Chronic deep venous thrombosis (DVT) of the inferior vena cava and the iliac veins, also known as chronic iliocaval thrombosis, is a cause of significant morbidity. This may lead to limb-threatening occlusive symptoms secondary to DVT in the lower extremities, phlegmasia cerulea dolens, and postthrombotic syndrome (PTS), and may be fatal secondary to development of pulmonary embolism. 1 2 3 4 5 6 7 8 9 10 PTS commonly presents with leg pain, swelling, venous claudication, cutaneous hyperpigmentation, varicose veins, and venous ulcers secondary to stasis. Iliocaval recanalization and reconstruction is a minimally invasive endovascular technique used in the management of chronic iliocaval thrombosis, which involves recanalization, angioplasty, and possibly stenting, with the ultimate aim of restoring in-line venous flow throughout the iliocaval system. This technique has been shown to provide significant symptomatic relief to patients while also potentially preventing recurrent thromboembolic disease.
Indications
The indications for endovascular iliocaval recanalization and reconstruction include deep venous occlusion secondary to recurrent DVT, severe PTS, and caval thrombosis leading to significant lifestyle limitations. 11 Acute thrombus is generally defined as those aged less than 2 weeks, while chronic thrombus is defined as being present for more than 4 weeks. Endovascular iliocaval reconstruction may be performed with both acute and chronic thrombosis; however, the indications and techniques for recanalization of acute iliocaval thrombosis differ from those used for chronic thrombosis. Acute thrombus is soft and pliable, allowing for early thrombolysis, recanalization, angioplasty, and stent placement. Subacute thrombosis (2–4 weeks old) may either be managed medically with anticoagulation, ambulation, and leg elevation or by endovascular recanalization. 12 Delaying the endovascular treatment of subacute thrombosis may lead to maturation of the thrombus. Subacute thrombus is large and bulky, which limits angioplasty and stenting and makes it less responsive to thrombolysis and thrombectomy. Chronic thrombus (>4 weeks) becomes organized and elicits inflammatory scarring in the vessel, which allows the vessel to be balloon dilated and stented more effectively after recanalization. 12 The classification of thrombus as acute, subacute, and chronic should be used as a rough guide, and clinical evaluation of the patient should ultimately guide intervention, especially in the subacute phase.
Clinical Evaluation
Preprocedural evaluation is critical for the appropriate management of patients with iliocaval thrombosis. 1 2 13 Relevant patient history should include the time course of symptoms, anticoagulation use, comorbidities which may affect anesthesia needs, personal or family history of thrombophilia (which may warrant further medical evaluation for hereditary thrombophilias 14 ), functional status related to deep venous occlusion, and prior interventions pertinent to the patient's current presentation such as inferior vena cava filter placement and surgical history.
A focused physical examination should be performed of the lower extremities, which may delineate the clinical extent of deep venous occlusion and the severity of disease. 2 13 Lower extremity edema, cyanosis, lipodermatosclerosis, varicosities, and tenderness are some of the clinical signs that are helpful in identifying the occluded venous segments. Laterality of signs and symptoms (unilateral or bilateral) is also important to assess. Nonspecific symptoms of venous occlusion include radiculopathy and back pain secondary to dilated epidural and lumbar collaterals, postural hypotension due to pooling of blood in collaterals and decreased venous return upon standing, abdominal and pelvic pain due to mesenteric collateral drainage, and dysphagia secondary to dilated azygous and hemiazygos veins causing mass effect on esophagus. Pertinent laboratory evaluation includes coagulation parameters, such as platelets, partial thromboplastin time, prothrombin time/international normalized ratio, and some specific laboratory tests relevant to the patient, such as anti-Xa levels, Ecarin chromogenic assay, clotting time, and dilute Russell's viper venom time. Additionally, a baseline complete blood count and basic metabolic panel should be obtained to evaluate for anemia and renal function. 13
Imaging Evaluation
A thorough review of all prior imaging examinations is critical to guide clinical decision making, plan potential interventions, gauge the location and extent of thrombosis, and evaluate the degree of collateralization and patency of access vessels. If not already available, appropriate imaging must be obtained prior to the procedure and is considered standard of care.
Duplex ultrasonography is a noninvasive imaging modality that obtains both anatomic and physiologic data regarding patency and flow characteristics of the deep venous system in the lower extremities. 15 Longitudinal gray scale sonographic imaging is important in addition to the transverse views, since color Doppler masks vessel wall thickening or webs within the vein lumen. Duplex sonography is limited in the complete evaluation of the iliac veins, but may be used for screening as appropriate. 13 The absence of spontaneous flow with respiration in the common femoral vein may raise suspicion for iliocaval thrombosis, which may not be directly visualized with ultrasound. Computed tomography venography (CTV) or magnetic resonance venography (MRV) are excellent noninvasive imaging modalities used to delineate the anatomy of the iliocaval venous system, demonstrate large collaterals, visualize calcified thrombotic segments, and determine the location of intravascular foreign bodies such as inferior vena cava filters. 16 CTV or MRV is particularly useful in cases where sharp recanalization is being planned or anticipated. 17
Anticoagulation
Anticoagulation is the standard of care for patients with both acute and chronic deep occlusion. Therefore, patients presenting for endovascular treatment of caval thrombosis are routinely on anticoagulation or antiplatelet medications. 18 19 Guidewire manipulation during recanalization carries the risk of extraluminal passage of the wire and subsequent hemorrhage; however, there are no specific guidelines for management of anticoagulation. Some practitioners suggest holding all anticoagulation and antiplatelet agents other than aspirin prior to endovascular interventions. 20 Warfarin and clopidogrel, for instance, should be held for 5 days; apixaban, fondaparinux, and rivaroxaban for 3 days; and enoxaparin for 24 hours. If the patient is on a heparin infusion, it may be held for 2 hours prior to intervention and restarted in the postprocedural care unit. Some authors, however, advocate continued heparin infusion during the procedure to eliminate the need for heparin titration during the procedure.
