Abstract
Objectives
The primary objective of this study was to determine the incidence and incurred morbidities of Respiratory syncytial virus (RSV)-related hospitalization (RSVH), the season following completion of prophylaxis.
Methods
A retrospective study was conducted of all infants enrolled in a prophylaxis clinic in one institution during the 2009 to 2014 RSV seasons. RSV infection was identified by Diseases codes and confirmed by RSV-positivity. Data were classified into five groups based on indications for prophylaxis. The incidence of RSVH was calculated. For each subgroup, differences in characteristics between children with and without RSVH were analyzed by independent t test or chi-square test.
Results
During five RSV seasons, 827 infants were enrolled. RSVH incidence the season following prophylaxis was 2.1% (n=17/827). Children with chronic lung disease (CLD) had the highest RSVH incidence (7.7%; n=4/52) followed by preterms 33 to 35 weeks gestation (2.5%; n=4/162), those with complex medical disorders (2.2%; n=3/135), those with congenital heart disease (1.5%; n=1/66) and preterms less than or equal to 32 weeks gestation (1.2%; n=5/412). There was no statistically significant association between indications for prophylaxis and RSVH (Fisher exact test, P=0.060). The odds of RSVH were 4.9 times greater (odds ratio [OR]=4.9; 95% CI: 1.53, 15.55; P=0.007) in CLD compared to those without CLD. The median length of RSVH stay was 4 days; 58.8% (n=10/17) required oxygen (median 1 day); 29.4% (n=5/17) required intensive care.
Conclusions
Infants with CLD are at highest risk for RSVH in the season postprophylaxis and may merit palivizumab for more than two seasons dependent on disease severity. However, larger prospective studies are necessary to confirm the findings before embarking on a strategy of providing prophylaxis for a third RSV season.
Keywords: Hospitalization, Outcomes, Palivizumab, Respiratory syncytial virus infection, Season after immunization
Respiratory syncytial virus (RSV) is a common cause of respiratory infection in infants and young children under the age of 2 years, with significant morbidity and mortality associated with lower respiratory tract illness (1–5). Children who are at high-risk for RSV infection, such as premature infants ≤ 35 weeks gestational age (GA) and those who have chronic lung disease (CLD) or hemodynamically significant congenital heart disease (CHD), receive palivizumab, a humanized monoclonal antibody, for prophylaxis during the RSV season (6,7). While the safety and efficacy of palivizumab has been firmly established in high-risk populations through randomized clinical trials and in the real-world setting (6–9), limited research has been conducted to examine the incidence and associated morbidities of RSV-related hospitalization (RSVH) once a course of prophylaxis has been completed (10,11).
The aim of this descriptive study was to examine the rate and severity of RSV infection requiring hospitalization in a cohort of children the season after receiving RSV prophylaxis. Specifically, the primary objectives were: (a) to determine the incidence of RSVH the season following completion of a recommended course of palivizumab and (b) to ascertain the RSVH incidence based on indications for RSV prophylaxis. The secondary objective was to examine morbidities incurred during RSVH in each subgroup of infants.
MATERIALS AND METHODS
A retrospective review was conducted of the medical records of all infants who were enrolled in a hospital-based, RSV prophylaxis clinic over a 5-year period from the 2009 to 2010 RSV season (defined as November 1st to March 31st of the following year) through to the 2013 to 2014 season. RSVH outcomes were sequentially collected on all patients starting in 2009; hence this was selected as the inception year for the study. The study cohort was assembled at a paediatric academic health sciences centre in the province of Ontario, Canada. The RSV prophylaxis clinic commenced in 1998 and receives referrals from a defined geographical region, which captures all admissions and respective hospitalizations through a hospital-based network. Criteria for enrolment are congruent with provincial guidelines, which targets high-risk children. These include children ≤35 completed weeks GA and aged ≤6 months at the commencement of or during the RSV season. Infants between 33 and 35 weeks GA qualify only if they are deemed moderate to high-risk for RSVH (risk assessment score of 49 to 100 based on the validated risk-scoring tool for RSV prophylaxis) (12,13). Other indications for prophylaxis include children less than 2 years with bronchopulmonary dysplasia/CLD who require oxygen and/or medical therapy within 6 months prior to the RSV season, and those less than 2 years of age with CHD requiring surgical repair or are on cardiac medication. Children classified as having complex medical disorders include those, for example, with neuromuscular impairment, immune deficiency and airway anomalies that were deemed to be at high risk for morbidity or mortality from RSV infection and are approved for prophylaxis on a case-by-case basis dependent on disease severity (14–16).
