blood pressure increased significantly but lipid profiles were similar after weight regain (Table 1). The inflammatory marker hs-CRP increased significantly after regaining body weight (Table 1). Among the OGTT derived IR index, only OGTT 2h glucose increased significantly after weight regain (Table 1). In terms of vascular stiffness indices, ABI did not change significantly throughout the weight loss and weight regain periods. baPWV decreased after weight loss (baseline 1414 ± 214 versus 1358 ± 168 cm/sec, P = .240, N = 18) but the difference did not reach statistical significance. Weight regain significantly increased the baPWV (from 3rd month, 1358 ± 168 to 60th month 1539 ± 264 cm/sec, P < .001, N = 18) (Table 1). In addition, baPWV at 60th month after weight regain was even worse than the baseline values in obese MetS (Table 1). Moreover, the increments of baPWV after weight regain in obese MetS were significantly higher than the increment of baPWV in lean healthy controls at 60th month (181 ± 146 versus 49 ± 100 cm/sec, P = .006).
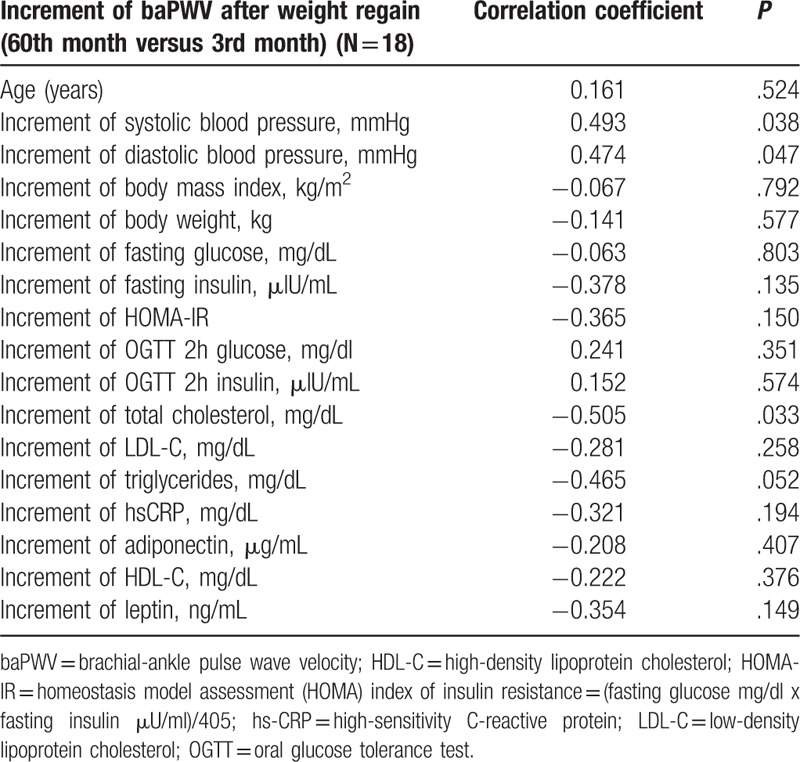
3.4. Correlation analysis of increment of PWV after weight regain at 60th month (versus at 3rd month, just finishing weight reduction) in obese non-diabetic men with MetS (N = 18)
Systolic blood pressure increment (r = 0.493, P = .038) and diastolic blood pressure increment (r = 0.474, P = .047) correlated with the increment of baPWV after weight regain (Table 2). However, body weight increment, hs-CRP increment and IR index changes did not correlate with the increment of baPWV after weight regain (Table 2).
Table 2.
Correlation analysis of increment of pulse wave velocity after weight regain at 60th month (versus 3rd month, just finishing weight reduction) in obese non-diabetic men with metabolic syndrome (N = 18).
4. Discussions:
Though, it is known that subjects with MetS or obesity have increased arterial stiffness and PWV.[9,13,30] However, there is lack of longitudinal study investigating time-sequential changes of PWV following weight loss and weight regain. Moreover, it is worthwhile to investigate the underlying mechanism inside arterial stiffness change after weight loss and weight regain in obese MetS. In this study, we found that obese non-diabetic men with MetS had a trend toward higher baPWV than the healthy controls (P = .083). Weight reduction with diet and exercise for 3 months decreased body weight and improved IR but did not reduce baPWV significantly. However, weight regain at 60th month worsened arterial stiffness with significant increase of baPWV (P < .001 versus weight reduction at 3rd month). Systolic or diastolic blood pressure increment but not weight, hs-CRP or IR change correlated with the increment of baPWV after weight regain.
