Abstract
CD19 chimeric antigen receptors (CARs) have demonstrated great efficacy against a range of B cell malignancies. However, antigen escape and, more generally, heterogeneous antigen expression pose a challenge to applying CAR therapy to a wide range of cancers. We find that low-dose radiation sensitizes tumor cells to immune rejection by locally activated CAR T cells. In a model of pancreatic adenocarcinoma heterogeneously expressing sialyl Lewis-A (sLeA), we show that not only sLeA+ but also sLeA– tumor cells exposed to low-dose radiation become susceptible to CAR therapy, reducing antigen-negative tumor relapse. RNA sequencing analysis of low-dose radiation-exposed tumors reveals the transcriptional signature of cells highly sensitive to TRAIL-mediated death. We find that sLeA-targeted CAR T cells produce TRAIL upon engaging sLeA+ tumor cells, and eliminate sLeA− tumor cells previously exposed to systemic or local low-dose radiation in a TRAIL-dependent manner. These findings enhance the prospects for successfully applying CAR therapy to heterogeneous solid tumors. Local radiation is integral to many tumors’ standard of care and can be easily implemented as a CAR conditioning regimen.
Keywords: CAR T cell, radiation, antigen escape, sialyl Lewis-A, pancreatic cancer
Graphical Abstract

Tumor antigen escape is a major challenge for CAR T cell therapy, especially for solid tumors. DeSelm et al. demonstrate how low-dose radiation conditioning sensitizes pancreatic cancer to CAR T cell killing and allows CAR T cells to eliminate heterogeneous tumors.
Introduction
CD19 chimeric antigen receptor (CAR) T cells achieve a complete response (CR) in a majority of patients with refractory, relapsed B cell malignancies.1 Responses to CAR therapy targeting solid tumors have to date been relatively scarce.2 One of the challenges to overcome in all cancers and especially solid tumors is antigen heterogeneity. Although all or most B cell malignancies express CD19,3 numerous potential CAR targets are only expressed in a fraction of all tumor cells within a patient, posing the risk of antigen escape. Low-level antigen expression may also result in resistance to CAR therapy.4 Targeting two or more antigens can be implemented in the event of a defined escape population or clone,5, 6, 7, 8, 9 but other approaches are needed to overcome greater or undefined target heterogeneity.
Strategies that improve antigen presentation, induce epitope spreading, or perpetuate existing antitumor T cell responses hold promise for combating tumor antigen escape. For example, cancer vaccines and “immunogenic” radiation therapy (RT) activate antigen-presenting cells (APCs) to improve tumor neoantigen display to endogenous T cells.10 However, the same neoantigens must still be expressed and presented in most, if not all, tumor cells to obtain a complete response. In patients who have pre-existing tumor-reactive T cells, which correlates with tumor mutational burden, immune checkpoint inhibitors can relieve T cell exhaustion and provide sustained responses. However, checkpoint inhibition cannot restore T cell responses against tumor cells that do not present the recognized antigens, just as CARs cannot direct a response against tumor cells devoid of the CAR target.
The improved tumor recognition that can occur after exposure to ionizing radiation, mediated by increased APC activation, improved T cell infiltration, and enhanced human leukocyte antigen (HLA) or CAR target expression on the tumor,10, 11 faces the same challenge of antigen escape because of antigen loss. However, we find that tumors that have been exposed to low-dose irradiation become more sensitive to CAR T cell activity, including tumor cells that lack the CAR target. Understanding this mechanism may be particularly valuable in overcoming solid tumor antigen escape.
We characterize this alternative mechanism by which tumor susceptibility to CAR T cell-mediated elimination is enhanced by radiation conditioning and exploit it to extend the reach of CAR T cells beyond the targeted antigen. Pancreatic cancer continues to carry a dismal prognosis with little improvement over the last decades, does not have uniformly expressed therapeutic target antigens, and is increasing in incidence. In an orthotopic pancreatic cancer model that is partially antigen-negative (Ag−), we provide a novel means to address the challenge of clonal antigen heterogeneity by combining low-dose radiation and CAR therapy.
Results
sLeA-Specific CAR T Cells Are Active against Pancreatic Tumor Cells In Vitro
Identifying a solid tumor target that is expressed on 100% of tumor cells and no critical normal tissues is challenging. Pancreatic cancer exemplifies this problem, with a number of attractive targets; however, none of these are clearly expressed on all tumor cells.12 Sialyl Lewis A (sLeA), a surface antigen expressed on 75%–90% of pancreatic tumors13 with low expression on normal human tissues13 is an active antibody target in clinical trials (NCT03118349, NCT02672917, and NCT02687230). The human monoclonal 5B1 antibody targeting sLeA has demonstrated specificity for pancreatic cancer in vitro and in vivo13 as well as safety and tolerability in pancreatic cancer patients at biologically active doses.14 We thus chose to construct a PDAC-targeting CAR using this sLeA-specific scFv. sLeA-specific LBBz CARs directed effective cytotoxicity against multiple pancreatic cancer tumor lines expressing sLeA but not sLeA− PC3 prostate cancer cells (Figure S1). Capan2 PDAC expressed an intermediate level of sLeA (Figure S1) and was selected for further experiments.
Low-Dose Radiation Sensitizes Tumor Cells to CAR T Cell Killing without Inducing Target Antigen Expression
To test an initial hypothesis that RT may induce sLeA expression and improve the ability of CAR T cells to eliminate tumors with heterogeneous target antigen expression, we irradiated tumor cells with 2 Gy and, 2 days later, performed a cytotoxic T lymphocyte (CTL) assay as well as fluorescence-activated cell sorting (FACS) analysis of surface target antigen expression. 2 Gy was chosen because higher RT doses induced small but significant tumor cell death, whereas 2 Gy resulted in no detectable difference in tumor viability (Figure 1A). We found that 2 Gy (hereafter called “low-dose RT”) increased the sensitivity of tumor cells to CAR T cell killing at every effector:target ratio (Figure 1B) but did not increase target antigen expression (Figure 1C).
Figure 1.
