Abstract
The design of vesicle microsystems as artificial cells (bottom-up synthetic biology) has traditionally relied on the incorporation of molecular components to impart functionality. These cell mimics have reduced capabilities compared with their engineered biological counterparts (top-down synthetic biology), as they lack the powerful metabolic and regulatory pathways associated with living systems. There is increasing scope for using whole intact cellular components as functional modules within artificial cells, as a route to increase the capabilities of artificial cells. In this feasibility study, we design and embed genetically engineered microbes (Escherichia coli) in a vesicle-based cell mimic and use them as biosensing modules for real-time monitoring of lactate in the external environment. Using this conceptual framework, the functionality of other microbial devices can be conferred into vesicle microsystems in the future, bridging the gap between bottom-up and top-down synthetic biology.
Keywords: giant lipid vesicles, synthetic biology, biosensing, microfluidics, artificial cells, cellular bionics
1. Introduction
In recent years, there has been an upsurge in interest surrounding the design and construction of functional vesicle-based systems. The application drivers for this area of research are numerous: from the use of vesicles for drug delivery [1–3], sensing agents [1,4] and microreactors [5–7] to their employment as chassis for artificial cells [8,9] and tissue mimics [10].
Vesicles are composed of a structural fabric of either phospholipids or fatty acids [11]. However, the components responsible for their functionality are more diverse. These include: ligands that decorate the membrane (e.g. DNA sticky ends, antibodies, tethered small molecules, nanoparticles, polymerizable amphiphiles) [7,12–15]; membrane-spanning structures (e.g. natural and engineered protein pores, pumps and channels, DNA origami) [16–18]; and biologically derived material encapsulated in the vesicle lumen (e.g. enzymes, cytoskeleton components, polymerases and coupled transcription/translation machinery) [6,19–22]. This varied repertoire of building blocks has allowed investigators to engineer vesicles capable of mimicking an assortment of cellular behaviours [23], and of successfully interfacing cell and vesicle communities together [24].
Recently, a new front in the construction of vesicle-based microsystems has opened up with the emergence of technologies allowing the encapsulation of whole cells within vesicles [25,26] using emulsion transfer vesicle generation methods [27]. Our previous work of encapsulating non-engineered cells in this context [25,26] paves the way for the rich and mature world of synthetic biology and genetic engineering to be tapped for the assembly of vesicles with enhanced functionality, using encapsulated cells as embedded functional modules. Encapsulated cells, for example, could be used in the design of compartmentalized systems capable of exhibiting sensor and bioreactor modalities, of harvesting energy and of dynamically responding to environmental stimuli.
Herein, we demonstrate this potential by designing genetically engineered microbes and using them as biosensing modules that are encapsulated within a vesicle chassis. Increasingly, synthetic biologists are exploiting the diverse array of sensors and regulators in nature that aid in survival to create devices that fill the growing need for low-cost diagnostic tools in medical [28], environmental [29] and manufacturing [30] applications. Demonstrating the validity of this conceptual framework shows that the functionality of other microbial devices can be conferred into vesicle microsystems in the future.
Encapsulating engineered organisms in vesicles allows them to be shielded from their external environment, which can be useful in several applications. For example, when using bacterial biosensors in co-cultures, encapsulation enables the sensor cells and the main culture cells to be physically separated, preventing unwanted interference and allowing the two species to exist in distinct optimized conditions. This is also true of potential medicinal applications; for example, in cell-based therapies, where encapsulation could shield the cell from the host's immune response. The potential for facile precision engineering of an external membrane using biological and synthetic components is also attractive for these applications; for example, the incorporation of molecular recognition modules, stimuli-responsive membranes, protein channels and functionalized nanoparticles. In short, hybrid systems such as these allow the advantages associated with both bottom-up and top-down synthetic biology to be combined.
There have been efforts in encapsulating engineered cells in droplets [31], gels [32], hydrogel microcapsules [33] and liquid core/shell structures [34], but not within cell-mimetic vesicles, which, owing to their biocompatibility, similarity to cell membranes and the potential for bilayer functionalization, are the most commonly used artificial cell chassis. We demonstrate this feature by introducing transmembrane pores into the vesicle membrane to facilitate the influx of analyte to the vesicle interior.
As a proof of concept, we created a whole-cell Escherichia coli lactate biosensor based on the transcriptional elements of the lldPRD operon that produces green fluorescent protein (GFP) in response to lactate. Lactate was chosen as an exemplar system because it is of interest in a variety of different industrial and biotechnological applications. For example, in bioprocessing, lactate is the primary waste metabolite and can negatively affect cell growth and productivity; therefore, it needs to be monitored to help control the culture trajectory and ensure product quality [30]. We show that the encapsulated biosensors can sense the external lactate concentration by incorporating membrane-spanning α-haemolysin (α-HL) pores embedded in the vesicle membrane, allowing diffusion of the analyte to the vesicle interior. Once the lactate diffuses across the cell membrane, the LldR regulatory protein binds to it and induces the lldPRD promoter, which then drives the expression of GFP leading to measurable fluorescence. This feasibility study demonstrates the potential of cellular bionics, where ‘living’ modules are fused with artificial cell microsystems for enhanced functionality.