Equipment
The equipment used for iliocaval recanalization and reconstruction includes standard micropuncture sets for internal jugular vein, common femoral vein, great saphenous vein, popliteal vein, or posterior tibial vein accesses: 6-French (F), 8-F, and 10-F sheaths; vertebral tip (angled) or braided tapered tip catheters; flush catheters; stiff straight- and angle-tipped hydrophilic guidewires (Glidewire; Terumo Corporation; Shibuya, Japan); high-pressure balloons; and loop snare devices as needed to begin the recanalization. Needles that may be used for sharp recanalization include BRK and BRK-1 transseptal (St. Jude Medical, Saint Paul, MN), Chiba (Cook Medical, Bloomington, IN), and Rösch-Uchida (Cook Medical) needles. Intravascular ultrasound (IVUS) may be useful for detecting residual mural thrombus, delineating the degree of deep venous involvement, identifying the proximity of critical structures, and determining stent sizes. 21
Currently, all stents used for caval or iliac deep venous reconstruction are used off-label, since there are no Food and Drug Administration–approved stents for iliocaval reconstruction. Large diameter and self-expanding stents are ideal for iliocaval system (e.g., Wallstents; Boston Scientific, Natick, MA). A 10-F sheath may accommodate up to a 20-mm Wallstent; 12-F sheath, however, may be required in cases where 24-mm Wallstents need to be placed. Both Wallstents and self-expanding nitinol stents have good long-term patency and clinical outcomes. 22 23 24 For venous closure, manual compression, pressure-assisted closure devices, or sutures may be used. 12
Anesthesia
General anesthesia is preferred for chronic iliocaval thrombosis recanalization, since angioplasty of stenosed veins may be painful and because these procedures are very time intensive. Sedation and analgesia may also be used in poor anesthetic candidates, or based on operator preference and experience. In these patients, generous use of local anesthesia at all vascular access sites is vital to ensure patient comfort. The patients' ability to follow commands is imperative, since frequent breath holds are needed for adequate quality diagnostic venograms. The patient should be typed and crossed for two units of blood prior to the procedure, to be administered in the event of serious hemorrhage. 12
Vascular Access
From a recent survey of 107 interventionalists, the most commonly used access sites are femoral (80.8%), internal jugular (68.7%), popliteal (54.5%), and saphenous vein (27.3%), with over half of all procedures (51.5%) using two access sites, and one-third (32.3%) using three. 25 The optimal access route, however, is open to debate, leading to considerable heterogeneity in practice patterns. Saphenous access may have the benefit of easier postprocedural hemostasis compared with femoral access, without being limited to smaller catheter calibers. This is particularly important in patients who are at high risk of postprocedural hemorrhage, such as fully anticoagulated patients. 2 Posterior tibial access may be appropriate in patients with distal occlusions in the lower extremity that may not be adequately approached with femoral or saphenous access. Distal access may also be more comfortable for patients and allows for early ambulation in recovery. The tibial approach, however, is limited to 7-F catheter sizes due to small vessel caliber.
Recanalization
Recanalization and reconstruction for lower extremity deep venous occlusive disease are shown in Figs. 1 , 2 , and 3 . Recanalization is performed from peripheral to central, to direct guidewire advancement into the true thin iliocaval conduit (referred to as “string sign”; Fig. 1b ) on subsequent contrast injections. 2 An 8-F sheath may be placed in the internal jugular vein and a 6-F sheath may be placed into the inferior access (such as common femoral, great saphenous, or popliteal vein), followed by advancement of a hydrophilic guidewire and vertebral catheter. Digital subtraction venographic images are obtained (following access) to determine the extent of deep venous occlusive disease and to plan the pathway for recanalization. Delayed venographic images are useful to visualize the reconstituted central segment. The string sign may be used as a guide if the identification of the channel becomes difficult to visualize among the surrounding collateral vessels. A short tapered “nub” will be present, which indicates the distal aspect of the completely occluded venous segment.
Fig. 1.
