Abstract
Anaphylactic reactions are triggered when allergens enter the blood circulation and activate IgE-sensitized mast cells (MCs) causing systemic discharge of prestored proinflammatory mediators. Since MCs are extravascular, how they perceive circulating allergens remains a conundrum. Here, we describe the existence of a CD301b+ perivascular dendritic cell (DC) subset that continuously samples blood and relays antigens to neighboring MCs, which vigorously degranulate and trigger anaphylaxis. DC antigen-transfer involves the active discharge of surface-associated antigens on 0.5-1.0-μm microvesicles (MVs) generated by vacuolar protein sorting 4 (VPS4). Antigen sharing by DCs is not limited to MCs, as neighboring DCs also acquire antigen-bearing MVs. This capacity of DCs to distribute antigens-bearing MVs to various immune cells in the perivascular space potentiates inflammatory and immune responses to blood-borne antigens.
One sentence summary
Anaphylaxis is triggered by dendritic cells relaying blood-borne allergen on microvesicles to mast cells.
Introduction
Currently, 4-5 persons per 100,000 suffer from anaphylaxis annually. These numbers continue to grow, particularly food-associated reactions (1, 2). These are especially frequent in the young, most of whom also present atopic diseases such as asthma, eczema, or allergic rhinitis (3). Acute anaphylaxis is associated with severe pathophysiological symptoms such as hives, loss in blood pressure, vasculature leakage, and a drop in body temperature, which can be fatal (4). These symptoms are triggered soon after allergens such as peanut antigens, insect venom, and certain medications enter the circulation of antigen-specific IgE-sensitized individuals (2). Mast cells (MCs) are primary effectors of anaphylaxis because of their unique ability to release large amounts of cytoplasmic granules enriched in inflammatory chemicals, upon allergen activation of their surface IgE. MCs are typically found lining blood vessels so when allergens enter the circulation, widespread MC degranulation is triggered resulting in rapid and systemic onset of anaphylaxis. Since MCs are located in the perivascular abluminal surface of relatively impregnable endothelial cells, it is unclear how blood-borne allergens contact MCs.
MCs possess the capacity to directly probe blood vessels with cellular protrusions to acquire IgE antibodies from the circulation (5). Dendritic cells (DCs) are also often observed alongside MCs at many sites. DCs are primarily immune surveillance cells with the unique capacity to extrude dendrites between cells that are connected via tight junctions (6). These probing dendrites allow DCs, lying underneath gut and respiratory epithelial tracts to sample luminal contents (6, 7). Additionally, epidermal Langerhans cells of the skin can penetrate the stratum corneum to sample external antigens (8). Here, we investigated how abluminal perivascular MCs detect circulatory antigens through cooperation with adjacent DCs to trigger anaphylaxis.
Results
MCs and CD11c+ cells are critical mediators of anaphylaxis.
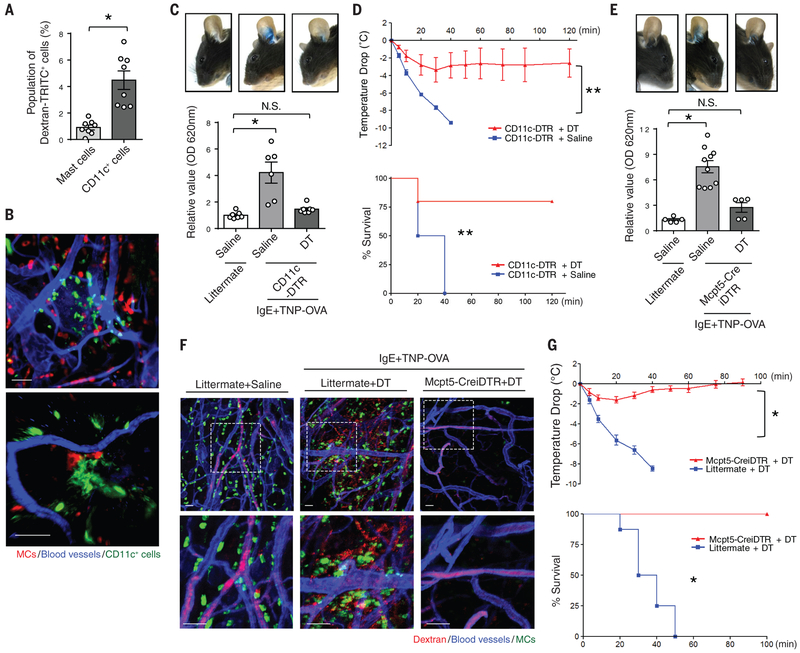
We investigated if MCs were able to bind and detect blood-borne antigens in a manner similar to circulating IgE antibodies (5). We intravenously (i.v.) injected TRITC-conjugated dextran (Dextran-TRITC), which is unable to enter the extravascular space. We then evaluated the ability of dermal abluminal MCs to acquire Dextran-TRITC by flow cytometry 30 min after injection. Only ~1% cKit+FcεRI+ skin MCs were positive for Dextran-TRITC (Fig. 1A, S1). Unexpectedly, up to 5% of CD11c+ cells in the skin cell preparation were positive for Dextran-TRITC (Fig. 1A, S1). To locate these CD11c+ cells within the tissue, we prepared whole mounts of the mouse ear and probed them for MCs and CD11c+ cells. Numerous CD11c+ cells were in close proximity to blood vessels and often in direct contact with both endothelial cells and MCs (Fig. 1B).
Fig. 1. PCA and PSA mediated by MCs and CD11c+ cells.
(A) Efficient antigen uptake by CD11c+ cells. CD11c-GFP mice were i.v. injected with TRITC-conjugated dextran (Dextran-TRITC). After 30 min, mice were sacrificed and their ears were dissected to generate a single-cell suspension. The dextran+ populations among CD45+FcεRI+cKit+ MCs and CD45+CD11c+ cells were compared. N=8 mice per group. Data are represented as the mean ± SEM. *P<0.001, unpaired Student's t-test. (B) CD11c+ cells lie in close proximity to blood vessels. The ears of CD11c-GFP (green) mice were dissected. A whole mount was then prepared and stained for MCs (avidin, red) and blood vessels (CD31, blue). Scale bars: 50 μm (left panel) and 15 μm (right panel). (C) CD11c+ cells mediate vascular leakage. CD11c-DTR or littermate mice were i.p. and i.v. injected with DT or vehicle every other day twice to deplete the population of CD11c+ cells. The day before antigen challenge, the ears of CD11c-DTR or WT mice were sensitized with TNP-specific IgE. TNP-conjugated ovalbumin (TNP-OVA) was i.v. injected along with Evans Blue dye into both groups. One hour post-injection, mouse ears were imaged, and dissected to extract dye for OD measurements. N=6-7 mice per group. Data are represented as the mean ± SEM. *P<0.001, one-way analysis of variance (ANOVA), Tukey's multiple comparisons test. (D) CD11c+ cells mediate anaphylaxis. After CD11c+ cells were depleted, IgE-sensitized CD11c-DTR mice were i.v. injected with TNP-OVA. Temperature changes were then monitored (upper panel). Survival during PSA was recorded and mice with temperature changes greater than 10 °C were sacrificed (lower panel). N=4-5 mice per group. Data are represented as the mean ± SEM. **P<0.05, two-way ANOVA (upper panel), Survival during PSA was recorded and analyzed by log-rank test (lower panel). (E, F) MC activation cause vascular leakage and anaphylaxis. Both Mcpt5-CreiDTR and littermate mice were i.v. injected with DT to deplete the population of MCs. The day before antigen challenge, the ears of each mouse were sensitized with TNP-specific IgE. TNP-OVA was i.v. injected to both group of mice along with (E) Evans Blue dye or (F) TRITC-conjugated dextran. One hour post-injection, (E) mouse ears were imaged and dissected to extract dye for OD measurements. Data are represented as the mean ± SEM. *P<0.001, one-way ANOVA, Tukey's multiple comparisons test. (F) Mouse ears were dissected and fixed for whole-mount imaging. Confocal microscopy was utilized to observe ears stained for MCs (avidin, green), blood vessels (CD31 antibody, blue), and dextran (red). The dotted squares in upper panels are magnified in the corresponding lower panels. Scale bar: 50 μm. (G) MC activation triggers a sharp drop in body temperature. After MCs were depleted, littermate or Mcpt5-CreiDTR mice were injected i.p. with TNP-specific IgE to sensitize them. The following day, mice were i.v. injected with TNP-OVA and temperature changes were monitored (upper panel). N=5-8 mice per group. Data are represented as the mean ± SEM. *P<0.001, two-way ANOVA. Survival during PSA was recorded and analyzed by log-rank test (lower panel). Two to three separate experiments were performed for each individual figure.
