Abstract
Efficient adaptation to environmental changes is pivotal for all bacterial cells. Almost all bacterial species depend on the conserved stringent response system to prompt timely transcriptional and metabolic responses according to stress conditions and nutrient depletion. The stringent response relies on the stress-dependent synthesis of the second messenger nucleotides and alarmones (p)ppGpp, which pleiotropically target and reprogram processes that consume cellular resources, such as ribosome biogenesis. Here we show that (p)ppGpp acts on the ribosome biogenesis GTPase A (RbgA) of Gram-positive bacteria. Using X-ray crystallography, hydrogen–deuterium exchange MS (HDX-MS) and kinetic analysis, we demonstrate that the alarmones (p)ppGpp bind to RbgA in a manner similar to that of binding by GDP and GTP and thereby act as competitive inhibitors. Our structural analysis of Staphylococcus aureus RbgA bound to ppGpp and pppGpp at 1.8 and 1.65 Å resolution, respectively, suggested that the alarmones (p)ppGpp prevent the active GTPase conformation of RbgA by sterically blocking the association of its G2 motif via their 3′-pyrophosphate moieties. Taken together, our structural and biochemical characterization of RbgA in the context of the alarmone-mediated stringent response reveals how (p)ppGpp affects the function of RbgA and reprograms this GTPase to arrest the ribosomal large subunit.
Keywords: GTPase, X-ray crystallography, ribosome assembly, inhibition mechanism, enzyme kinetics, (p)ppGpp, alarmone, cell stress, RbgA, stringent response
Introduction
Rapidly dividing bacterial cells depend on an effective translational machinery to maintain their fast growth rate. At the heart of this machinery, ribosomes translate mRNA into proteins. However, functional ribosomes have to be assembled in an efficient manner to meet the high demand on the translational machinery during cell proliferation. In Escherichia coli, ribosome assembly is estimated to take approximately 2 min with a corresponding assembly rate of 100,000 ribosomes/h (1). Bacterial ribosome biogenesis involves the initial transcription of a ∼5-kb primary rRNA transcript that is co-transcriptionally cleaved and modified to yield three mature rRNAs (23S, 16S, and 5S) that provide a platform for assembly of the large (50S) and small (30S) ribosomal subunits. Folding of the rRNA occurs co-transcriptionally and is accompanied by the hierarchically variable and block-wise incorporation of ∼50 ribosomal proteins (r-proteins) (2). The assembly process involves a set of ∼100 ribosome biogenesis factors to facilitate cleavage, modification, and chaperoning of intermediates in both the 50S and 30S biogenesis pathways (3). Therefore, ribosome biogenesis imposes a high metabolic load on bacterial cells and has to be precisely regulated during nutrient starvation to preserve cellular resources. In many bacterial species, ribosome biogenesis is regulated by the stringent response system that senses stress stimuli and signals the stress level via the pleiotropically acting nucleotide messenger alarmones (p)ppGpp (4, 5). Upon stress, such as restricted nutrient availability, RSH (RelA/SpoT homologue)-type proteins produce (p)ppGpp by transfer of pyrophosphate from ATP onto the 3′-OH moiety of GTP or GDP. Eventually, when environmental conditions ameliorate, (p)ppGpp is hydrolyzed by RSH-type hydrolases to retrieve GTP/GDP and consequently stress signaling declines. Alarmone-mediated regulation of ribosome biogenesis not only includes the repression of rRNA and r-protein gene transcription to shut down production of ribosomal components but may also involve the inhibition of ribosome biogenesis factors to block the assembly of ribosomal subunits (4, 6). In particular, the Staphylococcus aureus ribosome biogenesis associated GTPases RbgA, HflX, Era, RsgA, and ObgE have been recently shown to be directly targeted by (p)ppGpp to suppress GTPase activity (6). It has been hypothesized that the (p)ppGpp-mediated GTPase activity suppression prevents the final ribosome subunit maturation step and might therefore arrest subunits before they engage as matured subunits in 70S formation and translation (6).
The 50S subunit ribosome biogenesis GTPase RbgA (Ribosome biogenesis GTPase A; also called YlqF) has been shown to be essential for growth in Bacillus subtilis (7). Depletion of RbgA leads to a reduction of 70S ribosomes resulting from an arrest of large subunit biogenesis at premature 45S particles that lack the ribosomal proteins L16, L27, L28, L33, L36, and L37 and might be incompetent in 70S formation (8–12). RbgA homologues (YlqF-Related GTPase, YRG family) are evolutionarily widely distributed and can be found in all three kingdoms of life (13, 14). In Saccharomyces cerevisiae, the eukaryotic RbgA homologue Lsg1 acts late during the final ribosomal large subunit maturation and was shown to be involved in the GTPase-dependent release of the nuclear export adapter Nmd3 upon 60S subunit completion (15–17). RbgA belongs to the TRAFAC (translation factor) GTPase family and comprises a N-terminal Rossmann fold GTP-binding domain (G domain) and a C-terminal α-helical domain (18). The G domain features a K loop for K+ ion co-factor binding and is characterized by a circularly permuted GTPase fold (cpGTPase) in which the conserved G1–G2–G3–G4–G5 motif is rearranged to G4–G5–G1–G2–G3 in the protein sequence (18–21). It has been hypothesized that the cpGTPase fold might allow a nucleotide ligand-dependent movement of the C-terminal domain, which has also been suggested to be involved in rRNA contacts and might participate in rRNA remodeling (21). Crystal structures of guanosine nucleotide-bound RbgA homologues of Thermotoga maritima (18) and B. subtilis (PDB code 1PUJ) are available and reveal the N-terminal GTPase fold followed by an α-helical C-terminal putative RNA-binding domain. However, the molecular details of ribosome interaction and the mechanism of GTPase activation for 50S maturation have remained enigmatic. Furthermore, the mechanism by which the stringent response alarmone (p)ppGpp blocks the GTPase activation of RbgA to arrest the maturation of large ribosomal subunits is also unknown.
Here we present high-resolution X-ray crystal structures of S. aureus RbgA in complex with GDP, GMPPNP, ppGpp, and pppGpp. Dynamic and kinetic analysis shows that the alarmones (p)ppGpp act as competitive GTPase inhibitors of RgbA. Comparison of ribosome-free RbgA with the ribosome-associated GTPase active state of the eukaryotic RbgA homologue Lsg1 suggests how RbgA GTPase activation is triggered at the large subunit and inhibited by (p)ppGpp. Taken together, our structural and biochemical analyses of RbgA reveal how the GTPase active conformation is suppressed by (p)ppGpp to arrest large ribosomal subunits during the stringent response.
