Abstract
Dendritic cells (DCs) constantly sample peripheral tissues for antigens, which are subsequently ingested to derive peptides for presentation to T cells in lymph nodes. To do so, DCs have to traverse many different tissues with varying oxygen tensions. Additionally, DCs are often exposed to low oxygen tensions in tumors, where vascularization is lacking, as well as in inflammatory foci, where oxygen is rapidly consumed by inflammatory cells during the respiratory burst. DCs respond to oxygen levels to tailor immune responses to such low-oxygen environments. In the present study, we identified a mechanism of hypoxia-mediated potentiation of release of tumor necrosis factor α (TNF-α), a pro-inflammatory cytokine with important roles in both anti-cancer immunity and autoimmune disease. We show in human monocyte-derived DCs (moDCs) that this potentiation is controlled exclusively via the p38/mitogen-activated protein kinase (MAPK) pathway. We identified MAPK kinase kinase 8 (MAP3K8) as a target gene of hypoxia-induced factor (HIF), a transcription factor controlled by oxygen tension, upstream of the p38/MAPK pathway. Hypoxia increased expression of MAP3K8 concomitant with the potentiation of TNF-α secretion. This potentiation was no longer observed upon siRNA silencing of MAP3K8 or with a small molecule inhibitor of this kinase, and this also decreased p38/MAPK phosphorylation. However, expression of DC maturation markers CD83, CD86, and HLA-DR were not changed by hypoxia. Since DCs play an important role in controlling T-cell activation and differentiation, our results provide novel insight in understanding T-cell responses in inflammation, cancer, autoimmune disease and other diseases where hypoxia is involved.
Keywords: dendritic cells, hypoxia, inflammation, mitogen-activated protein kinases, tumour necrosis factors
Introduction
Dendritic cells (DCs) are the gatekeepers of the adaptive immune system, residing throughout peripheral tissues where they constantly sample for antigens [1,2]. When a DC encounters a foreign antigen, it secretes many inflammatory cytokines, including tumor necrosis factor α (TNF-α), that activate or repress the functions of other immune cells and contribute to an inflammatory disease state. Extracellular antigens can be ingested by endocytosis or phagocytosis, processed in the endolysosomal system and presented on MHC classes I and II on the surface of DCs. During this process, DCs traverse from the site of inflammation to the nearest draining lymph node, where they activate naive CD4+ and CD8+ T cells [3]. For the sampling of antigen and the traveling to the lymph node, a DC can pass many different tissues that display a wide range of oxygen levels, from almost atmospheric oxygen levels in the lung (20%) to hypoxic (<1%) oxygen tensions in the thymus and spleen [4]. On top of this, many disease states are characterized by low local oxygen levels. For example, the central region of solid tumors is very hypoxic due to a lack of vascularization [5,6]. Moreover, sites of infection and inflammation are often hypoxic microenvironments, due to the combination of reduced perfusion following physical damage and the high metabolic load of the activated inflammatory cells caused by the respiratory burst [7,8]. Hypoxia has been linked to many auto-inflammatory diseases, including diabetes and atherosclerosis [9–11]. In cancer, hypoxia induces the neovascularization that can promote tumor growth and possibly cancer cell metastasis [6]. Hypoxia can also be a direct driver of metastasis by inducing single-cell dissemination [12].
Oxygen levels are known to modulate many cellular processes, including metabolism, cell survival, and various signaling pathways [13,14]. The most important class of oxygen-sensitive signaling proteins is hypoxia-induced factor (HIF). HIFs are basic helix–loop–helix heterodimeric transcription factors involving three main subunits: HIF-1α, HIF-2α, and HIF-1β [13–15]. All three subunits are constitutionally expressed, but under normoxic conditions proline residues on the α-subunits are modified by prolyl hydroxylases (PHDs) in a process that consumes oxygen. After this hydroxylation, the von Hippel–Lindau E3 ligase (VHL) can trigger ubiquitination of the α-subunit leading to subsequent proteasomal degradation [13]. Under hypoxic conditions, PHD does not efficiently hydroxylate the α-subunit due to lack of oxygen, allowing HIF-1α or HIF-2α to dimerize with HIF-1β. These HIF complexes will then promote transcription of genes carrying hypoxic response elements (HREs) in their promoter regions [16–18].
Oxygen levels regulate the mammalian immune response, as it was recently shown that mice negative for HIF-1α fail to clear invasive bacterial infections [18]. Oxygen levels also modulate DC differentiation and the activation triggered by maturation stimuli such as lipopolysaccharide (LPS), a bacterial ligand of Toll-like receptor 4 (TLR4) [9,18–35]. Specifically, hypoxic DCs were shown to have increased levels of HIF-1α, metabolism shifted to glycolysis [30,31,33], and altered expression of chemokines and chemokine receptors affecting DC migration [27,28,30,32,34]. The effect of hypoxia on the expression of DC-maturation markers (e.g., CD80, CD83, CD86, MHC-II) is controversial, with both reductions [19,21,25–27,29,32], increases [30,31], and no effect [28,35] being reported. Similarly, both increased [31] and reduced [27,32] efficiencies of T-cell activation by hypoxic DCs, and both stimulatory [19,31,32] and inhibitory [21,25,27] effects of hypoxia on TLR-induced cytokine release (e.g., interleukin (IL)-6, IL-10, IL-12, TNF-α) have been reported.
The controversial findings of hypoxia on DC function likely arise from the fact that HIF not only modulates TLR signaling [36], but TLR stimulation in turn also affects HIF signaling [20,22,37]. TLR stimulation can promote the stabilization of HIF as well as up-regulate its expression [20,22]. The group of target genes regulated by HIF differs between activation of HIF via TLR stimulation or hypoxia [22] and this cross-talk between HIF and TLR signaling likely allows for fine-tuning of the cellular immune responses against different types of infections and disease states. However, the molecular mechanisms of the interplay between TLR and hypoxic signaling are still largely unknown. In the present study, we describe a pathway by which hypoxia potentiates TNF-α secretion by human monocyte-derived DCs (moDCs). MoDCs are differentiated from blood-isolated monocytes by culturing with IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF). Although their physiological role is unclear, moDCs are capable of migration to lymph nodes and of both MHC-I and II presentation [19,23–28,30,32–35]. We show an increase in LPS-induced secretion of TNF-α by moDCs upon hypoxia, in line with previous findings [31,32]. Our data show that this potentiation is caused by hypoxia-promoted expression of mitogen-activated protein kinase (MAPK) kinase kinase 8 (MAP3K8; TPL-2; COT), which is a kinase involved in the MAP3K signaling pathway downstream of TLR4. Given the essential roles of DCs in T-cell activation and differentiation, our results are important for understanding T-cell responses in cancer, infection, autoimmunity, and other diseases and disorders associated with hypoxia.