To investigate if these CD11c+ cells had any functional role in anaphylactic responses to allergens, we compared anaphylactic reactions in CD11c+-cell-depleted mice and non-depleted mice employing two classical assays: passive systemic anaphylaxis (PSA) and passive cutaneous anaphylaxis (PCA). Repeated injections of diphtheria toxin (DT) into CD11c-DTR mice depleted CD11c+ cells (9). Sixteen hours after locally sensitizing both WT and CD11c+-depleted mice with trinitrophenyl (TNP)-specific IgE, we challenged the mice i.v. with TNP-ovalbumin (TNP-OVA). To visualize the vascular leakage associated with PCA in the IgE sensitized mice, we co-injected Evans Blue dye i.v. along with TNP-OVA. There was strong vascular leakage in the non-depleted mice, whereas the extent of leakage in the CD11c+-depleted mice was significantly less (Fig. 1C). CD11c+cell depletion also protected mice from a severe PSA reaction upon systemic IgE sensitization and TNP-OVA challenge. There was a sharp drop in the body temperature of IgE-sensitized saline-treated CD11c-DTR mice but not in CD11c+-depleted mice following i.v. administration of TNP-OVA (Fig. 1D). Rather, CD11c+-depleted mice exhibited a modest drop in body temperature with no associated mortality (Fig. 1D). Thus, CD11c+ cells contribute significantly to the development of PCA and PSA in IgE-sensitized mice.
To confirm the role of MCs in our anaphylaxis model, we compared the PCA and PSA response in WT and MC-depleted mice (achieved by repeated administration of DT to Mcpt5-CreiDTR mice) (10). As expected, PCA challenge showed that TNP-specific IgE-sensitized control littermate mice experienced significantly increased blood vessel leakage than MC-depleted mice (Fig. 1E). To visualize the consequence of MC activation and vascular leakage at a local site, we examined the mouse ear in TNP-specific-IgE-sensitized control littermate and MC-depleted mice after i.v. injection of TNP-OVA along with dextran-TRITC. Whole-mount imaging of the mouse ear showed extensive MC degranulation and vascular leakage in control littermate mice but not in MC-depleted mice (Fig. 1F). When PSA was induced, TNP-specific-IgE-sensitized control littermate mice showed a significant drop in body temperature. In contrast, MC-depleted mice exhibited only limited responses with no associated mortality after TNP-OVA challenge (Fig. 1G). Thus, in addition to MCs, CD11c+ cells are important modulators of PCA and PSA in mice.
CD301b+ dermal DCs are critical mediators of anaphylaxis
We next established the identity of the perivascular CD11c+ cells that were collaborating with MCs to mediate both PCA and PSA responses. CD11c is expressed on both DCs and macrophages (11). A recent comprehensive analysis of skin immune cells showed high CD11c expression on dermal cDC1 (previously known as CD8α+ DCs) as well as cDC2 (previously known as CD11bhi dDCs). However, low levels of expression are also found on monocyte-derived DCs and some macrophages (11-13). The cDC2 subset constitutes the major DCs in the dermis and are defined as CD207−CD11bhiCD103−EpCAM−CD24−/loSirp+CD11c+ (13, 14) and function to promote the differentiation of Th2 and Th17 cells (15, 16).
Recently, a subset of CD301b expressing CD11bhi cDC2 was reported to be especially proficient in antigen uptake at the periphery following antigen challenge, and in mediating subsequent antigen presentation in the draining lymph nodes (17-19). Although certain monocyte-derived DCs and even macrophages reportedly share the CD301b marker (11, 13, 20), the subset of CD301b-expressing CD11bhi cells were designated dermal DCs because in addition to being CD301b+ and CD11c+, they were CD11bhiMHCIIhiCD64loLy6Clo (17, 21). Additionally, they express Zbtb46, a conventional DC-specific transcription factor expressed by cDCs and their committed progenitors, but not macrophages or monocytes (22-24).
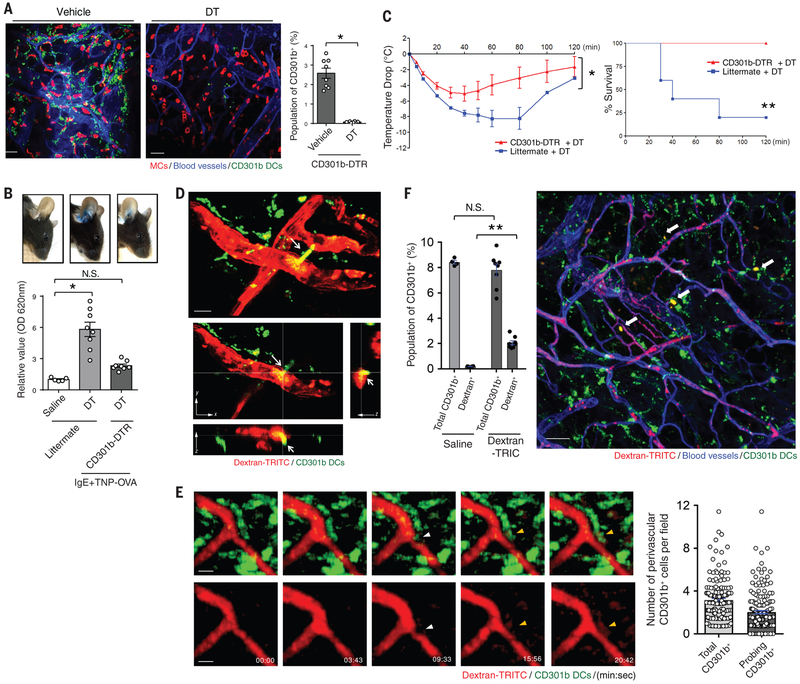
In view of their capacity to acquire antigen, we investigated whether the population of CD11c+ CD301b+ cells expressed markers characteristic of cDC2 and if they participated in PCA and PSA. Using transgenic CD301b-GFP-DTR mice (18), we found that the GFP+ CD301b+ cells in the mice were CD103−CD11bhiSirpa+EpCAM−CD24+ and also expressed Zbtb46 (Fig. S2). Whole-mount-imaged ears from CD301b-GFP-DTR mice harbored numerous GFP+ cells in the dermal perivascular space adjacent to both the vasculature and MCs (Fig. 2A, left panel). Moreover, DT injection into these mice selectively and almost completely depleted the GFP+ cells (Fig. 2A, middle and right panel). CD64+CD11c− cells (dermal perivascular macrophages) (11, 25) numbers were not altered after depletion of CD301b+ cells (Fig. S3).
Fig. 2. CD301b+ DCs mediate anaphylaxis after uptake of blood-borne antigen.
(A) Treatment of CD301b-DTR-GFP mice with DT results in the depletion of CD301b+ cells. CD301b-DTR-GFP mice were injected i.p. once with DT or vehicle to deplete CD301b+ cells. Depletion of CD301b+ cells were quantified by flow cytometry. The ears of CD301b-DTR-GFP mice were then dissected and a whole mount was prepared and examined by confocal microscopy: MCs (avidin, red), blood vessels (CD31, blue), and CD301b (green). N=8 mice per group, data are represented as the mean ± SEM. *P<0.001, unpaired Student's t-test. (B, C) CD301b+ cells mediate PCA and PSA. After CD301b+ cell depletion, the ears of CD301b-DTR mice or littermate controls were sensitized with TNP-specific IgE. TNP-OVA was injected i.v. along with Evans Blue dye. After 1 h, mouse ears were imaged and then dissected to extract dye for OD measurements. N=5-8 mice per group, data are represented as the mean ± SEM. *P<0.001, one-way ANOVA, Tukey's multiple comparisons test. (C) After CD301b+ cells were depleted, CD301b-DTR or littermate mice were i.p. injected with TNP-OVA and then temperature changes were monitored (left panel). Mice with changes in temperature greater than 10 °C were sacrificed. The survival rate is shown (right panel). N=5 mice per group. Data are represented as the mean ± SEM. *P<0.001, two-way ANOVA (left panel), **P<0.01, Survival during PSA was recorded and analyzed by log-rank test (right panel). (D, E) 3D visualization of CD301b+ DCs probing the vasculature. CD301b-GFP mice were i.v. injected with Dextran-TRITC. (D) After 30 min, mice ears were dissected. Whole mounts were prepared and imaged using two-photon microscopy. Arrows indicate lamellipodia-like structure protruding into the vasculature from CD301b+ cDC2. The upper panel is an xy-plane projection from a Z-stack view, whereas the lower panels are cross-sectional views of selected xy-, yz-, and xz-planes. Scale bars: 10 μm. (E) Immediately after injection, intravital two-photon microscopy of mouse ears was performed. Time-series events were captured and displayed. White arrowheads point to a dendrite protrusion into the vasculature from CD301b+ cDC2. Yellow arrowheads point to CD301b+ cDC2 taking up blood-borne dextran-TRITC. The probing activity of perivascular CD301b+ cDC2 was quantified from multiple images taken over a 20-minute time period (right panel). Scale bars: 10 μm. (F) A significant population of CD301b+ cDC2 takes up blood-borne antigens. CD301b-GFP mice were i.v. injected with Dextran-TRITC. After 30 min, the mice ears were dissected and processed as single cell suspension for flow cytometry where the Dextran-TRITC+ population was identified (left panel) or processed as whole mounts for microcopy where antigen-sampling cells were readily detectable (arrows, right panel). N=4-8 mice per group, data are represented as the mean ± SEM. **P<0.01, unpaired Student's t-test. Scale bar: 50 μm. Two to three separate experiments were performed for each individual figure.