2. Material and methods
2.1. Biosensor construction
The lactate biosensor was modified from Goers et al. [35], where the lldPRD promoter was modified to include the J23117 constitutive promoter within the O1 and O2 sites (electronic supplementary material, table S1). Strains were maintained on Luria–Bertani (LB) agar containing 100 µg ml−1 ampicillin and grown aerobically for 16 h at 37°C.
2.2. Biosensor characterization in liquid medium
Single colonies of E. coli containing the biosensor plasmid or a control plasmid (lacking the output module of the biosensor (figure 1a)) were used to inoculate 2 ml of LB medium containing 100 µg ml−1 ampicillin and grown at 37°C with shaking at 250 rpm for approximately 6 h. The starter culture was diluted 1 : 100 into 5 ml of M9 minimal medium (1× M9 salts, 2 mM MgSO4, 0.1 mM CaCl2, 0.34 g l−1 thiamine hydrochloride, 1 g l−1 NH4Cl and 0.4% glycerol) containing 100 µg ml−1 ampicillin and grown overnight at 37°C with shaking. The optical density of the overnight culture was adjusted to an OD600 nm of 1.0 in M9 minimal medium. In a 96-well plate, 25 µl of adjusted culture was added to a well containing 87.5 µl of M9 minimal medium containing 100 µg ml−1 ampicillin. The plate was covered with a Breathe-Easy® seal (Sigma Aldrich) and incubated at 37°C with shaking for 2 h for cells to reach exponential growth phase. Lactate (12.5 µl) of varying concentrations dissolved in M9 minimal medium was added to each well. The plate was covered with a Breathe-Easy® seal and incubated at 37°C with shaking for 2 h. Samples (20 µl) were taken from each well and mixed with 180 µl of filtered sterile water. Fluorescence in each well was measured using an Attune® Acoustic Focusing Cytometer (ThermoFisher; excitation 485 nm, emission 528 nm). Data were analysed using FlowJo software (FlowJo LLC, Ashland, OR).
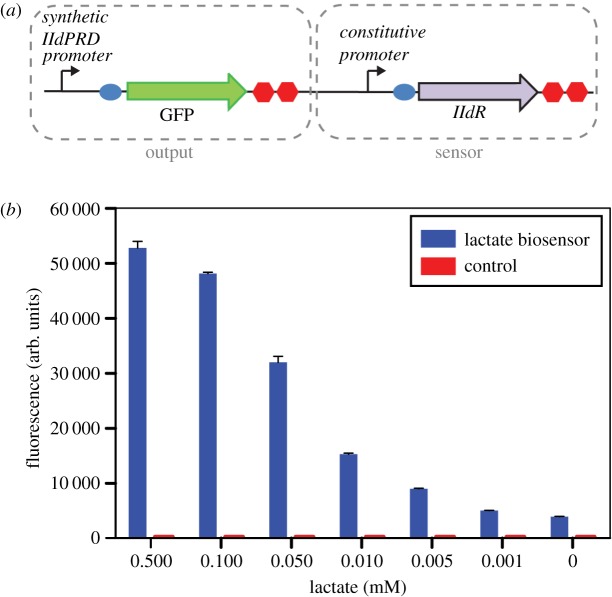
Figure 1.
Lactate biosensor used in this study. (a) Diagram of the lactate biosensor in E. coli. (b) Characterization of lactate biosensor in bulk (non-encapsulated form) using a flow cytometer. The whole-cell E. coli biosensor and control cells were spiked with different concentrations of l-lactate.
2.3. Biosensor preparation for encapsulation
The biosensor was grown under the same conditions as for the characterization studies (above). Following incubation for 2 h at 37°C with shaking, 2 ml of E. coli cells in exponential phase containing the biosensor were concentrated by centrifugation and resuspended in 600 µl of medium, yielding a concentration of approximately 5 × 108 cells ml−1.
2.4. Vesicle formation and biosensor assay
All lipids were purchased from Avanti Polar Lipids (USA) and were used without further purification. Lactate oxidase, horseradish peroxidase (HRP) and Amplex Red were purchased from Life Technologies (USA). Lactate, α-HL and all other chemicals were purchased from Sigma Aldrich (UK) unless otherwise stated. Giant unilamellar vesicles (GUVs) were generated using the phase transfer of individual droplets across a water–oil interface by modifying a procedure described elsewhere [36]. Sodium l-lactate, 99% purity, pH 7.4 in M9 minimal medium, was used.
A POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) lipid film was first prepared by dissolving 2 mg of lipid in chloroform and removing the chloroform under a stream of nitrogen and placing in a lyophilizer for 1 h. This was dissolved in mineral oil (2 mg ml−1) by placing the vial in a 60°C oven for 1 h.
