mamXY operon genes play an essential role in magnetite biomineralization, participate in redox reactions, and control magnetosome shape and size. However, mechanisms whereby the four MamXY proteins function together in iron oxidoreduction and transport are poorly understood. We used a combination of targeted cross-linking techniques and high-resolution mass spectrometry to elucidate the coordinated activity patterns of the MamXY proteins during magnetite biomineralization. Our findings indicate that the FtsZ-like protein undergoes polymerization and then recruits MamY, MamX, and MamZ in turn, and that these interactions depend on unique peptides present in the protein sequences. A hypothetical model of the functionalities of these proteins is proposed that accounts for the findings and provides a basis for further studies of coordination among magnetosome island (MAI) gene clusters during the process of magnetosome formation.
KEYWORDS: Magnetospirillum gryphiswaldense, magnetosome, biomineralization, MamXY proteins, unique peptides, protein interaction
ABSTRACT
The bacterium Magnetospirillum gryphiswaldense MSR-1 forms nanosized membrane-enclosed organelles termed magnetosomes. The mamXY operon, part of the magnetosome island (MAI), includes the mamY, mamX, mamZ, and ftsZ-like genes, which initiate gene transcription via the same promoter. We used a combination of molecular biological techniques (targeting of cross-linking reagents) and high-resolution mass spectrometry to investigate the coordinated activity of the four MamXY proteins in magnetite biomineralization. The FtsZ-like protein was shown by confocal laser scanning microscopy to be dispersed in the cytoplasm in the early stage of cell growth and then gradually polymerized along the magnetosome chain. Interactions of various pairs of MamXY proteins were observed using a bacterial two-hybrid system. We constructed a recombinant FtsZ-like-overexpressing strain, examined its growth patterns, and extracted magnetosome membrane proteins using a modified SDS/boiling method with BS2G-d0/d4 reagent, which helped stabilize interactions among MamXY proteins. In liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, MamY expression was detected first and remained highest among the four proteins throughout all stages of cell growth. MamX and MamZ expression was detected subsequently. The four proteins displayed coordinated expression patterns during the magnetosome maturation process. Unique peptides discovered in the MamXY protein sequences appeared to constitute “hidden” interaction sites involved in the formation of MamXY complex that helped control magnetosome shape and size.
IMPORTANCE mamXY operon genes play an essential role in magnetite biomineralization, participate in redox reactions, and control magnetosome shape and size. However, mechanisms whereby the four MamXY proteins function together in iron oxidoreduction and transport are poorly understood. We used a combination of targeted cross-linking techniques and high-resolution mass spectrometry to elucidate the coordinated activity patterns of the MamXY proteins during magnetite biomineralization. Our findings indicate that the FtsZ-like protein undergoes polymerization and then recruits MamY, MamX, and MamZ in turn, and that these interactions depend on unique peptides present in the protein sequences. A hypothetical model of the functionalities of these proteins is proposed that accounts for the findings and provides a basis for further studies of coordination among magnetosome island (MAI) gene clusters during the process of magnetosome formation.
INTRODUCTION
Magnetotactic bacteria (MTB) are ubiquitous worldwide in marine sediments and chemically stratified columns of freshwater (1). They have a unique ability to synthesize magnetosomes, intracellular organelles composed of membrane-enclosed chains of magnetite (iron oxide [Fe3O4]) or greigite (iron sulfide [Fe3S4]) crystals (2, 3). The presence of magnetosomes enables MTB to swim along the Earth’s geomagnetic field lines (magnetotaxis) (4). Magnetosomes have potential applications in early diagnosis and detection of pathogens (5), and as nanoscale drug carriers (6–9) for therapeutic targeting of cancer cells, with more effective biocompatibility than artificial magnetic particles. Therefore, many studies during the past two decades have been focused on mechanisms of magnetite biomineralization, improvement of MTB culture methods, and enhancement of magnetosome yield.
Magnetospirillum gryphiswaldense MSR-1, a frequently studied freshwater MTB strain, grows well under conditions involving a trace amount or high concentration (>20 μM) of iron; however, it synthesizes magnetosomes only in a microaerobic environment (dissolved oxygen concentration, <1%) with a high iron concentration (10, 11). The genes located in the magnetosome island (MAI) have specialized functions in magnetite biomineralization, and they belong to four operons (mms6, mamGFDC, mamAB, and mamXY) (12, 13). In MSR-1, the mamAB cluster is necessary for magnetosome formation (14), and the mms6 and mamGFDC clusters control crystal morphology and growth (15, 16). The mamXY cluster, which is conserved in all magnetospirilla (including MSR-1, Magnetospirillum magneticum AMB-1, Magnetospirillum magnetotacticum MS-1, and Magnetospirillum sp. strain SO-1) (see Fig. S1A in the supplemental material), includes mamY, mamX, mamZ, and ftsZ-like genes. In AMB-1, the N terminus of MamY is integrated in a magnetosome membrane (MM), and the number of small magnetosomes was greater in the ΔmamY mutant strain than in the wild-type (WT) strain (17). Because of the binding to biological membrane vesicles and liposome tubulation activity, MamY is hypothesized to be related to the invagination of the magnetosome vesicle (17). A recent study about the interaction of the MamY protein with cardiolipin (CL) suggests the presence of a unique protein-lipid interaction for magnetosome formation in magnetotactic bacteria (18). The MamX protein, located in the MM, plays important roles in the control of magnetosome size and maturation, and the deletion of mamX resulted in the presence of irregular superparamagnetic magnetite particles (19). MamZ (previously termed “MamH-like”) has 18 transmembrane domains. Its N-terminal domain is an ortholog of the major facilitator superfamily (MFS), and its C-terminal domain is similar to ferric reductase-like transmembrane component. In MSR-1, the deletion of mamZ or mamX resulted in an identical phenotype (20). ftsZ-like is a tubulin-like gene and encodes the FtsZ-like protein, located in the cytoplasm. In a structural comparison with FtsZ by the SWISS-MODEL program (www.swissmodel.expasy.org), the FtsZ-like protein lacked a C terminus but had high similarity in its N terminus (Fig. S1B). A deletion mutant of the ftsZ-like gene produced mainly superparamagnetic magnetite particles (21), and the defect of this mutant was reversed by nitrate (22), suggesting that the FtsZ-like protein participates indirectly in redox control of magnetite crystallization. In MSR-1, deletion of the whole mamXY cluster resulted in smaller crystals and reduced magnetism (14). The above-mentioned findings, taken together, indicate the functional redundancy of the four genes in the mamXY operon for magnetite biomineralization. The reasons for such redundancy, as well as the functionalities of the MamXY proteins, remain unclear.