To investigate the functional contribution of CD301b+ DCs to anaphylaxis, we performed PCA and PSA challenges in these mice. The depletion of CD301b+ cDC2 markedly reduced vascular leakage in the ears (Fig. 2B) and alleviated the drop in body temperature (Fig. 2C) in CD301b-GFP-DTR mice compared littermate controls, indicating that CD301b+ cDC2 contributed significantly to the development of anaphylaxis. A small subset of cDC1 (CD103+CD8α+CD11b−) known to be present in the dermis (26). Batf3−/− mice, which lack cDC1, also showed significantly reduced anaphylaxis (~ 50% of the effect observed by removing CD301b+ cDC2 (Fig. S4)). Thus, this capacity to sample blood-borne antigens is not unique to a particular DC subset.
To address whether this response involved the direct uptake of blood-borne antigens by perivascular CD301b+ cDC2, we injected i.v. dextran-TRITC into CD301b-GFP-DTR transgenic mice. Thirty minutes later, we generated whole-mount preparations of their ears, which were subjected to two-photon microscopy. We could visualize several of the fluorescent CD301b+ cDC2 adjacent to blood vessels in the process of taking up dextran-TRITC (Fig. 2D, Fig. S5, and video S1). In some cases, the CD301b+ cDC2 appeared to extrude long lamellipodia with multiple dendrites into the luminal surface of the blood vessel (Fig. 2D, top panel). Stacked images deployed in x-, y-, and z-axis planes revealed the movement of dextran-TRITC into CD301b+ cDC2. As shown in the yz- or xz-axis cross-sectioned planes, tips of protruding dendrites appeared to co-localize with dextran within the mouse blood vessel lumen (Fig. 2D, bottom panels). Dextran-TRIC uptake by CD301b+ cDC2 in the ear was also captured by intravital two-photon microscopy (Fig. 2E, Fig. S6, and video S2). Protruding dendrites (white arrowhead) from GFP-labelled CD301b+ cDC2 appeared to penetrate the vasculature within minutes and promptly become labeled with dextran-TRITC (yellow arrowhead) (Fig. 2E). Thus, the uptake of blood-borne dextran-TRITC by CD301b+ cDC2 appeared to be a rapid event. Additional intravital confocal microscopy of the ears of CD301b-GFP/Mcpt5-CretdTomato mice was undertaken. In these mice, CD301b+ cDC2 and MCs were fluorescently labeled with GFP and tdTomato, respectively. We were able to confirm that within 15 minutes of i.v. administration of Alexa 647-conjugated ovalbumin (OVA-A647), CD301b cDC2 but not MCs acquired antigen (Fig. S7 and video S3). To quantify antigen uptake by CD301b+ cells in vivo, we generated a single-cell preparation from the ears of CD301b-GFP-DTR mice 30 min after i.v. injection of dextran-TRITC and examined them by flow cytometry. Up to 30% of CD301b+ cDC2 were dextran-TRITC+ (Fig. 2F and Fig. S8), suggesting that antigen uptake by CD301b+ cDC2 occurs quickly, also corresponding to the rapid onset of anaphylaxis.
In vitro activation of IgE-sensitized MCs by antigen primed DCs
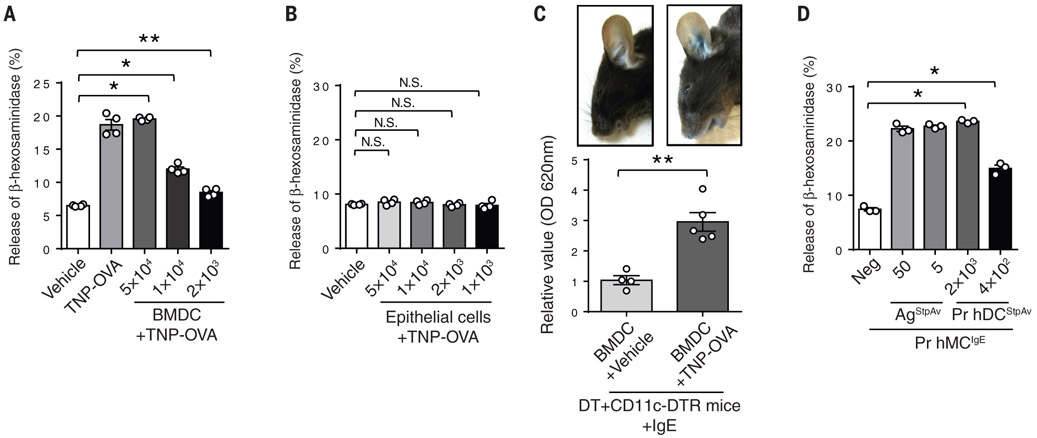
Although MCs are the primary effector cells of PCA and PSA, our data suggest a surprisingly critical role for CD301b+ DCs. These cells appear to be primarily responsible for acquiring antigens from the blood. If true, we hypothesized that the role of DCs in promoting anaphylaxis would be in relaying acquired antigens to adjacent IgE-sensitized MCs so as to activate them. Thus, we performed in vitro assays to investigate if mouse bone marrow-derived CD301b+ DCs (BMDCs), following acquisition of allergen, are capable of activating IgE-sensitized MCs. We first confirmed that our generated BMDCs expressed CD301b but not markers of macrophages (BMMs) such as CD115 (Fig. S9). BMDCs were pretreated with TNP-OVA for 20 min and then thoroughly washed to remove unbound antigen from the extracellular medium. Thereafter, the primed DCs were added in increasing number to cultures of the MC cell line (RBL-2H3) that had previously been sensitized with TNP-OVA-specific IgE (Fig. 3 A). We observed strong and dose-dependent MC degranulation indicating that antigens “presented” without any processing by the DCs were capable of activating MCs. When we replaced DCs with mouse epithelial cells in this assay, we failed to detect any MC activation, indicating that this activity was specific to DCs (Fig. 3B). To confirm that primed DCs have the capacity to activate sensitized MCs in vivo, we intradermally injected (i.d.) antigen-primed BM-derived CD301b+ DCs into DT-treated CD11c-DTR mice. Prior to reconstitution, MCs from these mice were sensitized with TNP-specific IgE directed at TNP-OVA. As shown in Figure 3C, i.d. delivery of antigen-primed BM-derived CD301b+ DCs evoked a strong PCA reaction in the mice. This confirmed that antigen-primed DCs can activate sensitized MCs in vivo. We also examined whether human DCs can activate sensitized human MCs following priming with antigen. Here, we utilized a human skin-derived DC preparation prepared from skin explants (27) and human peripheral blood-derived MCs (hPBMCs). The human DC preparation was exposed to streptavidin and then the primed DCs were co-incubated with hPBMCs sensitized with biotinylated IgE. As before, we observed dose-dependent MC degranulation (Fig. 3D), indicating that antigens bound onto human DCs possess the capacity to cross-link and degranulate IgE-sensitized human MCs.
Fig. 3. Antigen-primed DCs induce MC degranulation in vitro and in vivo.
(A) Antigen-primed BMDCs induce MC degranulation in a dose-dependent manner. BMDCs were primed with TNP-OVA for 15 min, rinsed three times and co-incubated with the TNP-specific-IgE-sensitized RBL-2H3 cells for 1 h, followed by measurement of β-hexosaminidase release. Data are represented as the mean ± SEM. *P<0.001, **P<0.05, one-way ANOVA, Tukey's multiple comparisons test. (B) Antigen-primed epithelial cells fail to induce MC degranulation. The same procedure as (A) was followed except that we replaced BMDCs with human bladder epithelial 5637 cells. Data are represented as the mean ± SEM. N.S. not significant, one-way ANOVA, Tukey's multiple comparisons test. (C) Antigen-primed BMDCs induce vascular leakage in vivo. CD11c+ cells were depleted in CD11c-DTR mice with i.p. and i.v. injections of DT 2 days before challenge with antigen-primed BMDCs. Ears of these mice were sensitized with TNP-specific IgE 1 day before challenge. For the challenge, BMDCs were primed with TNP-OVA or vehicle for 20 min, then washed before they were injected intradermally into the ears of the IgE-sensitized mice. Evans Blue dye was injected simultaneously i.v. into the same mice. After 1 h, the mouse ears were imaged and dissected to extract dye for OD measurements. N=4-5 mice per group, data are represented as the mean ± SEM. **P<0.05, unpaired Student's t-test. (D) Antigen-primed primary human skin DCs induce degranulation of primary human MCs. Primary human MCs cultured from peripheral blood were sensitized with biotinylated human IgE overnight. Primary human skin DCs were isolated from skin tissue as described in the methods section and primed with streptavidin for 20 min and rinsed three times. Both MCs and DCs were co-incubated for 1.5 h. β-hexosaminidase released into the extracellular medium was then measured. No β-hexosaminidase release was detected in control cultures containing only DCs. Data are represented as the mean ± SEM. *P<0.001, one-way ANOVA, Tukey's multiple comparisons test. Two to three separate experiments were performed for each individual figure.