RT Sensitizes Pancreatic Cancer to CAR T Cell Killing without Affecting Target Antigen Expression
(A) Tumor cell viability 48 hr after exposure to various doses of radiation. (B) Capan2 pancreatic cancer cells were exposed to low-dose RT (2 Gy) and, 48 hr later, incubated with CAR T cells at the indicated ratios for 18 hr, after which percent killing was determined. (C) Target antigen expression levels were unchanged 48 hr after RT. (D) Transcriptome analysis of target cells 6 hr after RT reveals a number of significantly affected apoptotic pathways. (E) TRAIL mRNA expression and protein levels in the media of CAR T cells after exposure to target antigen (sLeA-expressing Capan2 cells). (F) TRAIL protein was quantified in the media of LBBz and L(del) CAR T cells grown on target cells expressing or not expressing the target antigen. LFC, log2 fold change; E:T, effector:target. Error bars = SEM.
Low-Dose Radiation Affects Gene Sets Associated with Sensitivity to TRAIL-Mediated Death
To gain insight into potential mechanisms by which low-dose RT sensitizes tumor cells to CAR T cell killing, we performed RNA sequencing (RNA-seq) analysis on the tumor cells before and after low-dose RT. Although the RT itself was sublethal, gene set analysis revealed a high number of apoptotic pathways significantly affected by low-dose RT (Figure 1D). In particular, gene sets distinguishing tumor cells that are sensitive to TRAIL-mediated death from those that are not15 emerged with the lowest false discovery rate (FDR < 0.0000001 for each; 429 of 492 positive pathway members significantly induced, and 114 of 128 negative pathway members significantly downregulated) (Figure 1D).
CAR T Cells Produce TRAIL upon Target Antigen Encounter
TRAIL is a trimeric protein that induces death through two different receptors and a number of downstream signaling molecules that influence susceptibility; tumor cells are generally more sensitive to TRAIL-induced apoptosis than normal cells but still fall on a spectrum16. Gene set analysis suggested that low-dose RT may transcriptionally prime tumor cells to TRAIL-mediated death, which would only be relevant if the death ligand were present locally at sufficient levels. We thus analyzed TRAIL production from sLeA-specific CAR T cells and found that CAR T cells produce low levels of TRAIL at baseline but, upon target antigen encounter, significantly induce TRAIL mRNA and protein (Figure 1E). In contrast, TRAIL is not induced after tumor recognition by T cells expressing a truncated CAR that lacks the signaling domain (L(del)), establishing the dependence of TRAIL induction on CAR signaling (Figure 1F).
Ag− Tumor Cells Exposed to Low-Dose RT Are Susceptible to CAR T Cell TRAIL-Mediated Death
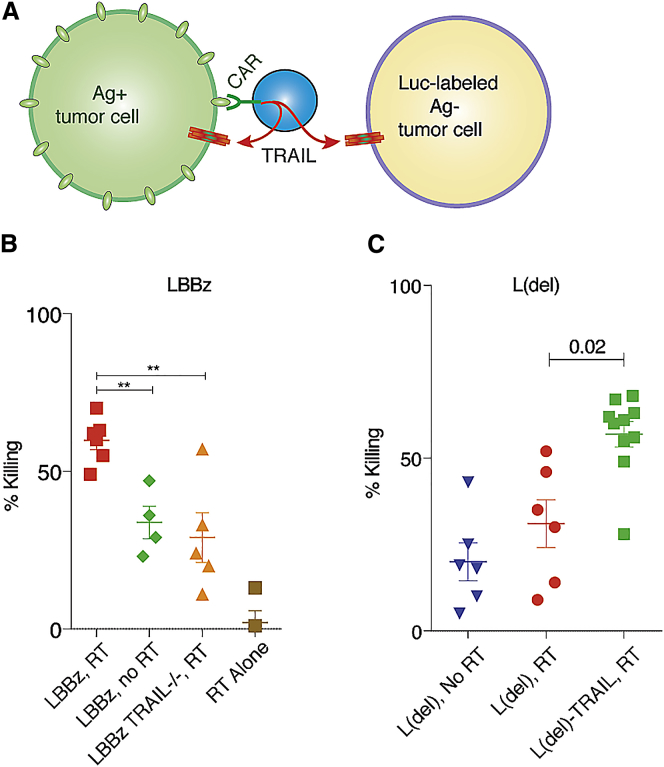
To test the functional significance of TRAIL produced by activated CAR T cells on Ag− tumors exposed to low-dose RT, we sorted tumor cells by FACS into antigen-positive (Ag+) and Ag− populations. Ag− cells were transduced with Firefly Luciferase (Luc) and remained stably Ag− over time (Figure S2). We next mixed 75% Ag+ with 25% Ag− Luc+ tumor cells, exposed them to low-dose or no RT, and incubated them with CAR T cells in which TRAIL was disrupted by CRISPR (Figures 2A, 2B, and S3). Using Luc activity to monitor Ag− cell killing, we found that wild-type (WT) CAR T cells on RT-exposed tumor cells produced the greatest magnitude of Ag− tumor cell death, which was significantly reduced by the absence of TRAIL in the CAR T cell or the absence of sensitizing RT to the tumor (Figure 2B). L(del) CAR T cells, which recognize the target cells but do not induce TRAIL, killed significantly more RT-sensitized Ag− tumor cells when they were made to constitutively express TRAIL (Figure 2C).
Figure 2.
TRAIL Expressed by Activated CAR T Cells Is Active against Antigen-Negative Tumor Cells in a Heterogeneous Tumor Population Exposed to Low-Dose Radiation
(A) CAR-activated T cells produce TRAIL, which acts upon radiation-sensitized antigen-positive and antigen-negative tumor cells. (B and C) Ag+ cells were mixed with luciferase-expressing Ag− cells at a ratio of 75:25, exposed to low-dose RT, and cocultured with LBBz (B) or L(del) (C) CAR T cells for 4 days, followed by luciferase-based quantification of cell killing. **p < 0.01.
