Abstract
The Mediator coactivator complex directs gene-specific expression by binding distal enhancer-bound transcription factors through its Med1 subunit while bridging to RNA polymerase II (Pol II) at gene promoters. In addition, Mediator scaffolds epigenetic modifying enzymes that determine local DNA accessibility. Previously, we found that deletion of Med1 in cardiomyocytes deregulates more than 5,000 genes and promotes acute heart failure. Therefore, we hypothesized that Med1 deficiency disrupts enhancer-promoter coupling. Using chromatin immunoprecipitation-coupled deep sequencing (ChIP-seq; n = 3/ChIP assay), we found that the Pol II pausing index is increased in Med1 knockout versus floxed control mouse hearts primarily due to a decrease in Pol II occupancy at the majority of transcriptional start sites without a corresponding increase in elongating species. Parallel ChIP-seq assays reveal that Med1-dependent gene expression correlates strongly with histone H3 K27 acetylation, which is indicative of open and active chromatin at transcriptional start sites, whereas H3 K27 trimethylated levels, representing condensed and repressed DNA, are broadly increased and inversely correlate with absolute expression levels. Furthermore, Med1 deletion leads to dynamic changes in acetyl-K27 associated superenhancer regions and their enriched transcription factor-binding motifs that are consistent with altered gene expression. Our findings suggest that Med1 is important in establishing enhancer-promoter coupling in the heart and supports the proposed role of Mediator in establishing preinitiation complex formation. We also found that Med1 determines chromatin accessibility within genes and enhancer regions and propose that the composition of transcription factors associated with superenhancer changes to direct gene-specific expression.
NEW & NOTEWORTHY Based on our previous findings that transcriptional homeostasis and cardiac function are disturbed by cardiomyocyte deletion of the Mediator coactivator Med1 subunit, we investigated potential underlying changes in RNA polymerase II localization and global chromatin accessibility. Using chromatin immunoprecipitation sequencing, we found that disrupted transcription arises from a deficit in RNA polymerase II recruitment to gene promoters. Furthermore, active versus repressive chromatin marks are redistributed within gene loci and at enhancer regions correlated with gene expression changes.
Keywords: chromatin immunoprecipitation-coupled deep sequencing, heart, histone H3 lysine 27, mediator, RNA polymerase II, enhancer
INTRODUCTION
It remains to be fully determined how the genome and its encoded information are able to direct the spatial, temporal, and lineage-specific gene expression patterns of a given cell type. The finding that protein-encoding genes constitute only 1.5% of the mammalian genome was initially surprising but has since led to the discovery that approximately half of the noncoding genome is likely important for governing transcriptional output (15, 41). Cis-acting regulatory enhancer elements impart transcriptional control irrespective of orientation and position, are frequently found at considerable distances from the genes they regulate, and are enriched in sequence-specific transcription factor (TF)-binding sites. Genes that are expressed in a cell fate- or tissue-specific manner are closely associated with larger “superenhancer” (SE) regions that are similar to previously described locus control regions or stretch enhancers (37). It is thought that large clusters of TF-bound SEs scattered throughout the genome determine cell fate by initiating and maintaining cell-specific gene expression (23).
The transcriptional output from enhancer elements is determined by the degree of local chromatin condensation and their ability to couple with the basal transcriptional machinery at gene promoters. Uncondensed and transcriptionally active chromatin is strongly correlated with the acetylation of histone H3 lysine 27 (H3K27ac), whereas condensed and repressed chromatin is enriched by H3K27 trimethylation (H3K27me3) (6, 10). In fact, the hallmarks of available and active enhancers and SEs are high levels of H3K27ac deposition, coenrichment of bromodomain and extraterminal domain (BET) family members such as Brd4, and the presence of the Mediator coactivator complex subunit Med1 (7, 23, 31, 49). Mediator is localized to enhancers through various interactions, such as Med1-dependent TF association (8) and recruitment by Brd4 (7, 31). Interestingly, Mediator reciprocally modifies chromatin accessibility by serving as a scaffold for histone acetyltransferases and methyltransferases (1, 4, 13, 48).
Enhancer-promoter coupling is facilitated by the ability of Mediator to physically and functionally bridge with RNA polymerase II (Pol II) and the general transcription apparatus at gene promoters (39). In vivo, Mediator associations with Pol II are transient in nature, and defining its precise role in transcriptional regulation at gene loci has been difficult (24, 38). However, Mediator has been proposed to both stabilize the preinitiation complex (16) and promote the transition of paused Pol II at transcriptional start sites (TSSs) into productive elongating species (14, 17, 29).
There is accumulating evidence that modified enhancer-promoter coupling correlates with and may be responsible for pathological cardiac responses. Pressure overload restructures the interactions found between cardiac enhancers and genes (42). The pattern of active enhancers is augmented during both stress (21) and normal development (12). Furthermore, inhibition of BET protein interactions with acetylated chromatin reverses the increase in Pol II pause/release from pressure overload stress and reduces the accompanying structural remodeling and cellular hypertrophy (3, 45). We recently found that Med1 and Mediator are required for cardiac homeostasis and pathological stress responses (5, 18, 19, 46). Deletion of Med1 in postnatal and adult cardiomyocytes leads to the dysregulation of more than 5,000 genes that induce acute heart failure (25, 46) and implicates Med1-dependent enhancer-promoter coupling in the pathogenesis of heart failure. Here, we used chromatin immunoprecipitation-coupled deep sequencing (ChIP-seq) to examine the consequences of cardiomyocyte Med1 knockout on Pol II recruitment and occupancy at gene loci and potential changes in chromatin accessibility at transcribed and enhancer regions.
METHODS
Animals.
All animal procedures were conducted according to principles detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Iowa. Med1 conditional floxed mice (Med1fl/fl, fl/fl) backcrossed into the C57Bl/6 background (The Jackson Laboratory, Bar Harbor, ME) were kindly provided by Dr. J. K. Reddy. Med1 cardiac-specific knockout (cKO) mice were generated by crossing Med1fl/fl female mice with Med1fl/+;αMHC-Cre male mice as previously reported (46). Mice were fed a standard chow diet and given water ad libitum.
ChIP-seq processing and analysis.
After euthanasia of 3-wk-old male mice with CO2 and cervical dislocation, hearts were rapidly harvested, rinsed in cold PBS, and snap frozen in liquid nitrogen. ChIP-seq was performed in triplicate using 1 heart/sample through the EpiQuest service from Zymo Research (Irvine, CA) with the Zymo-Spin ChIP Kit (catalog no. D5210) according to the manufacturer’s directions with minor modifications. Tissue was minced in 1× PBS and homogenized on ice. Samples were cross-linked with 1% formaldehyde for 10 min with gentle shaking and quenched with 0.125 M glycine. ChIP-grade antibodies against Pol II (catalog no. 9201012, lot no. B200433, Bethyl Laboratories, Montgomery, TX), H3K27ac (ab4729, lot no. GR2322896-1, Abcam, Cambridge, MA), H3K27me3 (ab6002, lot no. GR130002-4, Abcam), and normal mouse IgG (C15410206, lot no. RIG001L, Diagenode; Denville, NH, and 12-371B, lot no. 2430393, Millipore, Burlington, MA) were used. Approximately 10 µg of chromatin and 5 µg of antibody were used in each ChIP assay. Three independent ChIP assays were verified by quantitative PCR using positive control promoter primers and demonstrated a >70-fold ChIP DNA enrichment over IgG control (data not shown). Libraries from pooled ChIP DNA were prepared using a proprietary library preparation protocol from Zymo Research. ChIP-seq libraries were quantified using Qubit fluorometric and 2200 Tape Station, had an average size range of 371–564 bp, and were normalized based on Qubit measurements and quantified by quantitative PCR using the same primers as described above. Pol II, H3K27ac, and H3K27me3 libraries were enriched >75-, 28-, and 7.5-fold, respectively, over their input libraries; 50-bp single end reads were run on a HiSeq 1500 Next-Gen sequencing platform (Illumina, San Diego, CA). Raw sequences were preprocessed using the TrimGalore wrapper. ChIP-seq reads were aligned to the mouse mm10 genome by Bowtie with at most two mismatches. Reads that appeared more than twice at the same position on the same strand were discarded to remove PCR duplication. ChIP-seq data are accessible through GEO (accession no. GSE118965).