DCs activate MCs by discharging microvesicles with surface-bound allergens
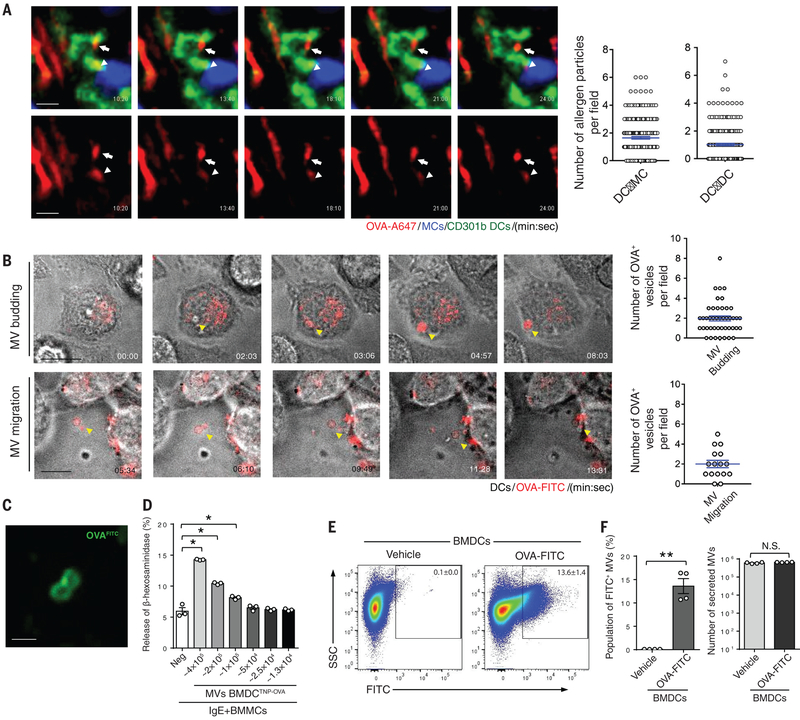
Next, we sought to investigate how antigen-primed DCs subsequently relay antigen to MCs. Live confocal imaging of blood-borne antigen-uptake by perivascular DCs undertaken in CD301b-GFP/Mcpt5-CretdTomato mice revealed the trafficking of “allergen particles” (~1 μm in size) between different perivascular CD301b+ cDC2 and between CD301b+ cDC2 and MCs (Fig. 4A, Fig. S10, and video S4). This raised the possibility that antigen-primed DCs may shed antigen-bearing microvesicles (MVs), which traffic to neighboring MCs and DCs. Since the secretion of extracellular vesicles occurred relatively quickly (~15 min), it was unlikely they were multivesicular body-generated exosomes, which require markedly more time for their generation (28). We transiently exposed a readily transfectable DC line (29) to OVA-A647, to examine whether DCs have the capacity to shed antigen-bearing MVs after exposure to antigen. Consistent with this hypothesis, we observed that within minutes of washing off unbound antigen, ~1-μm MVs budded off from the plasma membrane (Fig. 4B, top panels, Fig. S11A, and video S5). Shed MVs traveled randomly in the extracellular medium and bound neighboring immune cells (Fig. 4B, bottom panels, Fig. S11B, Figure S12, and video S6, S7). To examine whether these MVs were capable of relaying antigens, we harvested shed MVs from OVA-FITC-treated DCs employing differential ultracentrifugation and viewed them by microscopy. These MVs contained fluorescent OVA and most of this OVA appeared to be localized on the surface of the vesicles (Fig. 4C).
Fig. 4. Release of microvesicles (MVs) by DCs triggers degranulation in neighboring MCs.
(A) Time-lapse release of antigen-loaded vesicles from perivascular CD301b+ cDC2 to neighboring MCs and DCs in vivo. CD301b-GFP/Mcpt5-CretdTomato mice were injected i.v. with OVA-A647. The ears of live mice were then imaged using intravital confocal microscopy. MCs (blue), CD301b cDC2 (green), and A647-OVA (red). The arrowhead points to transfer of antigen-bearing vesicle from a CD301b+ DC (green) to a MC (blue). The arrow depicts transfer of antigen-bearing vesicles from a CD301b+ DC to another CD301b+ DC. Quantification of fluorescent particles was achieved by viewing multiple images and fields. Movement of particles from DCs to MCs (DC→MC) and from DCs to other DCs (DC→DC) for a 25-minute time period was quantitated (right panels). Scale bars: 10 μm (B) MV formation and secretion from a DC line (JAWSII). After the DC line was primed with antigen OVA-A647 (red), the cells were imaged using differential interference contrast (DIC). Yellow arrowheads point to the formation and release of antigen-loaded MVs. The budding MVs or their migration over a 20-minute time period were quantified from viewing multiple images and fields (right panels). Scale bars: 10 μm. (C) Fluorescent antigens are enriched on the membrane of recently formed MVs. After the DC line was primed with OVA-FITC (green), MVs released from these cells were collected through differential centrifuge and subjected to confocal microscopy. Scale bar: 1 μm. (D) TNP-OVA-loaded MVs induce MC degranulation. After the BMDCs were treated with TNP-OVA for 15 min, the MVs were collected and MVs numbers were calculated as described in materials and methods. Collected MVs were applied to IgE sensitized BMMCs in a dose-dependent manner (two-fold dilution) for 1 hr, followed by a β-hexosaminidase-release assay. Data are represented as the mean ± SEM. *P<0.001, one-way ANOVA, Tukey's multiple comparisons test. (E) Flow cytometric analysis of OVA-FITC loaded MVs. BMDCs were primed with OVA-FITC for 15 min, and after thorough washing to remove unbound OVA-FITC, fresh media was added and the cells incubated for 2 hr to release MVs. These particles were collected by differential centrifugation and analyzed by flow cytometry. Gating on 0.5-1.0-μm size MVs was performed as described in Fig. S14. Pseudo-colored dot plots of OVA-FITC treated or vehicle treated MVs were displayed as x: FITC and y: SSC. (F) Quantification of FITC+ population (left panel) or total MVs (right panel) of OVA-FITC treated or vehicle treated MVs was demonstrated as bar graph. Data are represented as the mean ± SEM. **P<0.05, N.S. not significant, unpaired Student's t test. Two to three separate experiments were performed for each individual figure.
Since antigens appeared to be loaded on the surface of MVs, isolated MVs from antigen-primed DCs may have the potential to activate MCs. Thus, we examined whether MVs harvested from TNP-OVA-treated BMDCs exhibited the capacity to induce the degranulation of TNP-specific-IgE-sensitized MCs. MVs isolated from TNP-OVA-treated BMDCs by differential ultracentrifugation as described previously (30) were capable of activating bone marrow-derived MCs (BMMCs) in a dose-dependent manner (Fig. 4D). Notably, MVs from vehicle-treated DCs failed to activate MCs (Fig. S13).
We developed a flow cytometric assay to quantitate DC-derived MVs. First, we established a gating area that corresponded to the reported size of MVs (31) using latex beads ranging in size from 0.5 μm to 1.0 μm (x: FCS-H, y: FCS-W) (Fig. S14). Thereafter, we quantitated MVs generated by in vitro-cultured primary BMDCs (Fig. 4E) or by a DC line following exposure to fluorescent OVA-FITC (Fig. S14). In each case, two distinct populations of MVs were shed by OVA-FITC-primed BMDCs. One population of MVs (~14 %) was FITC+, whereas the remainder were FITC− (Fig. 4F) indicating that MV production by DCs was not dependent on bound antigen. Since the total number of MVs generated by vehicle-treated BMDCs during the same period of time was comparable to the total number of MVs generated after exposure to antigen (Fig. 4F), MV shedding by DCs appears to be a constitutive activity and not antigen-induced.