TRAIL exerts a number of context-dependent effects, including apoptosis and necroptosis of both tumor cells and T cells17, or pro-tumor effects, including myeloid-derived suppressor cell recruitment through tumor cell nuclear factor κB (NF-κB) activation,18 or survival, invasion, and metastasis through Rac1 and Akt activation within the tumor.19 To better understand how an RT-sensitized tumor might respond to increased TRAIL stimulation provided by CAR T cells, we compiled known mediators of the various downstream TRAIL signaling pathways. Many pathway mediators are regulated through transcription, cleavage, phosphorylation, ubiquitination, or other events, but gene expression analysis can provide general information regarding overall pathway activation states. Notably, gene expression changes from RNA-seq data before and after sensitizing RT revealed that the majority of individual members of both pro-tumor and anti-tumor mediators downstream of TRAIL were significantly altered by sensitizing RT (Figure 3A, red and green represent significant changes, gray represents non-significant changes). Pro-survival, migration, metastasis, and tumor-supportive inflammation TRAIL pathway members were almost uniformly downregulated, whereas pro-apoptotic molecules were overwhelmingly induced, suggesting that sensitizing RT may predispose tumor cells to TRAIL-mediated apoptosis (Figures 3A and S4). Because apoptosis and necroptosis levels can be monitored by phosphatidylserine (PS) expression on the cell membrane, we tested whether TRAIL produced by CAR T cells induced detectable membrane PS changes over time in Ag– cells using live video microscopy of cultures containing fluorescent annexin-V antibody. RT-sensitized Ag– tumor cells were labeled with CellTrace Violet (CTV) before mixing with unlabeled Ag+ tumor cells and TRAILWT or TRAIL−/− CAR T cells. Automated quantification of Ag– tumor cells undergoing apoptosis demonstrated that TRAIL−/− CAR T cells fail to induce Ag– tumor apoptosis over time, whereas TRAILWT CAR T cells effect steady and significant Ag– tumor cell apoptosis (p < 0.0001; Figure 3B).
Figure 3.
Sensitizing RT Transcriptionally Primes Pancreatic Cancer Cells for TRAIL-Induced Death
(A) RNA expression levels of signaling molecules known to mediate various TRAIL responses, including survival and migration, tumor-supportive inflammation, necroptosis, apoptosis, and death receptor endocytosis, were quantified by RNA-seq before and after RT exposure to Capan2 pancreatic cancer cells in three biologic replicates. Significantly induced and downregulated molecules are shown in red and green, respectively, with magnitude represented by color gradient. Molecules in gray were not significantly changed. (B) CTV-labeled Ag− cells were exposed to RT 2 days before coculture with unlabeled Ag+ cells, annexin-V 595, and TRAIL−/− or TRAILWT CAR T cells. Cultures were monitored by live video microscopy, and Ag− cell apoptosis was quantified over time. Error bars = SEM.
Pancreatic Tumors Containing a Resistant Ag− Population Can Be Eliminated In Vivo by CAR T Cells following Sensitizing RT
We next established a mouse model for the challenging but common clinical scenario of heterogeneous solid tumors partially devoid of target antigen. PDAC consisting of 25% Ag– cells was established in the mouse pancreas and, 9 days later, treated with CAR T cells (Figure 4A). CAR T cells that consistently eliminated Ag+ orthotopic PDAC were unable to completely eliminate any heterogeneous tumors (Figures 4B–4E). We then tested whether sensitizing RT afforded any meaningful benefit to heterogeneous tumors treated with CAR T cells in vivo. Mice with established heterogeneous PDAC treated with sensitizing RT before CAR T cells achieved more CR and partial response (PR) by imaging, autopsy exam, and pathology (Figures 4B and 4F). Because the major known mechanism of CAR-independent T cell killing is through the T cell receptor (TCR), and because RT can induce HLA expression on target cells, we investigated whether TCR-dependent tumor killing plays a significant role after sensitizing RT. CAR T cells lacking their TCR (TCR−/−) (Figure S5) maintained the capacity to eliminate RT-sensitized heterogeneous tumors (Figure 4G). RT initially resulted in moderately increased T cell accumulation within the tumor over the first 2 weeks (Figures 4I–K). Despite significant tumor influx (Figure S6), TRAIL−/− CAR T cells failed to consistently achieve a complete response in RT-sensitized tumor-bearing mice, as demonstrated by both a waterfall plot of response at the time of death (which occurred from either graft-versus-host disease [GVHD] or tumor progression) (Figure 4B) and weekly bioluminescence imaging (Figure 4H). Mice with tumors that relapsed or progressed still harbored CAR T cells in the blood, spleen, and tumor, as assessed by FACS, and exhibited significant T cells penetrating the tumor by immunohistochemistry (IHC) but demonstrated outgrowth of Ag– tumor cells (Figures 4L and S7).
Figure 4.
Sensitizing RT Allows CAR T Cells to Eliminate Heterogeneous PDAC In Vivo
(A) Capan2 tumor cells were mixed at 75:25 sLeA+:sLeA− and then injected into the pancreas of NSG mice. After tumors established for 9 days, mice were given RT, followed by CAR T cells. (B) Waterfall plot of tumor volume change at time of death among different treatment groups. (C–H) BLI was performed weekly on mice that were untreated (C) or treated with RT and 1928z (D), LBBz (E), RT and LBBz (F), RT and TCR−/− LBBz (G), or RT and TRAIL−/− LBBz (H) CAR T cells. (I–K) T cell infiltration of tumors from CAR-treated or RT with CAR-treated mice was determined using BLI T cell imaging (detecting G-Luc on the transduced T cell) over the first 19 days (I) and by IHC from mice sacrificed on day 21 (J and K, all not significant [ns]). (L) Tumors in mice that progressed displayed reduced target antigen expression over time by FACS. (M) BLI of mice treated with RT+L(del) or RT+L(del)-TRAIL CAR T cells. Error bars = SEM.
To uncouple the effects of TRAIL and the CAR, we treated RT-sensitized mice with L(del) CAR T cells, which bind tumors but do not induce CAR cytotoxicity or TRAIL upon recognition, and L(del)-TRAIL CAR T cells, which bind tumors and constitutively express TRAIL but exert no CAR-mediated cytotoxicity. Although the first strategy yielded no response despite local T cell accumulation (Figure 4M), targeting constitutive TRAIL-expressing T cells to the tumor using the external CAR domain modestly increased the response rate (Figure 4M).