We hypothesize that (i) because transcription of the four mamXY operon genes is controlled by the same promoter (Fig. 1A), their transcription occurs sequentially; and (ii) the four proteins encoded by these genes form some sort of interaction pattern, or protein complex, to participate (along with other proteins) in magnetosome synthesis. To test these hypotheses, we studied the localization of FtsZ-like-enhanced green fluorescent protein (FtsZ-like-EGFP), evaluated interactions among the four proteins in vitro, and investigated the functionalities of MamXY proteins during the process of magnetosome formation using a combination of targeted cross-linking techniques and high-resolution mass spectrometry (HRMS). Our findings help elucidate the characteristics and functional significance of mamXY operon-encoded proteins in magnetite biomineralization.
FIG 1.
Structures of mamXY operon and FtsZ-like protein, and sequence alignment of FtsZ and FtsZ-like proteins in various species. (A) Structure of mamXY operon. There is an ∼32-bp overlap between mamX and mamZ. (B) Predicted 3D structure of FtsZ-like protein with conservative nucleotide binding site and SulA site. (C) Sequence alignment of five cytoskeletal proteins (FtsZMSR-1, FtsZ-likeMSR-1, FtsZAMB-1, FtsZ-likeAMB-1, and FtsZE. coli). Dark blue, light blue, and green highlight indicate that five, four, and three proteins share the same amino acids at this site, respectively. Red box, conservative FtsZ C terminus. Homologous regions are concentrated mainly in ∼320 amino acids of the N terminus.
RESULTS
Bioinformatic analysis of FtsZ-like protein in MSR-1.
Genomic analysis revealed the presence of two ftsZ homologs (MGMSRv2_2503 and MGMSRv2_2324) in MSR-1. FtsZ (gene code MGMSRv2_2503), belonging to the category of bacterial cytoskeletal filaments, is a tubulin homolog essential for prokaryotic cell division; it forms a ring-like structure (Z-ring) and recruits other proteins to form a complex that promotes cell division at the cell midpoint (23, 24). The FtsZ-like protein (gene code MGMSRv2_2324) is a truncated form of FtsZ having high similarity to the FtsZ N terminus. It retains GTP-dependent polymerization ability but not the ability to participate in cell division because of its lack of a C terminus (Fig. S1B) (21). The predicted three-dimensional (3D) structure of the FtsZ-like protein includes a nucleotide-binding site and SulA site, which is highly identified with the N terminus of FtsZ (Fig. 1B). The SulA site is related to polymerization and can be bound by the SulA protein for inhibition of FtsZ polymerization in Escherichia coli (25).
ftsZ homologs are conserved in the well-studied MTB strains M. gryphiswaldense MSR-1 and M. magneticum AMB-1. Amino acid sequences of FtsZ and FtsZ-like proteins from MSR-1 and from AMB-1 (amb3854 and amb1015), and of the FtsZ protein from E. coli (b0095) were aligned using the Clustal X software program (Conway Institute, University College Dublin, Ireland). BLASTP analysis revealed that five bacterial cytoskeletal proteins (FtsZMSR-1, FtsZ-likeMSR-1, FtsZAMB-1, FtsZ-likeAMB-1, and FtsZE. coli) had identities of >69% and that homologous regions were concentrated mainly in ∼320 amino acids of the N terminus (Fig. 1C). Only a few amino acids were conserved in the C terminus (Fig. 1C, red box). These findings indicate that FtsZ-like is a distinctive cytoskeletal protein in MTB that is involved (along with other proteins) in biomineralization but not in cell division.
Localization of FtsZ-like-EGFP protein in MSR-1.
FtsZ-like protein is found in cytoplasm and can be localized using enhanced green fluorescent protein (EGFP). We constructed a fusion expression strain (termed MSR-1-pBB-fzl-egfp) useful for fluorescence localization of the FtsZ-like protein. This strain was cultured in sodium lactate medium (SLM) with 20 μM ferric citrate, and expression of the ftsZ-like-egfp gene was induced by adding isopropyl β-d-1-thiogalactopyranoside (IPTG) to the medium. Cells were observed by confocal laser scanning microscopy at early (6 h) and mature (18 h) stages of magnetosome formation. Dark-field observation revealed that fluorescence distributed symmetrically in 6-h cells but distributed as dots along the long axis in 18-h cells (Fig. 2A and B). The FtsZ-like protein was already distributed in cells prior to magnetosome formation and appeared to undergo GTP-dependent polymerization (21) during the gradual course of magnetosome formation; the direction of the dotted line was consistent with orientation of MamK filaments, but there was no interaction between the MamK and FtsZ-like proteins detected by the bacterial two-hybrid system. These findings suggest that the FtsZ-like protein can polymerize gradually during the maturation process of magnetosome formation, and this leads us to further speculate that during the polymerization, it may function as a scaffold protein to organize with the other three mamXY operon-encoded proteins involved in magnetite biomineralization.
FIG 2.
Localization of FtsZ-like protein and interaction analysis of MamXY proteins. (A) FtsZ-like protein dispersed in MSR-1 at 6 h. (B) FtsZ-like protein arranged as dots along the long axis of MSR-1 cells at 18 h. (C) MamZ-MamY, MamX-MamY, MamX-MamZ, FtsZ-like–MamY, FtsZ-like–MamX, and FtsZ-like–MamZ interactions assessed by bacterial two-hybrid system. (D) Intrinsic interactions among MamXY proteins as predicted by online tool STRING. “√” represents the interaction between two proteins, and “×” represents no interaction between two proteins. pBT-LGF2/pTRG-Gal4, positive control; pBT/pTRG, negative control. **, P < 0.01 (t test) for comparison of β-galactosidase activities in experimental group versus negative control.
Interactions among MamXY proteins.