Molecular determinants mediating DC shedding of MVs and acquisition of antigen
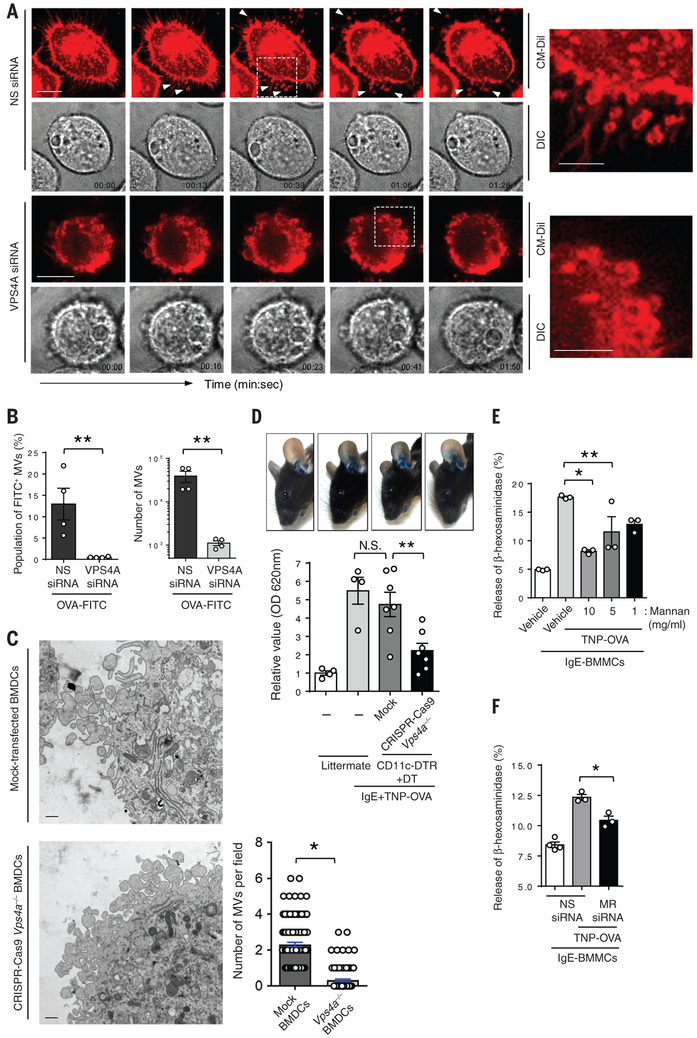
Finally, we investigated the molecular mechanism underlying antigen sharing and antigen acquisition by DCs. Specifically, we were interested in identifying critical cellular components mediating the shedding of antigen-bearing MVs as well as components involved in the initial acquisition of antigen. Since MCs become activated within minutes of the allergen entering the blood, the time for DCs to acquire and then present antigens to MCs is short. It is unlikely that there would be sufficient time for DCs to endocytose antigen and then export these antigens as exosomes within vesicles originating from multivesicular bodies (28). Even if this did occur, it is unlikely that the internalized antigen would still be bound on the outer surface of the vesicle membrane, which is necessary to activate MCs. Immune cells have the innate capacity to outwardly bud off plasma membrane fragments in the form of 0,2-1.0-μm-diameter MVs (32). Although these MVs lack cytosolic organelles and nuclear fragments (33), they are selectively enriched in various plasma membrane proteins including MHC class I, β1 integrin receptors, vesicle-associated membrane protein 3 (VAMP3) (30, 34), and presumably, any molecules that these receptors are bound to when budding occurs. Mechanistically, outward membrane budding requires the endosomal sorting complexes required for transport (ESCRT) machinery to assemble a spiral structure at the neck of the budding vesicle, which promotes MV budding (32). This budding event is specifically catalyzed by vacuolar protein sorting-associated protein 4 (VPS4) through its specific AAA-ATPase activity (35). We investigated if VPS4 was involved in relaying allergens from DCs to MCs by examining MV shedding from antigen-primed DCs after its VPS4A expression was markedly reduced (transfected with VPS4A siRNA) (Fig. S15) compared to control DCs (transfected with non-specific (NS) siRNA) using both microscopy and flow cytometry (Fig. 5A, 5B). To visualize MV shedding, we stained DCs with fluorescent Cell Tracker® CM-DIL, which selectively accumulates in the plasma membrane of the cell. We observed many fluorescent MVs shed from control antigen-primed DCs (Fig. 5A top panels, video S8). In contrast, there was complete abrogation of MV secretion in VPS4A-silenced DCs (Fig. 5 A bottom panels, video S9). Indeed, the surfaces of these cells appeared to be decorated with numerous vesicles in the process of budding but incapable of being released, consistent with the role of VPS4A in vesicle scission. We complemented these observations by quantitating MV shedding in control (NS siRNA-transfected) and OVA-FITC-primed murine DC line silenced in VPS4A (VPS4A siRNA-transfected) using flow cytometry. Compared to the controls, we observed that the population of MVs VPS4A-silenced DCs was reduced by up to 90% (Fig. 5B). Thus, the rapid relay of allergens by DCs following uptake was achieved via the budding of antigen-bound MVs from the plasma membrane in a VPS4A-dependent manner. To further support these observations, we sought to reproduce some of these observations using primary DCs in our in vitro and in vivo studies. Employing CRISPR-Cas9 technology (36), we knocked out Vps4a in primary DCs (Fig. S16 and S17). These Vps4a−/− BMDCs or mock transfected BMDCs were then exposed to OVA-FITC antigen and processed for transmission electron microscopy (TEM). Cross-sections of these DCs revealed numerous 0.5-1-μm extracellular MVs in close association with the mock-transfected BMDC (Fig. 5C and Fig. S18). This was in contrast to Vps4a−/− BMDCs, which exhibited a limited capacity to shed MVs (Fig. 5C). Instead, numerous vesicular structures incapable of detaching were observed to form on the plasma membrane of these BMDCs (Fig. 5C), consistent with the phenotype observed via live imaging (Fig. 5A). Next, we sought to demonstrate the in vivo contribution of MVs emanating from DCs by comparing cutaneous anaphylaxis in CD11c+ cell-depleted mice reconstituted with Vps4a−/− BMDCs or mock-transfected BMDCs. Compared to the extensive cutaneous anaphylaxis observed in the mock BMDC-reconstituted mice, Vps4a−/− BMDC-reconstituted mice exhibited greatly limited anaphylaxis (Fig. 5D). These in vivo studies support our in vitro observations and strongly suggest that VPS4A mediated MV secretion from DCs relays blood-borne antigen to MCs, leading to anaphylaxis.
Fig. 5. MVs shedding by DCs is VPS4-dependent and antigens borne by MVs are bound by the mannose receptor (MR).
(A) VPS4 mediates MV shedding. MV shedding by DC lines transfected with VPS4A siRNA (lower panels) or non-specific (NS) siRNA (upper panels). After 48 hrs after transfection, the DCs were stained with CM-Dil dye (red) that stains membranes and then exposed to antigen (OVA) followed by time lapse microscopy. The upper panels depict fluorescence images and the lower panels is the corresponding DIC image. The arrowheads indicate sites of MV shedding. Representative enlarged images from individual set of images (dotted region) demonstrate the shedding of MVs (upper right panel) and inhibition of shedding of formed vesicles (lower right panel). Scale Bars: 10 μm (time lapse images) and 5 μm (magnified images). (B) MV shedding requires VPS4A subunit. Quantitation of MVs produced by control and VPS4A knocked down DC lines using flow cytometry. Data are represented as the mean ± SEM. **P<0.05, unpaired Student's t test. (C) Ultrastructure of MV formation by BMDCs. CRISPR-Cas9-generated Vps4a−/− BMDCs or mock-transfected BMDCs were pre-treated with ovalbumin for 15 min and immediately fixed with 4% PFA and processed for transmission electron microscopy (TEM). MVs shed by mock transfected BMDCs or CRISPR-Cas9 Vps4a−/− BMDCs were quantified from viewing multiple images and fields (right panel). Scale bar = 500 nm, Data are represented as the mean ± SEM. *P<0.01, unpaired Student's t test. (D) MC-dependent anaphylaxis requires the secretion of MVs from nearby DCs. CRISPR-Cas9 generated Vps4a−/− BMDCs or mock-transfected BMDCs were generated. Confirmation of the specificity of Vps4a−/− is shown in Fig. S16 and S17. CD11c depleted mice (CD11c-DTR) were reconstituted with Vps4a−/− BMDCs or mock transfected BMDCs. The successful reconstitution of BMDCs is shown in Fig. S19. MCs in the reconstituted mice or littermate control mice were sensitized with i.d. injection of TNP-specific IgE antibody. On the following day, mice were i.v. challenged with TNP-OVA along with Evans Blue dye. After 30 min, the ears of mice were imaged (upper panels) and dissected for dye extraction and quantitation as shown in bar graph (lower panel). N= 4-7 mice per group, data are represented as the mean ± SEM. **P<0.05, N.S. not significant, one-way ANOVA, Tukey's multiple comparisons test. (E) The MR mediates OVA antigen acquisition by DCs. The DC line was pre-incubated with various concentrations of mannan (1, 5, and 10 mg/ml) or and then primed with TNP-OVA (0.1 μg/ml). This cell line was then added to IgE-sensitized BMMCs and MC degranulation was assessed. Data are represented as the mean ± SEM. *P<0.01, **P<0.05, one-way ANOVA, Tukey's multiple comparisons test. (F) The MR on DCs is responsible for binding OVA antigen. A DC line transfected with MR siRNA or NS siRNA was primed with TNP-OVA (0.1 μg/ml) and exposed to IgE sensitized BMMCs. The MC degranulation response was then evaluated. Data are represented as the mean ± SEM. *P<0.01, one-way ANOVA, Tukey's multiple comparisons test. Two to three separate experiments were performed for each individual figure.