Definitions and data collection
RSV infection was identified by the International Classification of Diseases-10 codes and cases were confirmed by RSV indirect fluorescent antibody test, culture or polymerase chain reaction. The season following prophylaxis was defined as the first RSV season (November 1st to March 31st) immediately following a child’s completion of RSV prophylaxis, regardless of whether the child received prophylaxis for one, two or three seasons. RSVH was defined as a hospital admission for severe RSV infection. A medically-attended RSV event was defined as any patient who experienced either a RSVH or any patient who was seen in the emergency department and was RSV-positive but discharged home. If a child was hospitalized for RSV the season following prophylaxis, data on hospital admission (paediatric ward or intensive care), respiratory course during hospitalization, length of hospital stay and outcome (alive or succumbed to RSV) were collected. Data were also assembled on all subjects who were RSV-positive and seen in the emergency room but did not require hospitalization. If an infant received prophylaxis for two consecutive seasons, only data from the most recent prophylaxis season was included in the analysis. However, data on the number of RSV seasons that infants were offered prophylaxis was retained.
Significant CHD was defined as uncorrected or palliated cyanotic or acyanotic disease with pulmonary hypertension (systolic pulmonary arterial pressure > 40 mmHg or mean pulmonary arterial pressure >25 mmHg) and/or a requirement for medication to manage congestive heart failure (17,18). CLD was determined by the established bronchopulmonary dysplasia collaborative criteria (19).
All infants meeting enrolment criteria for the provincial prophylaxis program, and who received palivizumab during the 2009/2010 through to the 2013/2014 RSV seasons, inclusive, were enrolled. All of the subjects who qualified for prophylaxis based on the governing criteria, received palivizumab. Children enrolled in the 2013 to 2014 RSV season were followed for outcomes until the 2015 to 2016 season if they had received more than one season of prophylaxis. There were no exclusion criteria. Data were extracted from medical records by a single nurse researcher using a standardized data collection tool. Data included baseline demographics, reason for enrolment in the RSV clinic, number of seasons offered prophylaxis and whether the child was hospitalized in the season following prophylaxis.
Statistical analysis
Statistical analyses were performed using IBM SPSS 24.0. Descriptive statistics were conducted on the variables extracted. Infants were classified into subgroups based on the indications for prophylaxis. A chi-square test of independence was conducted to determine if there was a significant association between indication for prophylaxis and RSVH. Odds ratios with corresponding 95% confidence intervals were computed. Differences in demographic and comorbidity variables between children with and without RSVH in each subgroup were examined using an independent t test for continuous variables and a chi-square test for nonparametric data. All inferential statistical tests were two-tailed and a P-value of less than 0.05 was considered statistically significant. The study (REB #13-072-D) was approved by the Hamilton Integrated Research Ethics Board. Informed consent was waived, since this was a medical chart review of patients incorporated under the circle of care without intervention.
RESULTS
A total of 827 children comprised the five-season study cohort, of which 106 children were seen in the clinic for two or more consecutive seasons because they met eligibility criteria for prophylaxis. There were 906 courses of palivizumab administered over the five RSV seasons, combined; 2009 to 2010 RSV season (n=206), 2010 to 2011 (n=174), 2011 to 2012 (n=181), 2012 to 2013 (n=184) and 2013 to 2014 (n=161). The clinic has a high patient adherence rate, consistent with results reported in the Canadian registry (20), with infants receiving 81.2% of their expected injections and 72% of their injections within the interdose injection intervals.
