Abstract
Background:
The metabolic syndrome unites three pathologies of the person – obesity, arterial hypertension and diabetes. In recent years the progressing of such distribution covering from 2.5% to 3.8% of the population with increase twice each 10-15 years is noted. Even at maintenance of level of sugar at diabetes accumulation of the secondary metabolites breaking small vessels isn't excluded. At the same time many life-endangering complications develop.
Objective:
To identify the possibilities of plasmapheresis in the prevention and treatment of complications of metabolic syndrome.
Method:
Analysis of the world literature data on complications of metabolic syndrome and methods of their treatment.
Results:
At metabolic syndrome the frequency of strokes and myocardial infarctions there is twice more often than in population. For 5-9 years the general life expectancy decreases. Disorders of microcirculation at diabetes lead to a retinopathy with total loss of sight, a nephropathy from the outcome in a renal failure, to polyneuropathy and diabetic foot syndrome with high risk of high level amputations of the lower extremities. At the same time medicamentous therapy is not able to prevent such complications and almost only way of removal of these pathological metabolites is therapeutic apheresis, mainly the plasmapheresis. Data from our own studies confirm the effectiveness of such tactics.
Conclusion:
Plasmapheresis has to be applied not only to the correction of already critical conditions, but also to their prevention.
Keywords: Metabolic syndrome, diabetes, diabetic foot, diabetic nephropaty, retinopathy, plasma-pheresis
1. Introduction
Metabolic syndrome or insulin resistance factor is clearly associated not only with the impairment of glucose tolerance and development of type II diabetes, but also with dyslipidemia with visceral type of adiposis, hypertension as well as with prothrombotic state. Moreover, metabolic syn-drome is the early stage of atherosclerosis devel-opment, diabetes mellitus type II, and endothelial dysfunction [1]. Its prevalence is from 2.5% to 3.8% of population with twofold increase in morbidity each 10-15 years. Among subjects over 70 years old diabetes mellitus appears to occur in 10% of cases. If in the end of the XX century there have been 135 million suffering from diabetes mellitus worldwide, by 2005 the amount will have increased up to 300 million [2, 3].
Accompanied by the accumulation of various pathological products infringing microcirculation and blood flow in the vessels of the heart, brain, kidney, eyes and peripheral arteries. Drug therapy is not always able to eliminate such complications and to the forefront apheresis therapy mainly plasmapheresis [4].
With the development of obesity there are a number of interdependent processes. Thus, adipo-cytes contribute to the fatty acids output from chylomicrons, which, in turn, increases the yield of triglycerides of adipocytes. Chylomicrons affect endothelial cells and their triglycerides are rapidly hydrolyzed into lipoprotein lipase. Developing dislipoproteinemia with accumulation of low de-nsity lipoprotein, apo-B, triglycerides, non-esteri-fied fatty acids contributes to the development of insulin resistance, and that closes the vicious circle. At the same time, it must be kept in mind that abdominal obesity is associated with insulin resistance with high risk of metabolic syndrome more, then general obesity [5].
In this case, on the first stage, while main-taining β-cells, overproduction of insulin is also possible to compensate insulin resistance [6]. There are evidences showing that hyperinsuli-nemia, associated with impaired lipid metabolism, in turn, also contributes both to high triglyceride levels (over 2.5 mmol/liter) and high density lipoproteins decrease with increasing tendency to arterial hypertension. Hyperinsulinemia is also associated with the increased thickness of the arterial wall [7, 8]. Higher levels of cholesterol and triglycerides also create favorable conditions for the development of cholelithiasis [9].
Obesity also contributes to the accumulation of “nonfat” tissues lipids, leading to its degeneration. This refers to the development of fatty liver and even of liver cirrhosis [10-13]. Free fatty acids along with triglycerides, accumulate in the paren-chymal cells of various organs, including cardio-myocytes, hepatocytes, β-cells of the pancreas, that results in chronic dysfunction of beta-cells [14].
In patients with cardiovascular diseases, coronary artery disease and myocardial infarction there is an increased level of “1 plasminogen activator inhibitor” (PAI-1). In syndrome of in-sulin resistance associated with obesity this level also increases because PAI-1 is formed and sec-reted from adipocytes, - mostly by visceral rather than subcutaneous ones. Level of PAI-1 is also increased in patients with Cushing's syndrome and receiving glucocorticosteroids [15].
It is remarkable that the disorders of vascular reactivity and appearance of biochemical markers of endothelial cell activation (endothelin-1, von Willebrand factor, soluble cell adhesion molecules and endothelial cell-cell adhesion) occur very early in individuals at risk of developing diabetes type II, even at the stage of normal glucose tolerance and absence of insulin resistance [16].
In those with the “central” type of obesity, high levels of total cholesterol, triglycerides and very low density lipoproteins, high blood pressure the risk of vascular lesions arises even in the phase of “pre-diabetic” (less than 6.1 mmol/l) glucose level [17]. At the same time, as in pre-diabetic phase and when diabetes is being diagnosed, it is often possible to identify the presence of coronary heart disease already [8]. The study of 5522 diabetic patients, aged 55 years old and older, revealed a clear correlation between the metabolic syndrome and thromboembolism of the deep veins as well as of the lung vessels with a frequency of 0.30-0.40 for the 5-year observation period [18].
In metabolic syndrome the risk and frequency of ischemic strokes increases. At the same time, in such cases there are about 60% of patients with metabolic syndrome, while there are about 20% of such patients among the other neurologic patients. Women, suffering from a metabolic syndrome, are especially subjected to ischemic strokes [19, 20]. The same can be told about risk of coronary heart disease, especially in the senior age group which increases twofold in men and fivefold in women, suffering from metabolic syndrome [21].
On the other hand, in metabolic syndrome men tend more often to develop hypogonadism with sexual and erectile dysfunctions [22-25].
Microcirculatory disorders in diabetes are aggravated by higher blood viscosity due to an increase in fibrinogen, fibronectin, von Willebrand factor and C-reactive protein [26].
Nowadays, the prevalence of metabolic syn-drome takes on the character of an epidemics, especially when it starts in childhood, which further leads to the earlier development of athero-sclerosis [6, 12]. S.W. Ryder (2007) [27] also reports that in the United States 1/3 of adults suffer from obesity, and for the last 20-30 years there is a 2-3 fold increase in the number of children who are overweight. The higher risk of cardiovascular disease in diabetes mellitus type II is associated with the accumulation of low-density lipoprotein and triglycerides due to lower high-density lipo-proteins, which is the anti-atherogenic factor [28].
One of the causes of VLDL hyperproduction is both insulin resistance and reduction of lipoprotein lipase activity against the raised glucose [29]. After coronary bypass operations in patients with hyperglycemia and insulin resistance more intense progression of atherosclerotic lesions of the coronary vessels is observed [30]. Studies have shown a direct correlation of the insulin-depen-dence level with the increase of peripheral vascular resistance, blood pressure and decrease of blood flow of the peripheral vessels [31]. A signi-ficant relationship between the impaired glucose tolerance and vascular dementia has also been found [32]. Diabetes mellitus type II at age of 55 reduces the expected individual life span for about 5 years [33].
However, diabetic patients can develop severe cardiomyopathy associated mostly with disorders of microcirculation in the myocardium than with atheromatous narrowing of the coronary arteries. It has a non-specific functional and morphological changes including: cardiomyocyte hypertrophy, interstitial fibrosis, arteriolar thickening, capillary microaneurysms associated with their network decrease, disturbances of left ventricular diastolic and then systolic function.
The development of such diabetic cardiomyo-pathy appears multifactorial. Pathogenetic mecha-nisms include greater density and stiffness with loss of elasticity due to ventricular myocardial fibrosis, microvascular damages, disorders of energy metabolism in the myocardium, structural abnormalities of collagen, contractile proteins and cardiac muscle sarcolemma [34]. At the same time the prevalence of left ventricular diastolic dysfunction can reach 85% [35].
In this syndrome there are more pronounced rises in blood pressure and vascular resistance during stress than in the control group. This hyperreactivity is a marker for future hypertension in normotensive yet, but hyperinsulinemic obese patients. Diabetes mellitus type II and hyper-tension are often linked. At the age of 45 years, about 40% of these patients have hypertension, and in 75 years – already 60%. Hypertension, in turn, increases the risk of cardiovascular disease, retinopathy and microalbuminuria in diabetes mellitus type II [36].
It is interesting to note that 53-80% of patients with hemochromatosis develop diabetes type II. Iron is capable of catalyzing free radical stress while free radicals and lipid peroxidation plays a certain role in the etiology of this form of diabetes. Special studies have confirmed the role of iron in the delay the development of diabetes type II [37]. Patients with cystic fibrosis are also predisposed to the development of both diabetes type I and type II. The glucose production by the liver is more intense, as well as in diabetes type II [38].
There is a definite relationship of insulin and triglyceride levels. In patients with diabetes melli-tus who are treated without insulin, blood trigly-ceride concentration increases. Insulin helps re-duce triglyceride levels. Most insulin dependent diabetic patients are insulin resistant and may have a compensatory chronic hyperinsulinemia, which increases the production of triglyceride-containing lipoproteins.
These changes may reflect the balance of many activities – from the increasing number of Non-Esterified Fatty Acids (NEFA) prior to the initiation of the intrahepatic processes of Very Low Density Lipoproteins (VLDL) production. Removal of the latter is performed by lipoprotein lipase, which activity also increases blood levels of insulin. However, the degradation triglycerides products are atherogenic, so the growth of such degradation intensity in diabetic patients increases the risk of atherosclerosis. Under these conditions, lipemia after a fatty meal increases the activity both of hepatic lipase and cholesterol esters. These factors also contribute to the formation of small LDL particles – the major atherogenic agents [39, 40].
In recent years it has been shown that TNF-α also affects the metabolism of lipids and glucose. Adipose tissue is an important source of endo-genous TNF-α out-turn and the expression of such cytokine increases with obesity, which in turn contributes to the development of insulin resis-tance in diabetes type II [41]. Elevated levels of ketone bodies promote greater intensity of lipid peroxidation and hydroxyl radicals in the vascular endothelium and in erythrocytes in diabetes type I, which contributes to the development of vascular complications. Obesity is associated with meta-bolic lesions in different tissues. In particular, the maintenance of the endotoxin (lipopoly-sac-charide) allocated in intestines, that triggers a number of pro-inflammatory and oxidative pro-cesses, In this case a kind of “metabolic endotoxemia“ occuries [42]. There is a communication of a metabolic syndrome with gout at which the increased level of uric acid promotes also other metabolic disorders with higher mortality [43]. There is a distinct correlation between the metabolic syndrome and psoriasis [44, 45].