A 0.2 ml aliquot of POPC in mineral oil solution was then deposited above 0.2 ml of 500 mM glucose in M9 minimal medium in an Eppendorf tube to form a column. The ‘internal’ GUV solution was then prepared, which was composed of sucrose (450 mM), lactate (0–50 mM) and engineered E. coli (1 : 9 dilution of 5 × 108 cells ml−1); 20 µl of this solution was added to 200 µl of lipid in oil and an emulsion was made by vortexing for 30 s. This emulsion was then layered above the water–oil column, and the tube centrifuged to transform the emulsion drops into lipid vesicles. The non-encapsulated material was removed from the surrounding solution by pelleting the GUVs three times (500g; 5 min), removing the supernatant and re-suspending in medium. This process was repeated three times to remove the majority of non-encapsulated bacteria. α-HL was added to the GUVs at a 1 : 9 ratio to give a final α-HL concentration of 0.05 mg ml−1. Lactate was then added to the external solution at a 1 : 9 ratio to give final concentrations ranging from 0 to 50 mM. The effectiveness of the biosensor/vesicle hybrid was characterized using a fluorimeter to monitor GFP production (excitation 485/20 nm; emission 528/20 nm; Synergy HT; BioTek Instruments, Inc., Winooski, VT, USA) and five replicates were measured for each condition.
2.5. Lactate enzymatic assay
For the lactate enzymatic assay, GUVs were formed as before, but with different inner and outer solutions. All aqueous solutions were prepared in phosphate-buffered saline. The internal vesicle solution consisted of 1 U ml−1 lactate oxidase and 450 mM sucrose. The external solution was composed of 500 mM arabinose solution to form a column. This was then centrifuged at 9000g for 30 min, allowing the water droplets in the emulsions to transfer from the oil to water phase, thus forming vesicles that aggregated into a pellet. The supernatant was removed, the pellet resuspended in 500 µl arabinose, and three more centrifugation rounds applied to remove any non-encapsulated enzyme. The pellet was resuspended in 450 mM sucrose solution. α-HL was then added at a 1 : 9 ratio to give a final α-HL concentration of 0.05 mg ml−1; 80 µl of this GUV solution was placed in a single well of a 96-well plate, followed by addition of 5 µl HRP (10 U ml−1), 5 µl Amplex Red (10 mM) and 10 µl lactate to give final lactate concentrations ranging from 0 to 1000 µM. Fluorescence was monitored using a fluorimeter (excitation 485/20 nm; emission 528/20 nm; Synergy HT) and five repeats were obtained for each condition.
2.6. Microfluidic encapsulation of bacteria
Polydimethylsiloxane (PDMS) microfluidic devices consisting of a single flow-focusing junction were fabricated via soft lithography as described elsewhere [37]. Briefly, negative masters were produced using SU-8 (A-Gas Electronic Materials, UK) photoresist and standard lithography. After development using EC solvent (A-Gas Electronic Materials, UK), the negative masters were exposed to trichloro(1H,1H,2H,2H-perfluorooctyl)silane vapour to suppress permanent adhesion to moulded PDMS. PDMS prepolymer and curing agent were then thoroughly mixed in a 10 : 1 ratio, and the mixture was poured onto the master wafer. The mixture was cured at 65°C overnight, then peeled off the master and bonded on PDMS-coated microscope glass slides (75 × 25 mm; VWR, UK) via plasma oxidation (100 W, 1 min, 20 sccm O2 flow, 67 Pa pressure) using a Femto plasma cleaner (Diener Electronic, Germany).
The microfluidic device was set up with the two fluid inlets and one vesicle outlet. The internal aqueous phase consisted of bacteria diluted at a 1 : 9 ratio with lactate (0–50 mM) present in M9 medium solution containing 450 mM sucrose. The bacteria–lactate solutions were mixed immediately before being inserted into the microfluidic device. The external oil phase consisted of FluorinertR™ FC-70 oil with 0.5% Pico-Surf™ (5% in FC-40) in FC-70 (Dolomite Microfluidics, UK). The internal aqueous phase and oil phase were injected using 1 ml plastic syringes linked to 1.09 mm polytetrafluoroethylene tubing (Adtech Polymer Engineering Ltd, UK). Two syringe pumps (Chemyx Inc., UK) were necessary to pump the reagents into the microfluidic system at controlled flow rates (2 and 3 µl min−1 for the water and oil phase, respectively). Droplets were generated at the flow-focusing junction and carried through the meander channel to the large reservoir. To examine fluorescence from the droplets, both flows were simultaneously stopped and the outlet was plugged to immobilize further movement of the droplets.
2.7. Fluorescence microscopy
Fluorescence images of encapsulated bacteria were viewed with an Olympus IX81 inverted microscope. Fluorescence experiments used an illuminating mercury arc lamp and were imaged with a fluorescein isothiocyanate filter, at an exposure time of 1000 ms. The lamp was turned off after each acquisition event to minimize photobleaching. Images were taken with a QICAM camera (QImaging).