Experimental
Cells and culture conditions
MoDCs were derived from peripheral blood mononuclear cells (PBMCs) obtained from buffy coats of healthy individuals as described previously [38]. Approval to conduct experiments with human blood samples was obtained from the blood bank and all experiments were conducted according to national and institutional guidelines. Informed consent was obtained from all blood donors by the Dutch blood bank. Samples were anonymized and none of the investigators could ascertain the identity of the blood donors. Briefly, peripheral blood leukocytes (PBLs) were separated from monocytes by a 1-h adhesion step at 5% CO2 and 37°C in RPMI-1640 (Thermo Fisher Scientific, Waltham, U.S.A.) with 2% human serum. Monocytes were differentiated into DCs by culturing for 6 days at 5% CO2 and 37°C in RPMI-1640 (Thermo Fisher Scientific) with 300 U/ml IL-4, 450 U/ml GM-CSF, 10% FBS (Greiner Bio-one, Kremsmünster, Austria), 2 mM UltraGlutamine (Lonza, Basel, Switzerland), and 1% antibiotic-antimycotic (Gibco by Life Technologies, Kremsmünster, Austria). For hypoxic culture, moDCs were kept overnight at 1% O2, 5% CO2, and 94% N2 at 37°C (CB53 incubator, Binder, Tuttlingen, Germany) in complete RPMI-1640 (Thermo Fisher Scientific) with 10% FBS (Greiner Bio-one), 2 mM UltraGlutamine (Lonza, Basel, Switzerland), and 1% antibiotic-antimycotic (Gibco by Life Technologies). For normoxic culture, cells were kept at atmospheric O2 (20%) with 5% CO2. To activate the DCs, 1 µg/ml of LPS (LPSs from Escherichia coli 0111:B4, Sigma–Aldrich, St. Louis, U.S.A.) was added to the medium. To quantitate cytokine production, cell culture supernatants were collected and stored at −80°C until analysis with IL-6 and TNF-α-specific ELISA (eBioscience, San Diego, U.S.A.).
Inhibitors and antibodies
To inhibit p38 signaling, we used 1 µM SB203580 (Cell Signaling Technology, Danvers, U.S.A.). To inhibit IκB kinase (IKK), we used 100 nM IKK-16 (R&D Systems, Minneapolis, U.S.A.), to inhibit MAP3K8 we used 1 µM of Tpl2 kinase inhibitor (Cayman Chemical, Ann Arbor, U.S.A.), to inhibit Erk1/2 we used 250 nM of CAY10561 (Cayman Chemical). Primary antibodies used were: mouse-IgG1 anti-MAP3K8 (sc-373677, Santa Cruz Biotechnology, Dallas, U.S.A.), rabbit monoclonal IgG anti-p38/ MAPK (8690, Cell Signaling Technology), rabbit monoclonal IgG anti-p-p38/MAPK (Thr180/Tyr182) (4511, Cell Signaling Technology), rabbit polyclonal anti-p44/p42 MAPK (Erk1/2) (Cell Signaling Technology), mouse-IgG1 anti-p-p44/p42 MAPK (Erk1/2) (Thr202/Tyr204) (Cell Signaling Technology), rabbit monoclonal IgG anti-GAPDH (2118, Cell Signaling Technology). The following secondary antibodies were used: goat anti-rabbit or anti-mouse IgG (H+L) IRDye 800 CW (926-32211, 926-32210, LI-COR Biosciences, Lincoln, U.S.A.). Cell death was assessed with Zombie Violet fixable viability dye (BioLegend, San Diego, U.S.A.). The following directly labeled antibodies were used: CD11c-FITC (BD Biosciences, Franklin Lakes, U.S.A.), CD14-PE-Cy7 (Miltenyi Biotec, Bergisch Gladbach, Germany), CD16-APC (Miltenyi Biotec), CD11b-PE-Cy7 (Beckman Coulter, Brea, U.S.A.), CD68-APC (BioLegend), HLA-DR-PE (BD Biosciences), CD83-FITC (BD Biosciences), CD86-APC (BD Biosciences), TLR2-FITC (BioLegend), TLR4-APC (BioLegend).
Western blot
SDS/PAGE with Western blot was used to analyze MAP3K8 knockdown efficiency and phosphorylation of p38 and Erk1/2 using 10% acrylamide gels. Proteins were then transferred to PVDF membranes (Immobilon-FL 0.45 µm, Merck, Burlington, U.S.A.), blocked for 1 h with 5% BSA in Tris/HCl-buffered saline and incubated overnight with primary antibodies (1:200 to 1:500 dilution). These were labeled by incubating with IRDye800–conjugated secondary antibodies (1:5000 dilution; LI-COR Biosciences) and analyzed using the Odyssey CLx Infrared Imaging System and ImageStudio Lite 5.0 analysis software (LI-COR Biosciences).
Flow cytometry
For flow cytometry assays, moDCs were seeded at 100,000 cells per well in 100 µl of complete RPMI in a V-bottom 96-well plate. After incubation at hypoxic or atmospheric oxygen levels and with or without inhibitors, as described above, cells were centrifuged at 1500 rpm for 2 min at 4°C. Supernatant was discarded and cells were incubated for 30 min in the dark with PBS containing Zombie Violet fixable viability stain (BioLegend, 1:2000 dilution), then fixed with 4% PFA for 2 min and incubated for 10 min in the dark on ice with 50 µl of phosphate-buffered azide (PBA; PBS containing 0.5% BSA and 0.01% NaN3) containing 1% human serum to block Fc receptors. Then, cells were incubated for 30 min in the dark on ice with 50 µl of PBA containing directly labeled antibodies. To perform compensation and set gates, AbC Total Antibody Compensation beads (Thermo Fischer Scientific) were stained in the same way as the cell samples. Cells were washed with 100 µl PBA, resuspended in 60 µl PBA, and analyzed on a FACSCalibur or FACSVerse flow cytometer (BD Biosciences).
Transfections
DCs were electroporated with siRNA against MAP3K8 (a 1:1:1 mix of GGCGUGUAAACUGAUCCCAGUAGAU, GGAAGGAGCUGGAACUUCCUGAGAA, and UGGUUGUCAUCAGUCAGAUAUGGAA; Thermo Fischer Scientific) using a Neon electroporation system (Thermo Fischer Scientific). Briefly, DCs were thawed from liquid nitrogen storage, washed once with PBS and resuspended in R buffer (from the Neon kit) at 0.5–1.0 × 106 cells in 120 µl volume. Four micrograms of plasmid DNA or 20 pmol siRNA mix was added and the cells were electroporated with two pulses of 1000 V at 40 ms pulse width. Cells were then transferred to pre-warmed RPMI-1640 containing 2 mM UltraGlutamine. Four hours post transfection, an equal amount of complete RPMI-1640 was added to the cells. Experiments were performed 24 h post-transfection.