Thus, the presence of both immune and metabolic changes in this form of diabetes makes it reasonable to use apheresis therapy at all stages of the disease. Attempts to use drugs against hypercholesterolemia can lead to a number of adverse complications. So, clofibrate effectively reduced content of atherogenic lipids, but in patients with diabetes it increased mortality from non-cardiac diseases. In particular, there was a 68% mortality increased from tumor diseases [46]. Moreover, during the statins treatment of patients with type II diabetes the content of antiatherogenic HDL has decreased more significantly and triglycerides concentration increased to compare with the patients without diabetes [47].
The fact that the diet, drug therapy, insulin allow to keep blood sugar levels, but it does not prevent its oscillation, which leads to a variety of secondary metabolic disorders, mainly vascular. At the same time, one of the reasons of the angiopathy in diabetes is the increase of platelet aggregation, which depends not only on the magnitude of the concentration of sugar in the blood, but from some other pathological products, affecting platelet membrane [48].
Prolonged hyperglycemia leads to glycation of proteins. Glycation of collagen can provoke atherogenesis by receipt of lipoproteins into the extracellular matrix, making it more susceptible to oxidative modification. The end products of the process of proteins glycation promote migration of monocytes and macrophages expression, which is an important mechanism for development of the early stages of atherogenesis. Nonenzymatic gly-cation end products are one of the toxic factors that determine the development of vascular comp-lications in diabetes. Among them is pyraline, arising from the interaction of glucose with amino groups of proteins. One of the consequences of its effects is the suppression of phagocytic activity, predisposing to infectious complications, also characteristic to diabetes [49].
Recent studies show that diabetes develops “oxidative stress.” Due to the reduction of antio-xidant status production of free radicals increases, enzymatic disorders that largely determine the secondary organ complications from diabetes [50]. Even with a normal level of LOW DENSITY LIPO-PROTEIN (LDL) there may be detectable markers of oxidation, such as antibodies to oxidized LDL and LDL-containing immune complexes, which seems a predisposing factor for the development of coronary artery lesions [51].
Observed in the clinic faster progression of coronary artery disease in diabetic patients is largely determined by the higher oxidative modi-fication of lipoproteins on the background of the intensification of lipid peroxidation. However, attempts to improve the condition of vessels using long-term administration of antioxidants (vitamin E) did not lead to the desired result [52].
In diabetes, superoxide production by mono-cytes increases, which is probably due to hyper-triglyceridemia. Susceptible to oxidative modifi-cation lipoproteins become immunogenic, resul-ting in the formation of circulating immune comp-lexes lipoproteins promoting the progression of atherosclerosis as a result of formation of “foam cell” – macrophages engulfing these immune complexes, and catalytic atherogenic immune mechanisms in the walls of the arteries. In addition, there is a relationship of diabetes with increasing tendency to thrombosis in the field of atheromatous lesions. In diabetes, adhesiveness and aggregation of platelets increase, as well as different levels of coagulation factors and tissue plasminogen inhibitors anticoagulant, which does potentially contribute procoagulant state [53].
Occlusive vascular diseases with disorders of both central and peripheral circulations are almost constant and rather severe satellites of diabetes. According to the U.S. National Commission for diabetes, these patients is 25 times more likely to go blind, 17 times more likely to suffer from kidney disease, 5 times more often affected limb gangrene, 2 times more likely – heart disease, and life expectancy is 30% shorter.
Diabetic foot syndrome is the most frequent problem at patients with type 2 diabetes. The syndrome of “diabetic foot” occurs at 15% from 200 million patients with diabetes around the world. In the countries of the West more than 60% of not traumatic amputations are higher - or below a knee is carried out at patients with diabetes [54]. Rather big spread of diabetes is available in the Arab countries where the number of such patients reaches 20,5 million, and the frequency of development of diabetic foot up to 25-31% [55]. At the same time amputation below a knee has been carried out in 72% and above a knee – at 27% of patients with diabetes [56]. In North America frequency of diabetic foot reaches 13.0% (USA) and 14,8% (Canada), and in Belgium – 16.6% [57]. According to data of the International Working Group on Diabetic foot 72,8% have a small risk of development of diabetic foot, but the high risk at the same time is 17,5% [58].
In the USA where about 16 million people have diabetes, 50-60 thousand amputations of extre-mities are annually produced. And such tendency accrues. So, if in 2005 in the USA there were 1.6 million amputated, then by 2050 their doubling up to 3.6 ml of people is expected [59]. In the cohort research performed in Turku (Finland) during 1998-2002 the rate of amputations both above- and below the knee, in case of the occlusion diseases of arteries of the lower extremities is 24,1 on 100 000 population for 1 year. In the same research covering France, need of amputations of the lower extremities arose at 15,353 people among whom 7,955 had diabetes. In the latter case the rate of amputations was 378 on 100.000 of population. At the same time need of amputations was 12 times higher in patients with diabetes, than in other cases. And, in 40% of cases amputation was per-formed in patients, previously having no signs of arterial blood circulation disorders of the lower extremities; so, the main reason for the amputation was diabetes and polyneuropathy [60].
Often there is the development of “diabetic” gangrene of the distal segments of the lower extremities. There are subjective complaints of body aches and muscle pain. In the pathogenesis of these disorders the deposition of sorbitol in the peripheral nerves plays a key role along with the activation of the so-called graft polyol that reduces intraneural blood flow and leads to chronic hypoxia with functional and structural changes in the nerve trunks. There are also conditions for segmental demyelination of the nerve fibers asso-ciated with slowing-down of the neural excitation speed.
The formed trophic ulcers, as a rule, don't tend to healing, progress and inevitably lead to ampu-tation not only feet, but also shins, and is frequent also hips. At the same time more than a half of such patients within the next 5 years have a need of amputation and a contralateral extremity.
Accession of an infection and gangrene is often resulted at the same time in need of amputation [61-64]. Septic complications at the same time are also a frequent cause of death after operations [65].
In the presence of diabetic ulcers of the lower extremities the risk of mortality within 5 years fluctuates from 43% to 55% and reaches 74% for the patients, who have already undergone ampu-tations. Cardiovascular diseases at the same time are the main reasons for death [66]. And, in the next 10 months mortality in the patients who have already undergone high amputations is much higher, than after the sparing amputations [67].
Repeated amputation was required in 34% of the amputated patients. Annual mortality in this group (210 patients) was 52%, and the general mortality for this period was 80% [68]. The need of repeated amputation arose from 23% to 60.7% of patients in the next 3 years. And, reamputation of a counterlateral extremity was carried out more often than the ipsilateral one [69]. From 3565 patients within a year repeated amputation was required in 26%, and more than 30% of them have died. The general costs of treatment of such patients in the USA have made 4.3 billion dollars [70]. In total in the USA for 2007 17.5 million patients with diabetes have been registered, and the general costs of their treatment have made 174 billion dollars [71].
Necrotic processes of the lower extremities in patients with diabetes can be limited to hypo-dermic cellulose, development of the deeper tissues and even muscular necrosis. In such cases the need of high amputation arises from 7.3% to 20.9% and even 53.2%, respectively [72]. High amputations were necessary even after the sparing amputations of foot in cases of activation of soft tissues inflammation or osteomyelitis [73].
Operations for revascularization (peripheral angioplasty or artificial vascular prosthesis) allow to postpone amputations in the syndrome of diabetic foot, however, when it is impossible or isn't effective, indications for amputation arise much more often [74]. In such cases high amputations were necessary in 13.4% of patients, and later in 86.2% of cases they have been carried out after peripheral angioplasty, to 21.1% - after vascular shunting and to 59.2% - in group of patients who hadn’t undergone operations for revascularization. At the same time, restenosis have developed in 16.7% of patients, thromboses of shunts – in 6.4% and a recurrence of ulcers – in 12.6% of patients. Critical ischemia of the counterlateral extremity has developed in 39.9% of patients and high amputation was required in 6.7% of them. For the four-year period of observations 49.82% of these patients have died, mainly because of coronary insufficiency [75]. It should be noted that operations of revascularization are more effective in non-diabetic occlusion vessels damages of the lower extremities [76].
The risk of coronary lesions in diabetes is 10-20 times higher and lethal outcome after myocardial infarction in these patients is 2 times higher than those without diabetes [77, 78]. Coronary balloon angioplasty in patients with diabetes carries a greater risk of restenosis and mortality [79]. The increased risk of shunts occlusion increases the need for reoperation of the coronary vessels [80]. Diabetic patients also can have cerebral circulatory disorders, progressing as the disease develops.
Diabetic retinopathy results in irreversible loss of vision. Diabetes generally is the leading cause of blindness among working-age population. In the United States the number of newly blinded patients with diabetic retinopathy increased by 8,000 people, and in Germany as a result of diabetic retinopathy blindness rate reaches 2.01 per 100,000 of population [81]. The changes of the retina at different time from the onset of diabetes are found in 98.8% of cases [82].
At the same time there is also proliferation of vessels according to the chorio-vitreous type of the vitreous body neovascularization discovered as one of the manifestations of proliferative diabetic retinopathy [83]. In these patients, significantly more than in the control groups autoantibodies to phosphatidyl-ethanolamine were detected [84]. Besides, in these cases the content Vascular Endothelial Growth Factor (VEGF) increases in the intraocular liquid, which in addition to stimulating angiogenesis promotes the increased vascular permeability for plasma proteins output and extra-vasal organization of the fibrin gel. The role of VEGF is considered to be the key in the pathogenesis of diabetic retinopathy [85].
Such patients are advised to take systematic courses of plasmapheresis 2 times a year, which will delay the progression of diabetic retinopathy. Plasmapheresis allows 40.5% of patients to reduce the number of retinal hemorrhages, 85% of patients improve visual acuity with the disap-pearance of “fog” and “flies” before the eyes, in 14% of patients on 5-70 expand the field of view on the background of improving microcirculation of bulbar conjunctiva with the disappearance of “sludge” syndrome, increase of blood flow and restore its continuity, reduce perivascular edema, a significant reduction in levels of cholesterol, triglycerides, fibrinogen.