Results
Structures of S. aureus RbgA bound to GDP and GMPPNP
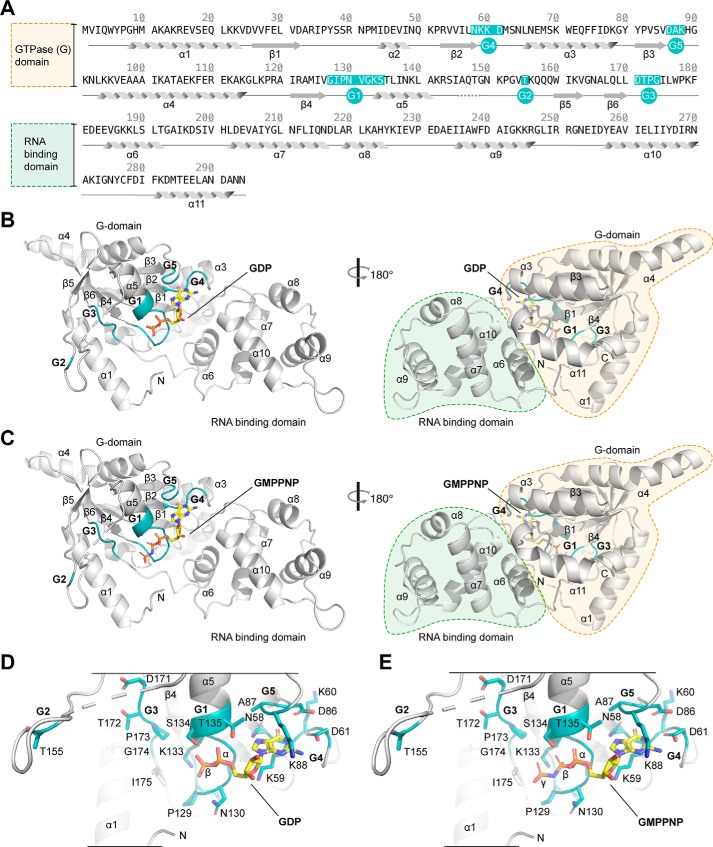
Typically, GTPases undergo conformational rearrangements upon hydrolysis of GTP to GDP and the subsequent release of Pi. To delineate nucleotide-dependent conformational changes of RbgA, we determined crystal structures of S. aureus RbgA bound to GDP and the nonhydrolysable GTP analogue GMPPNP3 at 2.15 and 1.93 Å resolution, respectively (Table 1). The structures revealed the two-domain architecture of RbgA consisting of the N-terminal G domain followed by the C-terminal α-helical putative rRNA interaction domain (Fig. 1, A–C). Both GDP and GMPPNP could be unambiguously identified in the unbiased electron density map within the GTPase active site of RbgA (Fig. S1, A and B). The guanine base is accommodated by stacking interactions of lysine 88 and lysine 59 and distinguished by hydrogen-bonding interactions of aspartate 86 and 61 and asparagine 58 of the G5 and G4 GTPase motifs (Fig. 1, D and E). Amino acids 129–134 of the G1 motif contribute to coordination of the α-, β-, and γ-phosphate moieties via hydrogen bonding and salt-bridge interactions (Fig. 1, D and E). The γ-phosphate of GMPPNP is furthermore surrounded by the nonpolar side chains of proline 129 and isoleucine 175 of the G1 and G3 motifs and is less well-defined in the electron density map than the α- and β-phosphate moieties (Fig. S1B). Thus, RbgA coordinates its GDP and GTP nucleotides similar to the T. maritima (18) and B. subtilis (PDB code 1PUJ) RbgA homologues. Despite the different nucleotide content, no significant structural differences between the GDP and GMPPNP-bound state could be observed. The root-mean-square deviation (r.m.s.d.) between both structures and the individual monomers in the unit cell was below 0.2 Å (Table S1 and Fig. S2, A–E). This observation is substantiated by the fact that both states of RbgA crystallized under different crystallization conditions but in the same space group with identical cell dimensions (Table 1).
Table 1.
Crystallographic table
Statistics for the highest-resolution shell are shown in parentheses.
| Structure | SaRbgA–GDP | SaRbgA–GMPPNP | SaRbgA–ppGpp | SaRbgA–pppGpp |
|---|---|---|---|---|
| PDB code | 6G0Z | 6G12 | 6G14 | 6G15 |
| Data collection | ||||
| Space group | P 21 21 21 | P 21 21 21 | P 21 21 21 | P 21 21 21 |
| Cell dimensions | ||||
| a (Å) | 71.932 | 71.899 | 71.781 | 72.084 |
| b (Å) | 77.812 | 77.71 | 74.512 | 78.467 |
| c (Å) | 124.667 | 124.36 | 125.215 | 125.022 |
| α (°) | 90.00 | 90.00 | 90.00 | 90.00 |
| β (°) | 90.00 | 90.00 | 90.00 | 90.00 |
| γ (°) | 90.00 | 90.00 | 90.00 | 90.00 |
| Energy (Å) | 0.97625 | 0.97903 | 0.97625 | 0.97625 |
| Resolution (Å) | 48.65–2.15 (2.23–2.15) | 62.24–1.93 (2.00–1.93) | 47.93–1.80 (1.864–1.80) | 48.89–1.65 (1.71–1.65) |
| No. unique reflections | 38,760 (3822) | 52,769 (5238) | 62,299 (6048) | 85,889 (8495) |
| Rmerge | 0.041 (0.277) | 0.056 (0.332) | 0.106 (0.555) | 0.035 (0.989) |
| I/σI | 8.79 (2.45) | 6.36 (2.08) | 7.88 (1.74) | 24.70 (1.85) |
| Completeness (%) | 99.9 (99.9) | 99.2 (99.5) | 99.0 (97.5) | 100.0 (99.9) |
| Redundancy | 2.0 (2.0) | 2.0 (2.0) | 4.2 (4.2) | 7.4 (7.4) |
| CC½ | 1.00 (0.88) | 1.00 (0.54) | 1.00 (0.94) | 1.00 (0.81) |
| Refinement | ||||
| Resolution (Å) | 48.65–2.15 | 62.24–1.93 | 47.93–1.80 | 48.89–1.65 |
| Rwork/Rfree | 18.2 | 22.7 | 21.7 | 21.8 |
| 21.7 | 25.6 | 25.6 | 24.4 | |
| No. atoms | 4989 | 5026 | 5020 | 5090 |
| Macromolecule | 4636 | 4636 | 4541 | 4611 |
| Ligand | 56 | 64 | 72 | 80 |
| Water | 297 | 326 | 407 | 399 |
| r.m.s.d. | ||||
| Bond lengths (Å) | 0.011 | 0.008 | 0.009 | 0.007 |
| Bond angles (°) | 1.30 | 1.12 | 1.19 | 1.11 |
| Ramachandran (%) | ||||
| Preferred | 98 | 98 | 99 | 97 |
| Allowed | 1.65 | 1.65 | 1 | 2.82 |
| Outliers | 0.35 | 0.35 | 0 | 0.18 |
Figure 1.
Crystal structures of S. aureus RbgA bound to GDP and GTP. A, amino acid sequence of RbgA illustrating the domain arrangement (N-terminal G domain and C-terminal RNA-binding domain). G motifs are highlighted in turquoise and labeled according to the identity (G1–G5). B and C, crystal structure of RbgA (gray cartoon representation) in complex with GDP (B) and GMPPNP (C) (yellow stick representation) in two 180° rotated views. Secondary structure elements are labeled according to their identity, and N and C indicate the respective termini. The G1–G5 motifs are colored turquoise and are labeled accordingly. D and E, detailed view on the GTPase active sites with the accommodated nucleotide GDP (D) and GMPPNP (E). Coloring is as in B. G motif and adjacent site chains are shown in stick representation and are labeled according to their identity.
Structures of ppGpp- and pppGpp-bound RbgA
The alarmones (p)ppGpp inhibit the GTPase activity of RbgA, yet the underlying molecular mechanism has remained unknown (6). Therefore, we determined the crystal structures of RbgA bound to ppGpp and pppGpp at 1.8 and 1.65 Å resolution, respectively (Fig. 2, A and B, and Table 1). Within the unbiased electron density maps, we could unambiguously identify either ppGpp or pppGpp (Fig. S1, C and D). The GDP and GTP moieties of ppGpp and pppGpp, respectively, associate to the active site in an identical fashion as their native nucleotide counterparts. The 3′-pyrophosphate moieties of both alarmones point away from the active site toward the solvent and seem to be stabilized only by the ϵ-amino group of lysine 88 of the G5 motif (Fig. 2, A and B). However, lysine 88 is not conserved among RbgA homologues arguing against a substantial role of this residue for the coordination of (p)ppGpp to RbgA (Fig. S3). This is further supported by comparable binding constants of RbgA for GDP, GTP, ppGpp, and pppGpp as determined by microscale thermophoresis (MST) (Fig. 2C and Fig. S4, A–D). Structural comparison of the alarmone-bound states of RbgA with its GDP/GMPPNP-bound states revealed no significant structural differences as indicated by the low r.m.s.d. (< 0.3 Å) (Table S1 and Fig. S2, A–E). These findings suggest that the alarmones do not alter the overall conformation of RbgA.