Primers and PCRs
MAP3K8 expression in untreated moDCs was checked with PCR on cDNA using primers for MAP3K8 (CTCCCCAAAATGGACGTTACC and GGATTTCCACATCAGATGGCTTA). Expression levels were quantitated with real-time quantitative PCR (RT-qPCR) using validated primers for MAP3K8 (GAGCGTTCTAAGTCTCTGCTG and GCAAGCAAATCCTCCACAGTTC), TBP (GAGCCAAGAGTGAAGAACAGTC and GCTCCCCACCATATTCTGAATCT) and GUSB (GACACGCTAGAGCATGAGGG and GGGTGAGTGTGTTGTTGATGG). Briefly, mRNA was isolated from DCs using a Quick-RNA MiniPrep Plus kit (Zymo Research, Irvine, U.S.A.) and cDNA was generated in a standard reverse transcriptase reaction. qPCRs were done on a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, U.S.A.), using FastStart SYBR Green Master mix (Hoffmann-La Roche, Basel, Switzerland) and analyzed in CFX Manager software version 3.1 (Bio-Rad, Hercules, U.S.A.). Gene expression was first normalized (ΔΔCq) to housekeeping genes (TBP and GUSB) validated with geNorm analysis, followed by normalization to untreated samples.
Statistical analysis
For datasets with two paired conditions, paired two-sided Student’s t tests were applied to assess significance when data followed a normal distribution, otherwise Wilcoxon’s matched pairs signed-rank tests were used. One-way ANOVA with Bonferroni’s post-hoc tests were applied for multiple comparisons. Box plots indicate mean with whiskers depicting minimal and maximal values. A value of P<0.05 was considered statistically significant (*P<0.05, **P<0.01, ***P<0.001).
Results
TLR4-triggered TNF-α secretion is potentiated by hypoxia
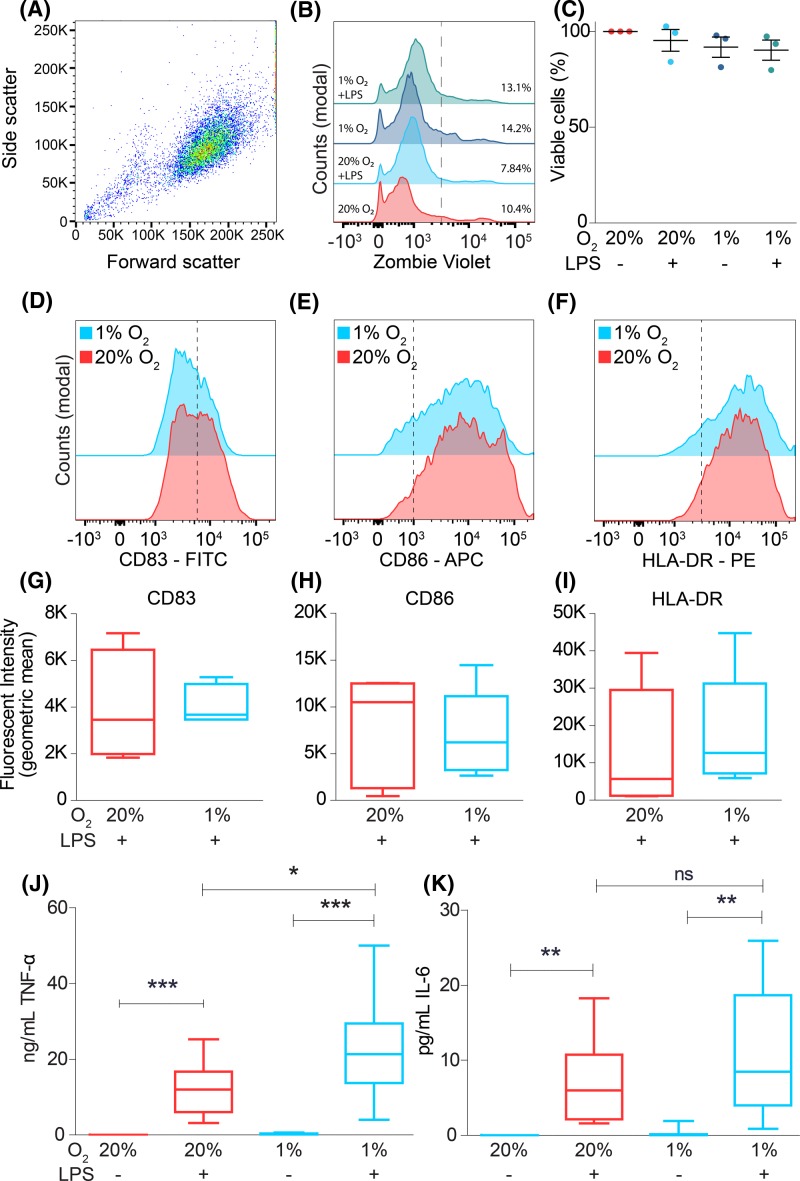
We started by studying the interplay of hypoxia and infection on the secretion of IL-6 and TNF-α. We used DCs differentiated from monocytes isolated from the blood of healthy volunteers by culturing in the presence of IL-4 and GM-CSF (moDCs). MoDCs are capable of endocytosis, phagocytosis, antigen (cross-)presentation and activation of T cells, and are a widely used model system for studying hypoxia on DC function [19,23–28,30,32–35]. Although moDCs are also found in vivo, they differ from conventional blood-circulating DC subsets and there has been an ongoing debate about their exact physiological role [39–41]. We characterized our moDC cultures by measuring expression of several DC, monocyte and macrophage surface markers. MoDCs were found to be a homogeneous population of CD11c+/CD11b+/CD16−/CD68− cells, of which ∼87% also expressed CD14+ (Supplementary Figure S1A–I). These results show that our cells are composed mainly of DC-like cells which express high levels of the DC marker CD11c. Monocytic lineage markers CD14 and CD11b were also highly expressed, whereas only low expression of the monocyte/macrophage markers CD16 and CD68 was observed. The moDCs were cultured in a hypoxic environment of 1% oxygen, or in an atmospheric environment of 20% oxygen. Similar to previous reports [19,25,28,31,35], flow cytometry experiments showed that culturing DCs overnight under hypoxic conditions did not significantly affect cell viability (Figure 1A–C). Culturing under hypoxic conditions trended toward decreased CD14 expression and a non-consistent increase in CD68 expression, however these differences are not significant. Expression of all other tested surface markers was unaltered (Supplementary Figure S1J–S).