Weight loss improves IR, decreases circulating leptin and increases adiponectin in obesity or MetS.[17,18,31] Weight reduction also improved arterial stiffness and decrease PWV.[14] Previous study in the SAVE trial showed that the amount of weight loss and the decrease of circulating insulin correlated with decrease of baPWV after weight loss in obese non-diabetic subjects.[12] Another study by Cooper et al [32] showed that reductions in heart rate and C-reactive protein were associated with reduced baPWV in obese young adults after weight reduction. In our study, weight reduction with diet and exercise for 3 months in obese non-diabetic men with MetS decreased baPWV but the difference did not reach significance (Table 1). Report by Gjevestad et al[33] showed significant weight reduction by either gastric bypass surgery or intensive life style intervention but no decrease of PWV in morbidly obese men at 1 year. The discrepancy of these studies on PWV changes after weight loss is difficult to explain. We postulated that studies that recruited younger obese subjects who did not have many metabolic abnormalities tended to show improvement of arterial stiffness after weight reduction. In contrast, obese subjects with MetS had many factors that contributed to arterial stiffness, which could not be reversed by short-term weight reduction program.
There were some studies addressing increase of PWV after weight gain in healthy subjects.[34,35] One study showed that increase of PWV after weight gain was associated with visceral abdominal fat increase.[34] Another study disclosed larger baseline body weight and greater annual weight gain were associated with larger annual pulse-wave velocity increases.[35] Our study also showed increase of baPWV after weight gain in lean and healthy controls but the difference did not reach statistical significance (Table 1). However, studies on temporal changes of arterial stiffness after weight regain in obese non-diabetic MetS, who had undergone weight reduction are lacking. Our study is novel in showing a significant increase of baPWV after weight regain at 60th month in comparison with PWV at 3rd month after weight reduction. Moreover, baPWV at 60th month after weight regain was even worse than the baseline values in obese MetS (Table 1). In addition, we reported that the increment of baPWV after weight regain was only associated with systolic or diastolic blood pressure increments but not with body weight, hs-CRP or insulin-resistance changes (Table 2). Moreover, our study disclosed that the increment of baPWV after weight regain at 60th month was significantly higher than the increment of PWV after weight gain in healthy lean healthy controls in the same time frame. This implies that the increment of baPWV after weight regain in obese MetS was beyond the aging-process or weight gain related increase of arterial stiffness within a 5-year period.
There are several limitations in this study. Among the study participants, only 18 obese non-diabetic men with MetS and 15 lean controls complied with call-backs at 60th month. The smaller sample size would limit its statistic impact. The other arterial stiffness indices, such as carotid-femoral PWV, central pulse wave analysis, and local arterial stiffness were not investigated in this study.[29] More studies on the other arterial stiffness indices are needed to corroborate our study findings on temporal changes of baPWV during weight reduction and weight regain. Finally, further cellular or animal studies are needed to address the molecular mechanism inside the changes of arterial stiffness after weight reduction and weight regain in obese MetS.
In conclusion, our study showed that a 3-month weight reduction program with diet and exercise in obese MetS decreased body weight and improved IR but did not reduce baPWV significantly. Weight regain at 60th month worsened arterial stiffness with significant increase of baPWV versus 3rd month after weight reduction program. Systolic or diastolic blood pressure increment but not weight, hs-CRP or IR change correlated with the increment of baPWV after weight regain. The increments of baPWV after weight regain in obese MetS were more than changes of arterial stiffness in lean controls during a 5-year period.
Acknowledgments
The authors would like to thank the Biostatistics Taskforce of Taichung Veterans General Hospital for statistical support.
Author contributions
Conceptualization: Kae-Woei Liang, Shih-Yi Lin, Wen-Lieng Lee, Wayne Sheu.
Data curation: Wen-Jane Lee, I-Te Lee, Shih-Yi Lin, Wen-Lieng Lee, Wayne Sheu.
Formal analysis: Wen-Jane Lee, I-Te Lee, Shih-Yi Lin, Jun-Sing Wang, Wen-Lieng Lee, Wayne Sheu.
Funding acquisition: Wen-Jane Lee, Shih-Yi Lin, Wen-Lieng Lee, Wayne Sheu.