Interactions among MamXY proteins were detected using a bacterial two-hybrid system. We constructed recombinant pBT and pTRG carrying four mamXY operon genes (Table 1) and cotransformed them into E. coli reporter strains in various combinations. The efficiency of interactions among the proteins was quantified based on measurement of β-galactosidase activity. Comparison by t test of activity differences in experimental group versus the negative control (pBT/pTRG) showed P values of <0.01 for pBT_FtsZ-like/pTRG_MamX versus pBT/pTRG, pBT_FtsZ-like/pTRG_MamZ versus pBT/pTRG, pBT_MamZ/pTRG_MamY versus pBT/pTRG, and pBT_MamX/pTRG_MamY versus pBT/pTRG but P values of >0.05 for pBT_FtsZ-like/pTRG_MamY versus pBT/pTRG and pBT_MamX/pTRG_MamZ versus pBT/pTRG. Thus, notable interactions occurred for MamX-MamY, MamZ-MamY, FtsZ-like–MamX, and FtsZ-like–MamZ but not for MamX-MamZ or FtsZ-like–MamY (Fig. 2C). A predicted network view of MamXY proteins showing intrinsic interactions with each other was generated using the online tool STRING (http://string-db.org) (19). The results of the two techniques combined indicate that the four mamXY operon-encoded proteins are linked end to end and form an interaction flow (Fig. 2D). The similarity of phenotypes of MSR-1 mamX mutant, ftsZ-like mutant, and mamXY operon deletion mutant suggest that magnetite biomineralization involves redundant functions based on the interactions. However, the interaction pattern of the four proteins remains unclear.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source |
|---|---|---|
| Strains | ||
| M. gryphiswaldense MSR-1 | Wild type | DSM 6361 |
| overexp-ftsZ-like strain | ftsZ-like-overexpressing strain, Kmr | This study |
| E. coli DH5α | endA1 hsdR17(rK− mK−) supE44 thi-1 recA1 gyrA (NalR) recA1 Δ(lacZYA-argF)U169 deoR[ϕ80dlacZΔM15] | Lab collection |
| E. coli S17-1 | thi endA recA hsdR with RP4-2-Tc::Mu-Km::Tn7 integrated in chromosome, Smr | Lab collection |
| Plasmids | ||
| pGEM-T | Cloning vector, Ampr | Promega |
| pBBR1MCS-2 | Broad-host-range cloning vector, Kmr | Lab collection |
| pBB-fzl-egfp | pBBR1MCS-2 derivative for FtsZ-like-EGFP expression, Kmr | This study |
| pBBR1MCS2-FtsZ-like | pBBR1MCS-2 containing ftsZ-like from M. gryphiswaldense MSR-1 | This study |
| pBT-LGF2/pTRG-Gal4 | Positive control in BacterioMatch II two-hybrid system | This study |
| pBT/pTRG | Negative control in BacterioMatch II two-hybrid system | This study |
| pBT_MamZ/pTRG_MamY | Used for MamZ-MamY interaction | This study |
| pBT_MamX/pTRG_MamY | Used for MamX-MamY interaction | This study |
| pBT_MamX/pTRG_MamZ | Used for MamX-MamZ interaction | This study |
| pBT_FtsZ-like/pTRG_MamY | Used for FtsZ-like-MamY interaction | This study |
| pBT_FtsZ-like/pTRG_MamX | Used for FtsZ-like-MamX interaction | This study |
| pBT_FtsZ-like/pTRG_MamZ | Used for FtsZ-like-MamZ interaction | This study |
Kmr, kanamycin resistance; Smr, streptomycin resistance; Ampr, ampicillin resistance.
Overexpression of FtsZ-like as a bait protein for protein recruitment.
In view of the predicted function of the FtsZ-like protein in MSR-1, we attempted to use it as a “bait protein” for recruiting the other three mamXY operon-encoded proteins in vivo. FtsZ-like is a cytoplasmic protein and therefore may be difficult to detect in a mixed solution of magnetosome proteins. We constructed an FtsZ-like-overexpressing strain (termed “overexp-ftsZ-like”) by biparental conjugation. A 6×His tag was added to the N terminus to facilitate detection by Western blotting. 6×His-FtsZ-like can be expressed in a preliminary experiment. The protein complex recruited by the FtsZ-like protein was expected to be revealed by Western blotting in later experiments, which would be helpful for the determination of associated bands in SDS-PAGE.
Phenotypic analysis of the FtsZ-like-overexpressing strain.
MSR-1 WT and overexp-ftsZ-like strains were cultured in SLM supplemented with 20, 40, or 60 μM ferric citrate, and the growth rate and magnetic response (Cmag) were monitored until the Cmag began to decline. The WT growth rate did not change notably under the three iron concentrations. With 20 and 40 μM ferric citrate, the growth rates of the overexp-ftsZ-like strain were slightly lower than those of the WT (Fig. 3A and B). The difference was more notable with 60 μM ferric citrate; in particular, the growth rate of overexp-ftsZ-like was 1.68-fold lower than that of the WT at 10 h (Fig. 3C). Cells cultured under high-iron conditions absorb more iron and display greater magnetism than those under low-iron conditions. Cmag is a measure of the average magnetic response. With 20 μM ferric citrate, overexp-ftsZ-like strain first showed a magnetic response at 10 h but lagged 2 h behind the WT and had a lower Cmag value than that of the WT (Fig. 3D). With 40 and 60 μM ferric citrate, the difference was greater, and the overexp-ftsZ-like strain lagged by 4 h and 12 h, respectively, in showing a magnetic response (Fig. 3E and F). These findings demonstrate that the magnetic response in overexp-ftsZ-like was inhibited at higher iron concentration; therefore, 20 μM ferric citrate was used in subsequent experiments.
FIG 3.
Growth rate and magnetic response (Cmag) of WT and overexp-ftsZ-like strains under various iron concentrations. (A to C) Growth rates (measured as OD565) of WT and overexp-ftsZ-like with 20, 40, and 60 μM ferric citrate. (D to F) Cmag values of WT and overexp-ftsZ-like with 20, 40, and 60 μM ferric citrate. Results indicate that 20 μM ferric citrate provides an optimal iron concentration for growth and magnetism of the overexp-ftsZ-like strain.
Based on the Cmag curve for overexp-ftsZ-like with 20 μM ferric citrate, we harvested cells with Cmag values of 0.2 to 0.9 for transmission electron microscopic (TEM) observation. Cells began to form magnetosomes at 8 h, and the number and size of magnetosomes increased gradually with time (Fig. 4A to H). Analyses using the ImageJ software program are shown in Fig. 4I and J and Tables S1 and S2. When the Cmag value was 0.2, the number of magnetosomes per cell was 6 to 10 (±4.7), and the magnetosome diameter was 11 to 15 (±4.2) nm. For Cmag values of 0.3 to 0.4, the number of magnetosomes was 6 to 15 (±4.5), and the diameter was 16 to 20 (±5.4) nm. For Cmag values of 0.5 to 0.6, the number of magnetosomes was 11 to 15 (±4.9), and the diameter was 16 to 25 (±5.9) nm. For Cmag values of 0.7 to 0.8, the number of magnetosomes was 16 to 20 (±5.3), and the diameter was 21 to 30 (±5.8) nm. For a Cmag value of 0.9, the number of magnetosomes was 21 to 25 (±6.4), and the diameter was 31 to 35 (±1.4) nm. The increase in the overexp-ftsZ-like Cmag value from 0.2 to 0.9 reflected the magnetosome maturation process, as the number of magnetosomes and diameter both gradually increased.
FIG 4.