Proximal diabetic neuropathy, accompanied by severe pain due to inflammatory lesions of the nerves in patients with insulin dependent diabetes mellitus type II at age over 50. Severe pain is not always amenable to steroid and cytostatic therapy [86]. This symmetrical sensory neuropathy asso-ciated with a range of structural changes in the peripheral nerves, including axonal degeneration, demyelination para-nodal with loss of myelinated fibers. The latter is possible due to the withering away of the distal axons as a result of phosphory-lation of proteins [87].
Upcoming polyneuropathy is accompanied by disturbances of both motor and sensory nerve fibers, as well as elements of the autonomic system. Motor neuropathy is a cause of muscle weakness, atrophy and paresis. Sensory neuro-pathy leads to weaken of the “watchdog” sensi-tivity to pain, compression and of thermal damage. Therefore minor injuries remain unnoticed. The patient does not respond to prolonged com-pression, even footwear that breaks the power of individual sections of the lower limb. Autonomic dysfunction is accompanied by a condition similar to sympathectomy with functional disorders of microcirculation. All the above mentioned greatly increases the risk of trophic ulcers and gangrene of the foot sections [88]. There is an evidence of a positive effect of plasmapheresis in trophic ulcers of the shins that developed due to the varicose veins dilatation or necrotizing vasculitis.
Diabetic microangiopathy is characterized by impaired capillary basement membrane structure, deposition of LDL in the vascular wall and proliferation of smooth muscle cells there. Related neuropathy contributes to narrowing of arterioles and precapillaries with increasing blood flow through the artery-venous shunts, which further depletes nutrition and gas exchange of peripheral tissues. This is accompanied by increased blood circulation in the skin with its increased surface temperature. Therefore, along with the reduction in sensitivity due to neuropathy there may be a feeling of the heat and burning of the skin and feet, night pain.
There is quite a clear relationship of diabetes and atherosclerosis. When this occurs it causes the formation of autoantibodies (anti-endothelial and “sclerotic”) and circulating immune complexes, accumulation of complement and its C3 fraction. If diabetes is accompanied by hypertension the described immunological disorders develop more rapidly and promote atherosclerotic lesions of both peripheral and coronary vessels. At the same time, these immunological changes precede the clinical vascular manifestations [89].
It should be taken into account that one of the pathogenic mechanisms of atherosclerosis develo-pment is considered to be low density lipoprotein oxidation, transforming them into a form that is available for capture by macrophages followed by the last generation of cytokines and other biologically active molecules which attract T-cells to increase their adhesion to the endothelium. Hyperglycemia, increasing the prooxidant status, thus, activates atherogenesis with an increased risk of vascular lesions. According to the pathological studies, coronary atherosclerosis in diabetic patients is found 1.7 times more frequently in men and 2.7 times more common in women, cerebral vascular lesions in 2.7 and 3.8 times more likely, and vascular pathology of the lower limbs are in 4 and 6.4 times more, respectively [90]. Diabetics are 2-5 times more likely to die from athero-sclerosis than nondiabetics. However, extremely long atherosclerotic vascular changes occur before the first symptoms of their involvement.
Vascular endothelium in diabetes has a smaller capacity for the vasodilators synthesis and pro-duces more vasoconstrictors and procoagulants. These features exacerbate vascular disorders in diabetes – retinopathy, neuropathy and ischemia. In particular, the vascular endothelium in type II diabetes is less potent of NO synthesis, which promotes local vasoconstriction [91]. Circulatory disorders due to the vascular lumen narrowing are aggravated by the increase of the thrombosis predisposition. The leading role in this process in case of diabetes belongs to the platelet activation along with the release of microparticles and procoagulants. This mechanism plays an important role in the pathogenesis of diabetic nephropathy [92].
There is a certain correlation of metabolic syndrome with psoriasis [44, 45].
Thus, apheresis therapy is pathogenetically justified to treat these secondary vascular disorders in diabetes and metabolic syndrome.
Due to plasmapheresis the aggregation blood cells inducers are removed (fibronectin, von Willebrand factor, fibrinogen, thrombospondin) [93]. Gavrilov et al. [94] described the reco-very of microcirculatory disorders after courses of plasmapheresis with increasing pain-free walking distance, healing of venous ulcers or amputations suspended with gangrene of the toes. Treatment of nonhealing venous ulcers of “diabetic foot” proved effective also using cascade plasmapheresis (reo-apheresis) [95, 96].
It is also supported by our own experience [97, 98]. There were 130 subjects observed having occlusal atherosclerotic conditions of the lower limbs arteries of various geneses and stage of manifestation. Plasmapheresis therapy course (4 sessions of membrane plasmapheresis with laser radiation of the blood) was performed in 34 subjects who had no indications or ability to provide operative revascularization of the lower limbs due to different reasons. In 14 subjects with decompensation of the lower limbs blood circulation, being unable to undergo reconstructive surgeries, plasmapheresis allowed avoiding amputations in 6 of them (43%); 4 (28.5%) of them had to undergo minor amputations and the other 4 subjects had to undergo high amputations (28.5%). At the same time, in 96 matched subjects the conservative therapy allowed keeping the limbs just in 22 (23%); minor amputations accounted for 11 subjects (11.5%) and 63 subjects (65.5%) had to undergo high amputations of the lower limbs.
There were also 19 subjects with diabetic foot syndrome associated with dramatic ischemia and toes necrosis who had undergone plasmapheresis therapies (4-5 sessions), which allowed eliminating trophic conditions; in case of necrotic process having emerged 6 subjects underwent necrectomy on the level of the demarcation line without mutilating high amputations of the lower limbs. In case of new ischemic and necrosis foci the repeated plasmapheresis therapies also enabled to be limited by the same minor amounts of the necrotized tissues removal.
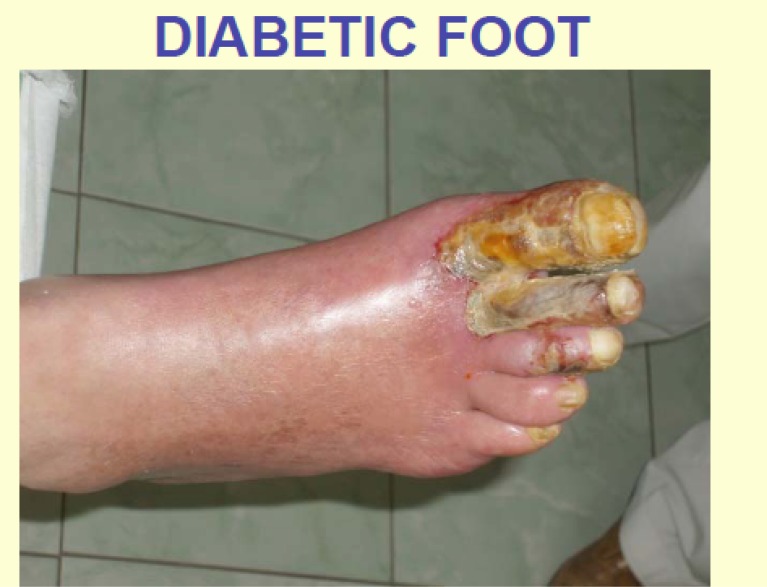
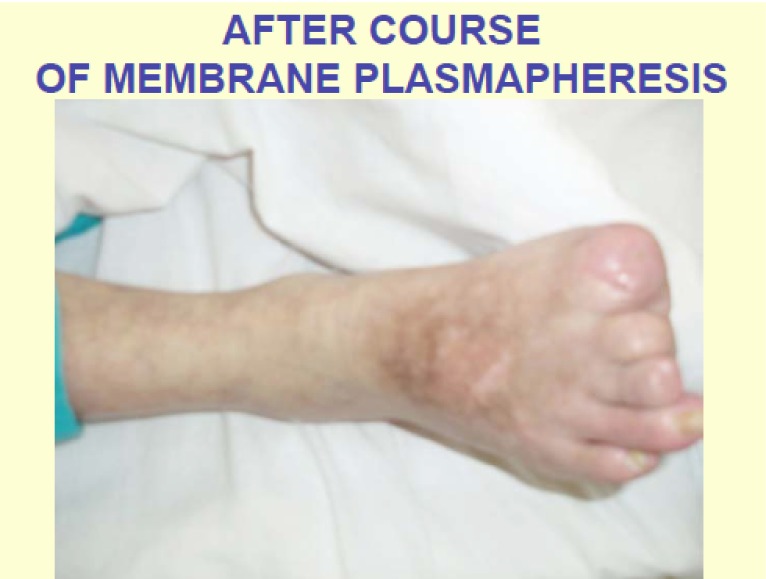
It can be illustrated by the photographs of the foot initial condition, showing necroses and inflammation irradiating to the adjacent parts of the foot. After plasmapheresis therapy with laser radiation of the blood the microcirculation improved, inflammation subsided, which allowed removing only distal sections of the I, II and III toes of the right foot followed by complete healing (Figs. 1 and 2).
Diabetic nephropathy is left on one of the first places among the causes of ESRD. About half of the diabetic patients in Europe and the U.S. are undergoing hemodialysis. It is the direct toxicity of the elevated glucose concentrations for the nephron structures associated with concomitant lipid disorders (frequent lipid deposits in the kidneys) and subsequent sclerotic changes of mesangium cells, together with deposits of circulating immune complexes that underlies renal parenchymal lesions in diabetes.
In the recent years, attention is paid to the role of “vascular endothelial growth factor” as multi-functional cytokine also known as vascular per-meability factor in the development of the micro- and macrovascular diabetic complications, parti-cularly diabetic retinopathy and nephropathy [99]. While the immune complex glomerulo-nephritis is characteristic for diabetes mellitus type I, atherosclerotic nephro-angiosclerosis is typical for diabetes type II.