Significantly enriched ChIP-seq peaks were identified and data were visualized with EaSeq software (version 1.05, www.easeq.net, University of Copenhagen, Copenhagen, Denmark) (32). After importation of sequencing files and alignment to to the mouse, mm10 genome ChIP peak regions were identified by the Identify Peaks tool, which compared each data set versus input using adaptive local thresholding. The EaSeq analysis procedure resembles that of MACS (52) with some modifications. The most abundant DNA fragment size of the samples was determined by finding the distance (>2 times the read length) between both strands, with the greatest correlation according to the Phantom Peak Coefficient method (30). Each data set was divided into 100-bp windows, and the reads within each window were scanned across the genome. A normalization coefficient (NCIS) serving to normalize the background levels of the two data sets was analyzed in accordance with Liang and Keles (33). Global thresholds were calculated based on a Poisson distribution using the genome-wide average number of reads in the window and a P value of 1e−5. Adaptive thresholds were modeled as follows: the average number of negative control reads in areas corresponding to 10×, 50×, and 250× window sizes was calculated for each position and used as the Poisson distribution λ. The most conservative threshold was chosen from the three local thresholds of the control and the global threshold of the sample. Thresholds from the negative control were scaled according with the NCIS normalization factor. The position and statistical analysis of windows passing the most conservative threshold having a NCIS-normalized log2-fold sample-to-control ratio of more than two and less than a threefold difference between the signal on the plus and minus strands were extracted into a separate list. This analysis was repeated four times, shifting windows 25 bp at a time with overlapping windows merged. For each region in the resulting list, the borders were refined by sliding a window of 100 bp from one window size upstream to downstream of the temporary border. The exact position where the number of sample reads within the window fell below the threshold was defined as new border of that region. Shoulders were excluded at values below µ + 2SD. After border refinement and peak merging, peaks were positively selected for an false discovery rate (32) value of 1e−5 or better and a minimum NCIS normalized log2-fold difference of two. Blacklisted genomic regions determined by the ENCODE project (15) found to produce false positive peaks were deleted from further analyses using the Colocalize and Gate tools.
Visualization and quantification of ChIP-seq signals at gene loci.
To visualize ChIP-seq peak regions within EaSeq that occur near genes, peaks distances from mouse mm10 reference gene TSSs were determined by the Annotate tool and associated with our Med1 fl/fl versus cKO RNA-seq results (GEO data set GSE84160) (46) using the Colocalize tool. Peaks were sorted as indicated and plotted using the Heatmap tool, where each line on the y-axis represents individual genomic regions with x-axis windows aligned to the annotated TSS or relative to the center of normalized gene body lengths. Heat maps and scatterplots were colorized by raw peak intensity (grayscale) of DNA fragment reads per kilobase per million mapped reads (RPKM) as indicated. Differences between fl/fl and cKO peak intensities were plotted as psuedocolored log2 ratiometric heat maps. Average peak intensity plots were created with the Average Track and Overlay tools. Two-dimensional scatterplot histograms of fl/fl versus cKO peak values within the promoter region (from −1,000 to −50 bp of the TSS), surrounding the TSS (−50 to +300 bp), and within the gene body [+300 bp to the transcriptional end site (TES)] were plotted using the Z-scatter tool pseudocolored according to the log2-fold change in RNA expression of that gene. Peak signal intensities were quantified using the Quantify tool.
Identification and analysis of enhancer regions.
Enhancers were identified similar to those reported by Whyte et al. (49) by first segregating H3K27ac peaks with centers positioned >2.5 kb from the nearest TSS and then concatenating (stitching) if within 12.5 kb of another peak. For fl/fl hearts, 62,632 such peaks clustered into 20,596 regions, whereas 64,932 cKO peaks clustered into 21,869 enhancers. Peaks were ranked ordered by signal, and the point at which the resulting cumulative distribution curves from fl/fl and cKO hearts reached the inflection point was used to delineate typical enhancers (TEs) from SEs. Unique enhancer regions from both genotypes were compiled using the Merge tool and associated with RNA-seq data (46) using the Colocalize tool. The log2 cKO-to-fl/fl ratio of the normalized K27ac signal across enhancers was determined by the Calculate tool. After SE regions were extracted into separate data sets using the Gate tool (1,487 for fl/fl and 1,694 for cKO, 1,852 total), SEs were subdivided into those that were unchanged (log2 cKO:fl/fl less than −0.1 and <0.1, n = 526), increased >1.25-fold (log2 cKO:fl/fl >0.32, n = 345), and decreased > 1.25-fold (log2 cKO:fl/fl less than −0.32, n = 175). Previously identified cardiac enhancers (12), SEs (27), and long noncoding RNAs (34) were downloaded and converted to mm10 coordinates using the UCSC Genome Browser liftover tool and colocalized to SEs, and overlapping fractions were determined by the Overlap tool.
TF motif identification.
SE regions were first divided into 500-bp fragments using the EaSeq Homogenize tool. Genomic coordinates of the fragments were imported into ChIPseek (chipseek.cgu.edu.tw) (9) for identification of known and potential novel TF motifs through its HOMER motif identification algorithm after stipulation of searches ±250 bp from fragment centers. For enriched de novo motifs, the known motifs with the highest matching scores from ChIPseek search output are listed. KEGG pathway enrichment analysis was performed using WebGestalt (webgestalt.org).
Statistics.
Summary data are presented as means ± SD or as box plots with 5−95% confidence intervals and means represented by “+” using GraphPad Prism (version 7). One-way or two-way ANOVA was used when comparing three or more groups with indicated post hoc comparisons. P values of <0.05 were considered as significant and further defined as indicated.
RESULTS
Med1 functions to establish Pol II occupancy at gene loci.
We previously reported that postnatal knockout of Med1 in cardiomyocytes (cKO) leads to heart failure with the differential expression of more than 5,000 genes by RNA sequencing compared with their floxed (fl/fl) controls (46). We further explored the transcriptomic profiles from these hearts and found that Med1 deletion leads to an cumulative decrease in average mRNA levels when all genes meeting the minimal threshold level of one fragment per kilobase per million mapped reads (FPKM) are considered (fl/fl: 54.6 ± 393.4 FPKM and cKO: 41.8 ± 188.0 FPKM, n = 11954; Fig. 1, A and B). Interestingly, the average expression level of significantly up- and downregulated genes with >1.5-fold expression appeared to normalize after Med1 disruption. Specifically, genes that are highly abundant in fl/fl hearts tend to be downregulated (135.5 ± 776.5 FPKM, n = 2852), whereas genes with relatively low expression tend to be upregulated (24.7 ± 60.9 FPKM; n = 2,891), resulting in comparable expression levels in cKO hearts (downregulated: 59.07 ± 293.6 FPKM and upregulated: 47.41 ± 167.3 FPKM). These results suggest that Med1 deletion may somehow uncouple transcriptional regulation in the heart.
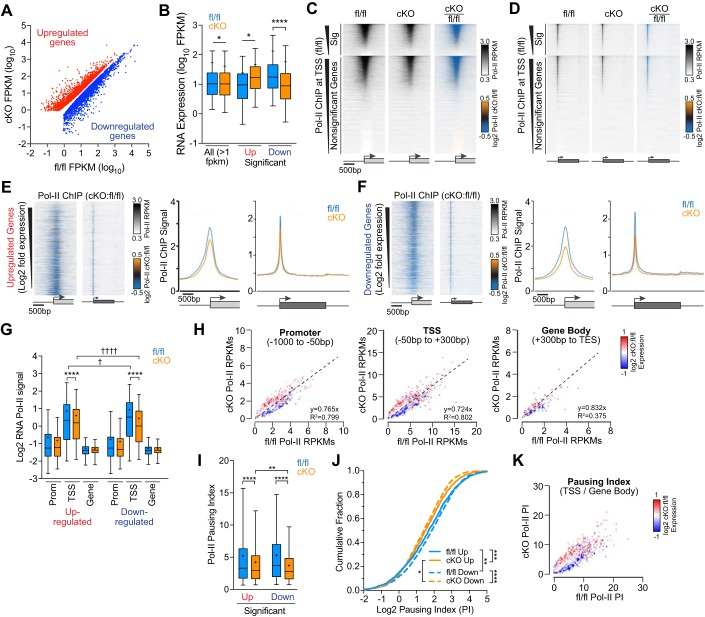
Fig. 1.