3. Results and discussion
3.1. Lactate biosensor characterization
The lactate biosensor was constructed using a synthetic lldPRD promoter (figure 1a) that combines the natural lldPRD operator sites with a weak constitutive promoter. We first assessed its ability to detect lactate in samples spiked with different concentrations of l-lactate in M9 minimal medium containing glycerol as the carbon source. The lactate biosensor containing the synthetic lldPRD promoter exhibited higher fluorescence levels than a previous version containing the natural lldPRD promoter [35], which resulted in an increased limit of detection of lactate (electronic supplementary material, figure S1). The biosensor displayed a clear response to lactate with separation between different concentrations ranging from 0.5 to 0.005 mM (figure 1b). Control cells that lacked the output part of the biosensor did not fluoresce in the presence of lactate.
3.2. Vesicle engineering
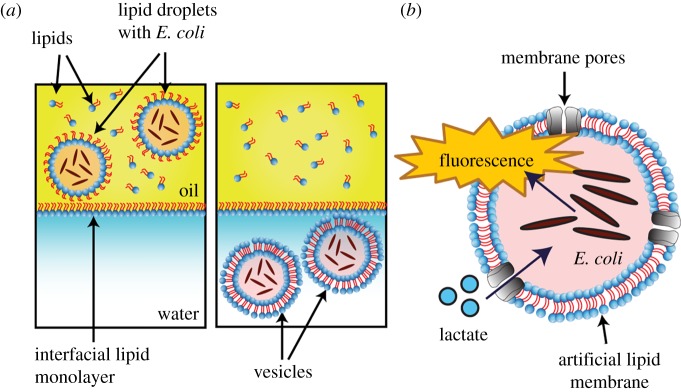
To encapsulate bacteria within giant vesicles, we took advantage of emulsion phase transfer technologies [27,36]. Lipid-stabilized water-in-oil emulsion droplets containing bacteria were transferred by gravity from an oil to an aqueous solution of a water–oil column, with a lipid monolayer present at the interface of the two phases. This process, driven by the higher density of the sucrose-containing droplets, led to the individual droplets being engulfed by a bilayer, resulting in a vesicle (figure 2a). The number of bacteria encapsulated in each GUV was controlled by adjusting the bacteria concentration beforehand (5 × 108 cells ml−1, yielding approx. 70 bacteria in a 25 nl GUV).
Figure 2.
Hybrid lactate biosensor construction via the phase transfer of water-in-oil droplets. (a) Bacteria were encapsulated in lipid-coated water-in-oil droplets loaded with a mixture of sucrose and cell medium, and were expelled above a water–oil column stabilized with a lipid monolayer. The bacteria-loaded droplets sank through the column due to their higher density and picked up another lipid monolayer, forming lipid vesicles. (b) Schematic of the vesicle system that was generated for the hybrid lactate biosensor. Lactate permeates the vesicle via the α-HL pores and initiates a fluorescence response (GFP) from the encapsulated E. coli.
The system required the construction of GUVs that are permeable to lactate, which otherwise cannot cross the vesicle membrane due to its negative charge. To do this, we decorated the GUVs composed of POPC lipid with transmembrane α-HL protein pores. This allowed lactate to reach the vesicle lumen and activate the bacterial biosensor (figure 2b) [38]. Protein monomers spontaneously insert into bilayers and aggregate to form heptameric protein pores of 1.4 nm diameter. In this way, the bacteria remain spatially separated from their external environment, but still retain their ability to sense and respond to the presence of lactate.
To test successful influx of lactate, we used an enzymatic assay based on the oxidation of lactate using lactate oxidase [39]. This yields H2O2, which subsequently oxidizes the non-fluorescent Amplex Red to the fluorescent molecule resorufin in the presence of HRP. GUVs were formed with lactate oxidase in the interior, α-HL embedded in the membrane, and HRP and Amplex Red and varying concentrations of lactate in the exterior (electronic supplementary material, figure S2a). The presence of α-HL allowed lactate oxidase in the vesicle interior to be exposed to lactate, with the H2O2 product (membrane permeable) diffusing out of the GUV to initiate subsequent steps of the cascade.
An increase in fluorescence was observed within approximately 20 min, with the reaction completing after approximately 120 min. This shows the successful flow of lactate through the pore, allowing it to be oxidized by lactate oxidase which is trapped in the GUV. With no α-HL, minimal fluorescence increase was seen, demonstrating that lactate oxidase was not present in significant amounts in the GUV exterior (e.g. due to vesicle rupture) and confirming that lactate cannot cross the vesicle membrane without α-HL pores. Larger lactate concentration gave higher fluorescence signals, with a linear relationship being present from 0 to 100 µM (electronic supplementary material, figure S2b). Above this concentration, the signal began to plateau (electronic supplementary material, figure S2c).