Due to the increase in vascular permeability in nephropathy the earliest sign of this kind of pathology development is the detection of micro-albuminuria (concentration – 30-200 mg/l, or with excretion rate 20-200 micrograms/min), which can be detected in 29-41% of diabetics with the disease duration over 5-7 years [100]. 70% of diabetics also suffer from microalbuminuria and hypertension, which strengthens the relationship between diabetes and nephropathy. In the United States, except for one million patients with diabetes type I and 13 million patients with diabetes mellitus type II, there are still about 6 million people who have this form of diabetes remaining undiagnosed. This is due to not yet streamlined screening diagnosis of micro-albuminuria preceding proteinuria, so it seems reasonable to be measured by radioimmunoassay or enzyme immunoassay techniques capable to measure the levels of 30-200 mg/l [101].
In patients with hyperinsulinemia in diabetes mellitus type II there is a greater risk of developing Alzheimer's disease (7.5% versus 1.4% in those with normal insulin) [102]. Perhaps this is due to the stimulating effect of glycoproteins on the development of neuritic plaques characteristic for Alzheimer's disease [103].
Diabetes mellitus type II is found 10-20 times more often than the insulin dependent diabetes. Cardiovascular diseases – coronary and peripheral vascular disease, stroke are the most common causes of death and morbidity in these patients [17]. Given the high prevalence of type II diabetes and the severity of its complications, it becomes clear that in 2002 in the U.S. has spent more than $ 90 billion to treat it [27].
In diabetes type II there are marked metabolic changes in these patients being prone to obesity. At the same time the increased concentration of TNF-α, excreted by fat cells when their amount is increased due to the obesity, predisposes the development of insulin resistance [104].
Not only insulin resistance and increased hepatic glucose production, but also β-cells dysfunction plays the key role in the pathogenesis of NIDDM. To some extent it is the hyperglycemia that contributes to the progressive dysfunction of β-cells. Accumulation of lipids and fatty acids in the islets also leads to accelerated apoptosis of β-cells, exacerbating the insulin secretion failure and inability to compensate the insulin resistance. This process is also affected by oxidative stress characteristic for diabetes. It is possible that the reason for this may be the autoantibodies to β-cells found in some patients with diabetes type II [105].
In type II diabetes the islet cells start to secrete amyloid polypeptides, or amylin, which is secreted as well as insulin. Besides, apolipoprotein E (apoE) and heparan sulfate proteoglycan (perlecan) are also secreted. These components are deposited in the structure of the pancreatic islet tissue, which contributes to gradual displacement of β-cells and reduction of insulin secretion. Amyloid deposition is enhanced by apoE and perlekan. These amyloid deposits in the pancreatic islets are increasing with age and are found in 90% of patients with diabetes mellitus type II and this factor plays the main role in the development of β-cell failure [106].
Assessment of the immune status shows changes of predominantly humoral component associated with the appearance of different special antibodies. Antibodies to insulin also play a pathogenic and sanogenic role, neutralizing its excess in the body in diabetes type II, which can lead even to an insulin deficiency.
Autoantibodies to the islet cell cytoplasm and glutamic acid decarboxylase are also identified in non-insulin dependent diabetes type II. These patients have marked hyperglycemia, and after a long, sometimes up to several years, use of oral hypoglycemic agents may become insulin-dependent. Thus, these options can be regarded as a slowly progressing form of diabetes type I – so-called latent (1.5 type) or latent autoimmune diabetes of adults. The presence of antibodies to glutamic acid decarboxylase is the most sensitive and specific marker for future insulin dependence in patients with latent diabetes [107]. Therefore performing apheresis therapy in diabetes type II may to some extent contribute to the prevention and its eventual transition to diabetes type I.
There is a separate problem of gestational diabetes, occurring in 2-8% of pregnant women mainly having some metabolic disturbances. It is accompanied by the development of hypertension without proteinuria and preeclampsia, but with a higher frequency of caesarean section. The child often develops macrosomia (overweight), hyper-bilirubinemia, and polycythemia, as well as hypoglycemia in the next few hours after birth [108]. It was first described by J.B. O'Sullivan and C.M. Mahan [109] in a group of 752 pregnant in Boston when excessive growth of glucose con-centration was detected during 3-4-hour “sugar curve” test.
Even a normal pregnancy is accompanied by some hyperinsulinemia and progressive sup-pression of insulin sensitivity. In addition, many women apparently have had undiagnosed and not manifested type I or type II diabetes revealed only during the pregnancy. However, between 24 and 28 weeks of pregnancy there may be tolerance disorders to carbohydrates even in previously healthy women. The risk of this gestational diabetes in pregnant women may increase in elder pregnant women, in case of obesity, of unex-plained death of a newborn or diabetes diagnosed in the previous pregnancy [110].
Pathogenetic mechanisms of gestational dia-betes mellitus development are associated with decreased insulin sensitivity of the tissues and an equivalent increase of its secretion due to the influence of hormones such as lactogen and progesterone together with cortisol and prolactin. Besides, disorders of lipid metabolism associated with increased concentration of free fatty acids and ketone bodies are also of significance. This type of disorders promotes the development of insulin dependent diabetes mellitus later in life in 50% of these women, particularly prone to obesity [111].
In case of gestational diabetes found in the III trimester of pregnancy, especially when it is persistent during 6 months after the birth, there is a high risk of insulin resistance development as well as diabetes type II 11-26 months after the delivery [112]. Hyperglycemia in gestational diabetes may be the cause of reducing the number of nephrons in the fetus, which is manifested in the development of kidney diseases later in life after birth. Such correlation was confirmed in special experiments on animals [113].
Furthermore, pre-gestational diabetes in pregnant women is 2-5 times more likely to lead to birth defects in fetal development. Special studies in vitro and in vivo have shown that elevated blood glucose levels contributed to the increase of the mutation frequency in embryos [114]. This diabetic embryopathy is the main reason for the higher perinatal mortality and neonatal morbidity of newborns of mothers with diabetes.
Fine et al. [115] have experimentally demon-strated the occurrence of genetic disorders in hyperglycemia pregnant animals. There were more incidences of congenital malformations in the fetus with gestational diabetes than in the general population. These defects can occur before the 7th week of pregnancy. They include such severe deformities, as micro- and even anence-phaly, spinal canal hernia, hydrocephalus, heart malfor-mations. Besides hyper- and hypoglycemia, hyper-ketonemia, hyperinsulinemia, these proce-sses are affected by the intracellular accumulation of glutathione in the conditions of oxidative stress during fetal organogenesis [116]. During the gestational diabetes there is accumulation of low-phosphorylated insulin like growth factor and the related protein-1, which interferes with the development of the fetus and leads to the body weight reduction [117]. In diabetes destructive, sclerotic and necrotic processes develop in the placenta, leading to placental insufficiency.
In addition, fatty acid, content of which increases in the blood of a pregnant woman with gestational diabetes, penetrate into the blood circulation and accumulate in fetal membranes of skeletal muscle cells, which in later life of the child becomes a predisposing factor of insulin resistance syndrome development [118].
In pregnant women with diabetes late toxicosis (with prevalence of hypertensive forms) develops more often. Diabetic microangiopathy promotes more severe preeclampsia [119]. Elevated levels of glucose in the fetus stimulate diuresis that promotes polyhydramnios, which poses a threat of pregnancy termination, weakness labor conractions, bleeding and fetal hypoxia.
There is a higher frequency of urogenital infections occurrence and activation as pyelone-phritis, cervicitis, and vaginitis develop. It is fraught with intrauterine infection of the fetus when ingested and aspiration of the infected amniotic fluid, being infected in its passage through the birth canal, and accompanied by the onset of pneumonia with a long and persistent cough, conjunctivitis, pustular skin lesions, upto the development of septic complications.
In the presence of diabetes in pregnant women, regardless of its form, the fetus develops diabetic fetopathy. When the body weight and the size of some organs (liver, heart, and spleen) tend to increase the development of functional systems slows down. Weight of the brain in the fetus is decreased due to degenerative changes of nerve and glial cells. And in the first days of life there are especially frequent neurological disorders – such as irritability and hypotonia, upto the development of cerebral palsy. In later life mental lag and physical disabilities, delayed speech and tongue-tied are often found. Neonatal respiratory distress syndrome and bronchopulmonary dys-plasia are the most common findings [120].
These findings allow justifying the preventive courses of plasmapheresis in pregnant women when gestational diabetes is found in order to reduce the risk of the process spreading on the fetus and to prevent the disease left untreated in women after the childbirth.
Therefore apheresis therapy becomes not only indicated in very advanced complications of dia-betes, but also in its very early period, including when the level of glucose is not achieved the “diabetic” level. Indeed, numerous studies indicate the favorable results of this treatment, its cor-rective influence on carbohydrate and lipid met-abolism, coagulation factors in patients with dia-betes, especially in conjunction with CHD [121]. Plasmapheresis can significantly reduce chole-sterol levels (from 353.8 ± 60.1 to 154.3 ± 32.6 mg/%), triglycerides (from 428.0 ± 142.6 to 178.3 ± 141.6 mg/%), fibrinogen (from 432.9 ± 97.4 to 255.5 ± 52.4 mg/%) with a reduced risk of possible acute vascular complications with 41.17 ± 7.17% to 11.7 ± 4.6% [122]. Enabling courses of plasma-pheresis in a secondary dyslipidemia in diabetes allowed more significantly reduce cholesterol to 4.03 ± 0.38 mmol / l (p = 0.025), low-density lipoprotein to 2.78 ± 0.28 (p = 0.017) and athero-genic coefficient to 3.31± 0.63 (p = 0.028). This improves the sensitivity to drugs, including hypoglycemic ones [123].
Methods of direct extracorporeal adsorption of fibrinogen and lipoprotein are also used [124-126]. Methods of cascade plasmapheresis are also perspective, given its greater efficiency and selectivity [127].
Plasmapheresis in diabetes reduces thirst, polyuria, pruritus, hyperglycemia, and glycosuria, improves blood rheology and microcirculation, and, most importantly, improves the sensitivity of cellular insulin receptors. Plasmapheresis is indicated in patients with an advanced picture of the disease in order to prevent a number of secondary complications of diabetes [128].