RNA polymerase II (Pol II) occupancy at transcription start sites (TSSs) and Pol II traveling into gene bodies is decreased in Med1 knockout hearts. A: scatterplot of significant differentially expressed upregulated (red) and downregulated (blue) genes from RNA-seq analysis of Med1 fl/fl versus Med1 cardiac-specific knockout (cKO) hearts [fragments per kilobase per million mapped reads (FPKM), n = 5, false discovery rate < 0.05) (46). B: box plot of summary data from A including average FPKM values for all gene expressed at >1 FPKM in either genotype. *P < 0.05 and ****P < 0.0001 by two-way ANOVA (n = 2,852–11,941, P < 0.0001) with Sidak’s multiple-comparison test. C and D: Pol II chromatin immunoprecipitation (ChIP) signal heat maps at TSSs (C) and across genes normalized for size (D) from Med1 fl/fl (left) and cKO (middle) hearts with ratiometric differences pseudocolored (right) as indicated. Genes were segregated for significant differential gene expression based on RNA sequencing and ranked in descending order of Pol II occupancy by ChIP-coupled deep sequencing (ChIP-seq). Boxes and arrows under the heat map widows denote genes and TSSs, respectively. RPKM, normalized reads per kilobase of sequence regions per million mapped reads. E and F, left: ratiometric heat maps of Pol II ChIP signals at TSSs and genes for significantly upregulated (E) and downregulated (F) genes >1.5-fold and sorted by descending log2 cKO:fl/fl fold changes in expression. E and F, right: average Pol II ChIP signals at TSSs and along gene bodies for up- and downregulated genes. G: box plot summary data of normalized Pol II ChIP-seq signal intensities at promoters (−1,000 to −50 bp), TSSs (−50 to +300 bp), and gene bodies [+300 bp to the transcriptional end site (TES)] for genes with 1.5-fold differential expression. n = 1,978–2,178. ****P < 0.0001 for fl/fl vs. cKO; †P < 0.05 and ††††P < 0.0001 for >1.5-fold up- vs. downregulated genes by two-way ANOVA with Sidak’s multiple-comparison test. H: scatterplots of normalized Pol II ChIP signals at promoters (left), TSSs (middle), and gene bodies (right) from Med1 fl/fl and cKO hearts ratiometric colored for relative gene expression. I–K: Pol II pausing index (PI) computed as the Pol II ChIP intensity ratio at TSSs versus gene bodies displayed as boxes and whiskers (n = 1,978–2,178, P < 0.0001 by one-way ANOVA with Tukey’s multiple comparisons test; I], cumulative values (by Kolmogorov-Smirnov tests; J), and scatter plots (K). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Because Mediator has been proposed to regulate Pol II recruitment to and pause/release from proximal gene promoters, we assessed Pol II occupancy at gene loci after cardiac disruption of Med1. Deletion of Med1 in postnatal cardiomyocytes results in early lethality, with no mice living past 7 wk of age (25, 46). Therefore, we isolated ventricles from 3-wk-old fl/fl and cKO mice. Consistent with our previous findings, cKO hearts were significantly hypertrophied at this age, with heart weight-to-body weight ratios of 8.82 ± 1.83 mg/kg (n = 15) compared with 7.03 ± 0.99 mg/g for fl/fl mice (n = 6, P = 0.036 by a two-tailed t-test). Using ChIP-seq, we identified 7,776 and 7,633 peaks that were significantly enriched for Pol II binding in fl/fl and cKO ventricular tissue, respectively. Of these peaks, 66.4% from fl/fl tissue and 62.5% from cKO tissue were localized to annotated gene TSSs (−50 to +300 bp), with the majority of the other peaks falling within intronic, intergenic, and 5′-untranslated regions. Ratiometric heat maps that enable the visualization of relative differences in Pol II occupancy suggested that Med1 deletion caused an overall decrease in Pol II abundance at gene 5′-ends (Fig. 1, C and D), consistent with an overall decrease in average transcript abundance. To determine whether this apparent decrease in Pol II binding correlates with differential gene expression differences, we segregated significantly upregulated genes from those downregulated. Somewhat surprisingly, the majority of genes that were >1.5-fold up- or downregulated in cKO myocardium had decreased Pol II binding (Fig. 1, E and F), arguing that differential expression in these hearts relies on factors other than relative Pol II occupancy. To localize where decreased Pol II association is occurring, we divided gene loci into proximal promoter (from −1,000 to −50 bp of the TSS), TSS (−50 to +300 bp), and gene body (+300 bp to the TES) regions. The region surrounding the TSS had significant reductions in Pol II occupancy for both upregulated (fl/fl: 1.82 ± 1.81 RPKM and cKO: 1.52 ± 1.38 RPKM, n = 1,978, P < 0.001 by ANOVA) and downregulated (fl/fl: 1.90 ± 1.74 RPKM and cKO: 1.36 ± 1.17 RPKM, n = 2,178, P < 0.001 by ANOVA) genes (Fig. 1G). Despite an average decrease in Pol II localization to gene loci in cKO ventricles, we found that upregulated genes bound significantly more Pol II than downregulated genes in cKO hearts. These relationships can be better seen when gene expression changes were superimposed on plots comparing cKO versus fl/fl Pol II ChIP reads (Fig. 1H). Upregulated genes after Med1 deletion tended to have higher Pol II reads, where downregulated genes had less. Although not significant, promoter and gene body regions contained similar Pol II occupancy trends to TSSs.
Decreased Pol II localization at TSSs from Med1 disruption could be either due to a failure to properly recruit Pol II or to facilitate the release of paused Pol II from the TSS for productive transcription elongation. Therefore, we used the pausing index or traveling ratio to determine the ratio of Pol II localized to the promoter versus the gene body known (3, 40). We found a significant decrease in the mean pausing index ratio in cKO versus fl/fl hearts for both up- and downregulated genes (P < 0.0001 by one-way ANOVA with Tukey’s multiple comparison test; Fig. 1I) and a significant leftward shift in their cumulative pausing index distribution curves (P = 0.0002 for upregulated and P < 0.0001 for downregulated cKO vs. fl/fl genes by Kolmogorov-Smirnov test; Fig. 1J). These data demonstrated less Pol II at the TSS relative to released Pol II within the gene body for cKO hearts. Because there were no differences in Pol II occupancy downstream of TSSs for either up- or downregulated genes (Fig. 1G), the decrease in pausing indexes in cKO samples likely reflects an overall defect in Pol II recruitment and/or recycling to TSSs rather than an increase in transcriptional pause/release. Interestingly, downregulated genes in cKO hearts had an additional decrease in pausing index relative to upregulated genes (P = 0.016 by Kolmogorov-Smirnov test), whereas the opposite was true in control hearts (P = 0.0067 by Kolmogorov-Smirnov test). Furthermore, genes with the greatest cKO-to-fl/fl pausing index ratios tended to be induced in cKO hearts (Fig. 1K). These data suggest that whereas Pol II accumulation at TSSs is generally hindered in cKO hearts, Pol II recruitment at upregulated genes overcomes this obstacle more readily.
Acetylation of histone H3 K27 strongly correlates with Med1-dependent gene expression.