3.3. Response of GUVs functionalized with Escherichia coli lactate biosensors
All biosensor experiments were conducted in M9 medium, and with 450 mM sucrose, as this was used to provide the density difference for vesicle generation via phase transfer. We first tested the system in monodisperse water-in-oil droplets on a microfluidic chip using bacteria that were premixed with varying concentrations of lactate. This was used to obtain the experimental conditions for subsequent experiments, and was particularly useful given that droplets are the precursors to the lipid vesicles themselves, and bacteria, therefore, exist in the same physiological environment. A PDMS microfluidic device with one flow-focusing junction was fabricated via standard soft lithography as described elsewhere [37]. The microfluidic device contained an observation chamber to accumulate droplets and facilitate the microscopy experiments (figure 3).
Figure 3.
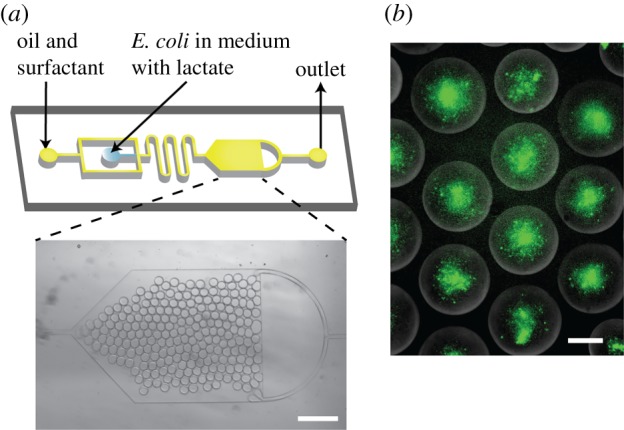
Microfluidic encapsulation of E. coli biosensor in droplets. (a) Microfluidic device design consisting of a flow-focusing junction and a large reservoir. Channel depth is 20 µm. Micrograph of the reservoir containing water-in-oil droplets. Scale bar, 400 µm. (b) Fluorescence images of bacteria encapsulated in water-in-oil droplets with 50 mM concentration after 300 min of incubation. Scale bars, 40 µm.
Bacteria were mixed with lactate (9 : 1) in M9 medium and were immediately encapsulated in droplets. Droplet sizes were monodisperse, with mean radii of 15 µm and the coefficient of variation less than 3%. Some droplets (less than 1%) were found to fuse with adjacent ones. Prototyping experiments revealed successful GFP production in the confined environment, with lactate concentrations ranging from 0.5 to 50 mM, at room temperature, over a period of 5 h.
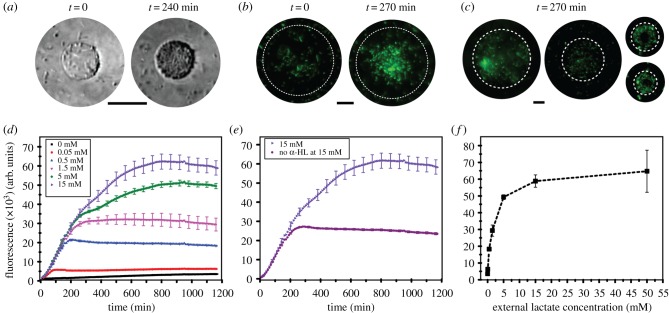
Following these experiments, we encapsulated bacteria in GUVs with embedded α-HL pores in their lipid bilayers. The GUVs were subsequently inserted into external solutions of varying lactate concentrations: 0, 0.05, 0.5, 1.5, 5, 15 and 50 mM. Lactate diffused through the α-HL pores and initiated a fluorescence signal increase. Figure 4a,b demonstrates bright field and fluorescence images of the GUV encapsulated biosensor at t = 0 and after approximately 4 h at 50 mM external lactate concentration. Microscopy images revealed non-encapsulated bacteria, which is a limitation of the system that can be improved upon in the future through microfluidic encapsulation strategies which offer greater encapsulation efficiencies [40,41], and through size-dependent filtration approaches. Nevertheless, the vesicles that encapsulated the microbes remained stable and the population of bacteria increased inside the vesicle over 4 h, as shown in figure 4a.
Figure 4.
Full operational system showing the hybrid lactate biosensor. (a) Bright field images showing encapsulated microbes in vesicles over 240 min. Scale bar, 20 µm. (b) Fluorescence images of the hybrid lactate biosensor at 50 mM concentration of lactate at t = 0 and t = 270 min. Brightness and contrasts enhanced equally across the fluorescence images. Scale bar, 20 µm. (c) Fluorescence images of hybrid biosensors at 50 mM lactate concentration at t = 240 min. Scale bar, 20 µm. (d) Graph showing fluorescence intensity over time of the produced GFP at different lactate concentrations as lactate penetrates the vesicle system via the α-HL pores. The full two-step reaction cascade takes place. (e) Fluorescence signal at 15 mM lactate in the presence and absence of α-HL pores. (f) Characterization data of the hybrid biosensor at different concentrations of lactate. Error bars represent the standard deviation of five technical replicates.