Investigation: Kae-Woei Liang, Wen-Jane Lee, Jun-Sing Wang, Wen-Lieng Lee, Wayne Sheu.
Methodology: Wen-Jane Lee, Jun-Sing Wang, Wen-Lieng Lee, Wayne Sheu.
Project administration: Wen-Jane Lee, Wayne Sheu.
Resources: Wayne Sheu.
Supervision: Wen-Lieng Lee, Wayne Sheu.
Validation: Wen-Jane Lee.
Writing – original draft: Kae-Woei Liang.
Writing – review & editing: Kae-Woei Liang, Jun-Sing Wang, Wayne Sheu.
Footnotes
Abbreviations: ABI = ankle-brachial index, DM = diabetes mellitus, hs-CRP = high-sensitivity C- reactive protein, IR = insulin resistance, MCP-1 = monocyte chemotactic protein-1, MetS = metabolic syndrome, OGTT = oral glucose tolerance test, PWV = pulse wave velocity.
K-WL and W-JL contributed equally to this work.
This study was supported in part by grants from Taichung Veterans General Hospital, Taiwan (TCVGH-1043104B, 1053104B, 1063102B, 1067328D, 1067314C).
Multi-faceted evaluations following weight reduction in subjects with metabolic syndrome NCT 01065753 on Feb 8, 2010-Retrospectively registered at www.clinicaltrials.gov.
The authors have no conflicts of interest to disclose.
References
- [1].van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke 2001;32:454–60. [DOI] [PubMed] [Google Scholar]
- [2].Webb DR, Khunti K, Silverman R, et al. Impact of metabolic indices on central artery stiffness: independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia 2010;53:1190–8. [DOI] [PubMed] [Google Scholar]
- [3].Toto-Moukouo JJ, Achimastos A, Asmar RG, et al. Pulse wave velocity in patients with obesity and hypertension. Am Heart J 1986;112:136–40. [DOI] [PubMed] [Google Scholar]
- [4].Yiming G, Zhou X, Lv W, et al. Reference values of brachial-ankle pulse wave velocity according to age and blood pressure in a central Asia population. PLoS One 2017;12:e0171737.1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shokawa T, Imazu M, Yamamoto H, et al. Pulse wave velocity predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima study. Circ J 2005;69:259–64. [DOI] [PubMed] [Google Scholar]
- [6].Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005;111:3384–90. [DOI] [PubMed] [Google Scholar]
- [7].Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006;113:664–70. [DOI] [PubMed] [Google Scholar]
- [8].Schram MT, Henry RM, van Dijk RA, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension 2004;43:176–81. [DOI] [PubMed] [Google Scholar]
- [9].Lin WY, Lai MM, Li CI, et al. In addition to insulin resistance and obesity, brachial-ankle pulse wave velocity is strongly associated with metabolic syndrome in Chinese—a population-based study (Taichung Community Health Study, TCHS). J Atheroscler Thromb 2009;16:105–12. [DOI] [PubMed] [Google Scholar]
- [10].Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. Jama 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Diehm C, Lange S, Darius H, et al. Association of low ankle brachial index with high mortality in primary care. Eur Heart J 2006;27:1743–9. [DOI] [PubMed] [Google Scholar]
- [12].Hughes TM, Althouse AD, Niemczyk NA, et al. Effects of weight loss and insulin reduction on arterial stiffness in the SAVE trial. Cardiovasc Diabetol 2012;11:114.1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rider OJ, Tayal U, Francis JM, et al. The effect of obesity and weight loss on aortic pulse wave velocity as assessed by magnetic resonance imaging. Obesity (Silver Spring) 2010;18:2311–6. [DOI] [PubMed] [Google Scholar]
- [14].Petersen KS, Blanch N, Keogh JB, et al. Effect of weight loss on pulse wave velocity: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol 2015;35:243–52. [DOI] [PubMed] [Google Scholar]
- [15].Barinas-Mitchell E, Kuller LH, Sutton-Tyrrell K, et al. Effect of weight loss and nutritional intervention on arterial stiffness in type 2 diabetes. Diabetes Care 2006;29:2218–22. [DOI] [PubMed] [Google Scholar]
- [16].Liang KW, Tsai IC, Lee WJ, et al. MRI measured epicardial adipose tissue thickness at the right AV groove differentiates inflammatory status in obese men with metabolic syndrome. Obesity (Silver Spring) 2012;20:525–32. [DOI] [PubMed] [Google Scholar]