TEM micrographs of overexp-ftsZ-like cells and frequency distributions of magnetosome number and magnetite crystal diameter. (A to H) overexp-ftsZ-like cells viewed by conventional TEM (scale bar = 500 nm) and at higher magnification (insets, scale bar = 200 nm). Arrows are magnetosomes. (I) Distribution of magnetosome number per cell in 400 cells. (J) Distribution of magnetite crystal diameter per cell in 400 cells.
Cross-linking reactions between MamXY proteins.
BS2G-d4/d0 (Pierce Biotechnology, Waltham, MA, USA), a deuteride/nondeuteride pairing, is a type of homobifunctional cross-linker and reacts efficiently with amino (-NH2) groups (26). To clarify the functionalities of MamXY proteins, we used BS2G-d4/d0 as a tool to probe interactions among the proteins and track their expression during the process of magnetosome formation. BS2G-d4/d0 was added to a mixture of magnetosome proteins to stabilize probable protein links, joinings among MamXY proteins were selected based on SDS-PAGE and Western blotting, as described above, and liquid chromatography-tandem mass spectrometry (LC-MS/MS) was performed for further detection and analysis.
Preliminary experiments showed that sufficient magnetosomes were purified from overexp-ftsZ-like cells in a 7.5-liter autofermentor (11) for optimization of cross-linking conditions, and these magnetosomes (Cmag, 1.92) were mature. Purified magnetosomes were recycled in a magnetic shelf (Fig. S2A) and freeze-dried by a vacuum. Cross-linking reaction mixtures were incubated with various concentrations of BS2G-d4/d0 (0.1, 0.2, 0.5, and 1 mM) for 5, 15, or 30 min. The SDS method was found to be more effective than the Triton X-100 method for extraction of MM proteins (data not shown). Major bands, assessed by position in SDS-PAGE and Western blotting, were cut out and analyzed by LC-MS/MS. We found that 0.2 mM BS2G-d4/d0 was suitable for MM protein cross-linking; specifically, the four mamXY cluster-encoded proteins were all visible after a 15-min cross-linking reaction, and the abundance of MamY was higher than that of the other three proteins (Fig. S2B and Table S3). Accordingly, subsequent experiments were performed using cross-linking conditions of 0.2 mM BS2G-d4/d0 for 15 min.
Expression patterns of MamXY proteins.
Based on phenotypic analysis of the overexp-ftsZ-like strain as described above, we collected cells with a Cmag value of 0.2 to 0.9 from multiple culture batches. MM proteins were exposed by a reaction with 0.2 mM BS2G-d4/d0 at 15 min and extracted by SDS-PAGE. Protein bands (Fig. 5A, black arrow) in silver gels were cut off and matched with position in Western blotting (Fig. 5B), and samples were detected by LC-MS/MS. Label-free quantitative analysis of specific MM proteins was performed using the peak area method of the MaxQuant software program, and label-free quantification (LFQ) intensity was normalized based on the ratio of each protein to the total identified proteins and termed normalized LFQ intensity value. Expression patterns of MamXY proteins were associated with Cmag changes based on normalized LFQ intensity values and are represented as a heat map with false-color view (Fig. 5C and Table S4). In the expression matrix, blue indicates low expression, red indicates high expression, and black indicates that the protein was not detected by LC-MS/MS. For the overexp-ftsZ-like Cmag value of 0.2, only MamY was detected; for a Cmag value of 0.3, the second detected protein was MamX; and for a Cmag value of 0.6, the third detected protein was MamZ.
FIG 5.
Expression patterns of MamXY proteins. (A) MM proteins of overexp-ftsZ-like with various Cmag values, analyzed by SDS-PAGE. In this experiment, we ensured the same mass of magnetosomes for all samples in the panels. Because of different straining time of target proteins in each panel, the gels were divided into three groups for silver staining after SDS-PAGE gels. Lanes 1 to 8, cross-linking MM proteins extracted from overexp-ftsZ-like with various Cmag values. Lane M, molecular marker; arrow indicates target bands. (B) Western blotting of MM proteins as in panel A. +, positive control. (C) Expression patterns of MamY, MamX, MamZ, and FtsZ-like proteins in samples with differing Cmag values, represented as a heat map with false-color view, created using the Multi Experiment Viewer tool (http://mev.tm4.org), version 4.8.1. In the expression matrix, blue indicates low expression, red indicates high expression, and black indicates that the protein was not detected by LC-MS/MS. (D) Positions of unique peptides and predicted protein interaction sites in MamXY protein. Green rectangles, unique peptides; orange circles, predicted protein interaction sites in unique peptides.
Although the overexpression strategy was adopted in order to enhance FtsZ-like production, this protein did not reach the HRMS detectable limit during flask culture of MSR-1. Label-free quantitative analysis revealed the following expression rules for the four mamXY-encoded proteins (Fig. 5C): (i) MamY expression did not change notably during the early stage of magnetosome formation (Cmag, 0.2 to 0.4), whereas MamY expression increased sharply (to its maximum) between a Cmag value of 0.4 and 0.5; (ii) MamX expression increased gradually from a Cmag value of 0.4 to 0.7 and then declined from a Cmag value of 0.7 to 0.9 but was maximal at a Cmag value of 0.3; and (iii) MamZ expression increased and then decreased from a Cmag value of 0.6 to 0.9 and was maximal at a Cmag value of 0.7. As Cmag increased, MamY, MamX, and MamZ functioned in turn in the maturation of magnetosomes, and their expression increased to differing degrees during the maturation stage (Cmag, 0.5 to 0.7), suggesting that the functions of the three proteins in this process are coordinated. The cytoplasmic FtsZ-like protein was detected only in submerged culture cells with a Cmag value of 1.9 (Fig. 5C), and the abundance of this protein is presumably increased during magnetosome maturation. When the FtsZ-like protein recruits the other three proteins and is anchored in MM, it may be more susceptible to detection.
Unique peptides may function as interaction joints.