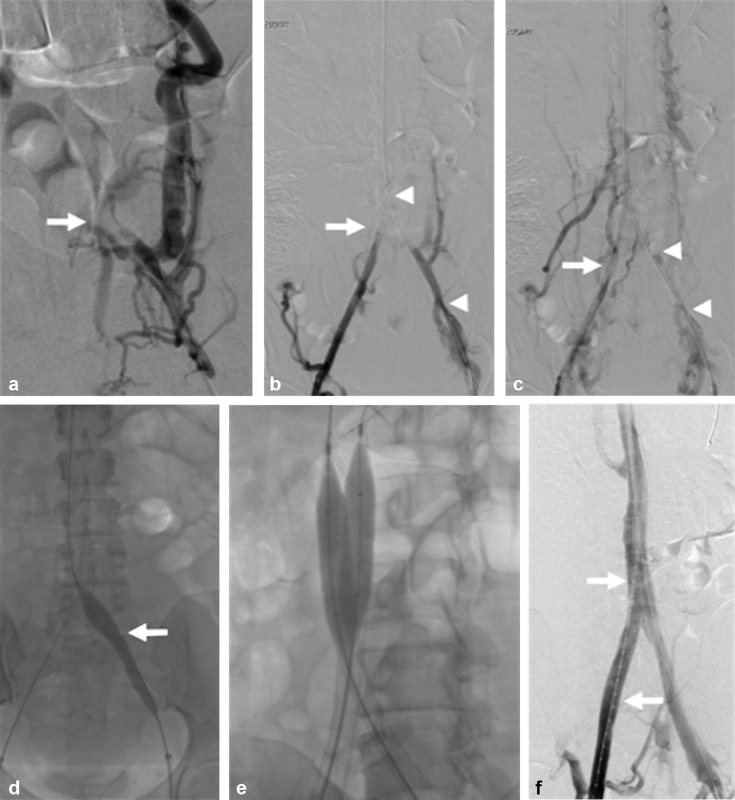
A 52-year-old female with quadriplegia, factor V Leiden, and antithrombin III deficiency referred for chronic iliocaval thrombosis and phlegmasia. ( a ) Left iliocaval venography demonstrating left common iliac occlusive disease with robust retroperitoneal collaterals. The iliocaval confluence is faintly seen (arrow). ( b ) Bilateral right (arrow) and left (arrowheads) iliocaval venography after blunt recanalization of the left iliac vein. ( c ) Bilateral right (arrow) and left (arrowheads) iliocaval venography after blunt recanalization of the right and left iliac veins. ( d ) Sequential angioplasty of the inferior vena cava was performed using 8 mm × 8 cm Mustang, 14 mm × 6 cm, and 18 mm × 4 cm Atlas balloons. Angioplasty of the bilateral right and left (arrow) common iliac veins was performed using 8 mm × 8 cm Mustang, 14 mm × 6 cm and 16 mm × 4 cm Atlas balloons. ( e ) Iliocaval reconstruction was performed using a 20 mm × 55 mm Wallstent within the inferior vena cava and 14 mm × 90 mm Wallstents within the bilateral common iliac veins. Postdeployment angioplasty was performed. ( f ) Completion venography showing brisk flow throughout the iliocaval venous reconstruction. A flush catheter is seen throughout the right iliocaval segments (arrows).
Fig. 2.
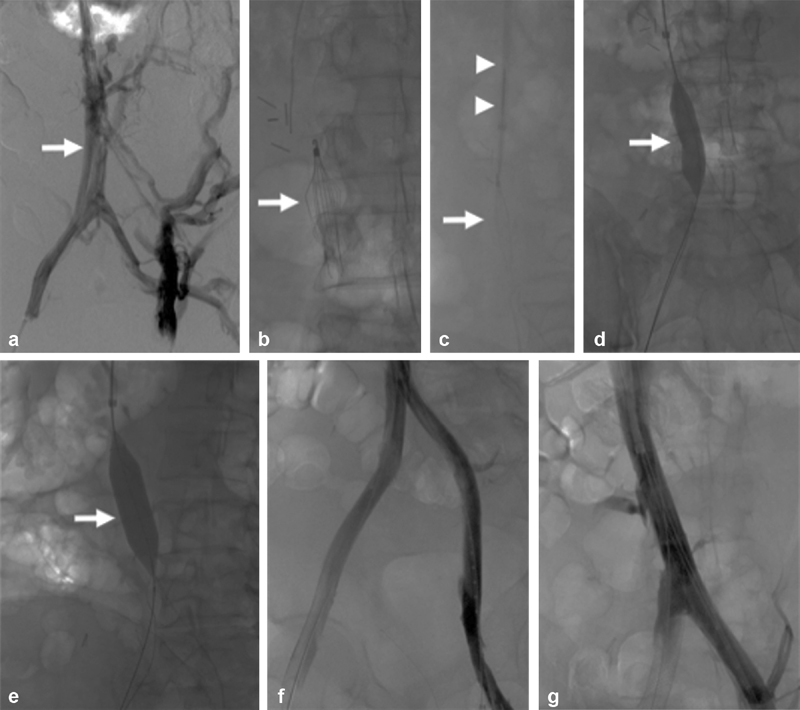
A 46-year-old male with esophageal cancer–associated hypercoagulability and inferior vena cava filter–associated chronic iliocaval thrombosis and left lower extremity ulceration (Clinical, Etiology, Anatomy, and Pathophysiology class 6 disease). ( a ) Bilateral iliocaval venography demonstrates chronic thrombotic changes throughout the iliac vein and inferior vena cava (arrow). Multiple lumbar and retroperitoneal venous collaterals are seen. ( b ) Fluoroscopic image showing a Denali inferior vena cava filter (arrow) causing chronic filter-associated iliocaval thrombosis. ( c ) The Denali inferior vena cava filter (arrow) was removed using the standard Cook retrieval set (arrowheads). ( d ) Angioplasty of the inferior vena cava using a 14-mm high-pressure balloon (arrow). ( e ) Angioplasty of the inferior vena cava using an 18-mm high-pressure balloon (arrow). ( f ) A 20 mm × 55 mm Wallstent was deployed in the inferior vena cava and 14 mm × 60 mm Wallstents were placed within the common iliac veins. Completion venography demonstrated robust in-line venous flow throughout both iliac veins. ( g ) Venography demonstrated in-line flow throughout the inferior vena cava.
Fig. 3.