Finally, we investigated how antigens were bound on the surface of MVs. Several receptors have been described on the surface of DCs capable of binding a wide range of antigens. These promiscuous receptors include scavenger and mannose receptors, which could potentially bind the OVA antigen employed in this study (37, 38). Indeed the mannose receptor (MR) on DCs, which binds a wide range of mannosylated compounds, has previously been shown to bind OVA (39). To investigate the possible role of the MR in antigen uptake, we examined if the pretreatment of mouse DCs with mannan, a linear polymer of mannose, would competitively block the binding of antigen (OVA-TNP) resulting in a loss of the ability of DCs to trigger MC degranulation. Mannan pretreatment significantly blocked the binding of antigen (OVA-TNP) as these cells subsequently failed to induce MC degranulation in a dose-dependent manner (Fig. 5E). To confirm that MRs on DCs were responsible for binding OVA-TNP, we knocked down the expression of MR in rodent DCs using siRNA transfection (Fig. S20). This significantly reduced the capacity DCs to activate MCs after priming with antigen (Fig. 5F). Immunoblots of isolated MVs generated by these DCs also revealed the presence of MR confirming their role in antigen transfer (Fig. S21). Thus, the MRs on DCs and MVs are important for the acquisition of OVA antigens, which are subsequently presented to MCs via MVs.
Discussion
Following the entry of allergens into the circulation, MCs are the primary effectors of the ensuing rapid and systemic allergic reactions. This is attributable, at least in part, to the large number of allergen-specific IgE molecules coating MCs. These cells pre-store a panoply of powerful inflammatory mediators, which, upon release, can work in concert to trigger various symptoms of anaphylaxis including vascular leakage, itchy rash, swelling of afflicted tissue, and a drop in blood pressure and body temperature. MCs can directly probe blood contents with protoplasmic protrusions to acquire circulatory IgE (5). It was initially hypothesized that this behavior also explained how these perivascular cells became so quickly activated following the entry of allergens into the circulation. However, we show that only a minority of MCs acquire blood-borne allergens in this manner. Rather, most perivascular MCs are activated in an indirect fashion by CD301b+ dermal DCs that are continuously probing blood contents. Live and fixed-tissue microscopy of the mouse ear vasculature revealed that blood-borne antigens were adsorbed by lamellipodia of CD301b+ cells that protrude through the endothelial wall into blood vessels. Following acquisition of antigens by the MR, CD301b+ DCs spontaneously shed antigen-bound MVs to neighboring IgE bearing MCs in the perivascular region. Upon degranulation, the many granule-associated MC mediators triggered both vascular leakage and a drop in body temperature. The functional importance of both MCs and CD301b+ DCs to anaphylaxis was demonstrated by the observation that anaphylaxis was abrogated when either one of these cells was depleted in mice. Although CD301b+ cDC2 have previously been reported to predominate in the dermis (14, 17, 18), we have noticed their preferential location in the abluminal surface of the dermal vasculature. We do not exclude a role for other DC subsets (26, 40) in anaphylaxis because CD103+CD8α+CD11b−cDC1s also contribute to this process, albeit to a lesser extent than CD301b+ cDC2.
That CD301b+ DCs and not MCs were primarily responsible for probing the vasculature for allergens is not surprising as DCs are the professional immune sampling cells in the body. Subepithelial DCs have been implicated in sampling various body sites that are exposed to the environment such as the airways, gut, and skin (6-8). Many environmental antigens can enter the circulation either when the vasculature is ruptured or when antigens including microorganisms are introduced into the blood (e.g., via insect bites) (41, 42). Thus, there is a need to continuously sample the blood for these extrinsic agents. Dermal CD301b+ DCs appear to perform this vital function. Although perivascular macrophages also appear capable of transendothelial sampling many hours after exposure to blood-borne antigens (25, 43), their functional relevance, particularly in the context of anaphylaxis, remains unknown. It is currently unclear how allergic reactions in the skin and mucosal surfaces are triggered. Since MCs are also found in the same subepithelial region where DCs probing these locations lie, it is conceivable that environmental allergens are also initially acquired by DCs and then delivered to adjacent sensitized MCs to trigger local allergic reactions. Indeed, this could be the explanation for the observation made over a decade ago showing that the depletion of lung CD11c+ cells during allergen challenge abrogates the characteristic features of asthma in mice (44).
APCs, including dermal CD301b+ cells, ingest extrinsic antigens in the periphery and then traffic to proximal draining lymph nodes (DLN) where they present processed antigens to T cells resulting in the development of antigen-specific immune responses (15). Here, we report a distinct form of antigen presentation by the CD301b+ DC subset, where antigens adsorbed onto the DC surfaces are promptly relayed to neighboring MCs in the skin to cause systemic anaphylaxis. Although some of the antigens obtained by DCs were internalized, a significant amount remained on the plasma membrane and were released to the extracellular medium associated with MVs. These vesicles are the products of specific budding and pinching-off activities occurring on the plasma membranes of DCs, because the secretion of MVs was abrogated when VPS4, which mediates scission of MVs from the DC-derived plasma membrane, was specifically knocked down. We confirmed the functional role played by MVs in anaphylaxis by showing the attenuated ability of Vps4a-deficient primary DCs to induce cutaneous vascular leakage. Since MCs are activated within minutes of allergens entering the blood, it is unlikely that there is sufficient time for the internalization of antigens to occur and for any packaging of antigens in vesicles analogous to exosomes. Rather, the budding of membrane vesicles containing surface-bound antigens appears to be a possible mechanism for the rapid transfer of these antigens.
During immune surveillance, DCs regularly utilize the broad binding specificity of the cell surface MR to capture and clear a wide range of extrinsic and endogenous antigens that they encounter. Although antigens bound by MR can be endocytosed (37), we show here that at least some OVA antigens bound by MRs are held onto their plasma membrane and subsequently presented to neighboring immune cells via MVs. The factors that determine endocytosis of antigens by the MR or re-distribution to neighboring cells via MVs remain unclear.
Our findings provide a mechanism for how blood-borne allergens trigger tissue MC degranulation through allergen acquisition from the circulation by perivascular CD301b+ cells and direct transfer to MCs through MVs. As CD301b+ DCs are capable of sampling extrinsic antigens in the dermis and trafficking to the DLNs (15, 18), this suggests that CD301b+ DCs are key mediators of tissue MC degranulation in response to blood-borne allergens in addition to their recognized role as migratory DCs capable of presenting processed antigen in DLN. The ability of CD301b+ DCs to acquire blood-borne extrinsic antigens and relay them to neighboring MC and other DCs may be a powerful but underappreciated mechanism for enhancing the magnitude of inflammatory and immune responses to blood-borne antigens. Since effective treatments for anaphylaxis remain elusive, recognizing the role of CD301b+ DCs in allergen sampling may serve as a target for future therapeutic strategies.
Material and methods
Mouse strains
Eight-to-twelve-week-old female and male mice were utilized for most of these studies. The following mouse strains were employed: C57BL/6 wild type (WT), CD11c-DTR-GFP, CD301b-DTR-GFP, iDTR, AI14, Batf3−/− (Jackson Faboratories), and Mcpt5-Cre+ (a gift from Dr. A. Roers, University of Technology, Dresden). CD11c+ cells were depleted as follows: 8-12-week-old CD11c-DTR-GFP mice and their littermates were given i.p. and i.v. injections of 500 ng of diphtheria toxin/mouse every other day, twice. To deplete CD11c+ cells in ear skin, CD11c-DTR mice were administered with DT i.p. 500 ng and 50 ng i.d. in both ears. Two days after DT injection, 1 × 106 cells of bone marrow-derived DCs (BMDCs) which were electroporated with Vps4a-specific CRISPR-Cas9 expressing plasmid, mock plasmid (PX458), or control vector pmaxGFP (lonza) were i.d. injected in the ears. CD301b+ cells were depleted as follows: 8-12-week-old CD301b-DTR-GFP mice and their littermates were administered i.p. and i.v. injections of 250 ng of diphtheria toxin (DT)/mouse every other day for 1 week, or i.p. and i.v. injection of 500 ng DT/mouse once. MCs in Mcpt5-CreiDTR mice were conditionally depleted as follows: 8-week-old Mcpt5-CreiDTR mice and Mcpt5-Cre/+ littermates were given five i.v. injections of 200 ng of diphtheria toxin/mouse within 1 week. Mcpt5-CreAI14 mouse and CD301b-GFP mouse were crossed to generate Mcpt5-CreAI14:CD301b-GFP mice for intravital imaging. All procedures related to mice were performed in strict accordance with the animal protocol approved by the Institutional of Duke University Animal Care and Use Committee.
Cell Culture
For bone marrow-derived MC (BMMC) culture, WT bone marrow was obtained from WT C57BF/6 mice in ice-cold Hank's Balanced Salt Solution (HBSS, Gibco), and cultured in complete RPMI containing 10% fetal bovine serum (FBS) (Hyclone), 1 mM nonessential amino acids, 25 mM HEPES, 2 mM L-glutamine, 1 mM sodium pyruvate, 1× Antibiotic-Antimycotic (all reagents from Gibco), 10 ng/ml SCF and 5 ng/ml IL-3 (BioLegend) for 8 to 12 weeks. For BMDC culture, bone marrow was obtained from WT C57BL/6 or CD301b-DTR-GFP mice and cultured in RPMI media containing 10% FBS, 1× GlutaMAX® (Gibco), 20 ng/ml granulocyte macrophage colony-stimulating factor (GM-CSF) (BioLegend), or 20 ng/ml GM-CSF and 50 ng/ml IL-4 for 6-7 days. For bone marrow-derived macrophage (BMM) culture, the same procedures were followed replacing the growth factors with 20 ng/ml macrophage colony-stimulating factor (M-CSF). The rat MC RBL-2H3 cells (ATCC) were cultured in minimum essential medium (MEM) medium (Gibco) containing 15% FBS and antibiotics. JAWSII cells (ATCC) were maintained in MEM α medium (Gibco) supplemented with 20% FBS, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cells were cultured at 37 °C in a humidified water-jacketed incubator under 5% CO2 / 95% air atmosphere.