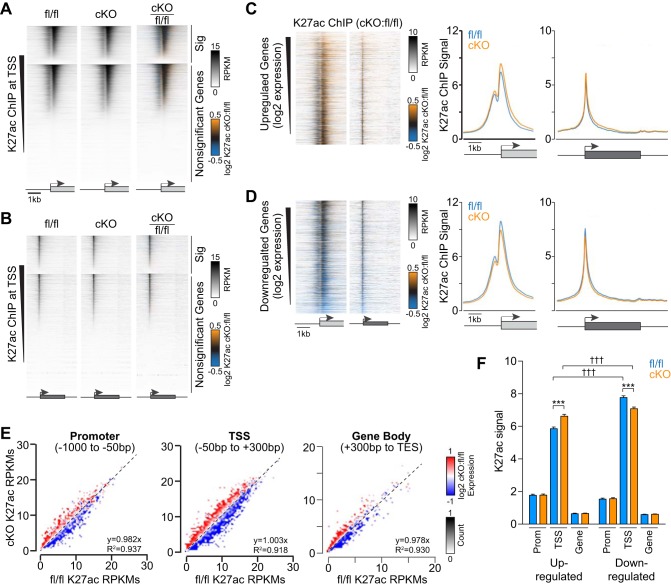
The changes in gene expression that result from Med1 deletion could arise from transcriptional processes in addition to Pol II occupancy and pause/release. Because Mediator serves as a scaffold for histone-modifying enzymes, we performed parallel ChIP reactions of reliable epigenetic marks that specify the degree of chromatin accessibility. We compared genome-wide levels of histone H3 Lys27 acetylation (K27ac), a marker for open or accessible DNA, with mutually exclusive K27 trimethylation (K27me3), an indicator of condensed and repressed chromatin. First, we identified significantly enriched genomic K27ac peaks and found that 5,254 of 73,623 fl/fl peaks and 5,087 of 75,422 cKO peaks were deposited as a doublet flanking TSSs (Fig. 2, A and B). These K27ac peaks correlated with the same TSSs as those occupied by Pol II (data not shown). When gene loci are separated into upregulated versus downregulated groups, we found that K27ac intensity correlated well with differential changes in gene expression (Fig. 2, C–F). This is evident by the results shown in Fig. 2E, where there was a striking demarcation between up- and downregulated genes at promoters, TSSs, and in gene bodies. Regression analyses showed near uniformity of the data and provided evidence showing that Med1 depletion does not affect total K27ac levels for differentially expressed genes. Consistent with this conclusion and the idea that acetylation correlates with DNA accessibility, we found that average K27ac ChIP-seq reads were substantially greater for all highly expressed genes (>50 FPKMs) than very low-expressing or silent genes (<1 FPKM) (data not shown). How Med1 deletion causes changes to intragenic K27ac levels that match fold changes in gene expression remains an open question.
Fig. 2.
Med1 deletion promotes the redistribution of active histone H3 K27 acetylation at genes to coincide with expression. A and B: heat maps of K27ac chromatin immunoprecipitation (ChIP) signal intensities from Med1 fl/fl and cardiac-specific knockout (cKO) ventricles within a 5-kb window flanking the transcriptional start site (TSS; A) and across gene bodies normalized for length (B). Genes were segregated for significant differential gene expression based on RNA sequencing and ranked by descending acetylation signal by ChIP-coupled deep sequencing (ChIP-seq). C and D, left: ratiometric heat maps of K27ac ChIP signals at TSSs and genes for upregulated (C) and downregulated (D) genes sorted by decreasing expression by RNA-seq. C and D, right: averaged K27ac ChIP signals at TSSs and along gene bodies for genes with >1.5-fold increase (C) or decrease (D) in gene expression. E: scatterplots of K27ac ChIP signals at promoters (left), TSSs (middle), and gene bodies (right) from fl/fl and cKO hearts ratiometric colored for relative gene expression. F: box plot summary data for normalized K27ac ChIP-seq signal intensities at promoters, TSSs, and gene bodies for genes with 1.5-fold differential expression. n = 1,978–2,178. ***P < 0.001 for fl/fl vs. cKO and †††P < 0.001 for up- vs. downregulated genes by two-way ANOVA (P < 0.0001) with Sidak’s multiple-comparison test.
Med1 deficiency induces widespread histone H3 K27me3 deposition.
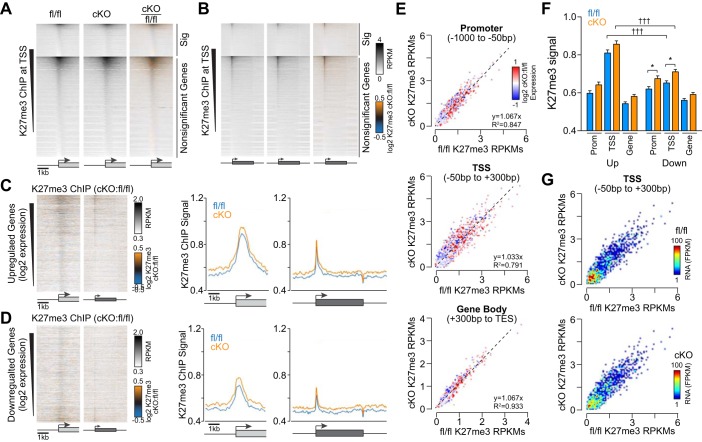
We next wondered whether repressive chromatin mark patterns would inform Med1-dependent transcriptional mechanisms and the degree to which they would reflect the K27ac or Pol II ChIP-seq patterns observed above. We identified a larger number of specific K27me3 peaks by ChIP-seq in Med1 fl/fl (n = 7,891) versus Med1-cKO mice (n = 5,093) that localized primarily to noncoding regions and at TSSs that were devoid of Pol II and K27ac (data not shown). However, we did observe an average increase in K27me3 deposition at both upregulated and downregulated genes in cKO ventricles (Fig. 3, A–D). In contrast to Pol II and K27ac, scatterplots of K27me3 levels in fl/fl versus cKO tissues did not show a strong demarcation between upregulated and downregulated genes (Fig. 3E). Summary data revealed significant increases in trimethyl deposition at promoters and TSSs for downregulated genes (Fig. 3F), with a similar trend for intragenic K27me3 levels in cKO hearts. There was also a significant difference in K27me3 levels at TSSs we compared upregulated (fl/fl: 0.81 ± 0.70 normalized RPKM and cKO: 0.86 ± 0.77 normalized RPKM) and downregulated (fl/fl: 0.65 ± 0.53 normalized RPKM and cKO: 0.71 ± 0.60 normalized RPKM, n = 1,978–2,178, P < 0.0001 by ANOVA) genes. Consistent with its mark for repressed chromatin, K27me3 levels inversely correlated with RNA expression (Figs. 1B and 3G) and were most abundant at silent genes in cKO hearts (data not shown).
Fig. 3.
Repressive histone H3 K27 trimethylation (K27me3) increases at gene loci after Med1 deletion. A and B.:K27me3 chromatin immunoprecipitation (ChIP) signal heat maps at transcriptional start sites (TSSs; A) and across genes normalized for size (B) from Med1 fl/fl and cardiac-specific knockout (cKO) hearts. Genes were segregated for significant differential gene expression based on RNA sequencing and ranked by descending methylation by ChIP-coupled deep sequencing (ChIP-seq). C and D, left: ratiometric heat maps of K27me3 ChIP signals at TSSs and genes for upregulated (C) and downregulated (D) genes sorted by decreasing expression. C and D, right: averaged K27ac ChIP signals at TSSs and along gene bodies for genes with >1.5-fold increase (C) or decrease (D) in gene expression. E: scatterplots of K27me3 ChIP signals at promoters (top), TSSs (middle), and gene bodies (bottom) from Med1 fl/fl and cKO hearts ratiometric colored for relative gene expression. F: box plot summary data for K27me3 ChIP-seq signal intensities at promoters, TSSs, and gene bodies for genes with 1.5-fold differential expression. n = 1,978–2,178. *P < 0.05 for fl/fl vs. cKO and †††P < 0.001 for up- vs. downregulated genes by two-way ANOVA (P < 0.0001) with Sidak’s multiple-comparison test. G: scatterplots of K27me3 ChIP-seq RPKMs at TSSs and ratiometric colored for RNA-seq reads of associated genes in Med1 fl/fl (top) and cKO (bottom) hearts.