Thus, the presence of both immune and metabolic shifts in this form of diabetes makes the use of apheresis therapy reasonable in all stages of the disease development. Plasmapheresis is almost the only way of correction of these complications – elimination of secondary metabolic disorders. Only by means of plasma exchange it is possible to remove the numerous damaging factors, such as CEC, glycoproteins, lipids, uric acid, endothelin, antibodies to insulin and others [129]. The plasma exchange at diabetes leads to reduction of thirst, a polyuria, skin itch, decrease in level of a glycemia, glucosuria, improvement of a rheology of blood and microcirculation, and that is especially important, to increase in sensitivity of cellular receptors to insulin [130].
And indeed, numerous works indicate favorable results of such treatment, its corrective influence on carbohydrate, lipidic exchange, and coagulative factors in patients with diabetes, especially in combination with ischemic heart disease [126]. By means of a plasmapheresis inductors of blood cells aggregation (fibronectin, Villebrand's factor, fibrinogen, thrombospondin) are removed [131]. At the same time the sensitivity to medicines including anti-hyperglycemics improves [123].
Gavrilov et al. [94] described restoration of microcirculation disorders after plasmapheresis courses with increase of pain-free walking distance, healing of trophic ulcers or delay of amputations in case of toes gangrenes. It confirms also our own experience of membrane plasmapheresis in diabetic angiopathy when in most cases it was possible to stop necrosis progression or to limit it only to toes phalanxes’ resections [97].
Treatment of poorly healing trophic ulcers in “diabetic foot” appeared effective by means of cascade plasma exchange as well (rheoapheresis) [95, 127]. Rheosorption in processing of plasma through special affine sepharose columns allowed significant reducing of concentration of fibrinogen, fibrin and other products of degradation that dramatically improved microcirculation in diabetic foot syndrome [73].
Unlike drug therapy (alprostadil, pentoxifylline), plasmapheresis promotes more considerable decrease in aggregation of erythrocytes and platelets due to removal of inductors of their aggregation (a fibronektin, von Willebrand's factor, fibrinogen, thrombospondin) [131].
Thus, the provided findings indicate the relevance of the “diabetic foot” problem and the perspective of apheresis therapy methods, mainly plasmapheresis, in treatment of this severe vascular pathology diabetic patients.
Conclusion
The provided findings show real dangers and serious complications of metabolic syndrome, which not always can be prevented by drug therapy. The accumulating large molecular secondary metabolites aren't removed by kidneys and damage the vessels endothelium, defining the further development of microcirculation disorders with ischemia of various tissues and organs. Only timely removal of them by means of plasmapheresis is capable to prevent irreversible damages of the latter.
Sufficiently high cost of plasmapheresis may stop its use, although in our country (Russia), it is much lower than in Europe or America. It is mainly provided due to more sparing plasmapheresis technique when only 30% of the circulating plasma is removed during one procedure instead of 100% of it, which enables to replace this volume by “water” only (isotonic solution of sodium chloride). Nevertheless, during all the set of four plasmapheresis procedures, performed every other day, up to 1-1.5 of the circulating plasma volume is removed. Such procedures are tolerated much better, takes about 1.5 and in half an hour of rest and observation the patient can go home. Risks of allergic reactions and viral diseases transmission is excluded, which otherwise is always possible when donor’s blood components are used.
Well-established Russian plasma filters such as “Rosa” and development of simple and safe single needle methods and devices for membrane plasmapheresis such as “Hemofenix” (“Trackpore Technology” Company) makes these important tasks quite realistic in almost all hospitals even on municipal levels. Possibility to perform such procedures in out-patient settings opens perspectives of their broader application in clinical practice. It is especially important, considering the increasing incidence of metabolic syndrome and diabetes in population.
Fig. (1).
Patient D, age of 50 years. Diabetic foot. View of foot before an initiation of treatment.
Fig. (2).
After course of plasmapheresis and resection of phalanxes of 1-2-3 fingers only.
Results of the treatment of patients with “diabetic foot”.
| Operations |
Without
Plasmapheresis N = 96 |
With
Plasmapheresis N = 34 |
|---|---|---|
| Without amputations | 23.0% | 76.4% |
| Small amputations | 11.5% | 11.8% |
| High level amputations | 65.5% | 11.8% |
Acknowledgements
Declared none.
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
No Animals/Humans were used for studies that are the basis of this research.
Consent for Publication
Not applicable.
Conflict of Interest
The author declares no conflict of interest, financial or otherwise.
References
- 1.Tune J.D., Goodwill A.G., Sassoon D.J., Mather K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017;183:57–70. doi: 10.1016/j.trsl.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis R.M., Wagner E.H., Groves T. Managing chronic disease. BMJ. 1999;318(7191):1090–1091. doi: 10.1136/bmj.318.7191.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goswami G., Shinkazh N., Davis N. Optimal pharmacologic treatment strategies in obesity and type 2 diabetes. J. Clin. Med. 2014;3(2):595–613. doi: 10.3390/jcm3020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarson C.L., Mahmud F.H., Baker J.E., Clark H.E., McKay W.M., Schauteet V.D., Hill D.J. Metformin in combination with structured lifestyle intervention improved body mass index in obese adolescents, but did not improve insulin resistance. Endocrine. 2009;36(1):141–146. doi: 10.1007/s12020-009-9196-9. [DOI] [PubMed] [Google Scholar]
- 5.Goh V.H.H., Hart W.G. Excess fat in the abdomen but not general obesity is associated with poorer metabolic and cardiovascular health in premenopausal and postmenopausal Asian women. Maturitas. 2018;107:33–38. doi: 10.1016/j.maturitas.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov N.A., Chubrieva S.Y. Metabolic syndrome and atherosclerosis. Med. Acad. J. 2007;7:45–60. [Google Scholar]
- 7.Haffner S.M. Diabetes, hyperlipidemia, and coronary artery disease. Am. J. Cardiol. 1999;83(9B):17F–21F. doi: 10.1016/s0002-9149(99)00213-1. [DOI] [PubMed] [Google Scholar]
- 8.Irace C., Carallo C., Crescenzo A., Motti C., De Franceschi M.S., Mattioli P.L., Gnasso A. NIDDM is associated with lower wall shear stress of the common carotid artery. Diabetes. 1999;48(1):193–197. doi: 10.2337/diabetes.48.1.193. [DOI] [PubMed] [Google Scholar]
- 9.Niemi M., Kervinen K., Rantala A., Kauma H., Päivänsalo M., Savolainen M.J., Lilja M., Kesäniemi Y.A. The role of apolipoprotein E and glucose intolerance in gallstone disease in middle aged subjects. Gut. 1999;44(4):557–562. doi: 10.1136/gut.44.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockenfeller P., Ring J., Muschett V., Beranek A., Buettner S., Carmona-Gutierrez D., Eisenberg T., Khoury C., Rechberger G., Kohlwein S.D., Kroemer G., Madeo F. Fatty acids trigger mitochondrion-dependent necrosis. Cell Cycle. 2010;9(14):2836–2842. doi: 10.4161/cc.9.14.12267. [DOI] [PubMed] [Google Scholar]
- 11.Firneisz G. Non-alcoholic fatty liver disease and type 2 diabetes mellitus: The liver disease of our age? World J. Gastroenterol. 2014;20(27):9072–9089. doi: 10.3748/wjg.v20.i27.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X.Y., Zhang X.H., Yao C.H., Zhu H.H., Zhang L. The impact of obesity and metabolic abnormality on carotid intima-media thickness and non-alcoholic fatty liver disease in children from an inland Chinese city. J. Clin. Med. 2014;3(1):323–333. doi: 10.3390/jcm3010323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duseja A., Singh S.P., Saraswat V.A., Acharya S.K., Chawla Y.K., Chowdhury S., Dhiman R.K., Jayakumar R.V., Madan K., Misra S.P., Mishra H., Modi S.K., Muruganathan A., Saboo B., Sahay R., Upadhyay R. Non-alcoholic fatty liver disease and metabolic syndrome – position paper of the Indian national association for the study of the liver, endocrine society of India, Indian college of cardiology and Indian society of gastroenterology. J. Clin. Exp. Hepatol. 2015;5(1):51–68. doi: 10.1016/j.jceh.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberg J.M. Lipotoxicity. Kidney Int. 2006;70(9):1560–1566. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 15.Morange P-E., Aubert J., Peiretti F., Lijnen H.R., Vague P., Verdier M., Négrel R., Juhan-Vague I., Alessi M.C. Glucocorticoids and insulin promote plasminogen activator inhibitor 1 production by human adipose tissue. Diabetes. 1999;48(4):890–895. doi: 10.2337/diabetes.48.4.890. [DOI] [PubMed] [Google Scholar]
- 16.Caballero A.E., Arora S., Saouaf R., Lim S.C., Smakowski P., Park J.Y., King G.L., LoGerfo F.W., Horton E.S., Veves A. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48(9):1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 17.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48(5):937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 18.Ray J.G., Lonn E., Yi Q., Rathe A., Sheridan P., Kearon C., Yusuf S., Arnold M.J., McQueen M.J., Pogue J., Probstfield J., Fodor G., Held C., Micks M., Genest J., Jr Venous thromboembolism in association with features of the metabolic syndrome. QJM. 2007;100(11):679–684. doi: 10.1093/qjmed/hcm083. [DOI] [PubMed] [Google Scholar]
- 19.Brola W., Sobolewski P., Fudala M., Goral A., Kasprzyk M., Szczuchniak W., Pejas-Dulewicz R., Przybylski W. Metabolic syndrome in Polish ischemic stroke patients. J. Stroke Cerebrovasc. Dis. 2015;24(9):2167–2172. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Liu L., Zhan L., Wang Y., Bai C., Guo J., Lin Q., Liang D., Xu E. Metabolic syndrome and the short-term prognosis of acute ischemic stroke: A hospital-based retrospective study. Lipids Health Dis. 2015;14:76. doi: 10.1186/s12944-015-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vishram J.K. Prognostic interactions between cardiovascular risk factors. Dan. Med. J. 2014;61(7):B4892. [PubMed] [Google Scholar]