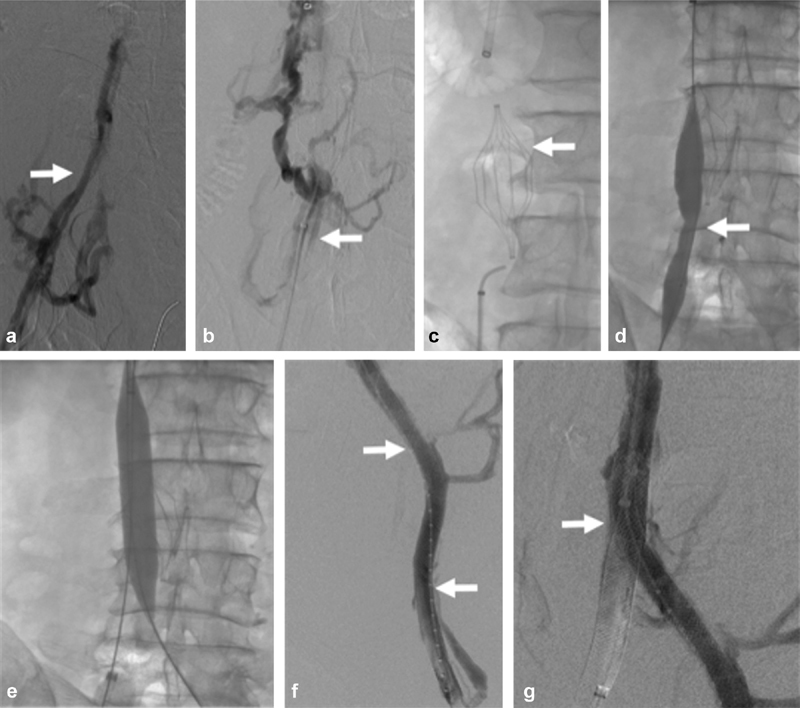
A 61-year-old male with methylenetetrahydrofolate reductase and smoking history with inferior vena cava filter–associated chronic iliocaval thrombosis and left lower extremity pain and swelling (Clinical, Etiology, Anatomy, and Pathophysiology class 4 disease). ( a ) Right iliocaval venography demonstrated venous irregularity consistent with chronic iliocaval thrombosis of the right iliocaval segment (arrow). ( b ) Initial attempts to cross the inferior vena cava filter–associated iliocaval thrombosis using a glidewire and glide catheter (arrow) were unsuccessful. ( c ) Fluoroscopic image demonstrating the Trapeze inferior vena cava filter (arrow) causing chronic filter-associated iliocaval thrombosis. Sharp recanalization was performed using a BRK needle and a loop snare. ( d ) Attempts to remove the inferior vena cava filter were unsuccessful. Angioplasty across the inferior vena cava filter was performed using a 4-mm Sterling balloon (arrow). ( e ) The infrarenal inferior vena cava was angioplastied using an 18-mm balloon) ( f ) A 20 mm × 55 mm Wallstent was deployed in the inferior vena cava and 12 mm × 90 mm Wallstents were deployed in the common iliac veins (arrows). ( g ) Completion venography demonstrated a briskly filling iliocaval reconstruction with Trapeze inferior vena cava filter sequestration (arrow).
Multiple recanalization techniques have been described for chronic venous stenosis and occlusions, the most common and straightforward of which is blunt recanalization. For this technique, the operator uses a guidewire and catheter to probe through the occlusion until the catheter reaches a patent segment. 11 12 21 Once the occlusion is crossed, through-and-through access may be obtained by capturing an exchange-length wire placed through the catheter with a loop snare device placed from another access site. Through-and-through wire access establishes a rigid rail that may be tightened from both ends to accomplish delivery of catheters, balloons, and stents across the obstructed veins, especially the recalcitrant segments. 21
Sharp recanalization techniques may be required when blunt recanalization techniques fail, for instance when the wire passage is not possible in areas of dense fibrosis and calcification, or when wires from central and proximal accesses may overlap in different planes and are unable to re-enter the reconstituted segment. Sharp recanalization of the obstruction involves the use of a recanalization needle or re-entry device and a loop snare. It can also be performed using a side-by-side balloon dilation in the inferior vena cava or iliofemoral segments, where two balloons are inflated in an attempt to create a common channel through which a wire may be passed to cross the obstruction and potentially to use a loop snare to entrap the wire.
In cases where standard blunt or sharp recanalization techniques fail to facilitate successful transversal of the occlusion, three-dimensional (3D) targeting of an AMPLATZER vascular plug (St. Jude Medical) or Wallstent endoprosthesis may be indicated. This technique uses a partially deployed AMPLATZER vascular plug or Wallstent endoprosthesis in the patent distal or proximal portion of the vessel which serves as a 3D target for transseptal needle localization from the opposite side. The partially deployed device may be targeted and punctured using rotational fluoroscopy which uses tactile and visual cues to help confirm the needle hitting the target. Following this, a 0.018-inch V-18 guidewire (Boston Scientific) is advanced through the interstices of the device before reconstraining the plug or stent, which establishes a through-and-through access when the device is removed from its respective sheath. 21
Thrombectomy
Several catheter-based mechanical and pharmacomechanical thrombectomy devices are available, which may be used in acute or subacute thrombosis and aid in clearing acute on chronic clots that appear during recanalization. Catheter-directed thrombolysis with tissue plasminogen activator, alteplase, or tenecteplase infusion may be performed using infusion catheters such as EkoSonic ultrasound-enhanced thrombolysis system (EKOS; BTG, London, UK), UniFuse (Angiodynamics, Latham, NY), or the Cragg-McNamara (Covidien Ltd, Dublin, Ireland) multi-sidehole infusion catheters. 26 Pharmacomechanical thrombectomy devices include the AngioJet rheolytic thrombectomy system (Boston Scientific) and the Trellis device (Covidien, previously discontinued). Pure mechanical thrombectomy may be performed using Indigo System (Penumbra, Alameda, CA), Arrow-Trerotola percutaneous thrombectomy device (Teleflex Inc., Wayne, PA), Cleaner 15 and XT (Argon Medical, Plano, TX), and AngioVac aspiration thrombectomy system (Angiodynamics). 27 28
Reconstruction