Med1 depletion augments cardiac enhancer accessibility.
Our findings that epigenetic chromatin modifications at transcribed regions are sensitive to Med1 deletion prompted us to consider whether similar changes may be occurring at more distant enhancer elements. K27ac is a reliable chromatin mark throughout active enhancers, with the largest clusters of K27ac correlating with SE regions bound by Mediator and Med1 (10, 49). Because active enhancers are regarded as regions of open chromatin and distinct from the proximal promoter, we examined DNA regions flanked by significantly enriched K27ac ChIP peaks >2.5 kb from TSSs (see Supplemental Tables S1 and S2 in the Supplemental Material; Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website). We found a broad distribution in enhancer sizes that exhibited a positive correlation between the K27ac signal and changes in the expression of the gene with the closest TSS (Fig. 4, A and B).
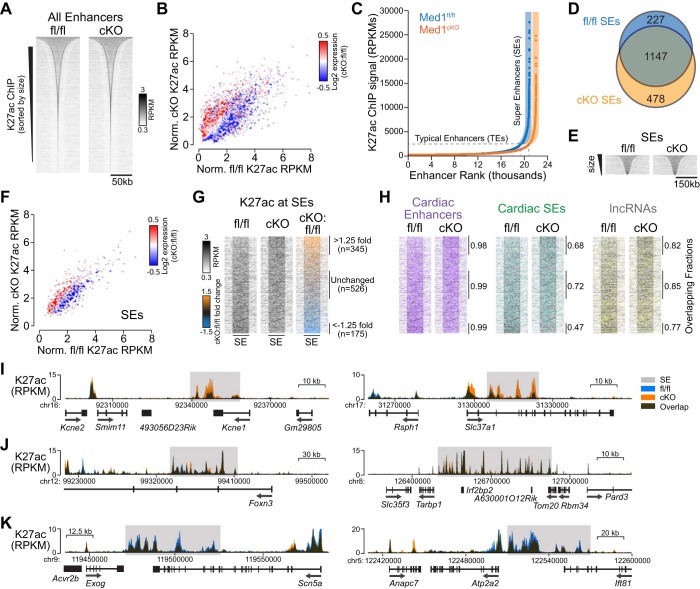
Fig. 4.
Cardiac Med1 depletion leads to superenhancer (SE) switching. A: heat map size distribution of Med1 fl/fl and cardiac-specific knockout (cKO) enhancers identified by concatenating K27ac chromatin immunoprecipitation-coupled deep sequencing (ChIP-seq) peaks residing >2.5 kb from transcriptional start sites (TSSs) and within 12.5 kb of one another. B: scatterplot of normalized K27ac ChIP-seq reads of enhancers and psuedocolored log2 fold change expression of the nearest gene determined by RNA-seq. C: rank order distribution of enhancer K27ac ChIP-seq peaks. SEs are distinguished from typical enhancers by those peaks with the greatest signal as defined by Whyte et al. (49). D: Venn diagram of the Med1 fl/fl and cKO SE overlap. E: heat map size distribution of SEs. Note that the window size was 3 times of that in A. F: scatterplot for normalized SE K27ac reads and pseudocolored by the differential expression of the nearest gene. G: heat map representation of SEs sorted by the log2 ratio of cKO to fl/fl SE K27ac intensity. SEs > 1.25-fold, less than −1.25-fold, and without changes in K27ac ChIP intensities after Med1 cKO are indicated. H: heat maps as in G indicating SEs that overlap with previously reported cardiac developmental enhancers (magenta) (12), cardiac SEs (green) (27), and long noncoding RNAs (ENCODE; yellow). I–K: example genomic tracks of SEs with stronger (I), unchanged (J), and weaker (K) K27ac ChIP-seq peak intensities after Med1 deletion.
Ranking enhancer regions by K27ac signal identified 1,374 fl/fl and 1,625 cKO unique candidate SEs of similar but significantly different sizes (fl/fl: 52,211 ± 32,887 bp and cKO: 55,275 ± 37,267 bp, P = 0.015 by t-test; Fig. 4, C–E, and Supplemental Table S3). We explored these potential cell fate-determining regulatory elements to assess whether they reflect Med1-dependent transcriptional changes and how well they correspond to known cardiac enhancers. As with enhancers, the TSSs of upregulated genes in cKO hearts tended to be in close proximity to SEs with greater K27ac reads, whereas TSSs of downregulated genes were associated with SEs with a decreased K27ac signal (Fig. 4F). The dynamic nature of Med1-dependent K27ac deposition within SEs was reflected by the >1.25-fold increase in cKO versus fl/fl levels for 345 of 1,852 total SEs and less than −1.25-fold decrease for 175 SEs (Fig. 4G). The genomic coordinates of the SEs we identified in 3-wk-old cardiac tissue agreed with previously reported cardiac gene regulatory elements, including conserved enhancers across multiple developmental time points (12), SEs from embryonic and 8-wk-old mice (27), and annotated long noncoding RNAs (ENCODE; Fig. 4H). This correlation validated our approach and suggests that SEs do not appreciably change once embryonic cardiac development commences. Furthermore, examination of genomic tracks with increased, relatively unchanged, and decreased cumulative K27ac levels across SE regions after Med1 deletion demonstrated that cKO K27ac peaks within SEs change in amplitude from established fl/fl peaks and do not arise de novo and/or are not eliminated (Fig. 4, I–K).
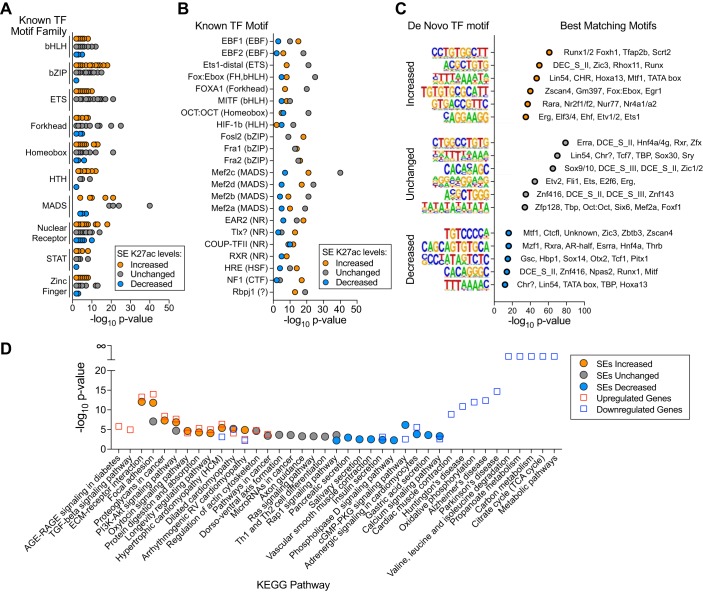
Because Med1 deletion modulates K27ac active enhancer marks, we wondered whether this apparent modulation of cardiac SEs might affect the accessibility of the specific TF DNA-binding motifs that these regions contain. We searched SE regions for known or otherwise de novo TF motifs (Fig. 5, A–C) (9). Perhaps not surprisingly, known TF motifs of Mef2 MADS box isoforms that regulate cardiomyocyte-specific transcription were the most significantly enriched and found within all three SE categories. Interestingly, binding motifs for known stress-responsive TFs with β-ZIP (e.g., Fos, Fra, and Jun), Ets (e.g., Ets, Erg, and Etv), helix-turn-helix (HLH; e.g., Myb and Rfx), and STAT domains were enriched in SEs with increased K27ac but were relatively absent in those with decreased levels. Development-related forkhead TF motifs (e.g., Foxa1) were prevalent in unchanged SEs with less significant enrichment in other SEs. These patterns suggest that transcriptional reprogramming from SEs after Med1 deletion may arise from stress-related pathways without contributions from fetal-like programs. We identified several novel motifs (Fig. 5C) that may represent new and potentially important cardiac TF-DNA interactions. It remains to be determined what the physiological relevance of these motifs are and with which TFs, known or uncharacterized, they interact.
Fig. 5.