Figure 1. Hypoxia potentiates LPS-induced TNF-α secretion.
(A–C) Cell viability of moDCs during atmospheric (20% O2) and hypoxic (1% O2) oxygen levels in presence or absence of LPS, determined by Zombie Violet as analyzed by flow cytometry. Representative dot plot (A), histograms (B), and quantitation for three donors ((C); average ± S.E.M.) are shown. (D–I) LPS-induced maturation of moDCs cultured at atmospheric or hypoxic oxygen levels, determined by expression levels of maturation markers CD83 (D,G), CD86 (E,H), and HLA-DR (F,I). Representative histograms showed quantitation for five donors. (J,K) Secretion by ELISA of the pro-inflammatory cytokines TNF-α (J) and IL-6 (K) from moDCs cultured at atmospheric or hypoxic oxygen levels. Quantitation from ten donors (average ± S.E.M.). *P<0.05; **P<0.01; ***P<0.001. Abbreviation: ns, not significant.
To stimulate an inflammatory response, we cultured the moDCs in the presence of LPS. Compared with LPS at atmospheric oxygen levels, LPS with hypoxia did not result in significant changes in surface expression of the maturation markers CD83 and CD86 (Figure 1D,E,G,H), as reported previously [26,28,30], but contrasting with other studies [19,21,25,31,32]. Surface levels of MHC-II were also not changed by hypoxia (Figure 1F,I), which again corresponds with some studies [28,30], while other studies report higher [31] or lower [21,25–27,29,32] MHC-II levels upon hypoxia. We observed the strongest potentiation for production of TNF-α, where hypoxia resulted in an approximately two-fold significant increase in LPS-triggered TNF-α secretion compared with atmospheric oxygen (Figure 1J). The increase in LPS-induced IL-6 secretion upon hypoxia was not significant compared with atmospheric oxygen levels (Figure 1K). A potentiation in TLR-stimulated TNF-α secretion by DCs upon hypoxia has been reported previously in some [31,32], but not all [21,27], studies. Moreover, hypoxia-mediated potentiation of TNF-α secretion has been observed for macrophages [17], osteoblasts [42], and human hepatocellular carcinoma cells [36].
SB203580, an inhibitor for p38/MAPK, blocks hypoxia-potentiated TNF-α secretion
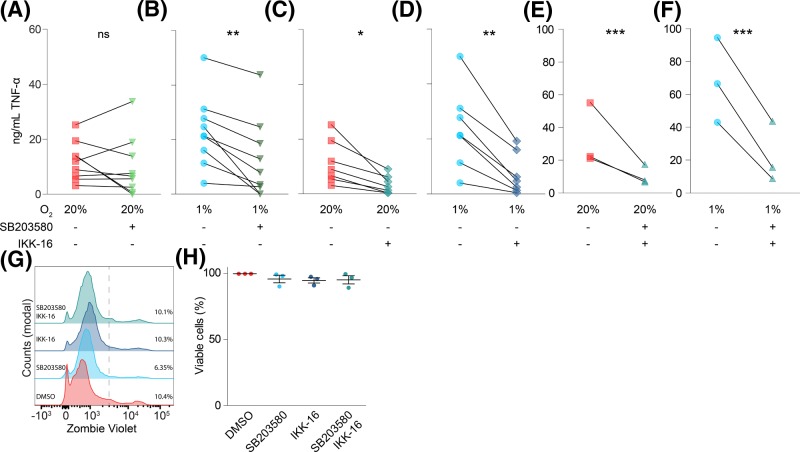
TLR4 stimulation triggers TNF-α secretion via two distinct signaling pathways. In the first pathway, TNF-α expression is up-regulated by activated nuclear factor κB (NF-κB) following IKK phosphorylation by TLR4. In the second pathway, TNF-α secretion is mediated via p38/MAPK signaling, which is activated in tandem with NF-κB following TLR4 stimulation [43,44]. We used specific inhibitors to elucidate the contribution of each pathway in the hypoxia-mediated potentiation of TNF-α secretion. Inhibition of p38/MAPK with SB203580, which inhibits the downstream catalytic activity of p38 [45,46], did not affect LPS-triggered TNF-α secretion under atmospheric oxygen levels (Figure 2A). In contrast, under hypoxic conditions, SB203580 resulted in a consistent and significant reduction in TNF-α secretion (Figure 2B). Blockage of IKK, which is responsible for activation of NF-κB [47,48], by IKK-16 blocked TNF-α secretion almost completely (to ∼20% of untreated) both under atmospheric and hypoxic conditions (Figure 2C,D). Simultaneous addition of both SB203580 and IKK-16 had a comparable effect (Figure 2E,F). The residual TNF-α secretion might be due to incomplete inhibition of the NF-κB and/or p38/MAPK pathways or due to the involvement of other signaling pathways. Under our conditions, SB203580 and IKK-16 did not affect the viability of the cells (Figure 2G,H). These findings suggest that the hypoxia-mediated potentiation of TNF-α release primarily occurs via increased activation of the p38/MAPK signaling pathway, while the IKK signaling pathway is activated by LPS irrespective of oxygen levels. In-line with this, hypoxia was reported to promote TLR2 and TLR4-induced pro-inflammatory activation of macrophages via the p38/MAPK pathway, but not via IKK signaling [49]. In contrast with macrophages [50], surface expression of TLR2 and TLR4 was unaffected by hypoxia (Supplementary Figure S2A–D), suggesting that the increased TNF-α secretion was not caused by increased expression of these pattern recognition receptors.
Figure 2. Hypoxia-mediated potentiation of TNF-α secretion is controlled by p38/MAPK.
(A–F) Effects of inhibitors of p38/MAPK (1 µM SB203580; (A,B,E,F)) and/or IKK (100 nM IKK-16; (C–F)) on LPS-induced TNF-α secretion by moDCs cultured under atmospheric (20% O2; (A,C,E)) or hypoxic (1% O2; (B,D,F)) oxygen levels. Individual donors shown. (G,H) Effects of SB203580 and/or IKK-16 on moDC viability, determined by flow cytometry labeling with Zombie Violet as analyzed by flow cytometry. Representative histograms (G) and quantitation for four donors ((H); average ± S.E.M.). *P<0.05; **P<0.01; ***P<0.001. Abbreviaton: ns, not significant.