Most of the infants were male (56.5%; n=467; Table 1). GA was the most frequent reason for enrolment, with more than two-thirds either ≤ 32 weeks GA (49.8%; n=412) or between 33 and 35 weeks GA (19.6%; n=162). Less than one-fifth of the children (16.3%; n=135) had complex medical disorders. Children with CHD (8.0%; n=66) and CLD (6.3%; n=52) comprised the remaining of the enrolled cohort. Collectively, infants had a mean GA of 32.4 weeks (SD=4.36; range = 23 to 42 weeks) and a mean birthweight of 1938.2 g (SD=898.10; range = 460 to 5083 g).
Table 1.
Demographic characteristics of infants enrolled, infants with RSVH and infants medically attended for RSV in the emergency department
| Characteristic | All Infants in RSV Prophylaxis Programa (n=827) |
Infants with RSVH the season after prophylaxis (n=17) |
Infants medically attended for RSV in the emergency departmentb (n=9) |
|---|---|---|---|
| Gender, Male; n (%) | 467 (56.5) | 12 (70.6) | 2 (22.2) |
| Reason for Enrolment; n (%) ≤32 completed weeks gestational age (GA) |
412 (49.8) | 5 (29.4) | 5 (55.6) |
| 33–35 completed weeks GA | 162 (19.6) | 4 (23.5) | 0 (0.0) |
| Chronic Lung Disease | 52 (6.3) | 4 (23.5) | 2 (22.2) |
| Congenital Heart Disease | 66 (8.0) | 1 (5.9) | 0 (0.0) |
| Complex Medical Disordersc | 135 (16.3) | 3 (17.6) | 2 (22.2) |
| Mean (SD); Range | Mean (SD); Range | Mean (SD); Range | |
| Gestational Age (weeks) | 32.4 (4.36); 23–42 | 31.9 (4.55); 25–40 | 29.7 (4.42); 25–37 |
| Birthweight (g) | 1938.2 (898.10); 460–5083 | 2033.7 (1061.91); 760–4360 | 1339.2 (687.49); 700–2640 |
aProgram enrolment during the 2009/2010 through to 2013/2014 RSV seasons, inclusive.
bInfants who were RSV-positive and were seen in the emergency department but not hospitalized.
cIncludes infants with conditions such as congenital airway anomalies, cystic fibrosis and neuromuscular impairment.
Incidence of RSVH the season after prophylaxis
Examination of infants’ medical records revealed that a total of 26 infants were medically attended for RSV-related events, of which 17 were hospitalized for RSV and nine were seen in the emergency room and discharged home, during the first season following completion of prophylaxis. This is an RSVH incidence of 2.1% (n=17/827) of the unique individuals enrolled over the five seasons. One of these 17 infants (5.9%) also had a breakthrough RSV infection during the previous RSV season while receiving prophylaxis. The overall incidence of medically attended breakthrough RSV infections in the total cohort while receiving prophylaxis during the respective RSV seasons was 4.0% (n=33/827). The characteristics of the infants requiring hospitalization for RSV (n=17) the season after prophylaxis compared to those infants who were seen in the emergency department but not hospitalized (n=9), and all infants enrolled in the prophylaxis clinic (n=827) are outlined in Table 1.