MS analysis provides information on unique peptides that form specific sequences in proteins. We obtained the information of unique peptides using the MaxQuant search engine, based on the M. gryphiswaldense refseq201412 database established in our lab. This analysis, taken together with previous findings regarding the properties of the mamXY cluster and stabilization by a cross-linking reagent of interactions among MamXY proteins, suggests that interaction sites may be in unique peptides of the MamXY proteins. Information regarding unique peptides is presented in Table S5 and Fig. 5D. MamY had the highest number of unique peptides, located at amino acid (aa) sequences 84 to 195, 199 to 233, 273 to 315, and 330 to 371 (Fig. 5D, green rectangle). The numbers of unique peptides were three for MamX (aa 63 to 99, 102 to 112, and 169 to 178), two for MamZ (aa 233 to 253; contiguous), and two for FtsZ-like (aa 171 to 178 and 234 to 253). Protein interaction sites were predicted using the online tool PredictProtein (www.predictprotein.org). The results revealed an overlap in MamX at aa 88 to 90, 96 to 101, and 177 to 198 and in MamZ at aa 220 to 221 (Fig. 5D, orange circle), suggesting connected functions of unique peptides in these two proteins. Unfortunately, bioinformatic analysis did not predict protein interaction sites for the MamY or FtsZ-like proteins, perhaps because of limited information in the protein library. Protein interactions in Magnetospirillum spp. may differ substantially from those in nonmagnetic bacteria. The unique peptides in the four MamXY proteins have common features of overlap and concentration in a particular region (Table S5 and Fig. 5D). Based on cross-linking reactions, frequently used unique peptides are strong candidates as interaction sites (joints). Future studies will focus on verification of unique peptides detected by LC-MS/MS as interaction joints.
DISCUSSION
We used protein cross-linking and HRMS methods in combination to study the functionalities of MamXY proteins and demonstrated that they function synergistically in magnetosome maturation and may form a complex for gradual recruitment of the FtsZ-like protein.
Targeted cross-linking method is useful for analysis of interactions among MM proteins.
In vitro experiments revealed that the FtsZ-like protein has self-polymerization ability and that MamXY proteins undergo interactions with each other. These observations, taken together, suggest that the FtsZ-like protein acts as an interactive recruiter. FtsZ-like is a cytoplasmic protein; we therefore used overexpression and 6×His tagging to increase its content and detectability by Western blotting, allowing accurate identification of a target protein in our constructed protein database. MamX, MamY, and MamZ are MM proteins. The amounts of membrane proteins extracted from magnetosomes are relatively low. We used BS2G-d0/d4 as a cross-linker and immunoblotting in the reaction system to create a targeted cross-linking method useful for the characterization of membrane proteins. In view of the complexity of total MM proteins as opposed to purified monomer, BS2G-d0/d4 should show reactions between other proteins. Although the average number of detected proteins by LC-MS/MS was ∼700 at various sampling times (Fig. S3A), analysis using the MaxQuant proteomic platform using our protein database provided accurate specific information about the proteins. Target proteins can be identified based on their unique peptides. However, information from the protein libraries of nonmagnetic bacteria is extremely limited. Combined with the results from analysis by using the online tool PredictProtein, it appears that protein-protein interaction sites are likely to be hidden in the unique peptides. Certain interaction sites among MamXY proteins further need to be confirmed. We will also continue to explore the MamXY interaction mechanism in MTB.
With regard to sample preparation, we focused on MM proteins. The FtsZ-like protein was found in a sample with high magnetic response (Cmag, 1.91). A small amount of cytoplasmic protein may have been mixed in, or the FtsZ-like protein interacting with MM proteins may itself have been anchored to MM. Our protein database was helpful for distinguishing specific proteins (e.g., MamXY) using sequences of unique peptides found only in MTB. The information gathered will be useful in future studies that target potential functionalities between other proteins.
Functionalities of MamXY proteins during magnetosome formation.
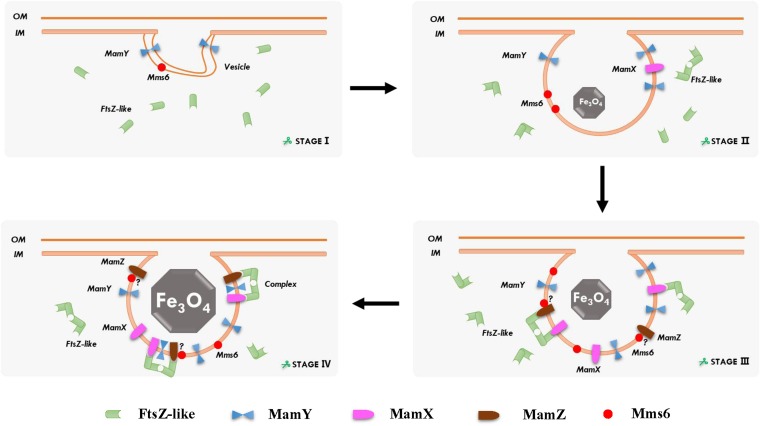
Prior to HRMS analysis, studies of multiple culture batches showed that the optimal iron condition for magnetosome formation in the overexp-ftsZ-like strain was 20 μM ferric citrate, supporting the functionalities of the MamXY proteins during this process. Based on the findings for gene function, protein interaction in vitro, and HRMS analysis, a hypothetical model of the functionalities of MamXY complex proteins in M. gryphiswaldense is presented in Fig. 6. In this model, the process of magnetosome synthesis is divided into four stages. In stage I, the cell membrane undergoes invagination to form MM. MamY is expressed on the MM surface and may help regulate magnetosome vesicle shape (17). The FtsZ-like protein disperses in the cytoplasm. In stage II, in association with crystal growth, the FtsZ-like protein begins self-polymerization and moves gradually closer to MM, but it does not interact with MamY. MamX becomes abundantly expressed and is recruited by polymerized FtsZ-like protein. For stage III, among the unique peptides in MamX, three predicted protein interactions as shown in Fig. 5C initiate interaction with the FtsZ-like protein. MamX has two conserved CXXCH heme-binding motifs, which were termed the “magnetochrome” by Pignol et al. (27). The MamX and FtsZ-like proteins form a complex that initiates electron transport on MM. For stage IV, in association with crystal maturation, MamZ is also recruited by polymerized FtsZ-like proteins; the aa 220 to 221 locus of MamZ may be the connection to the FtsZ-like protein. A MamX–FtsZ-like–MamZ complex is formed and facilitates reduction and associated transport of ferric iron. In addition, one of the MM proteins, Mms6, notably affects magnetosome composition, size, and distribution (28) and has similarity with the expression pattern of MamZ during the magnetosome maturation process. Mms6 may recruit MamZ through some unknown mechanism in order to assemble coherent Mms6 micelles for iron reduction/transport (29); however, it is unclear whether MamZ interacts with Mms6 before or after formation of the MamX–FtsZ-like–MamZ complex. In the late period of magnetosome maturation, building on MamX-MamY and MamY-MamZ interactions, all four proteins form a complex involved in the control of magnetosome size and shape. Although MamY protein interactions were not detected by bioinformatic analysis, frequently used unique peptides focus mainly on the aa 84 to 108, 109 to 136, and 273 to 291 loci, which may serve as interaction sites between MamY and other proteins.
FIG 6.