Figure 2.

Crystal structures of S. aureus RbgA bound to ppGpp and pppGpp. A and B, left panels, crystal structure of RbgA (gray cartoon representation) in complex with ppGpp (A) and pppGpp (B) (yellow stick representation). Secondary structure elements are labeled according to their identity, and N indicates the N terminus. The G1–G5 motifs are labeled accordingly. Right panels, close-up on the GTP binding site. Lys-88 (blue stick representation) of the G5 motif is in close proximity to the ϵ-phosphate moiety. C, binding and dissociation constants for GTP, GDP, ppGpp, or pppGpp and RbgA as determined by MST. Error bars represent the standard deviation of the calculated Kd or Ka values based on the fitting of the respective experimental data (Fig. S4).
RbgA binds nucleotides in the absence of magnesium
Our inspection of the electron density maps of the presented structures did not show electron density for magnesium, although magnesium was present in the final size-exclusion buffer at a concentration of 20 mm. The absence of magnesium is also true for the crystal structures observed for the T. maritima RbgA in complex with GDP, GTP, and GMPPNP (PDB codes 3CNN, 3CNO, and 3CNL (18)). Because the Mg2+ ion co-factor is essential for catalysis, we reasoned that our structures and the previously reported structure of the T. maritima homologue may not represent the GTPase active conformation of RbgA. In canonical GTPases, binding of Mg2+ in a tetragonal bipyramidal coordination sphere is facilitated by the G1, G2, and G3 motifs. Serine/threonine of the G1 motif GXXXXGK(S/T) (P loop) forms a direct contact, whereas aspartate of the G3 motif DXXG forms a water-mediated contact to the Mg2+, which is required for tight binding of the co-factor (22). The coordination sphere is completed by a contact via the conserved threonine of the G2 motif. However, our crystal structures revealed that although the G1 motif is in a position capable of interacting with a properly placed Mg2+ ion, the G2 (switch I) and G3 (switch II) motifs are positioned in a manner apparently not allowing interaction with the Mg2+ ion (Fig. 3A). Comparison of our structure and the T. maritima RbgA structure with the GTP-bound B. subtilis RbgA suggested that a G3 rearrangement would be required for GTP and Mg2+ co-factor binding (Fig. 3, B and C, and Fig. S5). It is noteworthy that the G3 motif is directly connected to the putative C-terminal RNA-binding domain via a linker and rearrangement of the C-terminal domain upon contact with the large subunit might allow proper positioning of the G3 motif and GTP and Mg2+ co-factor binding or vice versa. Mutation of the conserved phenylalanine at position 180 to alanine in the G3 linker region has been shown to be lethal for B. subtilis RbgA in vivo, underlining the importance of this region (21). In summary, displacement of the G2 and G3 motifs in absence of the proper RNA contact site might result in co-factor release and GTPase suppression.
Figure 3.
Binding of Mg2+ coincides with rearrangement of the G3 motif (switch II). A, upper panel, overview of the crystal structure of S. aureus RbgA (gray cartoon) in complex with GMPPNP (yellow stick representation). G motifs that participate in phosphate coordination and hydrolysis are colored turquoise and labeled according to their identity. Adjacent helices are labeled according to their identity for orientation (compare with fig. 1). Lower panel, close-up of the GTPase active site. The side chains of the G motifs G1–G3 are shown in blue stick representation. Magenta arrows emphasize the rearrangements that have to occur to locate the G2 motif (switch I; S-I) and G3 motif (switch II; S-II) in a position compatible with GTP hydrolysis. B, crystal structure of T. maritima RbgA (PDB code 3CNN (18)). Representations are as in A. C, crystal structure of B. subtilis RbgA (PDB code 1PUJ). Representations are as in A and B. In contrast to the structures of S. aureus and T. maritima, B. subtilis RbgA assumes a configuration in which the G3 motif is repositioned to allow for Mg2+ (green sphere) coordination.
Conformational dynamics of RbgA
As suggested by our crystal structures of RbgA, the conformation of RbgA might not necessarily be dictated by the identity of the bound nucleotide. However, crystal-packing contacts might indirectly affect the configuration of the G motifs by impacting the domain orientation of the N-terminal G domain and the adjacent C-terminal RNA-binding domain (Fig. S6). To investigate the role of GDP, GMPPNP, ppGpp, and pppGpp on the conformational dynamics of RbgA in solution, we performed hydrogen–deuterium exchange MS (HDX-MS). The HDX-MS analysis revealed that GDP, GMPPNP, and (p)ppGpp bind into the canonical guanosine nucleotide-binding site of RbgA (Fig. 4A). In particular, we observed a reduction in HDX of the nucleotide-bound RbgA when compared with apo-RbgA in the regions that contain the G motifs, demonstrating that the guanosine-binding site becomes stabilized upon nucleotide binding. It is noteworthy that we identified pronounced differences in HDX for GMPPNP compared with GDP and (p)ppGpp. Four regions (R1–R4) that contain the G4, G5, G1, and G3 motifs were less protected from HDX in complex with GMPPNP (Fig. 4, A and C), suggesting that GMPPNP-bound RbgA might exist in a different conformation than observed for the GDP- or (p)ppGpp-bound states. It is important to note that HDX-MS yields a time-averaged snapshot of multiple states and hence, different rates in ligand association and dissociation might affect the observed HDX. Interestingly, we also observed a difference in HDX between the 5′-diphosphate (GDP and ppGpp) and 5′-triphosphate (GTP and pppGpp) nucleotides in region 4, which includes the G3 motif (switch II). The increased protection of region 4 observed for the 5′-diphosphate nucleotides shows that the G3 motif is less stabilized in presence of the 5′-triphosphate nucleotides and is indicative for a conformational change in this region. This observation supports the idea that the G3 motif rearranges upon association of the 5′-triphosphate nucleotides (compare with Fig. 3 and Fig. S5). It is noteworthy that the G3 motif is directly connected to the RNA-binding domain of RbgA and consequently, the identity of the nucleotide bound might change the relative domain orientation of RbgA. However, we did not observe a difference in HDX for the C-terminal RNA-binding domain of RbgA. Summed up, the analysis by HDX-MS revealed that (p)ppGpp binds to the GDP/GTP binding site of RbgA as also observed in our crystal structures. Furthermore, the analysis showed that RbgA adopts different conformations in solution depending on the identity of the nucleotide, which is in contrast to the virtually identical conformation observed in the crystal structures and suggests that crystal packing might have influenced the conformation observed in our structures.
Figure 4.

Alarmones bind to the canonical GTP-binding site of RbgA. A, representative peptides of SaRbgA are colored according to their difference in HDX between nucleotide-bound (i.e. GDP, GMPPNP, ppGpp, and pppGpp) SaRbgA and apo-SaRbgA. The positions of the G-elements within the highlighted regions are indicated. B, time courses of deuterium uptake of regions R1–R4 of SaRbgA in the apo- and different nucleotide-bound states. C, location of regions R1–R4 displaying differences in HDX on the crystal structure of SaRbgA bound to pppGpp (this study).