The response of the encapsulated biosensor under different lactate conditions was monitored in a bulk GUV sample using a fluorimeter. Bacteria in different concentrations of lactate grew to a similar number over time and tripled over 3 h in all conditions (electronic supplementary material, figure S3). In all experiments, a plateau in fluorescence was eventually reached, which was taken as the end-point. The higher the lactate concentration, the larger the end-point fluorescence produced by the biosensor (figure 4d), with maximum intensity being reached earlier for lower lactate concentrations (approx. 1 h for 0.05 mM, approximately 12 h for 15 mM). The control case, which was identical apart from the absence of lactate, showed no fluorescence increase over time, as expected.
To test the effect of non-encapsulated bacteria, we ran the same experiment with 15 mM lactate externally, but with no α-HL pores present (figure 4e). In this scenario, only the non-encapsulated bacteria would be exposed to lactate. Compared with when α-HL was present, the fluorescence intensity after 12 h was 54–60% lower, showing that, although non-encapsulated bacteria are contributing to the signal, the majority of the response is derived from bacteria that are encapsulated in the vesicle.
Based on these experiments, we extrapolated a calibration curve based on the response of the hybrid biosensor to distinct lactate concentrations (figure 4c), which showed an increase in fluorescence signal up to 15 mM with lactate concentration and a linear measurement range up to 5 mM. The response range is, therefore, larger than the enzymatic-based sensor (linear up to 0.2 mM) under the conditions used in our experiments.
4. Conclusion
In conclusion, we show that engineered bacteria can be used as functional modules within lipid vesicles. In our system, the chosen functionality was lactate biosensing, although this work paves the way for the wide array of capabilities of synthetic biology systems to be incorporated in future. Whole-cell biosensors have a number of advantages over other types of sensors, including increased sensitivity, reduced cost and robustness. The use of engineered organisms encapsulated within vesicle compartments has several potential benefits compared with their use in open conditions, arising from the presence of a lipid vesicle barrier. First, the vesicle can provide protection against surrounding environments in instances where these are non-compatible. Second, the chemical environments inside the vesicle can be optimized for the performance of the bio-module, without the need to change the composition of the bulk solution. Third, the vesicle provides a two-dimensional surface that can be easily engineered to give added functionality, e.g. through the addition of molecular recognition modules, the addition of light-, temperature- and pH-responsive modalities, and responsive pores and channels [7,42–45]. These features may be desirable in applications ranging from co-culture (where the encapsulated ‘sensor’ cells are shielded from the main culture solution) to targeted binding in medicinal settings (e.g. by functionalizing the vesicle lipid membrane with appropriate receptors).
Supplementary Material
Data accessibility
All relevant data are available from the authors on reasonable request.
Authors' contributions
T.T. and Y.E. carried out the microfluidic experiments (device fabrication and testing), the GUV formation, the bacteria encapsulation and monitoring, and the corresponding analysis. L.D. prepared and characterized the E. coli construct. All authors contributed to designing the experiments, discussion and writing the manuscript. All authors gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
This work was supported by the EPSRC (grant nos. EP/K038648/1 and EP/J017566/1) and by an EPSRC Fellowship awarded to Y.E. (grant no. EP/N016998/1).
References
- 1.Bally M, Bailey K, Sugihara K, Grieshaber D, Vörös J, Städler B. 2010. Liposome and lipid bilayer arrays towards biosensing applications. Small 6, 2481–2497. ( 10.1002/smll.201000644) [DOI] [PubMed] [Google Scholar]
- 2.Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A. 2016. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 44, 381–391. ( 10.3109/21691401.2014.953633) [DOI] [PubMed] [Google Scholar]
- 3.Koçer A. 2007. A remote controlled valve in liposomes for triggered liposomal release. J. Liposome Res. 17, 219–225. ( 10.1080/08982100701528203) [DOI] [PubMed] [Google Scholar]