Hypothetical model of the functionalities of MamXY proteins in M. gryphiswaldense. The four mamXY cluster-encoded proteins interact with each other in a certain sequence and ultimately in the form of a protein complex which helps control magnetosome maturation. Mms6 may interact through some unknown mechanism with MamZ for magnetite biomineralization; however, it is unclear whether MamZ interacts with Mms6 before or after the formation of the MamX–FtsZ-like–MamZ complex. MamZ-Mms6 interactions are being further investigated. OM, outer membrane; IM, inner membrane. “?” represents the interaction between MamZ and Mms6 that needs to be proven further.
Mms6 is closely related to MamZ.
Because Mms6 was observed at a high frequency at each sampling point, we also performed HRMS analysis of the Mms6 expression profile. Cells lacking mms6 form magnetosomes that were poorly defined and smaller than those in AMB-1 or MSR-1 WT (15, 30) and show some phenotypic similarity to the mamXY mutant. High expression of mms6 has been observed in mamZ-deficient and complemented strains in our ongoing studies, suggesting some connection to MamZ (our unpublished data). In HRMS analyses, Mms6 was detected in all samples and showed significantly increased expression at a Cmag value of 0.7 (Fig. S3B). The expression pattern of Mms6 was similar to that of MamZ during the magnetosome maturation process, suggesting coordinated expression of these two proteins (Fig. S3B and C). The Mms6 N terminus most likely mediates contacts between Mms6 and other proteins (30), and the C terminus may bind both ferric and ferrous ions on the magnetosome surface (31, 32). In MamZ, the N terminus may be involved in iron transport by the major facilitator superfamily (MFS), and the C terminus may be involved in ferric reduction. Accordingly, we presumed that Mms6 may interact with MamZ in the magnetosome membrane, and control magnetosome size and shape in association with the MamXY cluster, by iron reduction/transport. This speculation is being validated.
The findings presented here provide strong evidence for interactions among MamXY proteins and define a dynamic orderly function of the MamXY complex in the process of magnetosome formation. The MamXY proteins have synergistic relationships with iron oxidoreduction reactions and transport. Our HRMS and bioinformatic analyses of unique peptides reveal interactions among the four MamXY proteins that depend on the unique peptides. The observed relationships among MamXY proteins and Mms6 provide a basis for more extensive studies of coordination among gene clusters in MAI. The combination of a targeted cross-linking method and HRMS as employed here will be useful for studies that address potential functionalities among other types of proteins.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. M. gryphiswaldense MSR-1 was cultured in sodium lactate medium (SLM) with 20 μM ferric citrate at 30°C with shaking (100 rpm). E. coli strains were cultured in Luria broth (LB) at 37°C. The antibiotics used were for E. coli, ampicillin (Amp) at 100 μg/ml and kanamycin (Km) at 50 μg/ml, and for M. gryphiswaldense, Km at 5 μg/ml and nalidixic acid (Nx) at 5 μg/ml.
Cellular localization of FtsZ-like-EGFP protein.
The ftsZ-like-egfp fusion gene was amplified by fusion PCR and cloned into pBBR1MCS-2 using XbaI and KpnI. The new fusion plasmid (termed pBB-fzl-egfp) was introduced into the MSR-1 WT by biparental conjugation. Cells in different growth stages were observed by confocal laser scanning microscopy.
Bacterial two-hybrid system.
Interactions between pairs of mamXY operon proteins were studied using the BacterioMatch two-hybrid system (Stratagene, La Jolla, CA, USA). Recombinant pBT (bait) and pTRG (target) were used simultaneously to transform E. coli, as per the manufacturer’s instructions. When the bait and target interacted, initial screening for the interaction was based on transcriptional activation of a ampicillin resistance gene in the E. coli reporter gene cassette. The activity of β-galactosidase, encoded by second reporter gene lacZ, was assessed in E. coli reporter strain XL1-Blue to validate bait-target interaction (33). The plasmids used in this study are listed in Table 1. Experiments were performed in triplicate. Differences between means were compared by Student’s t test, with a P value of <0.01 considered significant.
Construction of the ftsZ-like-overexpressing strain.
The MSR-1 ftsZ-like gene with a 6×His tag was amplified by PCR, cloned into pGEM-T vector, and confirmed by sequencing. The 6×His-ftsZ-like gene fragments from pGEM-T and pBBR1MCS-2 were digested by ApaI and SacI and ligated to form pBBR1MCS2-FtsZ-like. pUXF was introduced into the MSR-1 WT by biparental conjugation using E. coli S17-1 as the donor strain. Colonies were screened and selected by Km resistance (Kmr) and Nx resistance (Nxr) and confirmed by PCR. The ftsZ-like-overexpressing strain was termed “overexp-ftsZ-like”. During culturing of the overexp-ftsZ-like strain, expression of the 6×His-ftsZ-like gene was induced by adding isopropyl β-d-1-thiogalactopyranoside (IPTG) to the medium.
Cell growth and magnetic response.
The optical density at 565 nm (OD565) was measured by a UV-Vis spectrophotometer (model UNICO2100; Unico Instrument Co., Shanghai, China). Magnetic response (Cmag) was calculated based on the measurement of maximum and minimum scattering intensities, as described previously (10). The OD565 and Cmag values of the cell suspensions were measured in triplicate at 2-h intervals until 24 h.
Magnetosome numbers and diameters.
Cells with various Cmag values were coated on copper grids, washed twice with double-distilled H2O, and observed directly by TEM (model JEM-1230; JEOL, Tokyo, Japan). Statistical analysis of magnetosome numbers and diameters was performed using the ImageJ software program (National Institutes of Health, Bethesda, MD, USA) (10).
Preparation of magnetosomes and chemical cross-linking of magnetosome proteins.
MSR-1 magnetosomes were isolated and purified as in our previous study (5), freeze-dried, and stored at −20°C. Magnetosomes were mixed with various concentrations of cross-linking reagent BS2G-d0/d4 in a 1.5-ml Eppendorf tube and left for 5, 10, or 30 min, and the reaction was terminated by the addition of NH4HCO3 (final concentration, 20 mM). BS2G-modified magnetosomes were isolated using an NdFeB magnet, resuspended in 40 μl of 10 mM phosphate-buffered saline (PBS), mixed with 5×SDS loading buffer, boiled for 10 min, and centrifuged for 1 min at 12,000 × g. The supernatant was collected and subjected to SDS-PAGE and Western blotting.
LC-MS/MS and data analysis.