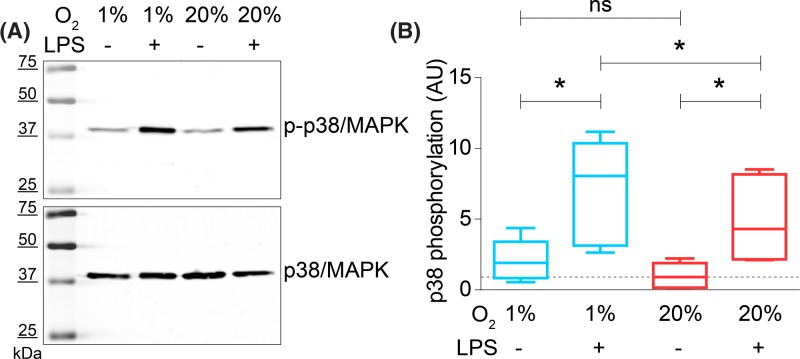
To directly investigate the activation of the p38/MAPK pathway by hypoxia and LPS, we determined activation of p38 by Western blot with phosphospecific antibodies (Figure 3A). As described previously, LPS treatment of DCs leads to activation of p38 by phosphorylation of the residues Thr180 and Tyr182 [51]. In-line with this, we observed a three- to four-fold increase in p-p38 upon LPS stimulation compared with unstimulated cells at atmospheric oxygen levels. Culturing the cells under hypoxic conditions in presence of LPS led to a further significant increase in p38 phosphorylation by ∼50%, whereas this increase was non-significant in absence of LPS (Figure 3B).
Figure 3. LPS-induced phosphorylation of p38/MAPK is increased at hypoxia.
(A) Representative Western blot of moDCs cultured at atmospheric (20% O2) or hypoxic (1% O2) oxygen levels and in presence or absence of LPS. The blot was stained with an antibody specific for p-p38/MAPK (top) and total p38/MAPK (bottom). (B) Quantitation of (A) for five donors. p38/MAPK phosphorylation during hypoxic inflammation, normalized to unstimulated cells at atmospheric oxygen levels (LPS-, 20% O2). *P<0.05; **P<0.01; ***P<0.001. Abbreviation: ns, not significant.
MAP3K8 is an HIF target gene and is overexpressed at hypoxic inflammation
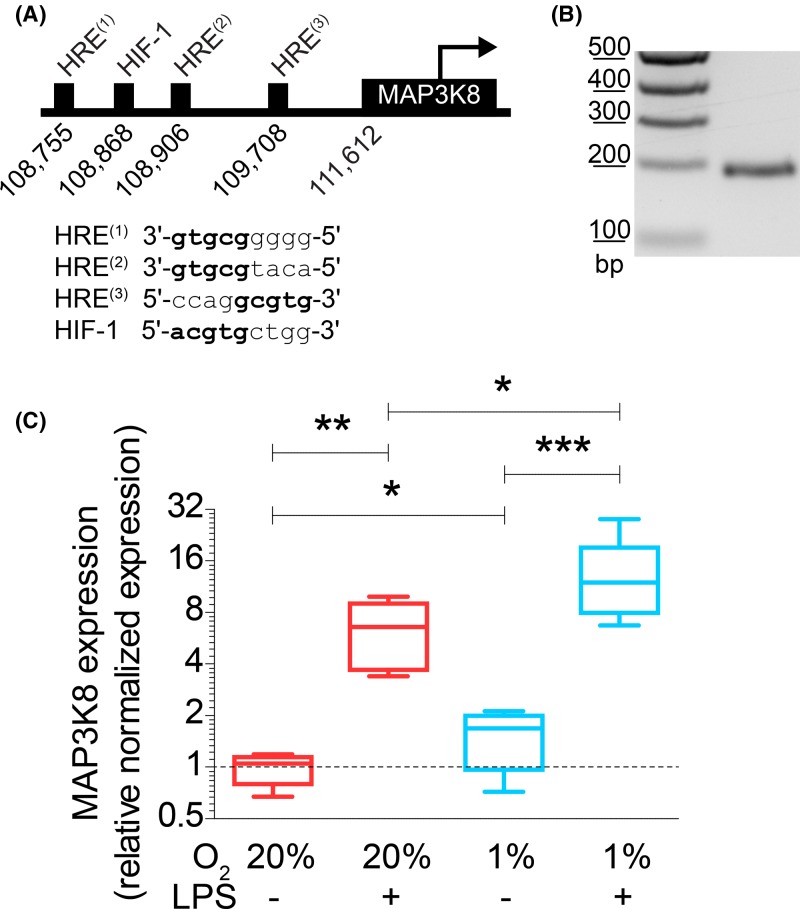
Hypoxia is well-known to increase expression of genes with an HRE in their promoter via the transcription factor HIF [15,16]. We therefore searched for genes coding for components in the p38/MAPK pathway carrying one or more HREs in their promoter. We identified MAP3K8 as a candidate gene downstream of HIF, since it is known to have a HIF-1β binding site and is up-regulated under hypoxia [17,52,53]. Indeed, we identified three HREs and an HIF-1-specific binding site upstream of the MAP3K8 gene on chromosome 10 (GenBank accession AL161651.13) using PROMO (Figure 4A) [54,55]. Reverse transcriptase (RT-) PCR showed that MAP3K8 is expressed in moDCs (Figure 4B).
Figure 4. Hypoxia increases expression of HIF target gene MAP3K8.
(A) Positions and sequences of HRE and HIF-binding site (HIF-1) in the promoter region of human MAP3K8. (B) MAP3K8 mRNA expression by moDCs as determined by PCR on cDNA obtained from unstimulated moDCs. Expected band size is 201 bp. (C) mRNA expression levels of MAP3K8 in moDCs cultured at atmospheric or hypoxic conditions in absence or presence of LPS determined by RT-qPCR. Quantitation from six donors. *P<0.05, **P<0.01, ***P<0.001.
Since MAP3K8 is a key mediator of p38/MAPK signaling, we hypothesized that the hypoxia-induced TNF-α potentiation occurred via increased expression of MAP3K8. To address this hypothesis, we cultured moDCs under atmospheric or hypoxic conditions and with or without addition of LPS, followed by mRNA isolation to analyze expression of MAP3K8 by RT-qPCR. Hypoxia led to a significant approximately two-fold increased expression of MAP3K8 in absence of LPS (Figure 4C). In the presence of LPS, this increase was even more pronounced and we observed an average ∼14-fold increase in MAP3K8 expression. Overall, the expression of MAP3K8 mirrors the potentiation of TNF-α secretion by a combination of LPS and hypoxia (Figure 1J) and is in qualitative agreement with the increased phosphorylation of p38 (Figure 3B). These findings support our hypothesis that MAP3K8 potentiates LPS-induced TNF-α secretion under hypoxic conditions.