Following wire recanalization of the occluded deep venous segments, the tract should be interrogated using angiography (with iodinated contrast or CO 2 ) or IVUS for integrity and transversal of adjacent arteries and organs prior to balloon angioplasty and stenting. IVUS may delineate the locations of vessel reconstitution or reentry, which may serve as landmarks for balloon inflation and stenting landing zones. 21 After confirmation of tract safety, systemic heparin should be initiated with a goal activated clotting time (ACT) of more than 230 (unless continuous heparin infusion is used). To insert the appropriately sized balloons for the next step, an 8-F sheath, at minimum, must be inserted. It may be necessary to predilate the tract with a 4- to 6-mm balloon to advance the 8-F sheath across the occlusion. The IVC is then sequentially angioplastied to 18 to 20 mm, the common iliac vein to 16 mm, and the external iliac and common femoral veins to 14 mm. 12 29 There is a risk of postprocedural rethrombosis in the recanalized lumen containing residual thrombus. The residual thrombus may be visualized using IVUS or outlined by contrast on angiography, and if present, there should be a low threshold for stenting, preferably using self-expanding stents. These stents have minimal foreshortening, good flexibility, and high resistive force. 30 31
Long-term stent patency requires maintenance of adequate inflow into the reconstructed inferior vena cava or iliac veins. 32 Inflow is evaluated in the common femoral, femoral, profunda femoris, and popliteal veins. If venography of these veins demonstrates significant stasis of flow below or within the stented segments, or significant flow through collaterals, additional angioplasty or stenting may be required in these segments. Venography of these veins may be performed from internal jugular, popliteal, or posterior tibial approaches. IVUS may be used as an adjunct to increase the sensitivity of postprocedural evaluation. 33 Patients who suffer from iliocaval thrombosis may also have thrombus extending inferiorly to the common femoral vein. For successful recanalization, stenting of this area may also be required. Note that the profunda femoris vein may serve as the primary or secondary drainage of lower extremity following iliocaval reconstruction. Preservation of its inflow is thus important whenever possible. 34
Technical Considerations
During recanalization of an obstruction, it is possible for the needle or guidewire to pass outside the vessel lumen. 35 36 Due to low pressures within the venous system, however, short segments of extraluminal passage of needle or guidewire in areas of chronic thrombosis have not been found to be clinically significant. Major venous tears, if encountered, may be managed with self-expanding stent grafts, such as conformable TAG or Viabahn (Gore Medical, Flagstaff, AZ). Alternatively, anticoagulation may be temporarily held and uncovered stents may be placed across the tear. 37
Recanalization at the left iliocaval confluence near the right iliac artery bifurcation and near the renal artery poses some risk of arterial injury, particularly when sharp recanalization is used. In these cases, IVUS or placement of arterial angiography catheter and intermittent injection of contrast may be used to assist in localizing the arteries to avoid injury when passing the guidewire or needle. Ureteral injuries may be prevented by nephroureteral stent placement preprocedurally, if sharp recanalization is required near the ureter crossing.
Inferior vena cava filter–associated iliocaval thrombosis presents an added obstacle for recanalization. Filter retrieval should be attempted for both retrievable and permanent filters. 38 39 40 Several studies have shown safe retrieval of permanent filters using advanced techniques. 41 42 43 44 If the filter cannot be retrieved, angioplasty and placement of a self-expanding 18- to 24-mm stent across the filter may be performed to exclude the filter from the reconstructed lumen. 45 46 Studies have shown similar long-term patency rates for both filter retrieval and exclusion techniques. 11
Complications
Immediate complications are related to vascular access and self-limiting access site hematomas or bleeding, which may be managed with manual compression or pressure-assisted devices. Arterial injuries leading to retroperitoneal or access site hematomas are rare and may lead to hemodynamic compromise, requiring blood transfusions. 12 Computed tomography angiography may be considered for clinical suspicion and arterial angiography and embolization as warranted. Antiplatelet agents such as clopidogrel and aspirin should be held if hemorrhage persists. Stent migration is the most common complication after stent placement, and if this does occur, an additional stent may be placed between the stents to reduce the risk of restenosis or interstent thrombosis. 29
Postprocedural Care
There are no specific anticoagulant recommendations regarding anticoagulation following iliocaval reconstruction for deep venous occlusive disease. Anticoagulant prophylaxis with enoxaparin or low-molecular-weight heparin may be considered during the immediate postprocedural period. Some authors opine the use of baby aspirin (81 mg) daily, clopidogrel 75 mg daily, and enoxaparin 1 mg/kg twice daily prior to discharge. 2 5 6 11 22 47 Enoxaparin is preferred for its rapid onset, reliable anticoagulation (unlike warfarin), and potential anti-inflammatory action. Some authors, on the other hand, use anticoagulants only during the acute phase within 6 months of a diagnosed venous thromboembolism or known thrombophilia. In cases without venous thromboembolism or thrombophilia, use of dual-platelet therapy with aspirin and clopidogrel with an anticoagulant is recommended. Patients should be seen in the outpatient clinic within 2 weeks postprocedure and may be transitioned to warfarin or direct oral anticoagulant. In patients with body mass index of greater than 40 or cachexia, low-molecular-weight heparin dose of 1 mg/kg may be supratherapeutic, and anti-Xa levels should be checked.