Med1-sensitive superenhancer (SE) regions differentially affect transcription factor (TF) DNA-binding motifs and correlated with the Med1 cardiac-specific knockout (cKO) phenotype. A and B: DNA-binding motif enrichments for known TFs within SEs of 1.25-fold increased, unchanged, and 1.25-fold decreased cKO-to-fl/fl K27ac chromatin immunoprecipitation (ChIP) ratios displayed by TF family (A) or individually (B). C: significantly enriched de novo TF motifs within SEs and their closest matching known TF motifs. D: KEGG pathway enrichment results for genes with >2-fold differential expression and nearest to SEs after Med1 deletion. The top 10 hits for each category are shown.
To compare how well changes in SE K27ac deposition correlate with overall differential gene expression and the heart failure phenotype of Med1 cKO animals, we performed in silico pathway enrichment analysis. Med1 cKO hearts had significant decreases in cardiac contractility and increased fibrosis and showed significant changes in metabolic and structural gene expression pathways (25, 46). Upregulated genes identified by RNA-seq and genes closest to SEs with increased K27ac deposition were similarly enriched in cardiomyopathy- and cell adhesion-related pathways (Fig. 5D). Downregulated genes and SEs with decreased K27ac are commonly enriched in Ca2+ handling as well as adrenergic cGMP/PKG and insulin signaling. Although there was considerable overlap in the predicted pathways affected by changes in RNA and SE composition, it was interesting that mitochondria-based metabolic pathways made up the most significantly enriched pathways for downregulated genes but were absent in SE-enriched pathways. It is likely that intervening genes lie between cardiac SEs and metabolic genes and, therefore, were not enriched in our analysis here. Whether metabolic genes are dependent on SEs remains a future avenue of exploration.
DISCUSSION
Our present work in cardiac tissue implicates the Med1 subunit of the Mediator coactivator complex in regulating gene expression by directing Pol II localization and determining epigenetic homeostasis. Using ChIP-seq assays against the Pol II holoenzyme and against open versus repressed chromatin marks, we provide an initial genomic landscape that illuminates how Med1 deficiency affects dramatic changes in cardiac transcription. At gene loci, deletion of Med1 reduces Pol II occupancy at the majority of TSSs, induces robust changes in H3K27 acetylation that correlate with Med1-dependent differential gene expression, and broadly induces H3K27 trimethylation that leads to a decrease in relative transcript abundance. At enhancer and SE regulatory regions, Med1 knockout leads to dynamic changes in established H3K27ac levels that also correlate with differential gene expression. Altered chromatin accessibility within individual enhancers likely impacts their association with predicted specific TFs and the regulation of their intended gene targets.
There is accumulating evidence that both preinitiation complex assembly with Pol II as well as Pol II pause/release from TSSs require enhancer integrity and Mediator function (for reviews, see Refs. 16 and 47). In the heart, it has been previously reported that genes can be grouped according to their Pol II occupancy profiles and further categorized by whether or not Pol II is proximally paused under homeostatic conditions and whether Pol II can be de novo recruited under pressure overload stress (43). When specifically measured, pressure overload induces Pol II pause/release that mechanistically depends on Brd4/BET protein interactions with acetylated enhancers (3). Although disruption of Mediator function by Med1 causes cardiac dysfunction and a similar leftward shift in the pausing index to that reported for pressure overload, the patterns of Pol II occupancy are distinct. Pressure overload induces a transition toward transcriptional elongation into gene bodies, whereas Med1 cKO hearts have reduced Pol II TSS accumulation without accompanying increases downstream (Fig. 1). These results suggest that the presence of Med1 within Mediator is required for proper Pol II recruitment at TSSs before any function it may have on pause/release. Pol II occupancy at gene loci is assumed to have reached equilibrium in our 3-wk-old postnatal cKO model, and the decrease in Pol II may reflect a deficiency in Pol II to recycle back to gene promoters. However, our unexpected finding that Med1-dependent upregulated genes have decreased Pol II occupancy suggests that additional specific readouts for Pol II activity (e.g., Ser2 vs. Ser5 phosphorylation or GRO-seq) are needed to more accurately assess the consequences of Med1 cKO on Pol II dynamics.
Gene expression depends largely on the degree of chromatin accessibility, and we found remarkable Med1-dependent differences in the magnitude and intragenic distribution of active H3K27ac and repressive H3K27me3 marks of differentially expressed genes. In agreement with ChIP-seq results from murine embryonic stem cells (26, 50), we found that expressed genes presented with a bimodal K27ac deposition pattern flanking the TSS (Fig. 2), whereas K27me3 levels were less prevalent with a broad peak just downstream of the TSS (Fig. 3). Med1 deficiency results in a similar proportion of upregulated versus downregulated genes (46), and it is quite apparent that the differences in promoter, TSS, and gene body levels of K27ac accurately reflect whether a particular gene is correspondingly induced or repressed compared with control samples. These striking results point to the possible existence of an intricate mechanism tying intragenic acetylation with Med1 function at a given gene for fine tuning its expression. Differential acetylation has been implicated in cardiovascular disease, with histone deacetylase inhibitors showing promising efficacy in reversing pathological gene expression and cardiac remodeling in animal models (36). Although altered K27ac levels and the enzymes that catalyze its deposition and removal are functionally associated with Mediator at enhancers (4, 44), we are unaware of Mediator-dependent coordination of both activities in the same system or at gene loci.
The mechanisms by which Mediator affects whether H3K27 is acetylated versus methylated may be inherently different given their distinctive patterns. Intragenic H3K27 trimethylation does not show the same correlation with differential gene expression as K27ac but rather reflects overall transcript abundance. Deletion of Med1 appears to broadly repress transcription, as supported by the overall decrease in mRNA levels for genes above a minimal threshold (>1 FPKM; Fig. 1B) and the trend for increased K27me3 across gene loci that was significant for downregulated genes (Fig. 3). As with its effects on acetylation, Med1-dependent regulation of H3K27me3 levels have yet to be elucidated, but histone demethylases can indirectly associate with Med1 (20, 51) and it is possible that Med1 disruption abrogates K27me3 homeostasis. Proper maintenance of K27me3 appears to be necessary in the heart, as Ezh2 K27 methyltransferase facilitates the repression of developmental genes in adult mice (11, 22), whereas its paralog, Ezh1, is required for regenerative capacity after myocardial infarction (2).
Consistent with the known genomic coordinates of cardiac enhancers, we defined putative SE regions based on the highest aggregates of K27ac peaks and determined their enrichment for known and de novo TF-binding sites. We found a number of motifs for pioneering TF members (e.g., Esrrb, Nrf1, Sox, Oct, and Fox) within SE regions of maintained or increased K27ac levels after Med1 depletion. Pioneering TFs are important in binding heterochromatin and initiating chromatin remodeling as well as stabilizing open enhancer regions (35). Because the coordinates of identified SEs did not change but rather K27ac levels within those regions, it is possible that the significant enrichment of these sites may reflect prior pioneering activity. Although de novo generation of K27ac peaks at enhancer regions has been observed in other systems (28), this process is associated with DNA methyltransferase activity and suggests that DNA methylation may be static in Med1 cKO hearts.
We also observed a number of activator protein (AP)-1 TF family member-binding motifs enriched in SE regions with increased but not decreased K27ac levels (e.g., Fosl2, Fra1, and Fra2). Induction of AP-1-dependent immediate-early gene responses is dependent on Mediator function through its kinase submodule that induces Pol II elongation (14). We speculate that increased acetylation of AP-1-binding regions may reflect a compensatory effect from decreased paused Pol II accumulation at TSSs in an attempt to increase target gene expression in these deteriorating hearts.
In summary, we found that cardiac disruption of Mediator function through deletion of its TF-interacting Med1 subunit alters seemingly unrelated cardiac transcription processes. Med1 cKO hearts lose their ability to properly maintain Pol II at TSSs through a potential defect in establishing preinitiation complexes. Furthermore, the lack of Med1 causes epigenetic changes that correspond with differential and absolute gene expression both with individual gene loci and at distinct TF-binding distal SE regions. Our results suggest that Med1 regulates transcriptional and epigenetic mechanisms that cumulatively determine gene expression output.