MAP3K8 directly links HIF signaling to the p38/MAPK pathway
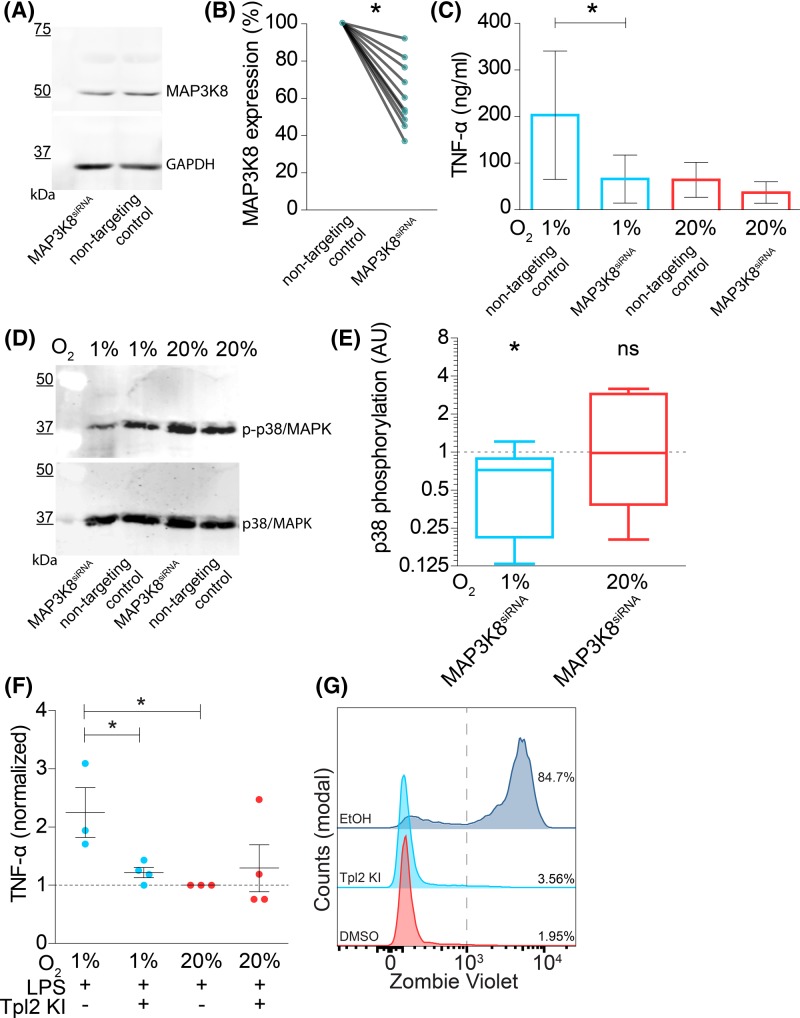
To obtain direct evidence that MAP3K8 is a key factor linking hypoxia to potentiation of LPS-stimulated TNF-α secretion, we performed knockdown experiments in moDCs where we targetted MAP3K8 with siRNA (MAP3K8siRNA). As a negative control, we transfected the moDCs with non-targetting siRNA. With this technique, we obtained an average reduction in ∼40% of MAP3K8 at the protein level, as quantitated by Western blot (Figure 5A,B). MAP3K8siRNA blocked the hypoxia-mediated potentiation of LPS-stimulated TNF-α secretion (Figure 5C). Phosphorylation of p38 was also reduced in MAP3K8siRNA moDCs following LPS stimulation at hypoxic, but not at atmospheric oxygen levels (Figure 5D,E).
Figure 5. MAP3K8 knockdown in moDCs blocks hypoxia-induced potentiation of LPS-induced TNF-α secretion.
(A) Representative Western blot of siRNA knockdown of MAP3K8 in moDCs (MAP3K8siRNA) stained with an antibody specific for MAP3K8 (top) and loading control (GAPDH; bottom). MoDCs were transfected with non-targetting siRNA as negative control. (B) Quantitation of (A) by band intensities normalized to GAPDH and shown relative to non-targetting control. Individual donors are shown. Compared using Wilcoxon’s matched-pairs signed rank test. (C) TNF-α secretion by moDCs with or without MAP3K8siRNA and cultured at atmospheric (20% O2) or hypoxic (1% O2) oxygen levels. Quantitation of 14 donors (average ± S.E.M.). (D) Phosphorylation of p38/MAPK in moDCs with or without MAP3K8siRNA and cultured at atmospheric (20% O2) or hypoxic (1% O2) oxygen levels. Representative Western blot probed for phosphorylated and total p38/MAPK shown. (E) Quantitation of (D) for six donors. Shown are the ratios of p38/MAPK phosphorylation for MAP3K8siRNA moDCs compared with non-targetting siRNA controls. (F) TNF-α secretion by moDCs following overnight incubation with LPS and Tpl2 kinase inhibitor (Tpl2 KI) normalized to atmospheric oxygen condition (average ± S.E.M.). (G) Representative flow cytometry histogram showing Zombie Violet cell viability staining on moDCs incubated overnight with Tpl2 KI, DMSO negative control, or 2 min 70% ethanol (EtOH) as positive control. *P<0.05. Abbreviation: ns, not significant.
We further validated the role of MAP3K8 in potentiation of TNF-α secretion with the small molecule inhibitor 4-[(3-chloro-4-fluorophenyl)amino]-6-[(3-pyridinylmethyl)amino]-1,7-naphthyridine-3-carbonitrile (Tpl2 kinase inhibitor). This molecule inhibits MAP3K8 by blocking binding of ATP to MAP3K8 [56] and did not affect cell viability (Figure 5G). In-line with the results obtained with siRNA knockdown, we observed that overnight incubation with this inhibitor together with LPS stimulation decreased the hypoxia-mediated potentiation of TNF-α secretion (Figure 5F). We also investigated phosphorylation of p38/MAPK following incubation with this inhibitor (Supplementary Figure S3A,B), and while we observed a reduction in LPS-mediated p38/MAPK phosphorylation under hypoxic conditions, this was not statistically significant. Together, these findings support that MAP3K8 promotes TLR-mediated TNF-α secretion under hypoxic conditions.