Localized RT Effectively Conditions Tumors for Subsequent CAR T Cell Administration
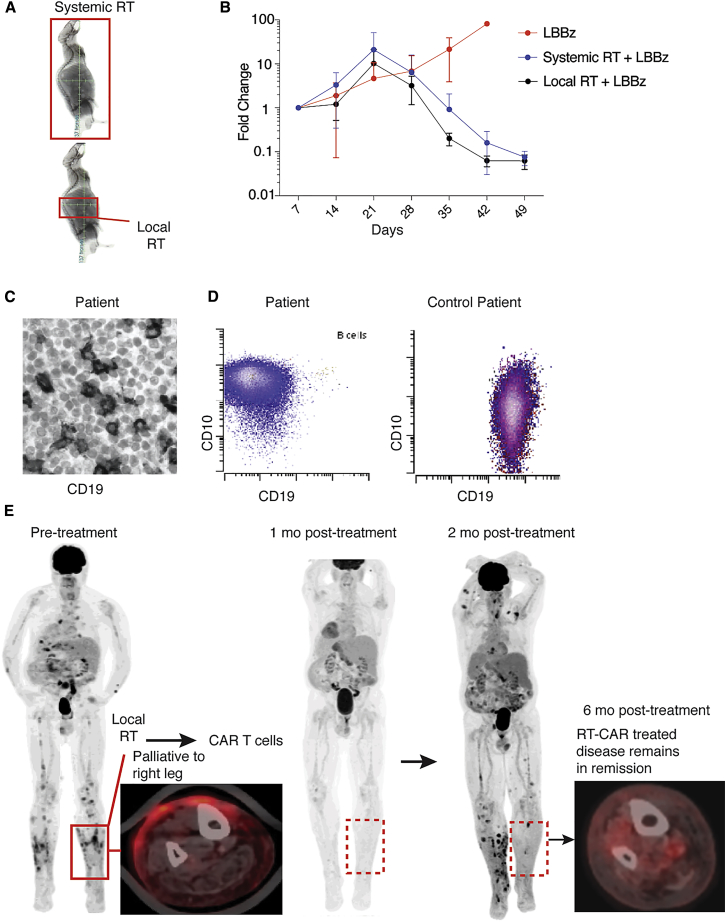
To determine whether systemic RT is required for CAR T cell sensitization or whether local RT to the tumor would suffice, we treated mice harboring orthotopic PDAC with RT to the whole body or only the pancreatic tumor, followed by CAR T cell administration (Figure 5A). Although total body RT-treated mice tended to have greater T cell tumor infiltration at early time points (Figure S8), both strategies resulted in similar tumor responses (Figure 5B). Thus, despite potentially different host effects between systemic and local low-dose RT, either approach effectively sensitizes heterogeneous tumor to CAR T cell killing.
Figure 5.
Outcome of a Large-Cell Lymphoma Patient with a Heterogeneous Tumor Treated with Palliative RT and CD19 CAR T Cells
(A) Total body or local RT was delivered to mice harboring heterogeneous tumors of the pancreas using image-guided radiation, followed by CAR T cells. (B) Tumor burden was monitored by BLI. (C and D) Patient biopsy before CAR T cell treatment examined for CD19 by IHC (C) and flow cytometry (D). (E) Fluorodeoxyglucose (FDG)-PET scan before and 1, 2, and 6 months after palliative leg RT and systemic 1928z CAR T cells. Error bars = SEM.
RT and CAR T Cell Treatment in Patients with Heterogeneous Tumors: A Case Report
Experience combining RT with CAR T cells is limited. Just as tumor cells transcriptionally primed for TRAIL-mediated killing by RT exhibited significantly more death in response to CAR T cells in our cell culture and mouse studies, it is conceivable that similar sensitization may occur in nearby Ag− normal tissue cells after RT. A patient with refractory diffuse large B cell lymphoma (DLBCL) bearing a large proportion of CD19– tumor cells in the sampled tumor masses (Figures 5C and 5D) presented for CD19 CAR therapy (NCT02631044). The patient had painful disease infiltrating the skin of his lower legs, particularly on the right. We offered palliative RT to his right leg (4 Gy × 5 fractions), and the patient then received CD19 CAR T cells as planned. In the days and weeks after CAR T cell therapy, the patient did not exhibit signs or symptoms of toxicity within the irradiated field. The patient exhibited a grade 1 cytokine release syndrome (CRS) without neurological symptoms. One month post-CAR T cells, the patient had an excellent response by positron emission tomography (PET)-computed tomography (CT) imaging (Figure 5E). Two months post-CAR T cell infusion, tumor rebounded in prior and new locations with CD19-low or -negative expression, with the exception of the diseased area that received palliative RT before CAR T cells. Currently, 1 year after treatment, the area of antigen-heterogeneous tumor subjected to palliative RT followed by CAR T cells remains disease-free (Figure 5E).
Discussion
Our initial choice to target CD19 in B cell malignancies was largely driven by the elevated and relatively homogeneous expression of CD19 in leukemia and lymphoma and its confinement to the B cell lineage in normal tissues.3 Based on the remarkable complete remission rates of 70%–90% in phase I acute lymphoblastic leukemia (ALL) trial patients,20, 21 the prospect of extending CAR therapy to a wide range of cancers is intriguing. Although CAR therapy has only recently begun to tackle solid tumors,2, 12, 21, 22 the results have so far been modest, with few occurrences of major responses.2, 23, 24 With escape and regrowth of Ag− tumor cells now being a well-documented mechanism of resistance to CAR therapy,24, 25, 26 novel approaches are needed to enable CAR T cells to effectively prevent antigen escape.
One approach to overcoming antigen escape from CAR T cells is to target two different antigens.8 Another makes use of “armored CARs” to recruit endogenous T cells via the secretion of activating cytokines such as interleukin-18 (IL-18)27 or the expression of costimulatory ligands.28 Checkpoint inhibitor therapy has since been added to CAR T cells, aiming to reinvigorate both CAR T cells and endogenous tumor-reactive T cells.29, 30 However, all of these approaches rely upon tumor cells to express a tumor-specific antigen that is recognized by either CARs or TCRs. More recently, FasL on CAR T cells has been shown to induce embryonal carcinoma death independent of target antigen expression, especially after Fas was ectopically expressed in the tumor.31 We found that another death-inducing ligand, TRAIL, produced by activated CAR T cells, exerts a significant apoptotic effect on native Ag− tumor cells that have been sensitized to TRAIL-mediated death by radiation conditioning.