Magnetosome samples (4 mg) were obtained from cells having various Cmag values, and then magnetosome proteins were cross-linked and extracted according to the above-described method. Cross-linked MM proteins of each sample were separated by SDS-PAGE, and target proteins were detected by Western blotting with mouse anti-His tag monoclonal antibody (Mab). Target bands of cross-linked MamXY proteins were cut out from the gel and digested overnight with trypsin (Promega, Fitchburg, WI, USA). When the protein-to-enzyme ratio reached 100:1, protein digestion was stopped by the addition of formic acid (final concentration, 0.1%).
Samples were analyzed by LC-MS/MS using HRMS (model Thermo Q Exactive; Thermo Fisher Scientific, Waltham, MA, USA). A nanoflow high-performance liquid chromatography (HPLC) instrument (model EASY-nLC 1000; Thermo Fisher) was coupled online to the mass spectrometer with a nanoelectrospray ion source (Thermo Fisher) for proteomic analysis (34). Chromatography columns were packed with Ultimate XB-C18 3-μm resin (Welch Materials, MD, USA). Peptide mixtures were loaded onto a C18 reversed-phase column (length, 10 cm; inner diameter [i.d.], 75 μm) with buffer A (99.5% water, 0.5% formic acid) and separated on a linear gradient of 3% to 100% buffer B (99.5% acetonitrile, 0.5% formic acid) for 75 min at a flow rate of 350 nl/min. The total time of an LC-MS/MS run, including loading and washing steps, was ∼90 min. The electrospray voltage was 2.0 kV. Peptides were analyzed by data-dependent MS/MS acquisition, with a dynamic exclusion duration of 18 s, using the following parameters: for MS1, resolution, 70,000; AGC target, 3e6; and maximum injection time, 20 ms; and for MS2, resolution, 17,500; AGC target, 1e6; maximum injection time, 60 ms; and scan range, m/z 300 to 1,400. The 20 most intense precursor ions were selected for MS/MS analysis.
Raw data were processed using the MaxQuant proteomics platform (35). Fragmentation spectra were searched against the M. gryphiswaldense database (refseq201412) established in our lab. Precursor and fragment mass tolerances were set, respectively, at 10 ppm and 20 millimass units (mmu) for the Q Exactive data, using the MaxQuant search engine (version 1.6.2.0). Two missed cleavage sites were allowed. The minimum peptide length was seven amino acids. Variable modifications included oxidation (M) and acetylation (protein N terminus), and the fixed modification was carbamidomethyl (C). Peptide ions were filtered using a cutoff score of 20. The false-discovery rate (FDR) was set to 1% for peptide identification. Label-free quantitative analysis of cross-linked proteins was performed using the LFQ intensity algorithm in MaxQuant (36).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (grant 31270093), the China Postdoctoral Science Foundation (grant 2015M570175), and the Project for Extramural Scientists of State Key Laboratory of Agro-biotechnology (grants 2015SKLAB6-24 and 2017SKLAB7-5).
We are grateful to Zhen Li (Mass Spectrometry Lab, Functional Genomic Technology Center, China Agricultural University) for help with analysis of HRMS data and to S. Anderson for English editing of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02394-18.
REFERENCES
- 1.Bazylinski DA, Frankel RB. 2004. Magnetosome formation in prokaryotes. Nat Rev Microbiol 2:217–230. doi: 10.1038/nrmicro842. [DOI] [PubMed] [Google Scholar]
- 2.Frankel RB, Blakemore RP, Wolfe RS. 1979. Magnetite in freshwater magnetotactic bacteria. Science 203:1355–1356. doi: 10.1126/science.203.4387.1355. [DOI] [PubMed] [Google Scholar]
- 3.Schübbe S, Kube M, Scheffel A, Wawer C, Heyen U, Meyerdierks A, Madkour MH, Mayer F, Reinhardt R, Schuler D. 2003. Characterization of a spontaneous nonmagnetic mutant of Magnetospirillum gryphiswaldense reveals a large deletion comprising a putative magnetosome island. J Bacteriol 185:5779–5790. doi: 10.1128/JB.185.19.5779-5790.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blakemore R. 1975. Magnetotactic bacteria. Science 190:377–379. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- 5.Li A, Zhang H, Zhang X, Wang Q, Tian J, Ying l, Li J. 2010. Rapid separation and immunoassay for low levels of Salmonella in foods using magnetosome-antibody complex and real-time fluorescence quantitative PCR. J Sep Sci 33:3437–3443. doi: 10.1002/jssc.201000441. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Duan J-H, Dai S-L, Ren J, Guo L, Jiang W, Ying l. 2008. Preparation and anti-tumor efficiency evaluation of doxorubicin-loaded bacterial magnetosomes: magnetic nanoparticles as drug carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol Bioeng 101:1313–1320. doi: 10.1002/bit.22011. [DOI] [PubMed] [Google Scholar]
- 7.Sun J-B, Duan J-H, Dai S-L, Ren J, Zhang Y-D, Tian J-S, Li Y. 2007. In vitro and in vivo antitumor effects of doxorubicin loaded with bacterial magnetosomes (DBMs) on H22 cells: the magnetic bio-nanoparticles as drug carriers. Cancer Lett 258:109–117. doi: 10.1016/j.canlet.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Han L, Zhang A, Wang H, Pu P, Jiang X, Kang C, Chang J. 2010. Tat-BMPs-PAMAM conjugates enhance therapeutic effect of small interference RNA on U251 glioma cells in vitro and in vivo. Hum Gene Ther 21:417–426. doi: 10.1089/hum.2009.087. [DOI] [PubMed] [Google Scholar]
- 9.Tang YS, Wang D, Zhou C, Ma W, Zhang YQ, Liu B, Zhang S. 2012. Bacterial magnetic particles as a novel and efficient gene vaccine delivery system. Gene Ther 19:1187. doi: 10.1038/gt.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Liu JX, Zhang WJ, Zhang TW, Yang J, Li Y. 2013. Expression patterns of key iron and oxygen metabolism genes during magnetosome formation in Magnetospirillum gryphiswaldense MSR-1. FEMS Microbiol Lett 347:163–172. doi: 10.1111/1574-6968.12234. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhang X, Jiang W, Li Y, Li J. 2011. Semicontinuous culture of Magnetospirillum gryphiswaldense MSR-1 cells in an autofermentor by nutrient-balanced and isosmotic feeding strategies. Appl Environ Microbiol 77:5851–5856. doi: 10.1128/AEM.05962-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ullrich S, Kube M, Schübbe S, Reinhardt R, Schüler D. 2005. A hypervariable 130-kilobase genomic region of Magnetospirillum gryphiswaldense comprises a magnetosome island which undergoes frequent rearrangements during stationary growth. J Bacteriol 187:7176–7184. doi: 10.1128/JB.187.21.7176-7184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Wang Q, Zhang W, Wang Y, Li L, Wen T, Zhang T, Zhang Y, Xu J, Hu J, Li S, Liu L, Liu J, Jiang W, Tian J, Li Y, Schuler D, Wang L, Li J. 2014. Complete genome sequence of Magnetospirillum gryphiswaldense MSR-1. Genome Announc 2:e00171-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohsse A, Ullrich S, Katzmann E, Borg S, Wanner G, Richter M, Voigt B, Schweder T, Schüler D. 2011. Functional analysis of the magnetosome island in Magnetospirillum gryphiswaldense: the mamAB operon is sufficient for magnetite biomineralization. PLoS One 6:e25561. doi: 10.1371/journal.pone.0025561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka M, Mazuyama E, Arakaki A, Matsunaga T. 2011. MMS6 protein regulates crystal morphology during nano-sized magnetite biomineralization in vivo. J Biol Chem 286:6386–6392. doi: 10.1074/jbc.M110.183434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheffel A, Gärdes A, Grünberg K, Wanner G, Schüler D. 2008. The major magnetosome proteins MamGFDC are not essential for magnetite biomineralization in Magnetospirillum gryphiswaldense but regulate the size of magnetosome crystals. J Bacteriol 190:377–386. doi: 10.1128/JB.01371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka M, Arakaki A, Matsunaga T. 2010. Identification and functional characterization of liposome tubulation protein from magnetotactic bacteria. Mol Microbiol 76:480–488. doi: 10.1111/j.1365-2958.2010.07117.x. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Suwatthanarak T, Arakaki A, Johnson BRG, Evans SD, Okochi M, Staniland SS, Matsunaga T. 2018. Enhanced tubulation of liposome containing cardiolipin by MamY protein from magnetotactic bacteria. Biotechnol J 13:e1800087. doi: 10.1002/biot.201800087. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Li S, Huang X, Li J, Li L, Pan Y, Li Y. 2013. MamX encoded by the mamXY operon is involved in control of magnetosome maturation in Magnetospirillum gryphiswaldense MSR-1. BMC Microbiol 13:203. doi: 10.1186/1471-2180-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raschdorf O, Muller FD, Posfai M, Plitzko JM, Schuler D. 2013. The magnetosome proteins MamX, MamZ and MamH are involved in redox control of magnetite biomineralization in Magnetospirillum gryphiswaldense. Mol Microbiol 89:872–886. doi: 10.1111/mmi.12317. [DOI] [PubMed] [Google Scholar]
- 21.Ding Y, Li J, Liu J, Yang J, Jiang W, Tian J, Li Y, Pan Y, Li J. 2010. Deletion of the ftsZ-like gene results in the production of superparamagnetic magnetite magnetosomes in Magnetospirillum gryphiswaldense. J Bacteriol 192:1097–1105. doi: 10.1128/JB.01292-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller FD, Raschdorf O, Nudelman H, Messerer M, Katzmann E, Plitzko JM, Zarivach R, Schüler D. 2014. The FtsZ-Like protein FtsZm of Magnetospirillum gryphiswaldense likely interacts with its generic homolog and is required for biomineralization under nitrate deprivation. J Bacteriol 196:650–659. doi: 10.1128/JB.00804-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan G, Dajkovic A, Wirtz D, Sun SX. 2008. Polymerization and bundling kinetics of FtsZ filaments. Biophys J 95:4045–4056. doi: 10.1529/biophysj.108.132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goley ED, Yeh YC, Hong SH, Fero MJ, Abeliuk E, McAdams HH, Shapiro L. 2011. Assembly of the Caulobacter cell division machine. Mol Microbiol 80:1680–1698. doi: 10.1111/j.1365-2958.2011.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee A, Cao C, Lutkenhaus J. 1998. Inhibition of FtsZ polymerization by SulA, an inhibitor of septation in Escherichia coli. Proc Natl Acad Sci U S A 95:2885–2890. doi: 10.1073/pnas.95.6.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinz A. 2014. The advancement of chemical cross-linking and mass spectrometry for structural proteomics: from single proteins to protein interaction networks. Expert Rev Proteomics 11:733–743. doi: 10.1586/14789450.2014.960852. [DOI] [PubMed] [Google Scholar]
- 27.Siponen MI, Adryanczyk G, Ginet N, Arnoux P, Pignol D. 2012. Magnetochrome: a c-type cytochrome domain specific to magnetotatic bacteria. Biochem Soc Trans 40:1319–1323. doi: 10.1042/BST20120104. [DOI] [PubMed] [Google Scholar]
- 28.Staniland SS, Rawlings AE. 2016. Crystallizing the function of the magnetosome membrane mineralization protein Mms6. Biochem Soc Trans 44:883–890. doi: 10.1042/BST20160057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Bu W, Wang L, Palo PE, Mallapragada S, Nilsen-Hamilton M, Vaknin D. 2012. Interfacial properties and iron binding to bacterial proteins that promote the growth of magnetite nanocrystals: X-ray reflectivity and surface spectroscopy studies. Langmuir 28:4274–4282. doi: 10.1021/la205074n. [DOI] [PubMed] [Google Scholar]
- 30.Lohsse A, Borg S, Raschdorf O, Kolinko I, Tompa E, Posfai M, Faivre D, Baumgartner J, Schuler D. 2014. Genetic dissection of the mamAB and mms6 operons reveals a gene set essential for magnetosome biogenesis in Magnetospirillum gryphiswaldense. J Bacteriol 196:2658–2669. doi: 10.1128/JB.01716-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawlings AE, Bramble JP, Hounslow AM, Williamson MP, Monnington AE, Cooke DJ, Staniland SS. 2016. Ferrous iron binding key to Mms6 magnetite biomineralisation: a mechanistic study to understand magnetite formation using pH titration and NMR spectroscopy. Chem Eur J 22:7885–7894. doi: 10.1002/chem.201600322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashyap S, Woehl TJ, Liu X, Mallapragada SK, Prozorov T. 2014. Nucleation of iron oxide nanoparticles mediated by Mms6 protein in situ. ACS Nano 8:9097–9106. doi: 10.1021/nn502551y. [DOI] [PubMed] [Google Scholar]
- 33.Slepenkin A, de la Maza LM, Peterson EM. 2005. Interaction between components of the type III secretion system of Chlamydiaceae. J Bacteriol 187:473–479. doi: 10.1128/JB.187.2.473-479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui Y, Liu X, Li X, Yang H. 2017. In-depth proteomic analysis of the hippocampus in a rat model after cerebral ischaemic injury and repair by Danhong injection (DHI). Int J Mol Sci 18:1355. doi: 10.3390/ijms18071355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox J, Mann M. 2008. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 36.Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, Mann M, Cox J. 2016. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods 13:731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.