Another major downstream target of MAP3K8 is the MEK/Erk pathway, which is described in literature to promote neutrophil development and LPS-mediated TNF-α secretion [57–59]. Therefore, we also probed for Erk1/2 phosphorylation in moDCs stimulated with LPS and cultured at hypoxic and atmospheric oxygen levels (Supplementary Figure S3C,D). We used inhibitors for Erk1/2 (CAY10561) [60] and MAP3K8 (Tpl2 kinase inhibitor) in this experiment and performed a TNF-α ELISA. MoDC viability was unaffected by these small molecule inhibitors (Supplementary Figure S3 and Figure 5G). However, we observed only low and inconsistent activation of Erk1/2 following LPS and this was not altered by hypoxic culture. In contrast, a positive control of starved HeLa cells exposed to serum showed clear Erk1/2 phosphorylation. Moreover, the increased TNF-α secretion under hypoxic conditions was not modified by inhibition of Erk1/2 (Supplementary Figure S3E). Phosphorylation of p38/MAPK was also unaffected by this inhibitor (Supplementary Figure S3A,B). These results show that LPS-mediated activation of the p38 pathway is specifically potentiated via MAP3K8 under hypoxic conditions.
Discussion
Hypoxia is capable of both potentiating and suppressing the response of immune cells and other cells to TLR stimuli. In primary human macrophages, the TLR2- and 4-induced expression and secretion of various pro-inflammatory cytokines (amongst others IL-6, IL-8, and IL-1β) was shown to be increased by hypoxia. In osteoclasts, hypoxia induced TNF-α expression and this depended on HIF-1α expression [42]. However, the effects of hypoxia on human DC function are more controversial, and both the inhibition [21,25,27,32] as well as stimulation [19,31,32] of TLR-induced cytokine secretion by hypoxia have been described (e.g., for IL-6, IL-10, IL-12, TNF-α). Antigen uptake by DCs was shown to be less efficient in hypoxic DCs [27,30], although Bosseto et al. [19] showed that uptake was not affected for Leishmania infections. The effects of hypoxia on the subsequent activation of T cells by DCs are equally controversial, and Jantsch et al. [31] described an increase in activation, while others reported a decrease [27,32]. The relatively modest effects observed in this study may explain the many contradictory reports on the effects of hypoxia on immune cells.
The divergent effects of hypoxia on immune cell regulation are probably caused by the complex interplay of hypoxia on intracellular signaling. In addition to the stabilization of HIF transcription factors, hypoxia causes a metabolic shift toward anaerobic glycolysis, as also shown for DCs [61]. This metabolic shift can further affect the secretion of cytokines, as shown for IL-1β secretion by macrophages [62]. Moreover, for immune phagocytes such as DCs, TLR signaling can cause local hypoxia due to the large oxygen consumption by NAPDH oxidases that produce reactive oxygen species (ROS) during the respiratory burst [20,24]. These ROS in turn can act as second messengers, further modulating the transcriptional profile of immune cells, and for instance the hypoxia-induced activation of NFκB is redox sensitive [63]. TLR-signaling can also directly affect HIF signaling via post-translational regulation and increased expression of HIF [22,64–66]. Additionally, hypoxia was shown to up-regulate both HIF and TLR4 expression in macrophages [50] and synergistic induction of HIF-1α activity by hypoxia and TLR4 stimulation has been shown in macrophages [67]. Finally, TNF-α can already induce HIF-1α expression under atmospheric conditions [68]. All these interacting pathways make it difficult to discern cellular responses to hypoxia from those caused by TLR signaling.
Because of the multifactorial responses of cells to hypoxia, the molecular mechanisms of hypoxia-mediated regulation of immune cell differentiation are still largely unknown. In corneal epithelial cells, the hypoxia-mediated attenuation of NF-κB and Erk1/2 activation and of IL-6 and IL-8 secretion are dependent on myeloid differentiation primary response gene 88 (MyD88) downstream of TLR4. In PC12 cells and primary human macrophages, the potentiation of inflammatory cytokine secretion (IL-1β, IL-6, IL-8) by hypoxia is controlled specifically via p38/MAPK and c-Jun N-terminal kinase (JNK) signaling, but not via IκBα [49,69]. In this study, we identified a new molecular link between hypoxia and TLR signaling in human moDCs (Figure 6). We found that MAP3K8 expression is directly regulated by hypoxia via several HREs, resulting in increased expression of MAP3K8 [17]. As the MAP3K8 kinase is involved in activation of the p38/MAPK pathway [70,71], its up-regulation results in increased transcription of pro-inflammatory cytokines [57]. In this way, hypoxia-induced MAP3K8 up-regulation sensitizes the cells to TLR stimulation. Since studies with MAP3K8 knockout mice demonstrated that long-term secretion of TNF-α, but not IL-6, is regulated by MAP3K8 [72], our findings explain why hypoxia leads to a potentiation of TLR-stimulated TNF-α secretion, but not or less of IL-6 secretion.
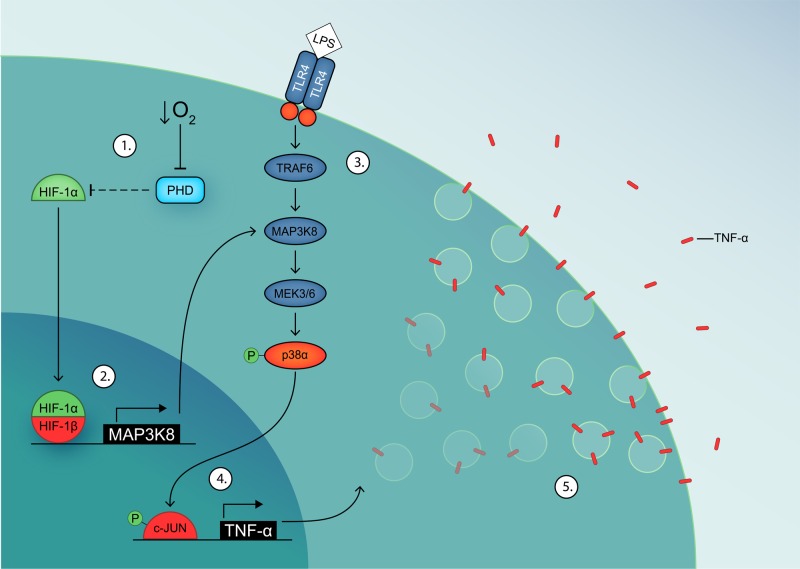
Figure 6. Model of MAP3K8-mediated potentiation of TNF-α secretion in hypoxic inflammation.