Primary human MC culture
Detailed procedures of primary human MC culture were explained in detail (45). For the culture of primary MC, Iscove’s Modified Dulbecco’s medium (IMDM, Gibco) containing 200 μM bovine serum albumin (BSA, Sigma), 1× Insulin-Transferrin-Selenium (ITS) supplement (Gibco), 75 μM β-mercaptoethanol (Sigma), 100 U/ml penicillin, and 100 μg/ml streptomycin was utilized. In brief, purified peripheral blood CD34+ peripheral blood cells (Allcells) were suspended at 5×105 cells/ml in supplemented IMDM containing 100 ng/ml recombinant human (rh) SCF, 10 ng/ml rhIL-6, and 1 ng/ml rhIL-3 for 0-3 weeks. For the subsequent 3-6 weeks, the cells were suspended at 5 × 105 cells/ml in supplemented IMDM containing 100 ng/ml rhSCF and 50 ng/ml rhIL-6. After 6 weeks of differentiation, cells were finally suspended at 5 × 105 cells/ml in supplemented IMDM containing 100 ng/ml rhSCF, 50 ng/ml rhIL-6, and 10% FBS. After differentiation of primary human MCs, we evaluated the maturation of primary human MCs via flow cytometry (>95% were double-positive for c-kit+ and FcεRI+) and toluidine blue staining (dark purple staining).
β-hexosaminidase assay
The day before the experiment, RBL-2H3 or BMMCs were sensitized with TNP-specific IgE antibody (BD Biosciences). For IgE-Ag stimulation, JAWSII cells or BMDCs were pretreated with TNP-OVA (Biosearch Technologies) for 15 min, followed by thorough washing with PBS 3 times. DCs and MCs were then co-incubated for 1 hr and supernatant was collected for β-hexosaminidase-release assay. Briefly, collected supernatant was incubated with p-nitrophenyl-N-acetyl-β-D-glucosaminide (3.4 mg/ml) dissolved in 0.1 M citrate buffer (pH 4.5) for 1 hr at 37 °C. 0.1 M carbonate buffer (pH 10) was then applied to the reaction wells to stop the reaction and develop color. The colorimetric measurement was performed at 405 nm using a microplate reader.
PCA and PSA
To elicit PCA, mice were injected with TNP-specific IgE antibody (100 ng/20 μl of PBS) (BD Biosciences) i.d. into the ears. After 16 hrs, mice were injected i.v. with 200 μg of TNP-OVA diluted in saline. For some experiments, 0.5% Evans blue dye or 1 mg Dextran-TRITC were injected along with 200 μg of TNP-OVA solution. To elicit PSA, mice were first sensitized with TNP-specific IgE (20 μg in saline) (BD Biosciences) by i.v. injection. After 24 hrs, mice were injected i.v. with 2 μg of TNP-OVA. After antigen challenge, body temperature was measured via rectal microprobe thermometer every 10 minutes to monitor systemic anaphylaxis.
Skin explant preparation and culture
Detailed procedures of skin explant preparation and culture are described previously (27). Skin explants were obtained from biopsies. Cut samples of approximately 1 cm × 1 cm × 0.5 cm were placed in 1 ml of Dulbecco's Modified Eagle Medium (DMEM) (Gibco) medium containing 10% FBS. Two to three days after culture, migrated cells were harvested and utilized for further experiments. Human skin specimens were collected from healthy patients undergoing plastic surgery at Duke University Medical Center and used in an anonymized fashion. All human samples for this study were obtained according to the protocols approved by the Institutional Review Board at Duke University.
Flow Cytometry
To produce single-cell suspensions for flow cytometry, dissected mouse ears were split open and the only dermis side lacking cartilage was digested with a digestion buffer (15% collagenase, 1% DNaseI, 10 mM 4-(2-Hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES), 1.5% FBS in HBSS). Single-cell suspensions were washed and stained with the fluorescently labeled antibodies against CD11b (clone M1/70), CD11c (clone N418), CD24 (clone M1/69), CD45 (clone 30-F11), CD64 (clone X54-5/7.1), CD103 (2E7), CD115 (clone AFS98), CD117 (clone 2B8), B220 (clone RA3-6B2), IA/IE (clone M5/114.15.2), FcεRI (clone MAR-1), Sirpα (clone P84), EpCAM (clone G8.8), and isotype controls (all antibodies from Biolegend) for 30 min at 4 °C. These were utilized to compare expression levels. Flow cytometric analysis was performed on a FACS CantoII or LSRII (BD) and was analyzed using FlowJo software (Tree Star, Ashland, OR).
Vps4a specific CRISPR-Cas9
To delete Vps4a in BMDCs and JAWSIIs, we used the genome-editing approach targeting the endogenous Vps4a gene through clustered regularly interspaced short palindromic repeats (CRISPR) and the endonuclease Cas9 (36). A CRISPR single guide RNA (sgRNA) targeting exon 2 of Vps4a gene was designed by utilizing the database (http://tools.genome-engineering.org) and was cloned into the pSpCas9(BB)-2A-GFP (PX458) plasmid (Addgene). The oligo sequence for Vps4a sgRNAs was GGATAAAGCCAAGAACTACG (20 nt sequence: 5' to 3') AGG (PAM). The CRISPR-Cas9 plasmid (px458) with gRNA targeting Vps4a exon 2 was electroporated with Amaxa Nucleofector (Lonza) into JAWSII cells or BMDCs. Forty-eight hours after electroporation in the JAWSII cells, the efficiency in generating site-specific double-strand breaks was measured through the SURVEYOR mutation detection assay (Integrated DNA Technologies) from purified genomic DNA derived from the JAWSII cells. After confirmation of the construction of CRISPR-Cas9 with sgRNA targeting Vps4a, BMDCs, which were matured with GM-CSF (20 ng/ml) and IL-4 (50 ng/ml) for 6 days, were electroporated with the constructed plasmid. Forty-eight hours after electroporation, immunoblotting with anti-VPS4A antibody (Santa Cruz Biotechnology) was performed to confirm the knock-out of endogenous VPS4A protein.
Electroporation
2 × 106 BMDCs were resuspended into 100 μl of Nucleofactor solution V (Lonza), mixed with Vps4a specific CRIPSR-Cas9 or mock plasmid and electroporated using Amaxa Nucleofector (Lonza) with program Y-001. Electroporated cells were cultured in media with 20 ng/ml of GM-CSF and 50 ng/ml of IL-4 for 48 or 72 hrs.
Immunofluorescent staining and microscopy
For co-culture visualization of MCs and DCs, BMMCs and BMDCs were fixed in 4% paraformaldehyde (PFA) and permeabilized in 0.1% saponin (Sigma) per 1% BSA in PBS. For whole-mount ear visualization, ears were excised and split open and immediately fixed in 4% PFA at room temperature for 2 hrs. To block and permeabilize the tissue, buffer containing 0.3 % Trion X-100 and 2.5% normal goat serum (Gibco) in 1% BSA-PBS was used.
To visualize blood vessels, 0.5 μg/ml of rat anti-mouse CD31 antibody (clone MEC 13.3, BD Biosciences) was incubated with mouse ears for overnight. A647-conjugated F(ab′)2 fragment anti-rat IgG (Jackson ImmunoResearch) as secondary antibody. To visualize MCs, FITC-avidin (BD Biosciences) were used. The samples were visualized by confocal microscopy or two photon microscopy.
For live imaging of MV secretion from DCs, JAWSII cells were grown on MatTek plates (MatTek Corporation) and treated with CellTracker® CM-DiI (Molecular Probes) for 20 min in serum-free MEMα media at 37 °C and 5% CO2 with humidification. After thoroughly washing with PBS, the pre-stained cells were treated with ovalbumin or PBS for 15 min in culture media. After thoroughly washing with PBS, cells were maintained at 37 °C while under Nikon ECLIPSE TE200 confocal microscope equipped with a 100×/1.49NA oil immersion objective lens was used to capture live moments for 15 min.
For intravital imaging by confocal microscopy, Mcpt5-CreAI14:CD301b-GFP mice were generated as described above and utilized. Mice were anesthetized using a mixture of ketamine and xylaxine and kept on a heating pad maintained at 38 °C. The inner side of mouse ears were taped onto a glass slide and immobilized with tape. 100 μl of 5mg/ml Alexa Fluor647-OVA (Molecular Probes) was injected i.v. and immediately imaged by Nikon ECLIPSE TE200 confocal microscope equipped with 20×/0.75NA multi-immersion objective lens.