GRANTS
This work was supported by generous research support from National Heart, Lung, and Blood Institute (NHLBI) Grant R01-HL-125436-02, the Fraternal Order of Eagles Diabetes Research Center, and the University of Iowa Carver College of Medicine (to C. E. Grueter) as well as NHLBI Postdoctoral Fellowship Training Grant 5-T32-HL-007121-38 (to K. M. Spitler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.D.H. analyzed data; D.D.H. and C.E.G. interpreted results of experiments; D.D.H. prepared figures; D.D.H. drafted manuscript; D.D.H., K.M.S., and C.E.G. edited and revised manuscript; D.D.H., K.M.S., and C.E.G. approved final version of manuscript; K.M.S. performed experiments; C.E.G. conception and design of research.
Supplemental Data
ACKNOWLEDMENTS
We thank Nicole Johnson (Zymo Research) for discussing and optimizing ChIP-seq experiments and Jessica Ponce and Rachel Minerath for helpful discussions regarding the manuscript.
REFERENCES
- 1.Acevedo ML, Kraus WL. Mediator and p300/CBP-steroid receptor coactivator complexes have distinct roles, but function synergistically, during estrogen receptor alpha-dependent transcription with chromatin templates. Mol Cell Biol 23: 335–348, 2003. doi: 10.1128/MCB.23.1.335-348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai S, Yu X, Li Y, Peng Y, Li C, Yue Y, Tao G, Li C, Pu WT, He A. Divergent requirements for EZH1 in heart development versus regeneration. Circ Res 121: 106–112, 2017. doi: 10.1161/CIRCRESAHA.117.311212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand P, Brown JD, Lin CY, Qi J, Zhang R, Artero PC, Alaiti MA, Bullard J, Alazem K, Margulies KB, Cappola TP, Lemieux M, Plutzky J, Bradner JE, Haldar SM. BET bromodomains mediate transcriptional pause release in heart failure. Cell 154: 569–582, 2013. doi: 10.1016/j.cell.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aranda-Orgilles B, Saldaña-Meyer R, Wang E, Trompouki E, Fassl A, Lau S, Mullenders J, Rocha PP, Raviram R, Guillamot M, Sánchez-Díaz M, Wang K, Kayembe C, Zhang N, Amoasii L, Choudhuri A, Skok JA, Schober M, Reinberg D, Sicinski P, Schrewe H, Tsirigos A, Zon LI, Aifantis I. MED12 regulates HSC-specific enhancers independently of mediator kinase activity to control hematopoiesis. Cell Stem Cell 19: 784–799, 2016. doi: 10.1016/j.stem.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baskin KK, Grueter CE, Kusminski CM, Holland WL, Bookout AL, Satapati S, Kong YM, Burgess SC, Malloy CR, Scherer PE, Newgard CB, Bassel-Duby R, Olson EN. MED13-dependent signaling from the heart confers leanness by enhancing metabolism in adipose tissue and liver. EMBO Mol Med 6: 1610–1621, 2014. doi: 10.15252/emmm.201404218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein E, Duncan EM, Masui O, Gil J, Heard E, Allis CD. Mouse polycomb proteins bind differentially to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Mol Cell Biol 26: 2560–2569, 2006. doi: 10.1128/MCB.26.7.2560-2569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhagwat AS, Roe J-S, Mok BYL, Hohmann AF, Shi J, Vakoc CR. BET bromodomain inhibition releases the mediator complex from select cis-regulatory elements. Cell Reports 15: 519–530, 2016. doi: 10.1016/j.celrep.2016.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borggrefe T, Yue X. Interactions between subunits of the Mediator complex with gene-specific transcription factors. Semin Cell Dev Biol 22: 759–768, 2011. doi: 10.1016/j.semcdb.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Chen TW, Li HP, Lee CC, Gan RC, Huang PJ, Wu TH, Lee CY, Chang YF, Tang P. ChIPseek, a web-based analysis tool for ChIP data. BMC Genomics 15: 539, 2014. doi: 10.1186/1471-2164-15-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, Boyer LA, Young RA, Jaenisch R. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci USA 107: 21931–21936, 2010. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado-Olguín P, Huang Y, Li X, Christodoulou D, Seidman CE, Seidman JG, Tarakhovsky A, Bruneau BG. Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nat Genet 44: 343–347, 2012. doi: 10.1038/ng.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickel DE, Barozzi I, Zhu Y, Fukuda-Yuzawa Y, Osterwalder M, Mannion BJ, May D, Spurrell CH, Plajzer-Frick I, Pickle CS, Lee E, Garvin TH, Kato M, Akiyama JA, Afzal V, Lee AY, Gorkin DU, Ren B, Rubin EM, Visel A, Pennacchio LA. Genome-wide compendium and functional assessment of in vivo heart enhancers. Nat Commun 7: 12923, 2016. doi: 10.1038/ncomms12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding N, Zhou H, Esteve P-O, Chin HG, Kim S, Xu X, Joseph SM, Friez MJ, Schwartz CE, Pradhan S, Boyer TG. Mediator links epigenetic silencing of neuronal gene expression with x-linked mental retardation. Mol Cell 31: 347–359, 2008. doi: 10.1016/j.molcel.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat Struct Mol Biol 17: 194–201, 2010. doi: 10.1038/nsmb.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature 489: 57–74, 2012. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eychenne T, Werner M, Soutourina J. Toward understanding of the mechanisms of Mediator function in vivo: Focus on the preinitiation complex assembly. Transcription 8: 328–342, 2017. doi: 10.1080/21541264.2017.1329000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galbraith MD, Allen MA, Bensard CL, Wang X, Schwinn MK, Qin B, Long HW, Daniels DL, Hahn WC, Dowell RD, Espinosa JM. HIF1A employs CDK8-mediator to stimulate RNAPII elongation in response to hypoxia. Cell 153: 1327–1339, 2013. doi: 10.1016/j.cell.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, Gautron L, Elmquist JK, Bassel-Duby R, Olson EN. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell 149: 671–683, 2012. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall DD, Ponce JM, Chen B, Spitler KM, Alexia A, Oudit GY, Song LS, Grueter CE. Ectopic expression of Cdk8 induces eccentric hypertrophy and heart failure. JCI Insight 2: e92476, 2017. doi: 10.1172/jci.insight.92476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harms MJ, Lim H-W, Ho Y, Shapira SN, Ishibashi J, Rajakumari S, Steger DJ, Lazar MA, Won K-J, Seale P. PRDM16 binds MED1 and controls chromatin architecture to determine a brown fat transcriptional program. Genes Dev 29: 298–307, 2015. doi: 10.1101/gad.252734.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He A, Gu F, Hu Y, Ma Q, Yi Ye LY, Akiyama JA, Visel A, Pennacchio LA, Pu WT. Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat Commun 5: 4907, 2014. doi: 10.1038/ncomms5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He A, Ma Q, Cao J, von Gise A, Zhou P, Xie H, Zhang B, Hsing M, Christodoulou DC, Cahan P, Daley GQ, Kong SW, Orkin SH, Seidman CE, Seidman JG, Pu WT. Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ Res 110: 406–415, 2012. doi: 10.1161/CIRCRESAHA.111.252205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-André V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell 155: 934–947, 2013. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeronimo C, Langelier M-F, Bataille AR, Pascal JM, Pugh BF, Robert F. Tail and kinase modules differently regulate core mediator recruitment and function in vivo. Mol Cell 64: 455–466, 2016. doi: 10.1016/j.molcel.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia Y, Chang H-C, Schipma MJ, Liu J, Shete V, Liu N, Sato T, Thorp EB, Barger PM, Zhu Y-J, Viswakarma N, Kanwar YS, Ardehali H, Thimmapaya B, Reddy JK. Cardiomyocyte-specific ablation of Med1 subunit of the Mediator complex causes lethal dilated cardiomyopathy in mice. PLoS One 11: e0160755, 2016. [Erratum in PLoS One 11: e0164316, 2016. 