RbgA GTPase inhibition by (p)ppGpp
Comparison of the crystal structure of alarmone-bound S. aureus RbgA, B. subtilis RbgA (PDB code 1PUJ), and the cryo-EM structure of ribosome associated S. cerevisiae Lsg1 (17) revealed that the δ- and ϵ-phosphates are placed in a position that would allow proper positioning of the G3 motif and Mg2+ coordination but may also prohibit full association of the switch I region contained G2 motif and could therefore impede Mg2+ and K+ coordination for hydrolase activation (Fig. 5). Hence, RbgA should not be capable of pppGpp hydrolysis. To test this hypothesis, we assayed RbgA GTPase for its ability to hydrolyze GTP, ppGpp, and pppGpp (Fig. 6A). Here we employed the RbgA homologue from B. subtilis that shares a 55% amino acid identity with S. aureus RbgA because B. subtilis 50S ribosomal subunits were readily accessible in high quality. As expected, RbgA could hydrolyze GTP, and this activity was stimulated by the presence of 50S ribosomal subunits. By contrast, RbgA did not hydrolyze ppGpp or pppGpp, regardless of the presence or absence of 50S ribosomal subunits (Fig. 6A), showing that the additional phosphate moieties prohibit the catalytically active conformation likely by blocking association of the G2 motif. Furthermore, we observed an ∼2-fold reduction in GTP hydrolysis when we included ppGpp or pppGpp at equimolar concentrations to GTP in the reactions (Fig. 6A).
Figure 5.
RbgA GTPase inhibition by (p)ppGpp. Shown is a comparison of the three different GTPase G1, G2, and G3 motif (turquoise) configurations observed in the crystal structures of S. aureus RbgA (left panel; this study), B. subtilis RbgA (middle panel; PDB code 1PUJ), and cryo-EM structure of S. cerevisiae Lsg1 (right panel; PDB code 5T62 (17)). RbgA/Lsg1 is shown in a gray cartoon representation in a close-up view on the GTPase active site. The associated nucleotides are shown in a yellow stick representation, and the coordinated Mg2+ ion is shown as a green sphere. Rearrangements of switches I and II required for GTPase activation are indicated by magenta arrows. The δ- and ϵ-phosphate moiety of (p)ppGpp sterically blocks the association of the G2 motif as suggested by the G motif configuration observed in the Lsg1 homologue (indicated as an orange oval).
Figure 6.

Alarmones competitively inhibit the GTPase activity of BsRbgA. A, BsRbgA was incubated with GTP, ppGpp, or pppGpp in the absence (gray bars) or presence (black bars) of 50S subunits, and its hydrolytic activity was determined by HPLC. Error bars indicate standard deviations derived from three individual measurements. B, Lineweaver–Burk plots of BsRbgA GTPase activity without or with purified 50S subunits in the presence of increasing concentrations of ppGpp (left panels) or pppGpp (right panels). The GTP concentration and initial velocity are given in mm−1 and min*nmol RbgA*nmol GTP−1, respectively. C, the initial velocities of GTPase activity of BsRbgA in presence of 1 mm GTP and increasing amounts of ppGpp (red line, squares) or pppGpp (green line, circles). Solid and dashed lines indicate the presence or absence of 50S subunits, respectively. Error bars represent standard deviations, derived from triplicates. The inhibitory constants (Ki) are shown on the right side.
Our crystal structures and HDX-MS analysis show that ppGpp and pppGpp bind to the same binding pocket as observed for GDP/GTP. Hence (p)ppGpp might act as a competitive inhibitor of GTP hydrolysis. To test this idea, we performed a kinetic analysis of RbgA's GTPase activity. We determined the initial velocity of GTP hydrolysis by RbgA at different GTP concentrations in presence of ppGpp or pppGpp with or without purified 50S ribosomal subunits. Visualization of the initial velocities of GTP hydrolysis in the Lineweaver–Burk diagram (1/initial velocity versus 1/GTP) revealed that increasing concentrations of ppGpp or pppGpp result in a strong shift of the x intercept toward the coordinate origin, while the y intercept does not change considerably, which is characteristic for a competitive mode of inhibition (Fig. 6B). Conversion of the x and y intercepts from the Lineweaver–Burk diagram into Km and Vmax values substantiates this notion. The apparent Km values increase in a dose-dependent manner with (p)ppGpp, whereas the maximum velocities remain largely unchanged, suggesting that (p)ppGpp acts as a competitive inhibitor (Table S2). Given the caveats of the Lineweaver–Burk representation, we also plotted the initial velocities of GTP hydrolysis by RbgA against the concentration of GTP (v/S characteristic, Fig. S7). Although Vmax was not reached, fitting of the data according to the equation v0 = (Vmax S)/(Km + S) resulted in a curve convincingly represented by our data. The derived values for Km and Vmax provide evidence for a strictly competitive inhibition as evidenced by Km values that increase with higher (p)ppGpp concentrations, whereas Vmax values remain constant (Table S3).
To determine the degree of inhibition, we extracted the inhibitory constants (Ki) for both alarmones from the v/S characteristic (compare with Fig. S7) using the equation v0 = (Vmax S)/(Km {1 + I/Ki} + S) yielding Ki values of 77 ± 6 and 337 ± 50 μm for ppGpp and 372 ± 23 and 829 ± 115 μm for pppGpp in absence or presence of 50S ribosomal subunits, respectively (Fig. 6C and Table S4). It is noteworthy that in presence of the 50S ribosomal subunits, RbgA activity is ∼2- and 5-fold less inhibited by pppGpp and ppGpp, respectively, as evidenced by the shift in their Ki values. Taken together, our results provide evidence that ppGpp and pppGpp act as competitive inhibitors of RbgA GTPase activity in the absence and presence of the 50S ribosomal subunits.
Discussion
Mechanism of RbgA GTPase inhibition by (p)ppGpp
In this study, we revealed how the stringent response alarmone (p)ppGpp suppresses the GTPase activity of the evolutionary widely conserved and essential large ribosomal subunit biogenesis cpGTPase RbgA. Our crystal structures of S. aureus RbgA suggest that ribosome-free RbgA might exist in a conformation that is incompatible with hydrolysis of GTP, characterized by the displaced G2 and G3 motifs (switch I + II) that leads to a deficiency of the active site coordination of Mg2+ and K+. However, our structural dynamics analysis by HDX-MS showed that RbgA undergoes structural rearrangements in solution depending on the identity of the associated nucleotide, which illustrates that the identical configuration observed in the crystal structures might not necessarily be the same in solution. We could show that the dynamics observed for GMPPNP-bound RbgA (GTPase active mimetic state) deviate from the dynamics observed for GDP, ppGpp, or pppGpp-bound RbgA, demonstrating that binding of the alarmones is not compatible with the GTPase active configuration. We furthermore demonstrate by our biochemical analysis that RbgA is incapable of hydrolyzing (p)ppGpp, suggesting that the δ- and ϵ-phosphate moieties prohibit the active conformation by precluding full association of the G2 motif. Association of the G2 motif to the active site, however, has previously been noted in a biochemical analysis of RbgA from B. subtilis to be crucial for efficient Mg2+/K+ co-factor coordination and hence for formation of the active configuration (19). From our data and based on a model of the RbgA switch loop (K loop), which was derived from a homology model using the transition state structure of the GTPase MnmE as a template (19), we also conclude that correct association of the loop structure is required to activate the GTPase. Moreover, we provide evidence for the (p)ppGpp alarmones acting in a GTP-competitive manner to reduce the GTPase activity of RbgA. This mode of GTPase inhibition has also been previously observed for a range of (p)ppGpp-targeted cellular GTPase with similar affinities and inhibitory constants for (p)ppGpp (reviewed in Ref. 23). Furthermore, the observed comparable binding affinities of GDP, GTP, and (p)ppGpp for RbgA are also a characteristic of other cellular GTPases that are affected by (p)ppGpp (reviewed in Ref. 23). Hence, the inhibition of the RbgA GTPase appears to be readily reversible when (p)ppGpp concentrations decline upon stress relief. Accordingly, inhibition of RbgA by (p)ppGpp should only occur in the context of high (p)ppGpp concentrations during the peak of stringent response signaling, when high (p)ppGpp concentrations arise in the millimolar range (24, 25).