Incidence of RSVH the season after prophylaxis based on indications for prophylaxis
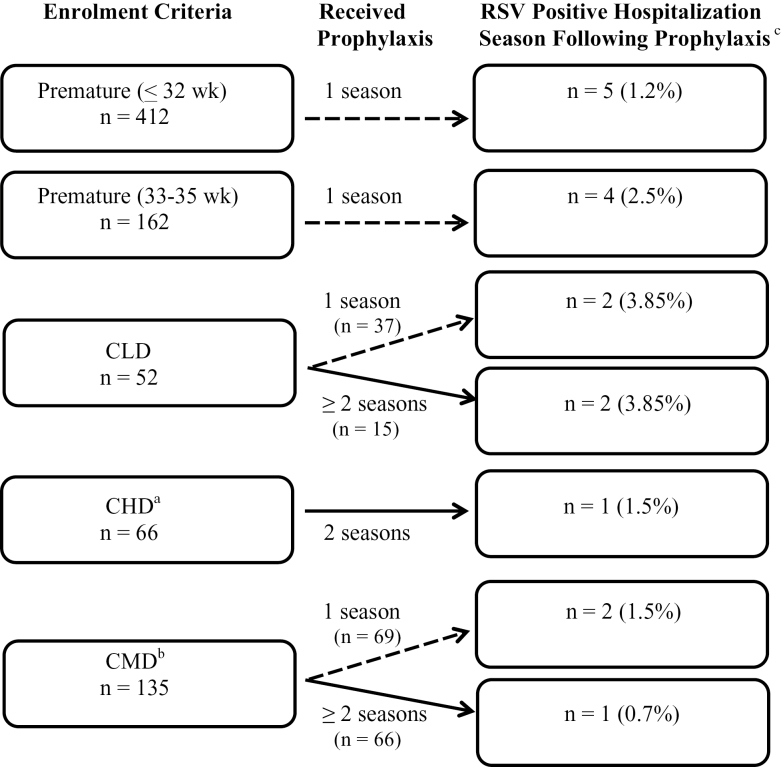
Examination of RSVH by indications for prophylaxis revealed that CLD children had a higher incidence of RSVH at 7.7% (n=4/52; Figure 1) than did the other four groups (RSVH range: 1.2 to 2.5%). Preterm infants 33 to 35 weeks GA had the second highest incidence of RSVH at 2.5% (n=4/162). Lower RSVH incidence was reported for children with complex medical disorders (2.2%; n=3/135), significant CHD (1.5%; n=1/66) and preterm infants ≤ 32 weeks GA (1.2%; n=5/412). However, there was no statistically significant association between indications for prophylaxis and RSVH (Fisher exact test, P=0.060). There was a significant association between indications for prophylaxis and all medically attended RSV events (n=26; Fisher exact test, P=0.034). The odds of RSVH was 4.9 times greater (OR=4.9; 95% CI: 1.53, 15.55; P=0.007) in children with CLD compared to those without CLD. None of the other comparisons were statistically significant.
Figure 1.
Number of respiratory syncytial virus (RSV) positive infants who were hospitalized the season following prophylaxis with palivizumab by indication for enrolment. aCHD = hemodynamically significant congenital heart disease; bCMD = complex medical disorders; cPercentages reported as a proportion of each enrolment criterion.
Demographically, infants who were hospitalized for their RSV illness (n=17) had a mean chronological age of 21.5 months (SD=10.54) at the time they required intervention and were hospitalized for RSV. At birth, the RSVH group (n=17) had a mean GA of 31.9 weeks (SD=4.55; range: 25 to 40 weeks), and a mean weight of 2033.7 g (SD=1061.91; range: 760 to 4360 g).
Incurred morbidities following RSVH
Most of the infants with RSVH the season after prophylaxis required admission to the general paediatric in-patient unit (70.6%; n=12) versus the paediatric intensive care (29.4%; n=5). Nine (34.6%) of the total RSV-positive children received treatment in the emergency department and were discharged home (five premature infants ≤32 weeks GA, two with CLD, one each with Down syndrome and cystic fibrosis). The median length of hospital stay for infants was 4 days (range: 1 to 60 days). Only one infant required ventilatory support for 3 days during hospitalization. Ten infants (58.8%) required oxygen without ventilator support for a median of 1 day (range: 1 to 60 days), one of whom was on home oxygen prior to hospitalization. None of the infants succumbed to their RSV infection.
Infants with RSVH versus those not hospitalized
A comparison of infants who experienced RSVH versus those that did not, revealed that the CLD infant group with RSVH had a significant heavier birthweight (mean birthweight = 2775 g [SD=1795.54]) than did infants with CLD who were not hospitalized (mean birthweight = 1218.2 g [SD=955.32]), P=0.012. All other groups of children who received prophylaxis based on indications were similar in birthweight between those hospitalized versus not-hospitalized for RSV infection. Other demographic characteristics were also similar between the children hospitalized for RSV and the groups who were not hospitalized (all P>0.05). Differences in GA and birthweight between RSVH and non-RSVH infants in the CHD group were not analyzed due to only one observation in the RSVH group.