Long-term treatment recommendations are variable; however, lifelong aspirin and several months of anticoagulation are recommended except in patients with underlying thrombophilia. Patients with recurrent venous thromboembolic disease or thrombophilia should be referred to a hematologist or vascular medicine physician, and are typically anticoagulated indefinitely. 48 Some authors discontinue clopidogrel after 2 months and maintain aspirin therapy indefinitely. Anticoagulation is typically maintained for at least 6 months and discontinued based on clinical status, laboratory values, and results of imaging studies, if available. For lower-risk patients, some operators may choose to limit anticoagulants for shorter periods.
There are no guidelines regarding postprocedure imaging. Duplex sonography of iliac veins and vena cava should be performed soon after the procedure to evaluate the stent patency and inflow. 49 Stent evaluation time periods by imaging may be performed at 2-month, 6-month, and 1-year intervals unless symptoms recur or worsen, although this is variable. Venographic evaluation may be performed at 6, 12, and 24 months, which may allow precise quantification of in-stent stenosis and potential for IVUS and intravascular biopsy for assessment of mural composition. Clinical follow-up is important to assess symptoms and compliance with antithrombotic regimens.
Outcomes
Iliocaval recanalization and reconstruction are considered the standard of care for chronic iliocaval occlusive disease, with multiple studies reporting favorable technical success rates and high patency. 49 50 51 52 53 54 55 56 The longest European study comprising 89 patients demonstrated primary, assisted primary, and secondary patency rates of 83, 89, and 93%, respectively. 7 In a recent study of 120 patients with inferior vena cava filter–associated iliocaval thrombosis, iliocaval reconstruction was shown to have 100% technical success rate and 2-year primary-assisted and secondary patency rates of 90 and 94%, respectively. 11 Additional studies are warranted.
Conclusion
Iliocaval recanalization stent reconstruction is the treatment of choice for symptomatic lower extremity chronic deep venous occlusive disease and is associated with high technical success rates, low complications, favorable clinical outcomes, and favorable long-term stent patency rates. It is an effective intervention for iliocaval thrombosis and has the potential to substantially improve the patient's quality of life.
References
- 1.Raju S. Treatment of iliac-caval outflow obstruction. Semin Vasc Surg. 2015;28(01):47–53. doi: 10.1053/j.semvascsurg.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Williams D M. Iliocaval reconstruction in chronic deep vein thrombosis. Tech Vasc Interv Radiol. 2014;17(02):109–113. doi: 10.1053/j.tvir.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 3.de Graaf R, de Wolf M, Sailer A M, van Laanen J, Wittens C, Jalaie H. Iliocaval confluence stenting for chronic venous obstructions. Cardiovasc Intervent Radiol. 2015;38(05):1198–1204. doi: 10.1007/s00270-015-1068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neglén P, Raju S. Balloon dilation and stenting of chronic iliac vein obstruction: technical aspects and early clinical outcome. J Endovasc Ther. 2000;7(02):79–91. doi: 10.1177/152660280000700201. [DOI] [PubMed] [Google Scholar]
- 5.Neglén P, Darcey R, Olivier J, Raju S. Bilateral stenting at the iliocaval confluence. J Vasc Surg. 2010;51(06):1457–1466. doi: 10.1016/j.jvs.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 6.Raju S, Owen S, Jr, Neglen P. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg. 2002;35(01):8–15. doi: 10.1067/mva.2002.121054. [DOI] [PubMed] [Google Scholar]
- 7.Hartung O, Loundou A D, Barthelemy P, Arnoux D, Boufi M, Alimi Y S. Endovascular management of chronic disabling ilio-caval obstructive lesions: long-term results. Eur J Vasc Endovasc Surg. 2009;38(01):118–124. doi: 10.1016/j.ejvs.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Hartung O, Otero A, Boufi Met al. Mid-term results of endovascular treatment for symptomatic chronic nonmalignant iliocaval venous occlusive disease J Vasc Surg 200542061138–1144., discussion 1144 [DOI] [PubMed] [Google Scholar]
- 9.Knipp B S, Ferguson E, Williams D M et al. Factors associated with outcome after interventional treatment of symptomatic iliac vein compression syndrome. J Vasc Surg. 2007;46(04):743–749. doi: 10.1016/j.jvs.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 10.Amin V B, Lookstein R A. Catheter-directed interventions for acute iliocaval deep vein thrombosis. Tech Vasc Interv Radiol. 2014;17(02):96–102. doi: 10.1053/j.tvir.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Chick J FB, Jo A, Meadows J M et al. Endovascular iliocaval stent reconstruction for inferior vena cava filter-associated iliocaval thrombosis: approach, technical success, safety, and two-year outcomes in 120 patients. J Vasc Interv Radiol. 2017;28(07):933–939. doi: 10.1016/j.jvir.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Hage A N, Srinivasa R N, Abramowitz S D, Chick J FB. Management and treatment of iliocaval thrombosis using endovascular recanalization, stenting, and reconstruction: what all practitioners should know. J Radiol Nurs. 2017;36(04):218–223. [Google Scholar]