Our data support the idea that (p)ppGpp likely sequesters RbgA-containing 45S and 50S particles because of GTPase suppression, which is substantiated by the observation that pppGpp increases the affinity of RbgA for mature 50S subunits (19). This, in turn, might withdraw mature 50S subunits from the formation of translationally active 70S ribosomes and consequently shuts down not only ribosome maturation but also protein production to economize cellular resources during starvation (6, 19). Interestingly, we demonstrated in our enzyme kinetic analysis that pppGpp and ppGpp become less potent inhibitors in the presence of the 50S ribosomal subunits. A plausible reason for this behavior might be that the rRNA contact–mediated rearrangement of the G domain and RNA-binding domain in a GTPase active conformation competes with the association of (p)ppGpp by clashing via the G2 motif with the δ- and ϵ-phosphate moieties. Taken together, our crystal structures, structural dynamics, and enzyme kinetic analyses of RbgA suggest that the alarmones (p)ppGpp prohibit formation of the GTPase active configuration by sterically precluding association of the G2 motif via the δ- and ϵ-phosphate moieties in a GTP-competitive manner.
Implications for final 50S ribosomal subunit maturation
Final maturation of the large subunit is to some extent conserved between prokaryotic and eukaryotic ribosomes and incorporation of uL16 coincides with Lsg1/RbgA GTPase activation and release (12, 17). However, the process appears unequally more intricate in eukaryotes than in prokaryotes. In brief, delivery and incorporation of uL16 has been shown in S. cerevisiae to require the dedicated chaperone Sqt1 that shields uL16's N-terminal domain before incorporation at the central protuberance close to the P site (26). Incorporation of uL16 into mature the large subunit appears to be concerted with the release of Sqt1, the activation and dissociation of the GTPase Lsg1, and the release of large subunit export adapter Nmd3 (15–17, 26). However, it is not precisely understood whether the GTPase activity of Lsg1 is required to assemble uL16 or whether assembly of uL16 leads to activation of Lsg1 and Nmd3 release to signal for subunit maturation. A similar but less complex scenario has been observed in the prokaryotic 50S maturation. RbgA-depleted cells enrich uL16 deficient pre-45S particles, and the presence of uL16 is required for stimulation of the GTPase activity and release of RbgA (12). Cryo-EM of pre-45S particles from RbgA-depleted cells revealed that four 45S subunit regions have a particularly high degree of conformational flexibility: the central protuberance; helix 38 (A-site finger); helices H89–93 of the peptidyl-transferase center (PTC); and helices H67–71, which are required for ribosomal intersubunit contacts (11, 12). Because the binding interface of Lsg1 is located in the same region of the large subunit and the ribosome maturation function is conserved between the homologues, we speculate that the RbgA-binding interface on the 23S rRNA also encompasses helices 67–71. It is noteworthy that the distance between the putative binding site of RbgA and the incorporation site of uL16 between helix 38 and 89 of the 23S rRNA are ∼40 Å apart. Hence, RbgA might not be activated by a direct contact with uL16. It seems more conceivable that incorporation of uL16 induces structural rearrangement in the 23S rRNA, which propagates toward H68–71 of the large subunit. The mature 50S arrangement of H69 and H71 might then allow RbgA to assume the GTPase active configuration by positioning its flexibly linked N-terminal G domain and C-terminal putative RNA-binding domain to rearrange the G motifs capable of hydrolyzing GTP for RbgA release, as suggested by the structural homology to the G domain and RNA-binding domain of Lsg1 (Fig. 7). In conclusion, the structural homology of RbgA and Lsg1, combined with the available structural data on 45S particles from RbgA-depleted cells (11, 12), suggests that RbgA associates to H68–71 of 45S particles, which in turn allows proper positioning of the surrounding PTC helices 89–93, the A-site finger, and the adjacent central protuberance compatible with recruitment of the final r-proteins. Incorporation of the final r-protein set may then establish the mature configuration of the central protuberance and peptidyl transferase center, which leads to the mature configuration of H68–71 and eventually the GTPase activation and release of RbgA to signal for 50S completion.
Figure 7.

Configuration control of YRG-type GTPase G motifs by the relative arrangement of H69/H71 of the large subunit. Shown are the crystal structures of S. aureus (yellow, this study) and B. subtilis (green, PDB code 1PUJ) aligned to the G1 motif of the cryo-EM structure of S. cerevisiae Lsg1 (blue, PDB code 5T62 (17)). The relative position of the flexibly connected N-terminal G domain and C-terminal RNA-binding domain of the YRG-type GTPases depends on the configuration of H69/H71. The mature configuration of H69/H71 might eventually signal for large ribosomal subunit completion to allow for GTPase activation by proper positioning of the G motifs (G1–G5) and subsequent GTPase release.
Experimental procedures
Cloning of expression constructs
S. aureus rbgA was amplified by PCR from S. aureus USA300 genomic DNA using a forward primer that contained a NcoI restriction site and the coding sequence for a hexahistidine tag and a reverse primer, which contained a BamHI restriction site. The B. subtilis rbgA homologue was amplified from B. subtilis 168 genomic DNA using a forward primer that contained a NcoI restriction site and a reverse primer, which contained a BamHI restriction site after a 4xGS linker and a hexahistidine tag coding sequence. The fragments were digested with NcoI and BamHI and cloned into pET24d (Novagen).
Production and purification of RbgA
Constructs were transformed in E. coli BL21(DE3) (Novagen) for overexpression. Cells were grown in 2 liters of lysogeny broth medium, supplemented with 25 g of lactose and kanamycin (50 mg/liter). The cells were incubated at 30 °C overnight under rigorous shaking (180 rpm). The cells were harvested by centrifugation (3,500 x g, 20 min, 4 °C) and resuspended in 20 ml of buffer A (20 mm HEPES-Na, pH 8.0, 250 mm NaCl, 20 mm KCl, 20 mm MgCl2, 40 mm imidazole) before lysis in a M-110L Microfluidizer (Microfluidics). The lysate was cleared at 47,850 x g for 20 min at 4 °C, and the supernatant was applied onto two 1-ml HisTrap FF columns (GE Healthcare) for nickel–nitrilotriacetic acid affinity chromatography. After a wash step with 15 column volumes of buffer A, proteins were eluted with three column volumes of buffer B (20 mm HEPES-Na, pH 8.0, 250 mm NaCl, 20 mm KCl, 20 mm MgCl2, 500 mm imidazole). Proteins were concentrated to 1 ml and further purified by size-exclusion chromatography (SEC). SaRbgA was purified using a HiLoad 26/600 Superdex 75 column (GE Healthcare) equilibrated in buffer C (20 mm HEPES-Na, pH 7.5, 200 mm NaCl). The main peak fractions were concentrated to 1.5 ml and dialyzed overnight at 4 °C against 200 ml of buffer C containing 10 g of HCl-activated charcoal and 1 mm EDTA to remove Mg2+ and co-purified nucleotides. RbgA was subsequently subjected to a second SEC step using a HiLoad 26/600 Superdex 75 column (GE Healthcare) equilibrated in buffer D (20 mm HEPES-Na, pH 7.5, 20 mm KCl, 20 mm MgCl2, 200 mm NaCl). BsRbgA was purified using a HiLoad 16/600 Superdex 200 column (GE Healthcare) equilibrated in buffer E (50 mm Tris-HCl, pH 7.5, 750 mm KCl, 5 mm MgCl2, 5% glycerol). The main peak fractions were concentrated, and concentrations were determined using a NanoDrop Lite spectrophotometer (Thermo Scientific).