- 4.Mason JT, Xu L, Sheng Z-M, O'Leary TJ. 2006. A liposome-PCR assay for the ultrasensitive detection of biological toxins. Nat. Biotechnol. 24, 555 ( 10.1038/nbt1201) [DOI] [PubMed] [Google Scholar]
- 5.Bolinger PY, Stamou D, Vogel H. 2008. An integrated self-assembled nanofluidic system for controlled biological chemistries. Angew. Chem. Int. Ed. 47, 5544–5549. ( 10.1002/anie.200801606) [DOI] [PubMed] [Google Scholar]
- 6.Elani Y, Law RV, Ces O. 2014. Vesicle-based artificial cells as chemical microreactors with spatially segregated reaction pathways. Nat. Commun. 5, 5304 ( 10.1038/ncomms6305) [DOI] [PubMed] [Google Scholar]
- 7.Hindley JW, Elani Y, McGilvery CM, Ali S, Bevan CL, Law RV, Ces O. 2018. Light-triggered enzymatic reactions in nested vesicle reactors. Nat. Commun. 9, 1093 ( 10.1038/s41467-018-03491-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luisi PL, Ferri F, Stano P. 2006. Approaches to semi-synthetic minimal cells: a review. Naturwissenschaften 93, 1–13. ( 10.1007/s00114-005-0056-z) [DOI] [PubMed] [Google Scholar]
- 9.Salehi-Reyhani A, Ces O, Elani Y. 2017. Artificial cell mimics as simplified models for the study of cell biology. Exp. Biol. Med. 242, 1309–1317. ( 10.1177/1535370217711441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villar G, Graham AD, Bayley H. 2013. A tissue-like printed material. Science 340, 48 ( 10.1126/science.1229495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walde P, Cosentino K, Engel H, Stano P. 2010. Giant vesicles: preparations and applications. ChemBioChem. 11, 848–865. ( 10.1002/cbic.201000010) [DOI] [PubMed] [Google Scholar]
- 12.Parolini L, Mognetti BM, Kotar J, Eiser E, Cicuta P, Di Michele L. 2015. Volume and porosity thermal regulation in lipid mesophases by coupling mobile ligands to soft membranes. Nat. Commun. 6, 5948 ( 10.1038/ncomms6948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manjappa AS, Chaudhari KR, Venkataraju MP, Dantuluri P, Nanda B, Sidda C, Sawant KK, Ramachandra Murthy RS. 2011. Antibody derivatization and conjugation strategies: application in preparation of stealth immunoliposome to target chemotherapeutics to tumor. J. Control Release. 150, 2–22. ( 10.1016/j.jconrel.2010.11.002) [DOI] [PubMed] [Google Scholar]
- 14.Gopalakrishnan G, Danelon C, Izewska P, Prummer M, Bolinger PY, Geissbühler I, Demurtas D, Dubochet J, Vogel H. 2006. Multifunctional lipid/quantum dot hybrid nanocontainers for controlled targeting of live cells. Angew. Chem. Int. Ed. 45, 5478–5483. ( 10.1002/anie.200600545) [DOI] [PubMed] [Google Scholar]
- 15.Bolognesi G, Friddin MS, Salehi-Reyhani A, Barlow NE, Brooks NJ, Ces O, Elani Y. 2018. Sculpting and fusing biomimetic vesicle networks using optical tweezers. Nat. Commun. 9, 1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koçer A, Walko M, Meijberg W, Feringa BL. 2005. A light-actuated nanovalve derived from a channel protein. Science 309, 755–758. ( 10.1126/science.1114760) [DOI] [PubMed] [Google Scholar]
- 17.Miller D, Booth PJ, Seddon JM, Templer RH, Law RV, Woscholski R, Ces O, Barter LM. 2013. Protocell design through modular compartmentalization. J. R. Soc. Interface 10, 20130496 ( 10.1098/rsif.2013.0496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns JR, Stulz E, Howorka S. 2013. Self-assembled DNA nanopores that span lipid bilayers. Nano Lett. 13, 2351–2356. ( 10.1021/nl304147f) [DOI] [PubMed] [Google Scholar]
- 19.Elani Y, Law RV, Ces O. 2015. Protein synthesis in artificial cells: using compartmentalisation for spatial organisation in vesicle bioreactors. Phys. Chem. Chem. Phys. 17, 15534–7. ( 10.1039/C4CP05933F) [DOI] [PubMed] [Google Scholar]
- 20.Noireaux V, Libchaber A. 2004. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl Acad. Sci. USA 101, 17 669–17 674. ( 10.1073/pnas.0408236101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberholzer T, Albrizio M, Luisi PL. 1995. Polymerase chain reaction in liposomes. Chem. Biol. 2, 677–682. ( 10.1016/1074-5521(95)90031-4) [DOI] [PubMed] [Google Scholar]
- 22.Merkle D, Kahya N, Schwille P. 2008. Reconstitution and anchoring of cytoskeleton inside giant unilamellar vesicles. ChemBioChem. 9, 2673–2681. ( 10.1002/cbic.200800340) [DOI] [PubMed] [Google Scholar]
- 23.Trantidou T, Friddin M, Elani Y, Brooks NJ, Law RV, Seddon JM, Ces O. 2017. Engineering compartmentalized biomimetic micro- and nanocontainers. ACS Nano 11, 6549–6565. ( 10.1021/acsnano.7b03245) [DOI] [PubMed] [Google Scholar]