DISCUSSION
This is the first study to systematically evaluate RSVH and incurred morbidities in the season postprophylaxis among children who received palivizumab for a variety of indications. Fanos et al. (10) evaluated preterm infants ≤ 35 weeks GA during the second RSV season after receiving prophylaxis in the first season (October 2004 to April 2005). Among 260 infants, 32.3% experienced a respiratory event of which 3.8% (n=10) were hospitalized. Only one patient was tested for RSV and was proven negative. Kim et al. (11) conducted a retrospective review of 466 Korean infants with CHD who had received palivizumab, only in the first year of life, from 2009 to 2015. Breakthrough RSVH occurred in 57 (12.2%) patients during a mean follow-up period of 24.3 ± 16.4 months, with 86% experiencing RSVH between October and January of the following RSV season. Only three infants (0.6%) were hospitalized with RSV after 1 year of age. Similarly, in our study, of the 66 subjects with CHD who received prophylaxis for two consecutive seasons, only 1 (1.5%) was hospitalized with RSV in the season following the completion of prophylaxis. This is in keeping with the epidemiology of RSV-related illness that steadily declines in childhood after 2 years of age (3,21,22). In addition, our current provincial guidelines offer prophylaxis to patients with significant, unresolved CHD up to 2 years of age, unlike the Korean guidelines that permit prophylaxis for only those aged less than 1 year (11). In an abstract, Campbell et al. (23) reported RSVH rates during the second respiratory season in preterm children who had (n=972) or had not received RSV prophylaxis (n=872). There was no statistical difference in RSVH in the palivizumab arm of the study (2.06%) versus the control group (1.26%). Although the abstract lacks details regarding the preterm cohort and the duration of the study, the results are similar to our cohort of healthy premature infants ≤ 35 weeks gestation in which the RSVH rate was 1.6% (n=9/574) in the season following prophylaxis.
Children with CLD, who are less than 2 years of age, comprise a very high-risk population for both respiratory-related hospitalization and RSVH. In a systematic review of CLD children conducted from 1995 to 2015 which included 39 articles, RSVH rates in children who did not receive prophylaxis across Europe, the USA and Canada range from 12 to 46%, with 8 of 10 studies reporting an incidence of between 12 and 21% (24). Moreover, following RSVH these children remain at risk for obstructive lung function that may persist into childhood, adolescence and adulthood, setting the stage for long-term chronic obstructive lung disease (25–27). Based on high-quality evidence from randomized trials (6,28), the majority of guidelines support the use of palivizumab for CLD children aged less than 2 years (29–31). In the Canadian prospective, cohort study of palivizumab, without a control arm, the hazard for breakthrough RSVH in all CLD children, irrespective of CLD disease severity (mild, moderate, or severe) in the first year of life is similar to those aged 1 to 2 years, who received prophylaxis specifically for more severe CLD (32). This may imply that children in the latter group remain at risk because of alterations in lung or airway growth during fetal development that are exacerbated postnatally with invasive ventilation or that prematurity itself is a precursor of reduced lung function (33). Our study confirms that children with CLD are still at highest risk for RSVH (7.7%) compared to those who are premature, have CHD or complex medical disorders during the season postprophylaxis and may merit palivizumab for more than two seasons dependent on disease severity. If the findings are proven in future, prospective studies, the benefits, efficacy, number needed to treat and incurred costs of the strategy will need to be carefully weighed prior to adoption.
Overall, the morbidities experienced in the season following prophylaxis were relatively mild since 12 children (70.6%) were admitted briefly to the paediatric ward while 9 (34.6%) of the total RSV-positive children were seen in the emergency room and discharged home. This may reflect older age at the time of RSV-related infection or that repetitive exposure to episodes of RSV results in enhanced immunity that ameliorates disease severity (34,35).