- 22.Huhtaniemi I. Late-onset hypogonadism: Current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J. Androl. 2014;16(2):192–202. doi: 10.4103/1008-682X.122336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corona G., Vignozzi L., Sforza A., Mannucci E., Maggi M. Obesity and late-onset hypogonadism. Mol. Cell. Endocrinol. 2015;418(Pt 2):120–133. doi: 10.1016/j.mce.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Wickramatilake C.M., Mohideen M.R., Pathirana C. Association of metabolic syndrome with testosterone and inflammation in men. Ann. Endocrinol. (Paris) 2015;76(3):260–263. doi: 10.1016/j.ando.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Owiredu W.K.B.A., Alidu H., Amidu N., Obirikorang C., Gyasi-Sarpong C.K., Bawah A.T., Dapare P.P.M., Luuse A.T. Sexual dysfunction among diabetics and its impact on the SQoL of their partners. Int. J. Impot. Res. 2017;29(6):250–257. doi: 10.1038/ijir.2017.32. [DOI] [PubMed] [Google Scholar]
- 26.Solerte S.B., Piovella F., Viola C., Schianca G.C., Gamba G., Fioravanti M., Ferrari E. Plasma fibronectin, von Willebrand factor antigen, and blood rheology. Association with diabetic microvascular disease. Acta Diabetol. Lat. 1985;22(3):239–246. doi: 10.1007/BF02590775. [DOI] [PubMed] [Google Scholar]
- 27.Ryder S.W. Innovation and improvement in the treatment of obesity and diabetes. Clin. Pharmacol. Ther. 2007;81(5):615–619. doi: 10.1038/sj.clpt.6100189. [DOI] [PubMed] [Google Scholar]
- 28.Riemens S., van Tol A., Sluiter W., Dullaart R. Elevated plasma cholesteryl ester transfer in NIDDM: relationships with apolipoprotein B-containing lipoproteins and phospholipid transfer protein. Atherosclerosis. 1998;140(1):71–79. doi: 10.1016/s0021-9150(98)00111-7. [DOI] [PubMed] [Google Scholar]
- 29.Erkelens D.W. Diabetic dyslipidemia. Eur. Heart J. 1998;19:27–30. [PubMed] [Google Scholar]
- 30.Korpilahti K., Syvänne M., Engblom E., Hämäläinen H., Puukka P., Rönnemaa T. Components of the insulin resistance syndrome are associated with progression of atherosclerosis in non-grafted arteries 5 years after coronary artery bypass surgery. Eur. Heart J. 1998;19(5):711–719. doi: 10.1053/euhj.1997.0869. [DOI] [PubMed] [Google Scholar]
- 31.Fossum E., Høieggen A., Moan A., Rostrup M., Nordby G., Kjeldsen S.E. Relationship between insulin sensitivity and maximal forearm blood flow in young men. Hypertension. 1998;32(5):838–843. doi: 10.1161/01.hyp.32.5.838. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh A., Biswas A.K., Banerjee A. A study on cognitive decline with respect to metabolic syndrome and inflammation in elderly Indians. Neurol. India. 2015;63(4):537–541. doi: 10.4103/0028-3886.162037. [DOI] [PubMed] [Google Scholar]
- 33.Vilbergsson S., Sigurdsson G., Sigvaldason H., Sigfusson N. Coronary heart disease mortality amongst non-insulin-dependent diabetic subjects in Iceland: the independent effect of diabetes. The Reykjavik Study 17-year follow up. J. Intern. Med. 1998;244(4):309–316. doi: 10.1046/j.1365-2796.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- 34.Perrone-Filardi P., Paolillo S., Costanzo P., Savarese G., Trimarco B., Bonow R.O. The role of metabolic syndrome in heart failure. Eur. Heart J. 2015;36(39):2630–2634. doi: 10.1093/eurheartj/ehv350. [DOI] [PubMed] [Google Scholar]
- 35.Ametov A.S., Sokareva E.V., Giljarovsky S.R., Dikova T.E. Left ventricular diastolic dysfunction in patients with type 2 diabetes. Sah. Diabetes. 2008;1:40–44. [Google Scholar]
- 36.Turner R. UK prospective diabetes study group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 37.Salonen J.T., Tuomainen T-P., Nyyssönen K., Lakka H.M., Punnonen K. Relation between iron stores and non-insulin dependent diabetes in men: Case-control study. BMJ. 1998;317(7160):727. doi: 10.1136/bmj.317.7160.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardin D.S., LeBlanc A., Para L., Seilheimer D.K. Hepatic insulin resistance and defects in substrate utilization in cystic fibrosis. Diabetes. 1999;48(5):1082–1087. doi: 10.2337/diabetes.48.5.1082. [DOI] [PubMed] [Google Scholar]
- 39.Hadj Ahmed S., Kaoubaa N., Kharroubi W., Zarrouk A., Najjar M.F., Batbout F., Gamra H., Lizard G., Hammami M. Association of plasma fatty acid alteration with the severity of coronary artery disease lesions in Tunisian patients. Lipids Health Dis. 2017;16(1):154. doi: 10.1186/s12944-017-0538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang F., Lu H., Liu F., Cai H., Song Z., Guo F., Xie Y., Shu G., Sun G. Effects of a liquid high-fat meal on postprandial lipid metabolism in type 2 diabetic patients with abdominal obesity. Nutr. Metab. (Lond.) 2017;14:54. doi: 10.1186/s12986-017-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coppack S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001;60(3):349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 42.Boutagy N.E., McMillan R.P., Frisard M.I., Hulver M.W. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie. 2016;124:11–20. doi: 10.1016/j.biochi.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharaf El Din U.A.A., Salem M.M., Abdulazim D.O. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J. Adv. Res. 2017;8(5):537–548. doi: 10.1016/j.jare.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furue M., Tsuji G., Chiba T., Kadono T. Cardiovascular and metabolic diseases comorbid with psoriasis: beyond the slin. Intern. Med. 2017;56(13):1613–1619. doi: 10.2169/internalmedicine.56.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodríguez-Zúñiga M.J.M., Cortez-Franco F., Quijano-Gomero E. Association of psoriasis and metabolic syndrome in Latin America: A systematic review and meta-analysis. Actas Dermosifiliogr. 2017;108(4):326–334. doi: 10.1016/j.ad.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Boumendil E.F. Mortality from non-cardiovascular disease and diabetes mellitus. Lancet. 1998;351(9102):598–599. doi: 10.1016/s0140-6736(05)78588-3. [DOI] [PubMed] [Google Scholar]
- 47.Bruckert E., Baccara-Dinet M., Aschwege E. Low HDL-cholesterol is common in European Type 2 diabetic patients receiving treatment for dyslipidemia. Diabet. Med. 2007;24(4):388–391. doi: 10.1111/j.1464-5491.2007.02111.x. [DOI] [PubMed] [Google Scholar]
- 48.Mazzanti L., Rabini R.A., Fumelli P., Martarelli D., Staffolani R., Salvolini E., Curatola G. Altered platelet membrane dynamic properties in type 1 diabetes. Diabetes. 1997;46(12):2069–2074. doi: 10.2337/diab.46.12.2069. [DOI] [PubMed] [Google Scholar]
- 49.Saleh J. Glycated hemoglobin and its spinoffs: Cardiovascular disease markers or risk factors? World J. Cardiol. 2015;7(8):449–453. doi: 10.4330/wjc.v7.i8.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahmoodnia L., Aghadavod E., Beigrezaei S., Rafieian-Kopaei M. An update on diabetic kidney disease, oxidative stress and antioxidant agents. J. Renal Inj. Prev. 2017;6(2):153–157. doi: 10.15171/jrip.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orchard T.J., Virella G., Forrest K.Y-Z., Evans R.W., Becker D.J., Lopes-Virella M.F. Antibodies to oxidized LDL predict coronary artery disease in type 1 diabetes: A nested case-control study from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes. 1999;48(7):1454–1458. doi: 10.2337/diabetes.48.7.1454. [DOI] [PubMed] [Google Scholar]
- 52.Simons L.A., von Konigsmark M., Simons J., Stocker R., Celermajer D.S. Vitamin E ingestion does not improve arterial endothelial dysfunction in older adults. Atherosclerosis. 1999;143(1):193–199. doi: 10.1016/s0021-9150(98)00287-1. [DOI] [PubMed] [Google Scholar]
- 53.Santilli F., Marchisio M., Lanuti P., Boccatonda A., Miscia S., Davì G. Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb. Haemost. 2016;116(2):220–234. doi: 10.1160/TH16-03-0176. [DOI] [PubMed] [Google Scholar]
- 54.Dalla Paola L., Faglia E. Treatment of diabetic foot ulcer: An overview strategies for clinical approach. Curr. Diabetes Rev. 2006;2(4):431–447. doi: 10.2174/1573399810602040431. [DOI] [PubMed] [Google Scholar]
- 55.Meo S.A., Usmani A.M., Qalbani E. Prevalence of type 2 diabetes in the Arab world: Impact of GDP and energy consumption. Eur. Rev. Med. Pharmacol. Sci. 2017;21(6):1303–1312. [PubMed] [Google Scholar]
- 56.Hazmy W., Mahamud M., Ashikin N., Jamilah S., Yee L.E., Shong H.K. Major limb amputations in Seremban Hospital: A review of 204 cases from 1997-1999. Med. J. Malaysia. 2001;56(Suppl. C):3–7. [PubMed] [Google Scholar]
- 57.Zhang P., Lu J., Jing Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017;49(2):106–116. doi: 10.1080/07853890.2016.1231932. [DOI] [PubMed] [Google Scholar]
- 58.Malgrange D., Richard J.L., Leymarie F. Screening diabetic patients at risk for foot ulceration. A multi-centre hospital-based study in France. Diabetes Metab. 2003;29(3):261–268. doi: 10.1016/s1262-3636(07)70035-6. [DOI] [PubMed] [Google Scholar]
- 59.Varma P., Stineman M.G., Dillingham T.R. Epidemiology of limb loss. Phys. Med. Rehabil. Clin. N. Am. 2014;25(1):1–8. doi: 10.1016/j.pmr.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fosse S., Hartemann-Heurtier A., Jacqueminet S., Ha Van G., Grimaldi A., Fagot-Campagna A. Incidence and characteristics of lower limb amputations in people with diabetes. Diabet. Med. 2009;26(4):391–396. doi: 10.1111/j.1464-5491.2009.02698.x. [DOI] [PubMed] [Google Scholar]
- 61.Stone P.A., Back M.R., Armstrong P.A., Flaherty S.K., Keeling W.B., Johnson B.L., Shames M.L., Bandyk D.F. Midfoot amputations expand limb salvage rates for diabetic foot infections. Ann. Vasc. Surg. 2005;19(6):805–811. doi: 10.1007/s10016-005-7973-3. [DOI] [PubMed] [Google Scholar]
- 62.Zgonis T., Stapleton J.J., Girard-Powell V.A., Hagino R.T. Surgical management of diabetic foot infections and amputations. AORN J. 2008;87(5):935–946. doi: 10.1016/j.aorn.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 63.Hunt D. Foot ulcers and amputations. 2009.