The approach we report here delineates a clinically feasible mechanism by which a tumor can be eliminated by CAR T cells in trans, irrespective of immunogenicity. This approach is thus relevant to preempt antigen escape and may be particularly beneficial in tumors with low mutational burden, where the probability of neoantigen presentation and recognition is low.
The spatial and temporal specificity achieved here relies upon physiologic responses of the CAR T cell to target antigen and of the tumor cell to radiation. Induction of TRAIL after target encounter ensures active and maximal production within the tumor microenvironment. The ability to escalate the TRAIL sensitivity of Ag+ and Ag− tumor cells through targeted RT provides a window of opportunity to enhance site-specific CAR T cell efficacy against heterogeneous tumors. The effect of this interaction has multiple implications. We found that both systemic and localized RT sensitize the tumor to CAR T cell killing. Most importantly, in antigen-heterogeneous pancreatic cancer, we show that Ag− tumor cells that would otherwise escape CAR recognition can be eliminated by CAR T cells after low-dose RT in vivo. In the case of systemic disease, low-dose total body irradiation may effectively sensitize tumor cells and results in elimination at a lower CAR T cell dose, potentially reducing the risk for cytokine release syndrome while increasing the efficacy.
The early observation that tumor cells are highly sensitive to TRAIL-induced apoptosis relative to normal cells16 generated enthusiasm for recombinant TRAIL or agonistic TRAIL receptor-based therapies. Unfortunately, this therapy has encountered multiple limitations, including downstream resistance to apoptosis through tumor gene expression changes,32 the short half-life of the TRAIL protein,32, the reduced apoptotic ability of the bivalent antibody,33 and limited local tumor penetration when administered systemically. CAR T cells as the source of TRAIL offer several potential advantages, such as concentrated TRAIL synthesis within the tumor, continuous production as long as tumor and T cells are present, and a supply of native trimeric protein rather than a potentially less apoptotic bivalent antibody.33 Although TRAIL may exert a pro-apoptotic effect on CAR T cells through death receptor 5,17 this activity is not increased by radiation conditioning prior to CAR T cell infusion.
Several other forms of immunotherapy are commonly combined with RT under certain circumstances. An immunogenic, ablative, high dose of radiation induces tumor death and, in some contexts, leads to increased antigen presentation, subsequent T cell activation, and potentially an “abscopal” or secondary immune response against unirradiated tumors.10 Because of the infrequency of the abscopal effect in clinical practice, predictably harnessing this phenomenon remains an active area of investigation. Unlike endogenous T cells, CAR T cells do not rely on antigen processing and presentation, and, unless radiation induces expression of the particular CAR target molecule,11 it is not intuitive whether radiation may have an immunogenic, immunosuppressive, or irrelevant effect on CAR T cell therapy. We describe a fundamentally different effect of radiation in the context of CAR T cell therapy, one by which a sublethal, low dose of radiation locally sensitizes tumors to CAR T cell killing in trans. Unlike its ablative counterpart, sensitizing radiation is not limited by location or size of disease and, given the much lower dose, may be applied to wider areas for patients with diffuse metastases, with less concern for RT-related side effects.
A patient with heterogeneous tumor treated with palliative (non-curative) RT before CAR T cell therapy exhibited results consistent with our mouse data without signs of excess toxicity. Although this clinical correlate aligns with animal findings, it does not test the hypothesis. In particular, the effect of RT alone on the lasting complete response of his heterogeneous tumor cannot be ignored. However, the administered radiation dose is roughly half the standard locally curative dose of more than 45 Gy for gross disease in this type of aggressive lymphoma.34 Further, although toxicity was not observed in the RT field of the leg, it is possible that other normal tissues, such as the gastrointestinal (GI) system, may exhibit heightened RT sensitivity to activated CAR T cell-produced TRAIL.35 A clinical trial incorporating RT with CAR T cells is planned to assess the effect on clonal antigen heterogeneity, the safety of RT conditioning, and systemic effects of local RT on CAR T cell-mediated disease response.
RT is currently used at some point in the treatment of about half of metastatic cancer patients for palliation and is commonly utilized in almost all non-metastatic cancer types as an alternative or an addition to surgery to increase local control.36 Implementing CAR therapy within current RT regimens may further increase local and systemic tumor control. Our findings suggest that integrated delivery of these two therapies warrants coordination among disease management teams.
Our findings support the concept that multimodality CAR therapy with RT conditioning may improve responses in solid tumors. Most importantly, we provide a mechanistic platform by which engineered T cells may be further enhanced to eliminate clonally heterogeneous solid tumors.
Materials and Methods
Cell Culture
Tumor cells expressing firefly luciferase-GFP were described previously.28 The 293T cell line and H29 and retroviral packaging cell lines were cultured in DMEM supplemented with 10% fetal calf serum (FCS).28 Capan2 cells were generously provided by Jason S. Lewis (Memorial Sloan Kettering Cancer Center [MSKCC]) and grown in RPMI medium supplemented with 10% FCS.
Buffy coats from healthy volunteer donors were obtained from the New York Blood Center. Peripheral blood mononuclear cells were isolated by density gradient centrifugation, and cells were then stimulated with phytohemagglutinin (PHA; Sigma) and cultured as described previously.28
Radiation
Radiation Dose
All experiments using PDAC used 2 Gy unless otherwise specified. For in vitro RT studies, all RT sensitization experiments were performed with RT given to the tumor cells 2 days prior to tumor analysis or coculture with T cells unless specified otherwise.