- [17].Fu CP, Sheu WH, Lee IT, et al. Effects of weight loss on epicardial adipose tissue thickness and its relationship between serum soluble CD40 ligand levels in obese men. Clin Chim Acta 2013;421C:98–103. [DOI] [PubMed] [Google Scholar]
- [18].Liang KW, Lee WJ, Lee IT, et al. Persistent elevation of paraoxonase-1 specific enzyme activity after weight reduction in obese non-diabetic men with metabolic syndrome. Clin Chim Acta 2011;412:1835–41. [DOI] [PubMed] [Google Scholar]
- [19].Liang KW, Tsai IC, Lee WJ, et al. Correlation between reduction of superior interventricular groove epicardial fat thickness and improvement of insulin resistance after weight loss in obese men. Diabetol Metab Syndr 2014;6:115.1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002;10625:3143–421. [PubMed] [Google Scholar]
- [21].Sheu WH, Chang TM, Lee WJ, et al. Effect of weight loss on proinflammatory state of mononuclear cells in obese women. Obesity (Silver Spring) 2008;16:1033–8. [DOI] [PubMed] [Google Scholar]
- [22].Sheu WH, Chin HM, Lee WJ, et al. Prospective evaluation of folic acid supplementation on plasma homocysteine concentrations during weight reduction: a randomized, double-blinded, placebo-controlled study in obese women. Life Sci 2005;76:2137–45. [DOI] [PubMed] [Google Scholar]
- [23].Reilly MP, Wolfe ML, Rhodes T, et al. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation 2004;110:803–9. [DOI] [PubMed] [Google Scholar]
- [24].Soyama A, Nishikawa T, Ishizuka T, et al. Clinical usefulness of the thickness of preperitoneal and subcutaneous fat layer in the abdomen estimated by ultrasonography for diagnosing abdominal obesity in each type of impaired glucose tolerance in man. Endocr J 2005;52:229–36. [DOI] [PubMed] [Google Scholar]
- [25].Liang KW, Lee WJ, Lee WL, et al. Diabetes exacerbates angiographic coronary lesion progression in subjects with metabolic syndrome independent of CRP levels. Clin Chim Acta 2008;388:41–5. [DOI] [PubMed] [Google Scholar]
- [26].Liang KW, Sheu WH, Lee WL, et al. Decreased circulating protective adiponectin level is associated with angiographic coronary disease progression in patients with angina pectoris. Int J Cardiol 2008;129:76–80. [DOI] [PubMed] [Google Scholar]
- [27].Liang KW, Lee WJ, Lee WL, et al. Decreased ratio of high-molecular-weight to total adiponectin is associated with angiographic coronary atherosclerosis severity but not restenosis. Clin Chim Acta 2009;405:114–8. [DOI] [PubMed] [Google Scholar]
- [28].Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol 2003;91:1519–22. A1519. [DOI] [PubMed] [Google Scholar]
- [29].Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–605. [DOI] [PubMed] [Google Scholar]
- [30].Ferreira I, Henry RM, Twisk JW, et al. The metabolic syndrome, cardiopulmonary fitness, and subcutaneous trunk fat as independent determinants of arterial stiffness: the Amsterdam growth and health longitudinal study. Arch Intern Med 2005;165:875–82. [DOI] [PubMed] [Google Scholar]
- [31].Dengel DR, Kelly AS, Olson TP, et al. Effects of weight loss on insulin sensitivity and arterial stiffness in overweight adults. Metabolism 2006;55:907–11. [DOI] [PubMed] [Google Scholar]
- [32].Cooper JN, Buchanich JM, Youk A, et al. Reductions in arterial stiffness with weight loss in overweight and obese young adults: potential mechanisms. Atherosclerosis 2012;223:485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gjevestad E, Hjelmesaeth J, Sandbu R, et al. Effects of intensive lifestyle intervention and gastric bypass on aortic stiffness: a 1-year nonrandomized clinical study. Obesity (Silver Spring) 2015;23:37–45. [DOI] [PubMed] [Google Scholar]
- [34].Orr JS, Gentile CL, Davy BM, et al. Large artery stiffening with weight gain in humans: role of visceral fat accumulation. Hypertension 2008;51:1519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wildman RP, Farhat GN, Patel AS, et al. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension 2005;45:187–92. [DOI] [PubMed] [Google Scholar]