- 64.Nielson D.L., Ali Y. Diabetic foot infections: Time to change the prodnotic concept. J. Am. Podiatr. Med. Assoc. 2009;99(5):454–458. doi: 10.7547/0990454. [DOI] [PubMed] [Google Scholar]
- 65.Sié Essoh J.B., Kodo M., Djè Bi Djè V., Lambin Y. Limb amputations in adults in an Ivorian teaching hospital. Niger. J. Clin. Pract. 2009;12(3):245–247. [PubMed] [Google Scholar]
- 66.Robbins J.M., Strauss G., Aron D., Long J., Kuba J., Kaplan Y. Mortality rates and diabetic foot ulcers: Is it time to communicate mortality risk to patients with diabetic foot ulceration? J. Am. Podiatr. Med. Assoc. 2008;98(6):489–493. doi: 10.7547/0980489. [DOI] [PubMed] [Google Scholar]
- 67.Izumi Y., Satterfield K., Lee S., Harkless L.B., Lavery L.A. Mortality of first-time amputees in diabetics: A 10-year observation. Diabetes Res. Clin. Pract. 2009;83(1):126–131. doi: 10.1016/j.diabres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 68.Remes L., Isoaho R., Vahlberg T., Hiekkanen H., Korhonen K., Viitanen M., Rautava P. Major lower extremity amputation in elderly patients with peripheral arterial disease: Incidence and survival rates. Aging Clin. Exp. Res. 2008;20(5):385–393. doi: 10.1007/BF03325142. [DOI] [PubMed] [Google Scholar]
- 69.Skoutas D., Papanas N., Georgiadis G.S., Zervas V., Manes C., Maltezos E., Lazarides M.K. Risk factors for ipsilateral reamputation in patients with diabetic foot lesions. Int. J. Low. Extrem. Wounds. 2009;8(2):69–74. doi: 10.1177/1534734609334808. [DOI] [PubMed] [Google Scholar]
- 70.Dillingham T.R., Pezzin L.E., Shore A.D. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch. Phys. Med. Rehabil. 2005;86(3):480–486. doi: 10.1016/j.apmr.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 71.Dall T., Mann S.E., Zhang Y., Martin J., Chen Y. Economic costs of diabetes in the U.S. In: 2007. Diabetes Care. 2008;31(3):596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 72.Aragón-Sánchez J., Quintana-Marrero Y., Lázaro-Martínez J.L., Hernández-Herrero M.J., García-Morales E., Beneit-Montesinos J.V., Cabrera-Galván J.J. Necrotizing soft-tissue infections in the feet of patients with diabetes: Outcome of surgical treatment and factors associated with limb loss and mortality. Int. J. Low. Extrem. Wounds. 2009;8(3):141–146. doi: 10.1177/1534734609344106. [DOI] [PubMed] [Google Scholar]
- 73.Stücker M., Moll C., Rudolph T., Robak-Pawelczyk B., Jünger M., Schultz-Ehrenburg U., Altmeyer P. Fibrinogen adsorption--a new treatment option for venous leg ulcers? Vasa. 2003;32(3):173–177. doi: 10.1024/0301-1526.32.3.173. [DOI] [PubMed] [Google Scholar]
- 74.Ferraresi R., Centola M., Ferlini M., Da Ros R., Caravaggi C., Assaloni R., Sganzaroli A., Pomidossi G., Bonanomi C., Danzi G.B. Long-term outcomes after angioplasty of isolated, below-the-knee arteries in diabetic patients with critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 2009;37(3):336–342. doi: 10.1016/j.ejvs.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Faglia E., Clerici G., Clerissi J., Gabrielli L., Losa S., Mantero M., Caminiti M., Curci V., Quarantiello A., Lupattelli T., Morabito A. Long-term prognosis of diabetic patients with critical limb ischemia: A population-based cohort study. Diabetes Care. 2009;32(5):822–827. doi: 10.2337/dc08-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dick F., Diehm N., Galimanis A., Husmann M., Schmidli J., Baumgartner I. Surgical or endovascular revascularization in patients with critical limb ischemia: Influence of diabetes mellitus on clinical outcome. J. Vasc. Surg. 2007;45(4):751–761. doi: 10.1016/j.jvs.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 77.Lopez-Candales A., Hernández Burgos P.M., Hernandez-Suarez D.F., Harris D. Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J. Nat. Sci. 2017;3(4):e341. [PMC free article] [PubMed] [Google Scholar]
- 78.Mohan M.L., Vasudevan N.T., Naga Prasad S.V. Proinflammatory cytokines mediate GPCR dysfunction. J. Cardiovasc. Pharmacol. 2017;70(2):61–73. doi: 10.1097/FJC.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joseph T., Fajadet J., Laurent J-P., Jordan C., Cassagneau B., Laborde J.C., Marco J. Implantation coronary endoprosthese is diabetic patients. Heart. Vessel. Dis. Arch. 1998;91(6):715–720. [PubMed] [Google Scholar]
- 80.Hoogwerf B.J., Waness A., Cressman M., Canner J., Campeau L., Domanski M., Geller N., Herd A., Hickey A., Hunninghake D.B., Knatterud G.L., White C. Effects of aggressive cholesterol lowering and low-dose anticoagulation on clinical and angiographic outcomes in patients with diabetes: The Post Coronary Artery Bypass Graft Trial. Diabetes. 1999;48(6):1289–1294. doi: 10.2337/diabetes.48.6.1289. [DOI] [PubMed] [Google Scholar]
- 81.Krumpaszky H.G., Lüdtke R., Mickler A., Klauss V., Selbmann H.K. Blindness incidence in Germany. A population-based study from Württemberg-Hohenzollern. Ophthalmologica. 1999;213(3):176–182. doi: 10.1159/000027415. [DOI] [PubMed] [Google Scholar]
- 82.Sdobnikova S.V., Stolyarenko G.E. The role of the back of the hyaloid membrane in the pathogenesis and transciliare surgery proliferative diabetic retinopathy. Vet. Ophthalmol. 1999;1:11–13. [PubMed] [Google Scholar]
- 83.Ishibashi T., Murata T., Kohno T., Ohnishi Y., Inomata H. Peripheral choriovitreal neovascularization in proliferative diabetic retinopathy: Histopathologic and ultrastructural study. Ophthalmologica. 1999;213(3):154–158. doi: 10.1159/000027411. [DOI] [PubMed] [Google Scholar]
- 84.Gargiulo P., Goldberg J., Romani B., Schiaffini R., Ciampalini P., Faulk W.P., McIntyre J.A. Qualitative and quantitative studies of autoantibodies to phospholipids in diabetes mellitus. Clin. Exp. Immunol. 1999;118(1):30–34. doi: 10.1046/j.1365-2249.1999.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Syroedova O.N., Tretiak E.B., Chentsova O.N., Suchkov S.V. The role of vascular endothelial growth factor in the pathogenesis of diabetic retinopathy. Allergology Immunol. 2006;7:424. [Google Scholar]
- 86.Said G., Elgrebly F., Lacroix C. Painful proximal diabetic neuropaty: inflammatory nerve lesions and spontaneously favorable outcome. Ann. Neurol. 1997;41(6):762–770. doi: 10.1002/ana.410410612. [DOI] [PubMed] [Google Scholar]
- 87.Fernyhough P., Gallagher A., Averill S.A., Priestley J.V., Hounsom L., Patel J., Tomlinson D.R. Aberrant neurofilament phosphorylation in sensory neurons of rats with diabetic neuropathy. Diabetes. 1999;48(4):881–889. doi: 10.2337/diabetes.48.4.881. [DOI] [PubMed] [Google Scholar]
- 88.Reiber G.E., Lipsky B.A., Gibbons G.W. The burden of diabetic foot ulcers. Am. J. Surg. 1998;176(Suppl. 2A):5S–10S. doi: 10.1016/s0002-9610(98)00181-0. [DOI] [PubMed] [Google Scholar]
- 89.Akhter F., Khan M.S., Alatar A.A., Faisal M., Ahmad S. Antigenic role of the adaptive immune response to d-ribose glycated LDL in diabetes, atherosclerosis and diabetes atherosclerotic patients. Life Sci. 2016;151:139–146. doi: 10.1016/j.lfs.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 90.Shantaram V. Pathogenesis of atherosclerosis in diabetes and hypertension. Clin. Exp. Hypertens. 1999;21(1-2):69–77. doi: 10.3109/10641969909068650. [DOI] [PubMed] [Google Scholar]
- 91.Poston L. Endothelial control of vascular tone in diabetes mellitus. Diabetologia. 1997;40(Suppl. 2):S113–S114. doi: 10.1007/s001250051421. [DOI] [PubMed] [Google Scholar]
- 92.Omoto S., Nomura S., Shouzu A., Hayakawa T., Shimizu H., Miyake Y., Yonemoto T., Nishikawa M., Fukuhara S., Inada M. Significance of platelet-derived microparticles and activated platelets in diabetic nephropathy. Nephron. 1999;81(3):271–277. doi: 10.1159/000045292. [DOI] [PubMed] [Google Scholar]
- 93.Weiss N. A critical review on the use of lipid apheresis and rheopheresis for treatment of peripheral arterial disease and the diabetic foot syndrome. Semin. Dial. 2012;25(2):220–227. doi: 10.1111/j.1525-139X.2011.01036.x. [DOI] [PubMed] [Google Scholar]
- 94.Gavrilov A.O., Yepifanova N.Y., Korolev M.L. Regulation and other systemic disorders of blood aggregation in patients of elderly and senile age. Moscow: 2003. [Google Scholar]
- 95.Agishi T., Kaneko I., Hasuo Y., Hayasaka Y., Sanaka T., Ota K., Amemiya H., Sugino N., Abe M., Ono T., Kawai S., Yamane T. Double filtration plasmapheresis. Ther. Apher. 2000;4(1):29–33. [PubMed] [Google Scholar]