Radiation Method
Local RT to the pancreas was performed by identifying the pancreatic tumor using intraperitoneal contrast and cone beam CT imaging on an X-Rad 225Cx machine, which combines high-accuracy cone beam CT imaging with 3D image-guided radiation treatment under general anesthesia. Local RT was delivered using either anterior-posterior, or anterior-posterior and lateral beams. Experiments requiring less target precision (total body RT) were performed using the small animal irradiator with open jaws in the anterior-posterior (AP) direction.
Flow Cytometry
We used fluorochrome-conjugated antibodies to CD3 (UCHT1), CD4 (S3.5), CD8 (3B5), and sLeA (7LE, AF405-conjugated, Novus Biologicals). We used Alexa 647-conjugated goat anti-human F(ab)2 (Thermo Fisher Scientific) to detect CARs. Flow cytometry was performed on a BD LSRII, and data were analyzed with FlowJo software version 9.5.2 (Tree Star). Fc Receptor Binding Inhibitor Antibody Human (eBioscience) was used to block Fc receptors. In some cases, CountBright beads (Invitrogen) were added to samples to count cell numbers.
TRAIL Measurements
For RNA and ELISA experiments, CAR T cells were exposed Capan2 expressing the target antigen for 4 hr, followed by removal and monoculture of T cells, which were replated in new medium daily. Cells were removed and analyzed for TRAIL mRNA expression at given time points, and the medium was collected at the end of each day for TRAIL ELISA (MyBiosource, MBS335491). qPCR was performed using the TaqMan system (Thermo Fisher Scientific) using primers Hs00921974 (TRAIL), Hs00366278 (DR5), and Hs04194366 (RPL13A, housekeeping).
Vector Constructs
The 1928ζ and 19BBζ CARs, which comprise the SJ25C1 CD19-specific scFv, have been described previously.37 LBBz and L28z were constructed by replacing the CD19-specific scFv with the human 5B1 scFv targeting sLeA. All constructs were designed to express Gaussia Luciferase for T cell imaging as described previously.38 L(del) mutants were created by removing the intracellular costimulatory and signaling domains from the specified construct while retaining the extracellular and transmembrane portion. Constructs expressing TRAIL were created by adding the TRAIL cDNA sequence following the specified CAR and a P2A sequence.
Retrovirus Production and Transduction
Plasmids encoding the SFG γ-retroviral (RV) vector39 were prepared as described previously.20 Vesicular stomatitis virus G protein (VSV-G)-pseudotyped retroviral supernatants derived from transduced gpg29 fibroblasts (H29) were used to construct stable retrovirus-producing cell lines as described previously.40 T cells were transduced by centrifugation on plates coated with RetroNectin (Takara). In T cell knockout studies, CAR transduction was performed directly after Cas9 and guide RNA (gRNA) electroporation as described previously.41
CTL Assays
CTL Assays Using 100% Ag+ Tumor Cells
The cytotoxicity of T cells transduced with a CAR was determined by standard luciferase-based assays. Tumor cells expressing firefly luciferase-GFP served as target cells. The effector and tumor cells were co-cultured at the indicated effector/target (E:T) ratio in black-walled 96- or 384-well plates in triplicate. Target cells alone were plated at the same cell density to determine baseline luciferase expression (no T cell control). 18 hr later, luciferase substrate (Bright-Glo, Promega) was directly added to each well. Emitted light was measured by luminescence plate reader or Xenogen IVIS imaging system (Xenogen) with Living Image software (Xenogen) for acquisition of imaging datasets. Lysis was determined as [1 − (RLUsample)/(RLUmax)] × 100. Assays were performed using CAR T cells transduced within the previous week.
CTL Assays Using 75% Ag+ and 25% Ag− Tumor Cells
In experiments involving pre-stimulated CAR T cells, all CAR T cells were grown for 10–12 days at a constant concentration of 1 million cells/mL in the presence of 20 U/mL IL-2, reconstituted every other day. CAR T cells were stimulated 3 days before CTL by adding the CAR T cells to adherent cells containing the target antigen (sLeA+ Capan2). CAR T cells were then cocultured with RT-sensitized 75% Ag+ Capan2 PDAC at an E:T ratio of 1:3 in 48-well plates in which only the Ag− cells expressed luciferase. Percent killing relative to no treatment controls was determined at the pre-specified time points of 4 days for LBBz CAR T cell cultures and 5 days for L(del) CAR T cell cultures.
In all cytotoxicity assays where RT was used, tumor cells (Capan2) were exposed to RT and grown for 2 days in culture, and then live cells were counted and incubated with CAR T cells.
Video Microscopy
Ag− cells labeled with CTV (Fisher Scientific, C34571) were mixed with unlabeled Ag+ cells at a ratio of 75% sLeA+ in addition to CAR T cells in 8-well microscopy slides, and annexin-V 595 (Fisher Scientific, A13203). Confocal images were acquired every 7 min over 18 hr in culture at optimal imaging parameters with an LSM 880 confocal microscope (Carl Zeiss). Data were 3D-rendered and visualized using Imaris (Bitplane). Percent killing of sLeA− cells was determined at every time point using a custom macro made in ImageJ FIJI (NIH), which automatically quantified total Ag− cells (blue cells) and dead and dying Ag− cells (double red- and blue-positive).
Gene Disruption
48 hr after initiating T cell activation, the cells were transfected by electrotransfer of Cas9 mRNA and gRNA using an AgilePulse MAX system (Harvard Apparatus). 3 × 106 cells were mixed with 5 μg of Cas9 and 5 μg of gRNA into a 0.2-cm cuvette. Following electroporation, cells were diluted into culture medium and incubated at 37°C, 5% CO2. To obtain TCR-negative T cells, TCR-positive T cells were removed 3–5 days after gRNA transfection using magnetic biotin anti-TCRαβ and anti-biotin microbeads and LS columns (Miltenyi Biotec). To obtain TRAIL-negative cells, TRAIL-positive T cells were removed using magnetic PE-anti-TRAIL (R&D Systems, FAB687P) and anti-phycoerythrin (PE) microbeads in LS columns (Miltenyi Biotec). To obtain DR5-negative cells, FACS was performed using PE-anti-DR5 staining.