Alarmone preparation
pppGpp was produced as described previously with minor modifications (27). 5 μm SAS1 from B. subtilis was incubated in 10 mm HEPES-Na, pH 7.5, 10 mm KCl, 10 mm MgCl2, and 100 mm NaCl together with 10 mm ATP and GTP each for 3 h at 37 °C. The reaction was stopped by addition of an equal volume chloroform followed by centrifugation (17,300 × g, 5 min, 4 °C). The aqueous phase was subjected to anion-exchange chromatography using a ResourceQ 6-ml column (GE Healthcare) at a flow rate of 6 ml/min and pppGpp eluted with a gradient of LiCl. pppGpp-containing fractions were pooled, and lithium chloride was added to a final concentration of 1 m. After addition of four volumes of ethanol, the suspension was incubated at −20 °C for 20 min and centrifuged (5000 × g, 20 min, 4 °C). The resulting pellet was washed with ethanol, dried, and stored at −20 °C.
Crystallization
Purified S. aureus RbgA was concentrated to 20 mg/ml. Nucleotides (GDP, GMPPNP, ppGpp, or pppGpp) were added at a final concentration of 5 mm, and RbgA was subsequently subjected to crystallization by sitting-drop vapor diffusion at 20 °C. Block shaped crystals grew within 2 days in drops containing 1 μl of RbgA-GDP and 1 μl of crystallization buffer (0.2 m lithium sulfate, 0.1 m MES, pH 6.0, 35% (v/v) 2-methyl-2,4-pentanediol (MPD)); 1 μl of RbgA-GMPPNP and 1 μl of crystallization buffer (0.2 m potassium fluoride, 0.1 m MES, pH 6.0, 20% PEG 3350); 1 μl of RbgA-ppGpp and 1 μl of crystallization buffer (0.2 m potassium sulfate, 20% PEG 3350); and 1 μl of RbgA-pppGpp and 1 μl of crystallization buffer (0.2 m potassium fluoride, 0.1 m MES, pH 6.0, 20% PEG 3350). The crystals were transferred into crystallization buffer containing 20% (v/v) glycerol as cryo-protectant, subsequently flash-frozen, and stored in liquid nitrogen. No cryo-protectant was added to the crystals of RbgA-GDP because of the presence of 35% MPD in the crystallization buffer.
Data collection and structure determination
Diffraction data were collected at Beamlines ID-30B, ID29, and ID23-1 of the European Synchrotron Radiation Facility (Grenoble, France) (28). Data were processed with the XDS program package for data reduction (29), and merging and scaling was performed using the AIMLESS program as implemented in the CCP4 package (30). The RbgA-GDP data set was solved by molecular replacement using the crystal structure of B. subtilis RbgA (PDB code 1PUJ) via the CCP4 implemented program Phaser (31). Coot (32) in combination with Refmac5 (CCP4 package) and phenix.refine (PHENIX package (33)) was used for iterative model building and refinement. The GMPPNP, ppGpp, and pppGpp RbgA state data sets were subsequently solved by molecular replacement using the GDP cleared S. aureus RbgA crystal structure (this study) via the CCP4 implemented program Phaser and refined using the phenix.refine software. The figures were prepared in PyMOL.
Affinity measurements using microscale thermophoresis
MST was performed on a Monolith NT.115 (NanoTemper Technologies GmbH, Munich, Germany) at 21 °C (red LED power was set to 70% and IR laser power to 25%) (34). RbgA (50 μm) was labeled with the dye NT 647 according to the supplier's protocol (NanoTemper Technologies). 200 nm RbgA was titrated with GTP, GDP, ppGpp, or pppGpp starting from a concentration of 0.5 mm in buffer C (20 mm HEPES-Na, pH 7.5, 200 mm NaCl). To each measurement Tween 20 (Sigma) was added to a final concentration of 0.05 mm. At least nine independent MST experiments were recorded at 680 nm and processed by NanoTemper analysis 1.2.009. For fitting of the experimental data and Kd determination, Origin8G was used.
Hydrogen–deuterium exchange MS (HDX-MS)
Prior HDX-MS SaRbgA (40 μm) was incubated without any nucleotide or in presence of 5 mm GDP, GMPPNP, ppGpp, or pppGpp. Preparation of samples for HDX-MS analysis was aided by a two-arm robotic autosampler (LEAP Technologies). Hydrogen–deuterium exchange was started by 10-fold dilution of SaRbgA in D2O-containing SEC buffer followed by incubation at 25 °C for 10/30/95/1000/10,000 s. The reaction was stopped by mixing the HDX reaction with an equal volume of ice-cold quench buffer (400 mm KH2PO4/H3PO4, 2 m guanidine HCl, pH 2.2) and subsequently injected into an ACQUITY UPLC M-class system with HDX technology (Waters) (35). Undeuterated samples of SaRbgA were prepared similar by 10-fold dilution in H2O-containing SEC buffer followed by quench and injection into the LC-MS system. SaRbgA was digested online using immobilized porcine pepsin at 12 °C at 100 μl/min flow rate of water + 0.1% (v/v) formic acid, and the resulting peptides were trapped on a C18 column kept at 0.5 °C. After 3 min, the C18 trap column was placed in line with an ACQUITY UPLC BEH C18 1.7-μm 1.0 × 100-mm column (Waters), and the peptides were eluted at 0.5 °C using a gradient of water +0.1% (v/v) formic acid (A) and acetonitrile +0.1% (v/v) formic acid (B) at 30 μl/min flow rate as follows: 0–7 min/95–65% A, 7–8 min/65–15% A, 8–10 min/15% A, 10–11 min/5% A, 11–16 min/95% A. Mass spectra were acquired on a G2-Si high-definition MS (HDMS) (Waters) mass spectrometer in HDMS or enhanced HDMS positive ion mode for deuterated and undeuterated samples, respectively (36, 37). Continuous lock mass correction was performed using [Glu1]-Fibrinopeptide B standard (Waters). Three replicates were measured for each incubation time. To reduce peptide carryover, the pepsin column was washed three times with 80 μl of 4% (v/v) acetonitrile and 0.5 m guanidine hydrochloride during each LC run, and an additional blank run was performed between each sample. Peptide identification and determination of deuterium uptake was carried out as described previously (38–40) aided by the PLGS and DynamX 3.0 software (Waters).
50S ribosome preparation for RbgA GTPase assay
B. subtilis 3610 cells were inoculated from an overnight culture in 200 ml of lysogeny broth medium and grown to an A600 of 0.5 at 37 °C. The cells were rapidly cooled down on ice and harvested at 4 °C for 20 min at 4000 x g. The cells were resuspended in ribosome buffer 1 (25 mm HEPES pH 7.5, 15 mm Mg(OAc)2, 100 mm KOAc, 6 mm β-mercaptoethanol, 0.025% DDM) and lysed by the use of a microfluidizer (Microfluidics). Cell debris was removed at 60,000 x g for 30 min and the supernatant loaded on a 17.5% (w/v) sucrose cushion. Ribosomes were harvested by ultracentrifugation at 200,000 × g for 2 h at 4 °C. The ribosomal pellet was resuspended in 400 μl of ribosome buffer 1 by stirring on ice for 20 min. The ribosomes were transferred to a fresh reaction tube, and undissolved material was removed by centrifugation (8000 x g, 5 min, 4 °C). 100 μl of resuspended ribosomes were loaded on top of a 10–60% (w/v) sucrose gradient. Gradients were prepared with a Gradient station (Biocomp) using the 10–50% long protocol. Gradient ultracentrifugation was performed within Ultra-Clear thin-wall tubes (14 ml, 14*95 mm) in an SW40 Ti rotor at 200,000 x g for 3 h at 4 °C. The gradient was analyzed with the gradient station coupled to a Biocomp Triax UV cell and 50S subunits manually fractionated. Fractions were pooled and concentrated with an Amicon Ultra centrifugal filter (100,000 Da molecular mass cutoff) to an A260 nm of 10.