There are several limitations in this study. First, the retrospective design, despite the use of accurate diagnostic codes for the identification of the RSV cases, may underestimate the true incidence of RSVH in the study population. Second, polymerase chain reaction with improved sensitivity and specificity has been more recently adopted to confirm the presence of RSV and the use of previous, less accurate diagnostic tests may also influence our primary outcome. Third, although the sample size of 827 infants was relatively large, the sub-classification of the analysis into distinctive indications for palivizumab with smaller numbers of subjects may have reduced the power to more accurately determine the populations that are still at risk in the season following prophylaxis. Last, medically attended RSV did not include visits to physician’s offices as these were too difficult to capture.
CONCLUSIONS
Our results confirm that CLD children are the highest risk group for RSVH the season following prophylaxis, with a significant odds of RSVH that is 4.9 times greater in children with CLD compared to those children without CLD, despite receiving prophylaxis for 2 years. Larger, prospective studies are necessary to determine the need for a third season of prophylaxis for the CLD population, recognizing that repetitive RSV infections may inflict greater damage on already compromised airways and pose a greater threat to future long-term respiratory function in these children.
Conflicts of interest
BAP has received research funding from AbbVie Corporation and compensation as advisor or lecturer from AbbVie Corporation. MLB and LAE have no conflicts to declare.
Informed consent: Informed consent was waived by the local IRB, since this was a medical chart review of patients incorporated under the circle of care without intervention.
Funding source: None.
Institutions of Origin of the Work: McMaster Children’s Hospital and McMaster University.
Ethics Board Approval: Hamilton Integrated Research Ethics Board.
References
- 1. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009;360(6):588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010;375(9725):1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bont L, Checchia PA, Fauroux B, et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther 2016;5(3):271–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stein RT, Bont LJ, Zar H, et al. Respiratory syncytial virus hospitalization and mortality: Systematic review and meta-analysis. Pediatr Pulmonol 2017;52(4):556–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Byington CL, Wilkes J, Korgenski K, Sheng X. Respiratory syncytial virus-associated mortality in hospitalized infants and young children. Pediatrics 2015;135(1):e24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduced hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998;102:531–7. [PubMed] [Google Scholar]
- 7. Feltes TF, Cabalka AK, Meissner HC, et al. ; Cardiac Synagis Study Group Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003;143(4):532–40. [DOI] [PubMed] [Google Scholar]
- 8. Chen JJ, Chan P, Paes B, Mitchell I, Li A, Lanctôt KL; CARESS investigators Serious adverse events in the Canadian registry of children receiving palivizumab (CARESS) for respiratory syncytial virus prevention. Plos One 2015;10(8):e0134711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wegzyn C, Toh LK, Notario G, et al. Safety and effectiveness of palivizumab in children at high risk of serious disease due to respiratory syncytial virus infection: A systematic review. Infect Dis Ther 2014;3(2):133–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fanos V, Scarcella A, Puddu M, et al. Respiratory disorders and hospitalization rates during the second RSV season in preterm infants who received palivizumab prophylaxis during their first RSV season. J Chemother 2009;21(3):302–10. [DOI] [PubMed] [Google Scholar]
- 11. Kim AY, Jung SY, Choi JY, et al. Retrospective multicenter study of respiratory syncytial virus prophylaxis in Korean children with congenital heart diseases. Korean Circ J 2016;46(5):719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sampalis JS, Langley J, Carbonell-Estrany X, et al. Development and validation of a risk scoring tool to predict respiratory syncytial virus hospitalization in premature infants born at 33 through 35 completed weeks of gestation. Med Decis Making 2008;28(4):471–80. [DOI] [PubMed] [Google Scholar]
- 13. Paes B, Steele S, Janes M, Pinelli J. Risk-scoring tool for respiratory syncytial virus prophylaxis in premature infants born at 33-35 completed weeks’ gestational age in Canada. Curr Med Res Opin 2009;25(7):1585–91. [DOI] [PubMed] [Google Scholar]