For TCR knockout, we used a gRNA that targets a sequence in the first exon of the constant chain of the TCRα gene (TRAC) that is required for the TCRα and β assembly and addressing it to the cell-surface, as described previously.41 TRAIL was performed using synthetic modified gRNA kits (Synthego). Guide RNAs were reconstituted at 1 μg μl−1 in cytoporation T buffer (Harvard Apparatus). Cas9 mRNA was synthesized by TriLink Biotechnologies.
Pancreatic Cancer Tumor Model
We used 8- to 12-week-old non-obese diabetic (NOD)/severe combined immunodeficiency (SCID)/IL-2Rγ-null (NSG) male mice (Jackson Laboratory) under a protocol approved by the MSKCC Institutional Animal Care and Use Committee. Specified ratios of sLeA+ and sLeA− Capan2 PDAC tumor cells sorted by FACS were injected into the pancreas of NSG mice after surgically opening the mice and exposing the pancreas under an institutional review board (IRB)-approved mouse protocol. 75,000 tumor cells were injected per mouse in 50% Matrigel. Mice were randomized to treatment, and treatment groups were blinded to personnel performing treatment and tumor assessment. Tumors established in the pancreas for 9 days, and then mice were treated with RT followed by T cells. Tumor volume was measured by bioluminescence imaging (BLI) using retro-orbital D-luciferin injection followed by IVIS imaging. The tumor burden for each mouse was expressed over time relative to that mouse’s baseline tumor BLI at the beginning of treatment.
T Cell Imaging
CAR T cells containing Gaussia Luciferase were imaged using coelenterazine (3031-10 Coelenterazine-SOL in vivo, Nanolight) injected retro-orbitally.
Transcriptome Analysis
Cells were lysed in Trizol LS (Invitrogen) and then submitted to the Integrated Genomics Operation at MSKCC for RNA extraction. After Ribogreen quantification and quality control on a bioAnalyser, 500 ng of total RNA underwent library preparation using Truseq Stranded Total RNA library preparation chemistry (Illumina) with 6 cycles of PCR. Samples were barcoded and run on a Hiseq 2500 1T in a 50 bp/50 bp paired-end run using the TruSeq SBS Kit v3 (Illumina). An average of 51 million paired reads was generated per sample, and the percentage of mRNA bases was 58% on average.
Statistics
All experimental data are presented as mean ± SEM. No statistical methods were used to predetermine sample size. Groups were compared using unpaired, two-tailed t test. Statistical analysis was performed on GraphPad Prism 7 software.
Author Contributions
J.Y. and M.L.P provided clinical care and expertise. J.E. assisted with CRISPR-based assays. V.K.R. assisted with animal experiments. M.H. and C.D. performed the experiments. C.D and M.S. interpreted the results.
Conflicts of Interest
The authors have no conflicts of interest. Memorial Sloan Kettering has submitted a patent application based in part on findings reported in this manuscript.
Acknowledgments
We would like to thank Simon Powell for postdoctoral fellowship support and J. Mansilla-Soto for critical discussions as well as the SKI Molecular Cytology Core Facility, Animal Facility, Laboratory of Comparative Pathology, Flow Cytometry Core Facility, Integrated Genomics Operation Core, and Gertrude Gunset for skilled assistance. This work was supported by the Molecular Cytology Core Facility funded by core grants P30 CA008748 S5 and U54 OD020355-01 and the Integrated Genomics Operation Core funded by NCI Cancer Center support grant (CCSG) P30 CA08748. The XRAD225Cx animal irradiator was funded by a Geoffrey Beene Research Foundation shared resources grant (to J. Deasy and P.B. Zanzonico).
Footnotes
Supplemental Information includes eight figures and Supplemental Materials and Methods and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.09.008.
Supplemental Information
References
- 1.Sadelain M., Rivière I., Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June C.H., Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brentjens R.J., Latouche J.B., Santos E., Marti F., Gong M.C., Lyddane C., King P.D., Larson S., Weiss M., Rivière I., Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 4.Fry T.J., Shah N.N., Orentas R.J., Stetler-Stevenson M., Yuan C.M., Ramakrishna S., Wolters P., Martin S., Delbrook C., Yates B. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkie S., van Schalkwyk M.C., Hobbs S., Davies D.M., van der Stegen S.J., Pereira A.C., Burbridge S.E., Box C., Eccles S.A., Maher J. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J. Clin. Immunol. 2012;32:1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 6.Kloss C.C., Condomines M., Cartellieri M., Bachmann M., Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruella M., Barrett D.M., Kenderian S.S., Shestova O., Hofmann T.J., Perazzelli J., Klichinsky M., Aikawa V., Nazimuddin F., Kozlowski M. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J. Clin. Invest. 2016;126:3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegde M., Mukherjee M., Grada Z., Pignata A., Landi D., Navai S.A., Wakefield A., Fousek K., Bielamowicz K., Chow K.K. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zah E., Lin M.Y., Silva-Benedict A., Jensen M.C., Chen Y.Y. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol. Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiotto M., Fu Y.X., Weichselbaum R.R. The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Sci. Immunol. 2016;1 doi: 10.1126/sciimmunol.aag1266. EAAG1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss T., Weller M., Guckenberger M., Sentman C.L., Roth P. NKG2D-Based CAR T Cells and Radiotherapy Exert Synergistic Efficacy in Glioblastoma. Cancer Res. 2018;78:1031–1043. doi: 10.1158/0008-5472.CAN-17-1788. [DOI] [PubMed] [Google Scholar]
- 12.DeSelm C.J., Tano Z.E., Varghese A.M., Adusumilli P.S. CAR T-cell therapy for pancreatic cancer. J. Surg. Oncol. 2017;116:63–74. doi: 10.1002/jso.24627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viola-Villegas N.T., Rice S.L., Carlin S., Wu X., Evans M.J., Sevak K.K., Drobjnak M., Ragupathi G., Sawada R., Scholz W.W. Applying PET to broaden the diagnostic utility of the clinically validated CA19.9 serum biomarker for oncology. J. Nucl. Med. 2013;54:1876–1882. doi: 10.2967/jnumed.113.119867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Reilly E.M., Wang J.S.-Z., Yu K.H., Lowery M.A., Varghese A.M., Bendell J.C. Single agent HuMab-5B1 (MVT-5873), a monoclonal antibody targeting sLea, in patients with pancreatic cancer and other CA19-9 positive malignancies. J. Clin. Oncol. 2017;35:4110. [Google Scholar]