Analysis of GTPase activity of BsRbgA
In end-point measurements, 5 μm BsRbgA were incubated together with 1 mm of nucleotides (GTP, ppGpp, pppGpp, or combinations thereof) at 37 °C in a buffer containing 50 mm HEPES-K, pH 7.4, 100 mm KCl, 50 mm KOAc, 12.5 mm Mg(OAc)2, 5 mm MgCl2, and 1 mm DTT for 100 min. Where indicated, 50S were employed in a concentration of 5 μm. The reactions were quenched by addition of two volume parts chloroform, followed by vigorous mixing for 5 s, heating at 95 °C for 15 s, and flash-freezing in liquid nitrogen. While thawing, samples were centrifuged (17,300 x g, 30 min, 4 °C), and the aqueous phase analyzed by HPLC on an Agilent 1260 Series system (Agilent Technologies) equipped with a C18 column (EC 250/4.6 Nucleodur HTec 3 μm; Macherey–Nagel). The nucleotides were eluted with a buffer containing 50 mm KH2PO4, 50 mm K2HPO4, 10 mm tetrapentylammonium bromide, and 25% (v/v) acetonitrile and detected at 253 nm in agreement with standards.
Enzyme kinetics of GTP hydrolysis by BsRbgA was determined by incubating 5 μm enzyme together with varying concentrations of GTP (i.e. 0.1, 0.2, 0.3, 0.4, 0.5, 0.75, and 1 mm) at 37 °C. Where indicated, 50S ribosomes were present in a final concentration of 5 μm. Alarmones ppGpp and pppGpp were employed in concentrations of 25, 100, 250, or 1000 μm. Samples were taken after 25, 50, 75, and 100 min in the presence of 50S or after 75, 150, 225, or 325 min in the absence of 50S and subsequently quenched and analyzed as described above.
The initial velocities of GTP hydrolysis were obtained from the slope of the linear regression of the amount of GDP quantified at different incubation times. The so-obtained initial velocities were plotted against the concentration of GTP (v/S) or in the double-reciprocal plot (Lineweaver–Burk). Values of Km and Vmax ± standard deviation were obtained from the v/S plot using the equation v0 = (Vmax S)/(Km + S). Inhibitory constants were obtained from the v/S characteristic fitted with the equation v0 = (Vmax S)/(Km {1 + I/Ki} + S). Analysis of all kinetic data were carried out using GraphPad Prism version 6.04 for Windows (GraphPad Software, San Diego, CA).
Author contributions
P. P., D. N. W., and G. B. conceptualization; P. P. and M. W. data curation; P. P., D. N. W., and G. B. supervision; P. P., W.S., M. W., T. K., and S.-A. F. investigation; P. P. and M. W. visualization; P. P., W. S., M. W., and S.-A. F. methodology; P. P., M. W., D. N. W., and G. B. writing-original draft; F. A. resources; D. N. W. and G. B. funding acquisition; D. N. W. and G. B. project administration.
Supplementary Material
Acknowledgments
We gratefully acknowledge the core facility “Protein Biochemistry and Protein Spectroscopy” at Marburg. We acknowledge support from the DFG-core facility for interactions, dynamics and macromolecular assembly structure at the Philipps-University Marburg. We thank Luis Beck for his contribution in the beginning of the project.
This work was supported by Deutsche Forschungsgemeinschaft Program SPP1879 (to G. B. and D. N. W.). The authors declare that they have no conflicts of interest with the contents of this article.
The atomic coordinates and structure factors (codes 6G0Z, 6G12, 6G14, and 6G15) have been deposited in the Protein Data Bank (http://wwpdb.org/).
This article contains Tables S1–S3 and Figs. S1–S7.
- GMPPNP
- guanosine-5′-[(β,γ)-imido]triphosphate
- r.m.s.d.
- root-mean-square deviation
- HDX
- hydrogen–deuterium exchange
- MST
- microscale thermophoresis
- SEC
- size-exclusion chromatography
- HDMS
- high-definition MS
- PTC
- peptidyl-transferase center
- MPD
- 2-methyl-2,4-pentanediol.
References
- 1. Chen S. S., Sperling E., Silverman J. M., Davis J. H., and Williamson J. R. (2012) Molecular biosystems measuring the dynamics of E. coli ribosome biogenesis using pulse-labeling and quantitative mass spectrometry. Mol. Biosyst. 8, 3325–3334 10.1039/c2mb25310k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis J. H., Tan Y. Z., Carragher B., Potter C. S., Lyumkis D., and Williamson J. R. (2016) Modular assembly of the bacterial large ribosomal subunit. Cell 167, 1610–1622.e15 10.1016/j.cell.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shajani Z., Sykes M. T., and Williamson J. R. (2011) Assembly of bacterial ribosomes. Annu. Rev. Biochem. 80, 501–526 10.1146/annurev-biochem-062608-160432 [DOI] [PubMed] [Google Scholar]
- 4. Hauryliuk V., Atkinson G. C., Murakami K. S., Tenson T., and Gerdes K. (2015) Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 13, 298–309 10.1038/nrmicro3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steinchen W., and Bange G. (2016) The magic dance of the alarmones(p) ppGpp. Mol. Microbiol. 101, 531–544 10.1111/mmi.13412 [DOI] [PubMed] [Google Scholar]
- 6. Corrigan R. M., Bellows L. E., Wood A., and Gründling A. (2016) ppGpp negatively impacts ribosome assembly affecting growth and antimicrobial tolerance in Gram-positive bacteria. Proc. Natl. Acad. Sci. U.S.A. 113, E1710–E1719 10.1073/pnas.1522179113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morimoto T., Loh P. C., Hirai T., Asai K., Kobayashi K., Moriya S., and Ogasawara N. (2002) Six GTP-binding proteins of the Era/Obg family are essential for cell growth in Bacillus subtilis. Microbiology 148, 3539–3552 10.1099/00221287-148-11-3539 [DOI] [PubMed] [Google Scholar]
- 8. Uicker W. C., Schaefer L., and Britton R. A. (2006) The essential GTPase RbgA (YlqF) is required for 50S ribosome assembly in Bacillus subtilis. Mol. Microbiol. 59, 528–540 10.1111/j.1365-2958.2005.04948.x [DOI] [PubMed] [Google Scholar]
- 9. Matsuo Y., Oshima T., Loh P. C., Morimoto T., and Ogasawara N. (2007) Isolation and characterization of a dominant negative mutant of Bacillus subtilis GTP-binding protein, YlqF, essential for biogenesis and maintenance of the 50 S ribosomal subunit. J. Biol. Chem. 282, 25270–25277 10.1074/jbc.M703894200 [DOI] [PubMed] [Google Scholar]
- 10. Matsuo Y., Morimoto T., Kuwano M., Loh P. C., Oshima T., and Ogasawara N. (2006) The GTP-binding protein YlqF participates in the late step of 50 S ribosomal subunit assembly in Bacillus subtilis. J. Biol. Chem. 281, 8110–8117 10.1074/jbc.M512556200 [DOI] [PubMed] [Google Scholar]
- 11. Li N., Chen Y., Guo Q., Zhang Y., Yuan Y., Ma C., Deng H., Lei J., and Gao N. (2013) Cryo-EM structures of the late-stage assembly intermediates of the bacterial 50S ribosomal subunit. Nucleic Acids Res. 41, 7073–7083 10.1093/nar/gkt423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jomaa A., Jain N., Davis J. H., Williamson J. R., Britton R. A., and Ortega J. (2014) Functional domains of the 50S subunit mature late in the assembly process. Nucleic Acids Res. 42, 3419–3435 10.1093/nar/gkt1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mier P., Pérez-Pulido A. J., Reynaud E. G., and Andrade-Navarro M. A. (2017) Reading the evolution of compartmentalization in the ribosome assembly toolbox: the YRG protein family. PLoS One 12, e0169750 10.1371/journal.pone.0169750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Britton R. A. (2009) Role of GTPases in bacterial ribosome assembly. Annu. Rev. Microbiol. 63, 155–176 10.1146/annurev.micro.091208.073225 [DOI] [PubMed] [Google Scholar]