- 13.Mahnken A H, Thomson K, de Haan M, O'Sullivan G J. CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol. 2014;37(04):889–897. doi: 10.1007/s00270-014-0875-4. [DOI] [PubMed] [Google Scholar]
- 14.Rosendaal F R, Reitsma P H. Genetics of venous thrombosis. J Thromb Haemost. 2009;7 01:301–304. doi: 10.1111/j.1538-7836.2009.03394.x. [DOI] [PubMed] [Google Scholar]
- 15.Aurshina A, Ascher E, Hingorani A, Salles-Cunha S X, Marks N, Iadgarova E. Clinical role of the “venous” ultrasound to identify lower extremity pathology. Ann Vasc Surg. 2017;38:274–278. doi: 10.1016/j.avsg.2016.05.113. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Nakahara H. MR venography for the assessment of deep vein thrombosis in lower extremities with varicose veins. Ann Vasc Dis. 2014;7(04):399–403. doi: 10.3400/avd.oa.14-00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karande G Y, Hedgire S S, Sanchez Y et al. Advanced imaging in acute and chronic deep vein thrombosis. Cardiovasc Diagn Ther. 2016;6(06):493–507. doi: 10.21037/cdt.2016.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAree B J, O'Donnell M E, Fitzmaurice G J, Reid J A, Spence R A, Lee B. Inferior vena cava thrombosis: a review of current practice. Vasc Med. 2013;18(01):32–43. doi: 10.1177/1358863X12471967. [DOI] [PubMed] [Google Scholar]
- 19.Saha P, Black S, Breen K, Patel A, Modarai B, Smith A. Contemporary management of acute and chronic deep venous thrombosis. Br Med Bull. 2016;117(01):107–120. doi: 10.1093/bmb/ldw006. [DOI] [PubMed] [Google Scholar]
- 20.Arabi M, Vellody R, Cwikiel W B, Gemmete J J. Endovascular treatment of lower gastrointestinal bleeding from systemic-to-mesenteric venous collateral vessels caused by inferior vena cava occlusion: report of two cases. J Vasc Interv Radiol. 2011;22(07):1035–1038. doi: 10.1016/j.jvir.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Khaja M S, Chick J FB, Schuman A D et al. Fluoroscopic targeting of Wallstents and Amplatzer Vascular Plugs in sharp recanalization of chronic venous occlusions. Cardiovasc Intervent Radiol. 2017;40(11):1777–1783. doi: 10.1007/s00270-017-1724-z. [DOI] [PubMed] [Google Scholar]
- 22.Raju S, Neglen P.High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity J Vasc Surg 20064401136–143., discussion 144 [DOI] [PubMed] [Google Scholar]
- 23.Stoeckel D, Pelton A, Duerig T. Self-expanding nitinol stents: material and design considerations. Eur Radiol. 2004;14(02):292–301. doi: 10.1007/s00330-003-2022-5. [DOI] [PubMed] [Google Scholar]
- 24.DeRubertis B G, Alktaifi A, Jimenez J C, Rigberg D, Gelabert H, Lawrence P F. Endovascular management of nonmalignant iliocaval venous lesions. Ann Vasc Surg. 2013;27(05):577–586. doi: 10.1016/j.avsg.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Hage A N, Srinivasa R N, Abramowitz S D, Gemmete J J, Reddy S N, Chick J FB. Endovascular iliocaval stent reconstruction for iliocaval thrombosis: a multi-institutional international practice pattern survey. Ann Vasc Surg. 2018;49:64–74. doi: 10.1016/j.avsg.2018.01.076. [DOI] [PubMed] [Google Scholar]
- 26.Chen J X, Sudheendra D, Stavropoulos S W, Nadolski G J. Role of catheter-directed thrombolysis in management of iliofemoral deep venous thrombosis. Radiographics. 2016;36(05):1565–1575. doi: 10.1148/rg.2016150138. [DOI] [PubMed] [Google Scholar]
- 27.Lee K H, Han H, Lee K J et al. Mechanical thrombectomy of acute iliofemoral deep vein thrombosis with use of an Arrow-Trerotola percutaneous thrombectomy device. J Vasc Interv Radiol. 2006;17(03):487–495. doi: 10.1097/01.RVI.0000202611.93784.76. [DOI] [PubMed] [Google Scholar]
- 28.Behrens G, Bjarnason H. Venous thromboembolic disease: the use of the aspiration thrombectomy device AngioVac. Semin Intervent Radiol. 2015;32(04):374–378. doi: 10.1055/s-0035-1564792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy E H, Johns B, Varney E, Buck W, Jayaraj A, Raju S. Deep venous thrombosis associated with caval extension of iliac stents. J Vasc Surg Venous Lymphat Disord. 2017;5(01):8–17. doi: 10.1016/j.jvsv.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Duda S H, Wiskirchen J, Tepe G et al. Physical properties of endovascular stents: an experimental comparison. J Vasc Interv Radiol. 2000;11(05):645–654. doi: 10.1016/s1051-0443(07)61620-0. [DOI] [PubMed] [Google Scholar]
- 31.Neglén P, Tackett T P, Jr, Raju S. Venous stenting across the inguinal ligament. J Vasc Surg. 2008;48(05):1255–1261. doi: 10.1016/j.jvs.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 32.van Vuuren T M, Kurstjens R L, de Wolf M A, van Laanen J H, Wittens C H, de Graaf R.Stent extension into a single inflow vessel is a valuable option after endophlebectomy Phlebology 2017 10.1177/0268355517739766[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLafferty R B. The role of intravascular ultrasound in venous thromboembolism. Semin Intervent Radiol. 2012;29(01):10–15. doi: 10.1055/s-0032-1302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel D, Comerota A J, Al-Jabouri M, Assi Z I. Common femoral endovenectomy with iliocaval endoluminal recanalization improves symptoms and quality of life in patients with postthrombotic iliofemoral obstruction. J Vasc Surg. 2012;55(01):129–135. doi: 10.1016/j.jvs.