- 24.Lentini R, et al. 2014. Integrating artificial with natural cells to translate chemical messages that direct E. coli behaviour. Nat. Commun. 5, 4012 ( 10.1038/ncomms5012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elani Y, Trantidou T, Wylie D, Dekker L, Polizzi K, Law RV, Ces O. 2018. Constructing vesicle-based artificial cells with embedded living cells as organelle-like modules. Sci. Rep. 8, 4564 ( 10.1038/s41598-018-22263-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan Y-C, Hettiarachchi K, Siu M, Pan Y-R, Lee AP. 2006. Controlled microfluidic encapsulation of cells, proteins, and microbeads in lipid vesicles. J. Am. Chem. Soc. 128, 5656–5658. ( 10.1021/ja056641h) [DOI] [PubMed] [Google Scholar]
- 27.Pautot S, Frisken BJ, Weitz D. 2003. Production of unilamellar vesicles using an inverted emulsion. Langmuir 19, 2870–2879. ( 10.1021/la026100v) [DOI] [Google Scholar]
- 28.Wei TY, Cheng CM. 2016. Synthetic biology-based point-of-care diagnostics for infectious disease. Cell Chem Biol. 23, 1056–1066. ( 10.1016/j.chembiol.2016.07.016) [DOI] [PubMed] [Google Scholar]
- 29.Karig DK. 2017. Cell-free synthetic biology for environmental sensing and remediation. Curr. Opin. Biotechnol. 45, 69–75. ( 10.1016/j.copbio.2017.01.010) [DOI] [PubMed] [Google Scholar]
- 30.Dekker L, Polizzi KM. 2017. Sense and sensitivity in bioprocessing-detecting cellular metabolites with biosensors. Curr. Opin. Chem. Biol. 40, 31–36. ( 10.1016/j.cbpa.2017.05.014) [DOI] [PubMed] [Google Scholar]
- 31.Rakszewska A, Tel J, Chokkalingam V, Huck WT. 2014. One drop at a time: toward droplet microfluidics as a versatile tool for single-cell analysis. NPG Asia Mater. 6, e133 ( 10.1038/am.2014.86) [DOI] [Google Scholar]
- 32.Weaver JC, McGrath P, Adams S. 1997. Gel microdrop technology for rapid isolation of rare and high producer cells. Nat. Med. 3, 583–585. [DOI] [PubMed] [Google Scholar]
- 33.Lim F, Sun AM. 1980. Microencapsulated islets as bioartificial endocrine pancreas. Science 210, 908–910. ( 10.1126/science.6776628) [DOI] [PubMed] [Google Scholar]
- 34.Rabanel JM, Banquy X, Zouaoui H, Mokhtar M, Hildgen P. 2009. Progress technology in microencapsulation methods for cell therapy. Biotechnol. Prog. 25, 946–963. ( 10.1002/btpr.226) [DOI] [PubMed] [Google Scholar]
- 35.Goers L, Ainsworth C, Goey CH, Kontoravdi C, Freemont PS, Polizzi KM. 2017. Whole-cell Escherichia coli lactate biosensor for monitoring mammalian cell cultures during biopharmaceutical production. Biotechnol. Bioeng. 114, 1290–1300. ( 10.1002/bit.26254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii S, Matsuura T, Sunami T, Nishikawa T, Kazuta Y, Yomo T. 2014. Liposome display for in vitro selection and evolution of membrane proteins. Nat. Protoc. 9, 1578–1591. ( 10.1038/nprot.2014.107) [DOI] [PubMed] [Google Scholar]
- 37.Trantidou T, Elani Y, Parsons E, Ces O. 2017. Hydrophilic surface modification of PDMS for droplet microfluidics using a simple, quick, and robust method via PVA deposition. Microsyst. Nanoeng. 3, 16091 ( 10.1038/micronano.2016.91) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. 1996. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 274, 1859–1865. ( 10.1126/science.274.5294.1859) [DOI] [PubMed] [Google Scholar]
- 39.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. 1977. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 253, 162–168. ( 10.1006/abio.1997.2391) [DOI] [PubMed] [Google Scholar]
- 40.van Swaay D. 2013. Microfluidic methods for forming liposomes. Lab. Chip. 13, 752–767. ( 10.1039/c2lc41121k) [DOI] [PubMed] [Google Scholar]
- 41.Elani Y. 2016. Construction of membrane-bound artificial cells using microfluidics: a new frontier in bottom-up synthetic biology. Biochem. Soc. Trans. 44, 723–730. ( 10.1042/BST20160052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mura S, Nicolas J, Couvreur P. 2013. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991 ( 10.1038/nmat3776) [DOI] [PubMed] [Google Scholar]
- 43.Karamdad K, Hindley JW, Bolognesi G, Friddin MS, Law RV, Brooks NJ, Ces O, Elani Y. 2018. Engineering thermoresponsive phase separated vesicles formed via emulsion phase transfer as a content-release platform. Chem. Sci. 9, 4851–4858. ( 10.1039/C7SC04309K) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Ahmady ZS, Al-Jamal WT, Bossche JV, Bui TT, Drake AF, Mason AJ, Kostarelos K. 2012. Lipid–peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano 6, 9335–9346. ( 10.1021/nn302148p) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leung SJ, Romanowski M. 2012. Light-activated content release from liposomes. Theranostics 2, 1020 ( 10.7150/thno.4847) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available from the authors on reasonable request.