- 15.Hamaï A., Richon C., Meslin F., Faure F., Kauffmann A., Lecluse Y., Jalil A., Larue L., Avril M.F., Chouaib S., Mehrpour M. Imatinib enhances human melanoma cell susceptibility to TRAIL-induced cell death: Relationship to Bcl-2 family and caspase activation. Oncogene. 2006;25:7618–7634. doi: 10.1038/sj.onc.1209738. [DOI] [PubMed] [Google Scholar]
- 16.Walczak H., Miller R.E., Ariail K., Gliniak B., Griffith T.S., Kubin M., Chin W., Jones J., Woodward A., Le T. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 17.Tschumi B.O., Dumauthioz N., Marti B., Zhang L., Schneider P., Mach J.P., Romero P., Donda A. CART cells are prone to Fas- and DR5-mediated cell death. J. Immunother. Cancer. 2018;6:71. doi: 10.1186/s40425-018-0385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartwig T., Montinaro A., von Karstedt S., Sevko A., Surinova S., Chakravarthy A., Taraborrelli L., Draber P., Lafont E., Arce Vargas F. The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2. Mol. Cell. 2017;65:730–742.e5. doi: 10.1016/j.molcel.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Karstedt S., Conti A., Nobis M., Montinaro A., Hartwig T., Lemke J., Legler K., Annewanter F., Campbell A.D., Taraborrelli L. Cancer cell-autonomous TRAIL-R signaling promotes KRAS-driven cancer progression, invasion, and metastasis. Cancer Cell. 2015;27:561–573. doi: 10.1016/j.ccell.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005930. 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morello A., Sadelain M., Adusumilli P.S. Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors. Cancer Discov. 2016;6:133–146. doi: 10.1158/2159-8290.CD-15-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jindal V., Arora E., Gupta S. Challenges and prospects of chimeric antigen receptor T cell therapy in solid tumors. Med. Oncol. 2018;35:87. doi: 10.1007/s12032-018-1149-9. [DOI] [PubMed] [Google Scholar]
- 23.Louis C.U., Savoldo B., Dotti G., Pule M., Yvon E., Myers G.D., Rossig C., Russell H.V., Diouf O., Liu E. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner R., Wu D., Cherian S., Fang M., Hanafi L.A., Finney O., Smithers H., Jensen M.C., Riddell S.R., Maloney D.G., Turtle C.J. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–2410. doi: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson H.J., Brentjens R.J. Overcoming Antigen Escape with CAR T-cell Therapy. Cancer Discov. 2015;5:1238–1240. doi: 10.1158/2159-8290.CD-15-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avanzi M.P., Yeku O., Li X., Wijewarnasuriya D.P., van Leeuwen D.G., Cheung K., Park H., Purdon T.J., Daniyan A.F., Spitzer M.H., Brentjens R.J. Engineered Tumor-Targeted T Cells Mediate Enhanced Anti-Tumor Efficacy Both Directly and through Activation of the Endogenous Immune System. Cell Rep. 2018;23:2130–2141. doi: 10.1016/j.celrep.2018.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Z., Condomines M., van der Stegen S.J.C., Perna F., Kloss C.C., Gunset G., Plotkin J., Sadelain M. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suarez E.R., Chang K., Sun J., Sui J., Freeman G.J., Signoretti S., Zhu Q., Marasco W.A. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7:34341–34355. doi: 10.18632/oncotarget.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherkassky L., Morello A., Villena-Vargas J., Feng Y., Dimitrov D.S., Jones D.R., Sadelain M., Adusumilli P.S. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong L.K., Chen Y., Smith C.C., Montgomery S.A., Vincent B.G., Dotti G., Savoldo B. CD30-Redirected Chimeric Antigen Receptor T Cells Target CD30+ and CD30− Embryonal Carcinoma via Antigen-Dependent and Fas/FasL Interactions. Cancer Immunol Res. 2018 doi: 10.1158/2326-6066.CIR-18-0065. Published online August 7, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichikawa K., Liu W., Zhao L., Wang Z., Liu D., Ohtsuka T., Zhang H., Mountz J.D., Koopman W.J., Kimberly R.P., Zhou T. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat. Med. 2001;7:954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 33.Wajant H. Principles of antibody-mediated TNF receptor activation. Cell Death Differ. 2015;22:1727–1741. doi: 10.1038/cdd.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng A.K., Yahalom J., Goda J.S., Constine L.S., Pinnix C.C., Kelsey C.R., Hoppe B., Oguchi M., Suh C.O., Wirth A. Role of Radiation Therapy in Patients With Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2018;100:652–669. doi: 10.1016/j.ijrobp.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Finnberg N.K., Gokare P., Navaraj A., Lang Kuhs K.A., Cerniglia G., Yagita H., Takeda K., Motoyama N., El-Deiry W.S. Agonists of the TRAIL Death Receptor DR5 Sensitize Intestinal Stem Cells to Chemotherapy-Induced Cell Death and Trigger Gastrointestinal Toxicity. Cancer Res. 2016;76:700–712. doi: 10.1158/0008-5472.CAN-15-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller K.D., Siegel R.L., Lin C.C., Mariotto A.B., Kramer J.L., Rowland J.H., Stein K.D., Alteri R., Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 37.Sadelain M. CAR therapy: the CD19 paradigm. J. Clin. Invest. 2015;125:3392–3400. doi: 10.1172/JCI80010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos E.B., Yeh R., Lee J., Nikhamin Y., Punzalan B., Punzalan B., La Perle K., Larson S.M., Sadelain M., Brentjens R.J. Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nat. Med. 2009;15:338–344. doi: 10.1038/nm.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivière I., Brose K., Mulligan R.C. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc. Natl. Acad. Sci. USA. 1995;92:6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallardo H.F., Tan C., Ory D., Sadelain M. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90:952–957. [PubMed] [Google Scholar]
- 41.Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S.J., Hamieh M., Cunanan K.M., Odak A., Gönen M., Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.