- 15. Hedges J., West M., and Johnson A. W. (2005) Release of the export adapter, Nmd3p, from and the cytoplasmic GTPase Lsg1p. EMBO J. 24, 567–579 10.1038/sj.emboj.7600547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. West M., Hedges J. B., Chen A., and Johnson A. W. (2005) Defining the order in which Nmd3p and Rpl10p load onto nascent 60S ribosomal subunits. Mol. Cell Biol. 25, 3802–3813 10.1128/MCB.25.9.3802-3813.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malyutin A. G., Musalgaonkar S., Patchett S., Frank J., and Johnson A. W. (2017) Nmd 3 is a structural mimic of eIF 5 A, and activates the cpGTPase Lsg 1 during 60 S ribosome biogenesis. EMBO J. 36, 854–868 10.15252/embj.201696012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim D. J., Jang J. Y., Yoon H. J., and Suh S. W. (2008) Crystal structure of YlqF, a circularly permuted GTPase: Implications for its GTPase activation in 50S ribosomal subunit assembly. Proteins 72, 1363–1370 10.1002/prot.22112 [DOI] [PubMed] [Google Scholar]
- 19. Achila D., Gulati M., Jain N., and Britton R. A. (2012) Biochemical characterization of ribosome assembly gtpase RbgA in Bacillus subtilis. J. Biol. Chem. 287, 8417–8423 10.1074/jbc.M111.331322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anand B., Verma S. K., and Prakash B. (2006) Structural stabilization of GTP-binding domains in circularly permuted GTPases: implications for RNA binding. Nucleic Acids Res. 34, 2196–2205 10.1093/nar/gkl178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gulati M., Jain N., Anand B., Prakash B., and Britton R. A. (2013) Mutational analysis of the ribosome assembly GTPase RbgA provides insight into ribosome interaction and ribosome-stimulated GTPase activation. Nucleic Acids Res. 41, 3217–3227 10.1093/nar/gks1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wittinghofer A., and Vetter I. R. (2011) Structure-function relationships of the G domain, a canonical switch motif. Annu. Rev. Biochem. 80, 943–971 10.1146/annurev-biochem-062708-134043 [DOI] [PubMed] [Google Scholar]
- 23. Kanjee U., Ogata K., and Houry W. A. (2012) Direct binding targets of the stringent response alarmone (p)ppGpp. Mol. Microbiol. 85, 1029–1043 10.1111/j.1365-2958.2012.08177.x [DOI] [PubMed] [Google Scholar]
- 24. Varik V., Oliveira S. R. A., Hauryliuk V., and Tenson T. (2017) HPLC-based quantification of bacterial housekeeping nucleotides and alarmone messengers ppGpp and pppGpp. Sci. Rep. 7, 11022 10.1038/s41598-017-10988-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patacq C., Chaudet N., and Létisse F. (2018) Absolute quantification of ppGpp and pppGpp by double-spike isotope dilution ion chromatography–high-resolution mass spectrometry. Anal. Chem. 90, 10715–10723 10.1021/acs.analchem.8b00829 [DOI] [PubMed] [Google Scholar]
- 26. Pausch P., Singh U., Ahmed Y. L., Pillet B., Murat G., Altegoer F., Stier G., Thoms M., Hurt E., Sinning I., Bange G., and Kressler D. (2015) Co-translational capturing of nascent ribosomal proteins by their dedicated chaperones. Nat. Commun. 6, 7494 10.1038/ncomms8494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steinchen W., Vogt M. S., Altegoer F., Giammarinaro P. I., Horvatek P., Wolz C., and Bange G. (2018) Structural and mechanistic divergence of the small ( p) ppGpp synthetases RelP and RelQ. Sci. Rep. 8, 2195 10.1038/s41598-018-20634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gabadinho J., Beteva A., Guijarro M., Rey-Bakaikoa V., Spruce D., Bowler M. W., Brockhauser S., Flot D., Gordon E. J., Hall D. R., Lavault B., McCarthy A. A., McCarthy J., Mitchell E., Monaco S., et al. (2010) MxCuBE: a synchrotron beamline control environment customized for macromolecular crystallography experiments. J. Synchrotron Radiat. 17, 700–707 10.1107/S0909049510020005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kabsch W. (2010) XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winn M. D., Ballard C. C., Cowtan K. D., Dodson E. J., Emsley P., Evans P. R., Keegan R. M., Krissinel E. B., Leslie A. G., McCoy A., McNicholas S. J., Murshudov G. N., Pannu N. S., Potterton E. A., Powell H. R., et al. (2011) Overview of the CCP 4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 10.1107/S0907444910045749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., and Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 33. Adams P. D., Pavel V., Chen V. B., Ian W., Echols N., Moriarty N. W., Read R. J., Richardson D. C., Jane S., and Thomas C. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jerabek-Willemsen M., Wienken C. J., Braun D., Baaske P., and Duhr S. (2011) Molecular interaction studies using microscale thermophoresis. Assay Drug Dev. Technol. 9, 342–353 10.1089/adt.2011.0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wales T. E., Fadgen K. E., Gerhardt G. C., and Engen J. R. (2008) High-speed and high-resolution UPLC separation at zero degrees celsius. Anal. Chem. 80, 6815–6820 10.1021/ac8008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Geromanos S. J., Vissers J. P., Silva J. C., Dorschel C. A., Li G. Z., Gorenstein M. V., Bateman R. H., and Langridge J. I. (2009) The detection, correlation, and comparison of peptide precursor and product ions from data independent LC-MS with data depandant LC-MS/MS. Proteomics 9, 1683–1695 10.1002/pmic.200800562 [DOI] [PubMed] [Google Scholar]
- 37. Li G. Z., Vissers J. P., Silva J. C., Golick D., Gorenstein M. V., and Geromanos S. J. (2009) Database searching and accounting of multiplexed precursor and product ion spectra from the data independent analysis of simple and complex peptide mixtures. Proteomics 9, 1696–1719 10.1002/pmic.200800564 [DOI] [PubMed] [Google Scholar]
- 38. Schäper S., Steinchen W., Krol E., Altegoer F., Skotnicka D., Søgaard-Andersen L., Bange G., and Becker A. (2017) AraC-like transcriptional activator CuxR binds c-di-GMP by a PilZ-like mechanism to regulate extracellular polysaccharide production. Proc. Natl. Acad. Sci. U.S.A. 114, E4822–E4831 10.1073/pnas.1702435114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steinchen W., Schuhmacher J. S., Altegoer F., Fage C. D., Srinivasan V., Linne U., Marahiel M. A., and Bange G. (2015) Catalytic mechanism and allosteric regulation of an oligomeric (p)ppGpp synthetase by an alarmone. Proc. Natl. Acad. Sci. U.S.A. 112, 13348–13353 10.1073/pnas.1505271112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karniel A., Mrusek D., Steinchen W., Dym O., Bange G., and Bibi E. (2018) Co-translational folding intermediate dictates membrane targeting of the signal recognition particle receptor. J. Mol. Biol. 430, 1607–1620 10.1016/j.jmb.2018.04.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.