10.1371/journal.pone.0164316. 27690354.] doi: 10.1371/journal.pone.0160755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karmodiya K, Krebs AR, Oulad-Abdelghani M, Kimura H, Tora L. H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genomics 13: 424, 2012. doi: 10.1186/1471-2164-13-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan A, Zhang X. dbSUPER: a database of super-enhancers in mouse and human genome. Nucleic Acids Res 44: D164–D171, 2016. doi: 10.1093/nar/gkv1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King AD, Huang K, Rubbi L, Liu S, Wang CY, Wang Y, Pellegrini M, Fan G. Reversible regulation of promoter and enhancer histone landscape by DNA methylation in mouse embryonic stem cells. Cell Reports 17: 289–302, 2016. doi: 10.1016/j.celrep.2016.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knuesel MT, Taatjes DJ. Mediator and post-recruitment regulation of RNA polymerase II. Transcription 2: 28–31, 2011. doi: 10.4161/trns.2.1.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landt SG, Marinov GK, Kundaje A, Kheradpour P, Pauli F, Batzoglou S, Bernstein BE, Bickel P, Brown JB, Cayting P, Chen Y, DeSalvo G, Epstein C, Fisher-Aylor KI, Euskirchen G, Gerstein M, Gertz J, Hartemink AJ, Hoffman MM, Iyer VR, Jung YL, Karmakar S, Kellis M, Kharchenko PV, Li Q, Liu T, Liu XS, Ma L, Milosavljevic A, Myers RM, Park PJ, Pazin MJ, Perry MD, Raha D, Reddy TE, Rozowsky J, Shoresh N, Sidow A, Slattery M, Stamatoyannopoulos JA, Tolstorukov MY, White KP, Xi S, Farnham PJ, Lieb JD, Wold BJ, Snyder M. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res 22: 1813–1831, 2012. doi: 10.1101/gr.136184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J-E, Park Y-K, Park S, Jang Y, Waring N, Dey A, Ozato K, Lai B, Peng W, Ge K. Brd4 binds to active enhancers to control cell identity gene induction in adipogenesis and myogenesis. Nat Commun 8: 2217, 2017. doi: 10.1038/s41467-017-02403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerdrup M, Johansen JV, Agrawal-Singh S, Hansen K. An interactive environment for agile analysis and visualization of ChIP-sequencing data. Nat Struct Mol Biol 23: 349–357, 2016. doi: 10.1038/nsmb.3180. [DOI] [PubMed] [Google Scholar]
- 33.Liang K, Keleş S. Normalization of ChIP-seq data with control. BMC Bioinformatics 13: 199, 2012. doi: 10.1186/1471-2105-13-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matkovich SJ, Edwards JR, Grossenheider TC, de Guzman Strong C, Dorn GW II. Epigenetic coordination of embryonic heart transcription by dynamically regulated long noncoding RNAs. Proc Natl Acad Sci USA 111: 12264–12269, 2014. doi: 10.1073/pnas.1410622111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayran A, Drouin J. Pioneer transcription factors shape the epigenetic landscape. J Biol Chem 293: 13795–13804, 2018. doi: 10.1074/jbc.R117.001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol 52: 303–319, 2012. doi: 10.1146/annurev-pharmtox-010611-134712. [DOI] [PubMed] [Google Scholar]
- 37.Parker SC, Stitzel ML, Taylor DL, Orozco JM, Erdos MR, Akiyama JA, van Bueren KL, Chines PS, Narisu N, Black BL, Visel A, Pennacchio LA, Collins FS, Becker J, Benjamin B, Blakesley R, Bouffard G, Brooks S, Coleman H, Dekhtyar M, Gregory M, Guan X, Gupta J, Han J, Hargrove A, Ho S, Johnson T, Legaspi R, Lovett S, Maduro Q, Masiello C, Maskeri B, McDowell J, Montemayor C, Mullikin J, Park M, Riebow N, Schandler K, Schmidt B, Sison C, Stantripop M, Thomas J, Thomas P, Vemulapalli M, Young A; NISC Comparative Sequencing Program; National Institutes of Health Intramural Sequencing Center Comparative Sequencing Program Authors; NISC Comparative Sequencing Program Authors . Chromatin stretch enhancer states drive cell-specific gene regulation and harbor human disease risk variants. Proc Natl Acad Sci USA 110: 17921–17926, 2013. doi: 10.1073/pnas.1317023110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrenko N, Jin Y, Wong KH, Struhl K. Mediator undergoes a compositional change during transcriptional activation. Mol Cell 64: 443–454, 2016. doi: 10.1016/j.molcel.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poss ZC, Ebmeier CC, Taatjes DJ. The Mediator complex and transcription regulation. Crit Rev Biochem Mol Biol 48: 575–608, 2013. doi: 10.3109/10409238.2013.840259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA. c-Myc regulates transcriptional pause release. Cell 141: 432–445, 2010. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivera CM, Ren B. Mapping human epigenomes. Cell 155: 39–55, 2013. doi: 10.1016/j.cell.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosa-Garrido M, Chapski DJ, Schmitt AD, Kimball TH, Karbassi E, Monte E, Balderas E, Pellegrini M, Shih T-T, Soehalim E, Liem D, Ping P, Galjart NJ, Ren S, Wang Y, Ren B, Vondriska TM. High-resolution mapping of chromatin conformation in cardiac myocytes reveals structural remodeling of the epigenome in heart failure. Circulation 136: 1613–1625, 2017. doi: 10.1161/CIRCULATIONAHA.117.029430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sayed D, He M, Yang Z, Lin L, Abdellatif M. Transcriptional regulation patterns revealed by high resolution chromatin immunoprecipitation during cardiac hypertrophy. J Biol Chem 288: 2546–2558, 2013. doi: 10.1074/jbc.M112.429449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siersbæk R, Madsen JGS, Javierre BM, Nielsen R, Bagge EK, Cairns J, Wingett SW, Traynor S, Spivakov M, Fraser P, Mandrup S. Dynamic rewiring of promoter-anchored chromatin loops during adipocyte differentiation. Mol Cell 66: 420–435.E5, 2017. doi: 10.1016/j.molcel.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Spiltoir JI, Stratton MS, Cavasin MA, Demos-Davies K, Reid BG, Qi J, Bradner JE, McKinsey TA. BET acetyl-lysine binding proteins control pathological cardiac hypertrophy. J Mol Cell Cardiol 63: 175–179, 2013. doi: 10.1016/j.yjmcc.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spitler KM, Ponce JM, Oudit GY, Hall DD, Grueter CE. Cardiac Med1 deletion promotes early lethality, cardiac remodeling, and transcriptional reprogramming. Am J Physiol Heart Circ Physiol 312: H768–H780, 2017. doi: 10.1152/ajpheart.00728.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taatjes DJ. The human Mediator complex: a versatile, genome-wide regulator of transcription. Trends Biochem Sci 35: 315–322, 2010. doi: 10.1016/j.tibs.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsutsui T, Fukasawa R, Shinmyouzu K, Nakagawa R, Tobe K, Tanaka A, Ohkuma Y. Mediator complex recruits epigenetic regulators via its two cyclin-dependent kinase subunits to repress transcription of immune response genes. J Biol Chem 288: 20955–20965, 2013. doi: 10.1074/jbc.M113.486746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153: 307–319, 2013. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young MD, Willson TA, Wakefield MJ, Trounson E, Hilton DJ, Blewitt ME, Oshlack A, Majewski IJ. ChIP-seq analysis reveals distinct H3K27me3 profiles that correlate with transcriptional activity. Nucleic Acids Res 39: 7415–7427, 2011. doi: 10.1093/nar/gkr416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng X, Jedrychowski MP, Chen Y, Serag S, Lavery GG, Gygi SP, Spiegelman BM. Lysine-specific demethylase 1 promotes brown adipose tissue thermogenesis via repressing glucocorticoid activation. Genes Dev 30: 1822–1836, 2016. doi: 10.1101/gad.285312.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of ChIP-Seq (MACS). Genome Biol 9: R137, 2008. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.