For intravital imaging by two photon microscopy, CD301b-eGFP-DTR mice were anesthetized with a mixture of isoflurane and O2 supplied through a isoflurane vaporizer to a mask covering the snout. To prevent hypothermia of mice during intravital imaging, the imaging platform was heated and maintained at 38 °C. Ear pinnae were taped onto the imaging platform to secure their position and minimize movement during imaging. Immediately after injecting 200 μl of 10 mg/ml dextran-TRITC (150 kDa, Molecular Probes), time-lapse Z-stack imaging was initiated using Olympus FV1000 multiphoton upright microscope, characterizing in vivo movement of CD301b cDC2 near blood vessels at 30-sec intervals for a total 30 min. Olympus FV1000 Multiphoton equipped with 25×/1.05 NA water immersion objective lens was utilized for two photon microscopy. Collected series of images were reconstructed in 3D using Imaris software (Bitplane).
Preparation of microvesicles (MVs).
JAWSII cells or BMDCs plated on 100 cm2 culture dishes were treated with antigens for 15 min in culture media, followed by thorough washing with PBS to clear unbound antigens. After resupplying culture media, cells were incubated in culture conditions for 2 hrs. Collected culture supernatant was spun at 400 × g for 5 min, and resulting supernatant was re-spun at the same condition to remove cell debris. Thereafter, a final highspeed spin at 100,000 × g for 30 min was performed. A Optima L-90K ultracentrifuge (Beckman Coulter) was utilized for centrifugation. The collected MV pellet was resuspended in PBS and analyzed by flow cytometry, where particles ranging in size between 0.5 and 1.0 μm were counted. In our studies, we observed that DCs cultured in 100 cm2 culture dishes with 70 % confluency typically generated ~1 × 106 MVs.
Immunoblots.
Samples were mixed with RIPA buffer and then vortexed for 30 sec, three times. After 13,000 × g centrifugation, the lysates were mixed with sample buffer and separated on a 4%−20 % SDS-PAGE gels. Proteins on gels were transferred onto PVDF membranes (Bio-Rad) and blocked with 3% bovine serum albumin (BSA) in Tris-buffer saline with Tween-20 (TBST) for 2 hrs. Membranes were incubated with mouse anti-VPS4A monoclonal (clone A-11, 0.2 μg/ml, Santa Cruz Biotechnology) antibody or rabbit anti-MR1 polyclonal antibody (1 μg/ml, R&D Systems) overnight at 4 °C. Anti-mouse IgG antibody (1:2500, Bio-Rad) or anti-rabbit IgG antibody (1:1000, Bio-Rad) conjugated with horseradish peroxidase were used for secondary antibodies.
siRNA sequence
VPS4A: 5′-GUGGAAUGAUGUAGCUGGA[dT][dT]-3′
Mannose receptor, Ctype 1: 5′-CUCCUUACUGGGCAAUGCA[dT][dT]-3′
Statistical analyses
Statistical analyses were performed using GraphPad Prism v.6 (GraphPad Software). Unpaired Student’s t-test, two-way ANOVA and one-way ANOVA with Tukey's multiple comparisons tests were used to calculate statistical significance. Log-rank test were used to analyze survival during PSA. P<0.05 was considered statistically significant. Data are presented as mean ± SEM.
Supplementary Material
Acknowledgements
We thank A. Roers for the Mcpt5-Cre mice. B. Hayes is gratefully acknowledged for critical review of this manuscript. We thank Y. Jiao and B. Chen for assistance with two-photon microscopy. We thank R. Vancini and S. Miller for assistance with electron microscopy. We thankfully acknowledge the Duke Cancer Institute and Duke Human Vaccine Institute Flow Cytometry Facility for the use of FACSCanto II and LSR II.
Funding: This work was funded by U.S. National Institutes of Health grants (U01-AI082107; R01-AI096305; R56-DK095198). A. MacLeod receives funding from U.S. National Institute of Health grants R21-AI128727, the Duke Physician-Scientist Strong Start Award, Funds from the Department of Dermatology and the Dermatology Foundation.
Footnotes
Competing interests: The authors declare no competing interests associated with this work.
Data and materials availability: Plasmids and reagents described in this study are available to the scientific community upon request to S.N.A. All data are available in the manuscript or the supplementary materials.
References and Notes
- 1.Tejedor-Alonso MA, Moro-Moro M, Mugica-Garcia MV, Epidemiology of Anaphylaxis: Contributions From the Last 10 Years. J Investig Allergol Clin Immunol 25, 163–175; quiz follow 174–165 (2015). [PubMed] [Google Scholar]
- 2.Boden SR, Wesley Burks A, Anaphylaxis: a history with emphasis on food allergy. Immunol Rev 242, 247–257 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons FE, O. World Allergy, World Allergy Organization survey on global availability of essentials for the assessment and management of anaphylaxis by allergy-immunology specialists in health care settings. Ann Allergy Asthma Immunol 104, 405–412 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Sampson HA et al. , Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol 115, 584–591 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Cheng LE, Hartmann K, Roers A, Krummel MF, Locksley RM, Perivascular mast cells dynamically probe cutaneous blood vessels to capture immunoglobulin E. Immunity 38, 166–175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rescigno M et al. , Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2, 361–367 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Sung SS et al. , A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 176, 2161–2172 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M, External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med 206, 2937–2946 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung S et al. , In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudeck A et al. , Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 34, 973–984 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Tamoutounour S et al. , Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Merad M, Sathe P, Helft J, Miller J, Mortha A, The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 31, 563–604 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang B et al. , IL-27 Facilitates Skin Wound Healing through Induction of Epidermal Proliferation and Host Defense. J Invest Dermatol 137, 1166–1175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henri S et al. , CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med 207, 189–206 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang H et al. , The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol 11, 608–617 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlitzer A et al. , Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol 16, 718–728 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Kumamoto Y, Denda-Nagai K, Aida S, Higashi N, Irimura T, MGL2 Dermal dendritic cells are sufficient to initiate contact hypersensitivity in vivo. PLoS One 4, e5619 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumamoto Y et al. , CD301b(+) dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 39, 733–743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami R et al. , A unique dermal dendritic cell subset that skews the immune response toward Th2. PLoS One 8, e73270 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malissen B, Tamoutounour S, Henri S, The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol 14, 417–428 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Denda-Nagai K et al. , Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): efficient uptake and presentation of glycosylated antigens by dendritic cells. J Biol Chem 285, 19193–19204 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumamoto Y, Hirai T, Wong PW, Kaplan DH, Iwasaki A, CD301b(+) dendritic cells suppress T follicular helper cells and antibody responses to protein antigens. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meredith MM et al. , Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med 209, 1153–1165 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satpathy AT et al. , Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med 209, 1135–1152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barreiro O et al. , Pivotal role for skin transendothelial radio-resistant anti-inflammatory macrophages in tissue repair. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelson BT et al. , Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med 207, 823–836 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindenberg JJ et al. , IL-10 conditioning of human skin affects the distribution of migratory dendritic cell subsets and functional T cell differentiation. PLoS One 8, e70237 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ, Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113 Pt 19, 3365–3374 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Brossart P, Goldrath AW, Butz EA, Martin S, Bevan MJ, Virus-mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J Immunol 158, 3270–3276 (1997). [PubMed] [Google Scholar]
- 30.Muralidharan-Chari V et al. , ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 19, 1875–1885 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crompot E et al. , Avoiding false positive antigen detection by flow cytometry on blood cell derived microparticles: the importance of an appropriate negative control. PLoS One 10, e0127209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choudhuri K et al. , Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature 507, 118–123 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDaniel K et al. , Functional role of microvesicles in gastrointestinal malignancies. Ann Transl Med 1, 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolo V et al. , Selective localization of matrix metalloproteinase 9, beta1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Res 58, 4468–4474 (1998). [PubMed] [Google Scholar]
- 35.Lata S et al. , Helical structures of ESCRT-III are disassembled by VPS4. Science 321, 1354–1357 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ran FA et al. , Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8, 2281–2308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgdorf S, Lukacs-Kornek V, Kurts C, The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol 176, 6770–6776 (2006). [DOI] [PubMed] [Google Scholar]
- 38.McGreal EP, Miller JL, Gordon S, Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol 17, 18–24 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figdor CG, van Kooyk Y, Adema GJ, C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol 2, 77–84 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Mollah SA et al. , Flt3L dependence helps define an uncharacterized subset of murine cutaneous dendritic cells. J Invest Dermatol 134, 1265–1275 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues SF, Granger DN, Blood cells and endothelial barrier function. Tissue Barriers 3, e978720 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Handfield C, Kwock J, MacLeod AS, Innate Antiviral Immunity in the Skin. Trends Immunol 39, 328–340 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abtin A et al. , Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol 15, 45–53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rijt LS et al. , In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 201, 981–991 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holm M et al. , Seven week culture of functional human mast cells from buffy coat preparations. J Immunol Methods 336, 213–221 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.