- 96.Klingel R., Mumme C., Fassbender T., Himmelsbach F., Altes U., Lotz J., Pohlmann T., Beyer J., Küstner E. Rheopheresis in patients with ischemic diabetic foot syndrome: Results of an open label prospective pilot trial. Ther. Apher. Dial. 2003;7(4):444–455. doi: 10.1046/j.1526-0968.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 97.Voinov A.V., Voinov V.A., Karchevskii K.S., Libov I.V. Plasmapheresis in the treatment of diabetic angiopathy; Proceedings of the XI conference of Moscow hemapheresis society; Moscow. 2003. [Google Scholar]
- 98.Voinov A.V., Bedrov A., Voinov V.A. Syndrome of “diabetic foot”. Vestn. Hir. 2012;171:106–109. [PubMed] [Google Scholar]
- 99.Hanefeld M., Appelt D., Engelmann K., Sandner D., Bornstein S.R., Ganz X., Henkel E., Haase R., Birkenfeld A.L. Serum and plasma levels of vascular endothelial growth factors in relation to quality of glucose control, biomarkers of inflammation, and diabetic nephropathy. Horm. Metab. Res. 2016;48(8):529–534. doi: 10.1055/s-0042-106295. [DOI] [PubMed] [Google Scholar]
- 100.Mattock M.B., Barnes D.J., Viberti G., Keen H., Burt D., Hughes J.M., Fitzgerald A.P., Sandhu B., Jackson P.G. Microalbuminuria and coronary heart disease in NIDDM: An incidence study. Diabetes. 1998;47(11):1786–1792. doi: 10.2337/diabetes.47.11.1786. [DOI] [PubMed] [Google Scholar]
- 101.Sheth J.J. Diabetes, microalbuminuria and hypertension. Clin. Exp. Hypertens. 1999;21(1-2):61–68. doi: 10.3109/10641969909068649. [DOI] [PubMed] [Google Scholar]
- 102.Kuusisto J., Koivisto K., Mykkänen L., Helkala E.L., Vanhanen M., Hänninen T., Kervinen K., Kesäniemi Y.A., Riekkinen P.J., Laakso M. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: Cross sectional population based study. BMJ. 1997;315(7115):1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Curb J.D., Rodriguez B.L., Abbott R.D., Petrovitch H., Ross G.W., Masaki K.H., Foley D., Blanchette P.L., Harris T., Chen R., White L.R. Longitudinal association of vascular and Alzheimer’s dementias, diabetes, and glucose tolerance. Neurology. 1999;52(5):971–975. doi: 10.1212/wnl.52.5.971. [DOI] [PubMed] [Google Scholar]
- 104.Prins J.B., Niesler C.U., Winterford C.M., Bright N.A., Siddle K., O’Rahilly S., Walker N.I., Cameron D.P. Tumor necrosis factor-α induces apoptosis of human adipose cells. Diabetes. 1997;46(12):1939–1944. doi: 10.2337/diab.46.12.1939. [DOI] [PubMed] [Google Scholar]
- 105.Brooks-Worrell B.M., Juneja R., Minokadeh A., Greenbaum C.J., Palmer J.P. Cellular immune responses to human islet proteins in antibody-positive type 2 diabetic patients. Diabetes. 1999;48(5):983–988. doi: 10.2337/diabetes.48.5.983. [DOI] [PubMed] [Google Scholar]
- 106.Kahn S.E., Andrikopoulos S., Verchere C.B. Islet amyloid: A long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48(2):241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 107.Tuomi T., Carlsson A., Li H., Isomaa B., Miettinen A., Nilsson A., Nissén M., Ehrnström B.O., Forsén B., Snickars B., Lahti K., Forsblom C., Saloranta C., Taskinen M.R., Groop L.C. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999;48(1):150–157. doi: 10.2337/diabetes.48.1.150. [DOI] [PubMed] [Google Scholar]
- 108.Cruz J., Grandía R., Padilla L., Rodríguez S., Hernández García P., Lang Prieto J., Márquez-Guillén A. Macrosomia predictors in infants born to Cuban mothers with gestational diabetes. MEDICC Rev. 2015;17(3):27–32. doi: 10.37757/MR2015.V17.N3.6. [DOI] [PubMed] [Google Scholar]
- 109.O’Sullivan J.B., Mahan C.M. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–285. [PubMed] [Google Scholar]
- 110.Greene M.F. Screening for gestational diabetes mellitus. N. Engl. J. Med. 1997;337(22):1625–1626. doi: 10.1056/NEJM199711273372211. [DOI] [PubMed] [Google Scholar]
- 111.Friedman J.E., Ishizuka T., Shao J., Huston L., Highman T., Catalano P. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes. 1999;48(9):1807–1814. doi: 10.2337/diabetes.48.9.1807. [DOI] [PubMed] [Google Scholar]
- 112.Buchanan T.A., Xiang A.H., Kjos S.L., Trigo E., Lee W.P., Peters R.K. Antepartum predictors of the development of type 2 diabetes in Latino women 11-26 months after pregnancies complicated by gestational diabetes. Diabetes. 1999;48(12):2430–2436. doi: 10.2337/diabetes.48.12.2430. [DOI] [PubMed] [Google Scholar]
- 113.Amri K., Freund N., Vilar J., Merlet-Bénichou C., Lelièvre-Pégorier M. Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes. 1999;48(11):2240–2245. doi: 10.2337/diabetes.48.11.2240. [DOI] [PubMed] [Google Scholar]
- 114.Lee A.T., Reis D., Eriksson U.J. Hyperglycemia-induced embryonic dysmorphogenesis correlates with genomic DNA mutation frequency in vitro and in vivo. Diabetes. 1999;48(2):371–376. doi: 10.2337/diabetes.48.2.371. [DOI] [PubMed] [Google Scholar]
- 115.Fine E.L., Horal M., Chang T.I., Fortin G., Loeken M.R. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes. 1999;48(12):2454–2462. doi: 10.2337/diabetes.48.12.2454. [DOI] [PubMed] [Google Scholar]
- 116.Sakamaki H., Akazava S., Ishibashi M. Painful proximal diabetic neuropaty: inflammatory nerve lesions and spontaneously favorable outcome. Ann. Neurol. 1997;41:762–770. doi: 10.1002/ana.410410612. [DOI] [PubMed] [Google Scholar]
- 117.Gibson J.M., Westwood M., Lauszus F.F., Klebe J.G., Flyvbjerg A., White A. Phosphorylated insulin-like growth factor binding protein 1 is increased in pregnant diabetic subjects. Diabetes. 1999;48(2):321–326. doi: 10.2337/diabetes.48.2.321. [DOI] [PubMed] [Google Scholar]
- 118.Baur L.A., O’Connor J., Pan D.A., Storlien L.H. Relationships between maternal risk of insulin resistance and the child’s muscle membrane fatty acid composition. Diabetes. 1999;48(1):112–116. doi: 10.2337/diabetes.48.1.112. [DOI] [PubMed] [Google Scholar]
- 119.Ailamzyan E.K., Petrishchev N.N., Mozgovaja E.V. Treatment of vascular complications in patients with insulin-dependent diabetes mellitus during pregnancy. Obstet. Gynecol. 2000;2:18–22. [Google Scholar]
- 120.Evsyukova I.I., Kosheleva N.G. Pregnant women and newborns. 1996.
- 121.Sokolov E.I. Diabetic heart. 2002.
- 122.Khayutina T.L., Konovalov G.A., Borisov I.A. Therapeutic plasmapheresis in the correction of hy-percholesterolemia in diabetes mellitus type 2.; Proceedings of XII Conference; Moscow. Moscow: Hemapheresis Society; 2004. p. 74. [Google Scholar]
- 123.Khayutina T.L., Balabolkin M.I., Konovalov G.A., et al. Ways correction of lipid metabolism in patients with type 2 diabetes. Vestn. MEDSI. 2008-2009;2:38–43. [Google Scholar]
- 124.Richter W.O., Schneidewind J.M., Ramlow W., Jahn P., Jung N., Nielebock E., Tachezy H., Eulitz K., Koll R., Klinkmann J. Extracorporeal fibrinogen adsorption--efficacy, selectivity and safety in healthy subjects and patients with foot ulcers. Transfus. Apheresis Sci. 2002;26(1):15–27. doi: 10.1016/s1473-0502(01)00140-9. [DOI] [PubMed] [Google Scholar]
- 125.Julius U., Taseva K., Fischer S. The role of lipoprotein apheresis in diabetis mellitus and metabolic-vascular syndrome. Efferent Therapy. 2013;19:49–50. [Google Scholar]
- 126.Thompson G.R. Lipoprotein apheresis: Past, present and future. Efferent Therapy. 2013;19:50. [Google Scholar]
- 127.Bláha M., Rencová E., Bláha V., Malý R., Blazek M., Studnicka J., Andrýs C., Fátorová I., Filip S., Kasparová M., Procházková R., Malý J., Zimová R., Langrová H. The importance of rheological parameters in the therapy of microcirculatory disorders. Clin. Hemorheol. Microcirc. 2009;42(1):37–46. doi: 10.3233/CH-2009-1184. [DOI] [PubMed] [Google Scholar]
- 128.Voinov V.A. Therapeutic apheresis. Constanţa: Celebris; 2016. [Google Scholar]
- 129.Shelukhin V.A., Kostyuchenko A.L. Apheresis therapy at diseases and damages of kidneys. In: Kostyuchenko A.L., editor. Efferent Therapy. Saint Petersburg: Foliant; 2003. pp. 268–302. [Google Scholar]
- 130.Kivva V.N., Anufriyenko V.F., Redkina L.V., Cherkashina S.F. Application of a plasmapheresis in complex therapy of a metabolic syndrome X.; Proceedings of VII conference of Moscow society of hemapheresis; Moscow. 1999. [Google Scholar]
- 131.Kudritsky S.Y., Levin G.Y. The comparative analysis of influence of medicamentous therapy and a plasma exchange on aggregation of blood cells at patients with a syndrome of diabetic foot. Revista Ozonoterapia. 2009;3:202–205. [Google Scholar]