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Aborahma A M. Use of a percutaneous puncture needle for true lumen re-entry during subintimal recanalization of the superficial femoral artery. J Vasc Surg Cases Innov Tech. 2016;2(03):108–110. doi: 10.1016/j.jvscit.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang H L, Li M F, Chiang C L, Chen M CY, Wu C J, Pan H B. The use of a Colapinto TIPS Needle under cone-beam computed tomography guidance for true lumen re-entry in subintimal recanalization of chronic iliac artery occlusion. J Chin Med Assoc. 2017;80(06):371–375. doi: 10.1016/j.jcma.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Vedantham S, Kahn S R, Goldhaber S Z et al. Endovascular therapy for advanced post-thrombotic syndrome: proceedings from a multidisciplinary consensus panel. Vasc Med. 2016;21(04):400–407. doi: 10.1177/1358863X16650747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angel L F, Tapson V, Galgon R E, Restrepo M I, Kaufman J. Systematic review of the use of retrievable inferior vena cava filters. J Vasc Interv Radiol. 2011;22(11):1522–1.53E6. doi: 10.1016/j.jvir.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Sildiroglu O, Ozer H, Turba U C. Management of the thrombosed filter-bearing inferior vena cava. Semin Intervent Radiol. 2012;29(01):57–63. doi: 10.1055/s-0032-1302453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doshi M H, Narayanan G. Late endovascular removal of Günther-Tulip inferior vena cava filter and stent reconstruction of chronic post-thrombotic iliocaval obstruction after 4753 days of filter dwell time: a case report with review of literature. Radiol Case Rep. 2016;11(04):348–353. doi: 10.1016/j.radcr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iliescu B, Haskal Z J. Advanced techniques for removal of retrievable inferior vena cava filters. Cardiovasc Intervent Radiol. 2012;35(04):741–750. doi: 10.1007/s00270-011-0205-z. [DOI] [PubMed] [Google Scholar]
- 42.Al-Hakim R, Kee S T, Olinger K, Lee E W, Moriarty J M, McWilliams J P.Inferior vena cava filter retrieval: effectiveness and complications of routine and advanced techniques J Vasc Interv Radiol 20142506933–939., quiz 940 [DOI] [PubMed] [Google Scholar]
- 43.Al-Hakim R, McWilliams J P, Derry W, Kee S T. The hangman technique: a modified loop snare technique for the retrieval of inferior vena cava filters with embedded hooks. J Vasc Interv Radiol. 2015;26(01):107–110. doi: 10.1016/j.jvir.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Tamrazi A, Wadhwa V, Holly B et al. Percutaneous retrieval of permanent inferior vena cava filters. Cardiovasc Intervent Radiol. 2016;39(04):538–546. doi: 10.1007/s00270-015-1214-0. [DOI] [PubMed] [Google Scholar]
- 45.Kuo W T, Cupp J S, Louie J D et al. Complex retrieval of embedded IVC filters: alternative techniques and histologic tissue analysis. Cardiovasc Intervent Radiol. 2012;35(03):588–597. doi: 10.1007/s00270-011-0175-1. [DOI] [PubMed] [Google Scholar]
- 46.Neglén P, Oglesbee M, Olivier J, Raju S. Stenting of chronically obstructed inferior vena cava filters. J Vasc Surg. 2011;54(01):153–161. doi: 10.1016/j.jvs.2010.11.117. [DOI] [PubMed] [Google Scholar]
- 47.Raju S. Best management options for chronic iliac vein stenosis and occlusion. J Vasc Surg. 2013;57(04):1163–1169. doi: 10.1016/j.jvs.2012.11.084. [DOI] [PubMed] [Google Scholar]
- 48.Connors J M. Thrombophilia testing and venous thrombosis. N Engl J Med. 2017;377(12):1177–1187. doi: 10.1056/NEJMra1700365. [DOI] [PubMed] [Google Scholar]
- 49.Nazarian G K, Bjarnason H, Dietz C A, Jr, Bernadas C A, Hunter D W. Iliofemoral venous stenoses: effectiveness of treatment with metallic endovascular stents. Radiology. 1996;200(01):193–199. doi: 10.1148/radiology.200.1.8657909. [DOI] [PubMed] [Google Scholar]
- 50.Arabi M, Krishnamurthy V, Cwikiel W et al. Endovascular treatment of thrombosed inferior vena cava filters: techniques and short-term outcomes. Indian J Radiol Imaging. 2015;25(03):233–238. doi: 10.4103/0971-3026.161436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blättler W, Blättler I K. Relief of obstructive pelvic venous symptoms with endoluminal stenting. J Vasc Surg. 1999;29(03):484–488. doi: 10.1016/s0741-5214(99)70277-6. [DOI] [PubMed] [Google Scholar]
- 52.Hurst D R, Forauer A R, Bloom J R, Greenfield L J, Wakefield T W, Williams D M. Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34(01):106–113. doi: 10.1067/mva.2001.114213. [DOI] [PubMed] [Google Scholar]
- 53.Kurklinsky A K, Bjarnason H, Friese J L et al. Outcomes of venoplasty with stent placement for chronic thrombosis of the iliac and femoral veins: single-center experience. J Vasc Interv Radiol. 2012;23(08):1009–1015. doi: 10.1016/j.jvir.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neglén P, Hollis K C, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46(05):979–990. doi: 10.1016/j.jvs.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 55.Neglén P, Thrasher T L, Raju S. Venous outflow obstruction: an underestimated contributor to chronic venous disease. J Vasc Surg. 2003;38(05):879–885. doi: 10.1016/s0741-5214(03)01020-6. [DOI] [PubMed] [Google Scholar]
- 56.O'Sullivan G J, Semba C P, Bittner C A et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2000;11(07):823–836. doi: 10.1016/s1051-0443(07)61796-5. [DOI] [PubMed] [Google Scholar]