- 14. Paes B, Mitchell I, Li A, Harimoto T, Lanctôt KL. Respiratory-related hospitalizations following prophylaxis in the Canadian registry for palivizumab (2005-2012) compared to other international registries. Clin Dev Immunol 2013;2013:917068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manzoni P, Paes B, Lanctôt KL, et al. Outcomes of infants receiving palivizumab prophylaxis for respiratory syncytial virus in Canada and Italy: An international, prospective cohort study. Pediatr Infect Dis J 2017;36(1):2–8. [DOI] [PubMed] [Google Scholar]
- 16. Paes B, Mitchell I, Li A, Lanctôt KL. Respiratory hospitalizations and respiratory syncytial virus prophylaxis in special populations. Eur J Pediatr 2012;171(5):833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feltes TF, Sondheimer HM, Tulloh RM, et al. ; Motavizumab Cardiac Study Group A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatr Res 2011;70(2):186–91. [DOI] [PubMed] [Google Scholar]
- 18. Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: Guidelines from the American heart association and American thoracic society. Circulation 2015; 132:2037–99. [DOI] [PubMed] [Google Scholar]
- 19. Abman SH, Collaco JM, Shepherd EG, et al. ; Bronchopulmonary Dysplasia Collaborative Interdisciplinary care of children with severe bronchopulmonary dysplasia. J Pediatr 2017;181: 12–28.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan P, Li A, Paes B, Abraha H, Mitchell I, Lanctôt KL; CARESS investigators Adherence to palivizumab for respiratory syncytial virus prevention in the Canadian registry of palivizumab. Pediatr Infect Dis J 2015;34(12):e290–7. [DOI] [PubMed] [Google Scholar]
- 21. Schanzer DL, Langley JM, Tam TW. Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children. Pediatr Infect Dis J 2006;25(9):795–800. [DOI] [PubMed] [Google Scholar]
- 22. Ajayi-Obe EK, Coen PG, Handa R, et al. Influenza A and respiratory syncytial virus hospital burden in young children in East London. Epidemiol Infect 2008;136(8):1046–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campbell AL, Pollack PF, Fredrick LM, Groothuis JR. RSV hospitalization rates in preterm children in the season following palivizumab prophylaxis compared to children without palivizumab prophylaxis. Arch Dis Child 2008; 93(Suppl II): A339–46. [Google Scholar]
- 24. Paes B, Fauroux B, Figueras-Aloy J, et al. Defining the risk and associated morbidity and mortality of severe respiratory syncytial virus infection among infants with chronic lung disease. Infect Dis Ther 2016;5(4):453–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greenough A, Alexander J, Boit P, et al. School age outcome of hospitalisation with respiratory syncytial virus infection of prematurely born infants. Thorax 2009;64(6):490–5. [DOI] [PubMed] [Google Scholar]
- 26. Fauroux B, Simões EAF, Checchia PA, et al. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther 2017;6(2):173–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berry CE, Billheimer D, Jenkins IC, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med 2016;194(5):607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev 2013;4:CD006602. [DOI] [PubMed] [Google Scholar]
- 29. American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014;134:e620–38. [DOI] [PubMed] [Google Scholar]
- 30. Bollani L, Baraldi E, Chirico G, et al. ; Italian Society of Neonatology Revised recommendations concerning palivizumab prophylaxis for respiratory syncytial virus (RSV). Ital J Pediatr 2015;41:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Figueras Aloy J, Carbonell Estrany X; Comité de Estándares de la SENeo.. Update of recommendations on the use of palivizumab as prophylaxis in RSV infections. An Pediatr (Barc) 2015; 82:199.e1-2. [DOI] [PubMed] [Google Scholar]
- 32. Wang DY, Li A, Paes B, Mitchell I, Lanctôt KL; CARESS Investigators First versus second year respiratory syncytial virus prophylaxis in chronic lung disease (2005-2015). Eur J Pediatr 2017;176(3):413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kotecha SJ, Edwards MO, Watkins WJ, et al. Effect of preterm birth on later FEV1: A systematic review and meta-analysis. Thorax 2013;68(8):760–6. [DOI] [PubMed] [Google Scholar]
- 34. Yui I, Fujino M, Sawada A, Nakayama T. Novel clinical features of recurrent human respiratory syncytial virus infections. J Med Virol 2014;86(9):1629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohuma EO, Okiro EA, Ochola R, et al. The natural history of respiratory syncytial virus in a birth cohort: The influence of age and previous infection on reinfection and disease. Am J Epidemiol 2012;176(9):794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]