(1) Hypoxia inhibits PHD activity, leading to stabilization of HIF-1α. (2) HIF-1α dimerizes with HIF-1β and up-regulates MAP3K8 expression, which potentiates the p38/MAPK signaling cascade. (3) LPS-induced dimerization of TLR4 activates the p38/MAPK signaling cascade. (4) c-JUN is phosphorylated by p-p38α and up-regulates TNF-α expression. (5) TNF-α is transported to the plasma membrane and released.
Cancer cells express p38/MAPK, and this pathway is activated by hypoxia [73,74]. MAPK signaling can induce angiogenesis and metastasis, as well as tolerance by polarization of macrophages to the tolerogenic M2 subtype and of T cells to Th17 cells [12,75]. In this way, MAP3K8 expression promotes tumor growth and progression as well as immune suppression [76,77]. On the other hand, MAPK signaling can also have tumor suppressing functions: its expression is associated with lung cancer patient survival via a p38 signaling pathway downstream of JNK [78] and it suppresses colitis-associated tumorigenesis [79]. In-line with this, MAP3K8−/− mice show more tumor initiation and faster progression of chemically induced skin cancer [80].
MAP3K8 is also involved in autoimmunity. For example, MAP3K8 links IL-17 receptor signaling to TAK1 activation, which leads to increased JNK, p38, and NFκB activity in Th17 cells, promoting autoimmune neuroinflammation [81,82]. Moreover, MAP3K8 activity in T cells limits regulatory T cell (Treg) function by inhibiting FoxP3 expression and lowering production of the immunosuppressive cytokines IL-10 and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) [83]. In addition, TNF-α plays a pivotal role in a variety of autoimmune diseases, causing sustained inflammation and tissue damage [84]. In many autoimmune diseases, a negative feedback loop between tissue damage and inflammation exists, where MAP3K8 promotes myeloperoxidase activity, ROS production, and recruitment of neutrophils and macrophages, causing tissue damage [72]. This tissue damage and ROS production might subsequently lead to local hypoxia which further up-regulates MAP3K8 activity, contributing to the localized hypoxia observed in many autoimmune diseases. In-line with this, patients suffering from systemic sclerosis and cerebrovascular inflammation show clear signs of localized hypoxia, and fibroblasts from these patients show marked increases in activation of p38 [85–87]. In addition, MAP3K8−/− mice have reduced amounts of circulating neutrophils, which display impaired sensitivity to chemokines and lower TNF-α secretion [57,58]. Bone-marrow derived DCs of MAP3K8−/− mice also show impaired TNF-α secretion [88] and increased IL-12 [89]. It would be interesting to study DC function in these MAP3K8−/− mice under hypoxic conditions to confirm our in vitro findings.
In conclusion, we show that hypoxia potentiates LPS-stimulated TNF-α secretion in human moDCs by up-regulating expression of MAP3K8, a serine/threonine kinase capable of activating the p38/MAPK signaling pathway. Since our data provide a mechanistic link between hypoxia and immune cell function, and since these are important factors in infection, cancer, and autoimmune disease, MAP3K8 might be a promising therapeutic target for treatment of these diseases.
Supporting information
Supplementary Figure.
Supplementary Figure.
Supplementary Figure.
Abbreviations
- DC
dendritic cell
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HIF
hypoxia-induced factor
- HRE
hypoxic response element
- IL
interleukin
- IKK
IκB kinase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MAP3K8
MAPK kinase kinase 8
- moDC
monocyte-derived DC
- NF-κB
nuclear factor κB
- PBA
phosphate-buffered azide
- PHD
prolyl hydroxylase
- qPCR
quantitative polymerase chain reaction
- ROS
reactive oxygen species
- RT-qPCR
real-time quantitative PCR
- TLR4
toll-like receptor 4
- TNF-α
tumor necrosis factor α
Funding
This work was supported by the Hypatia Fellowship from the Radboud University Medical Center, the Starting Grant from the European Research Council [grant number ERC 336479]; the Career Development Award from the Human Frontier Science Program [grant number 00022/2014]; the Netherlands Organization for Scientific Research [grant number NWO-ALW VIDI 864.14.001]; and Gravitation [grant number 2013 ICI-024.002.009].
Author contribution
L.M.P., A.O., W.M., T.R.D.J.R. and G.v.d.B. designed and performed the experiments. M.t.B., M.B.B. and L.W.d.H contributed to the experiments. All authors contributed to writing the manuscript.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Baranov M.V, Ter Beest M., Reinieren-Beeren I., Cambi A., Figdor C.G. and van den Bogaart G. (2014) Podosomes of dendritic cells facilitate antigen sampling. J. Cell Sci. 127, 1052–1064 10.1242/jcs.141226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merad M., Sathe P., Helft J., Miller J. and Mortha A. (2013) The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 31, 563–604 10.1146/annurev-immunol-020711-074950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roche P.a. and Furuta K. (2015) The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 15, 203–216 10.1038/nri3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNamee E.N., Korns Johnson D., Homann D. and Clambey E.T. (2013) Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol. Res. 55, 58–70 10.1007/s12026-012-8349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Policastro L. and Ibañez I. (2013) The tumor microenvironment: characterization, redox considerations, and novel approaches for reactive oxygen species-targeted gene therapy. Redox Signal. 19, 854–895 10.1089/ars.2011.4367 [DOI] [PubMed] [Google Scholar]
- 6.Noman M.Z., Hasmim M., Messai Y., Terry S., Kieda C., Janji B.. et al. (2015) Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia.. Am. J. Physiol. Cell Physiol. 309, C569–C579 10.1152/ajpcell.00207.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flannagan R.S., Cosío G. and Grinstein S. (2009) Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 7, 355–366 10.1038/nrmicro2128 [DOI] [PubMed] [Google Scholar]
- 8.Shalova I.N., Lim J.Y., Chittezhath M., Zinkernagel A.S., Beasley F., Hernández-Jiménez E.. et al. (2015) Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1α. Immunity 42, 484–498 10.1016/j.immuni.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 9.Chaudhari S.M., Sluimer J.C., Koch M., Theelen T.L., Manthey H.D., Busch M.. et al. (2015) Deficiency of HIF1α in antigen-presenting cells aggravates atherosclerosis and type 1 T-helper cell responses in mice. Arterioscler. Thromb. Vasc. Biol. 35, 2316–2325 10.1161/ATVBAHA.115.306171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe S.S., Shin K.C., Ka S., Lee Y.K., Chun J.-S. and Kim J.B. (2014) Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes 63, 3359–3371 10.2337/db13-1965 [DOI] [PubMed] [Google Scholar]
- 11.Quiñonez-Flores C.M., González-Chávez S.A. and Pacheco-Tena C. (2016) Hypoxia and its implications in rheumatoid arthritis. J. Biomed. Sci. 23, 62 10.1186/s12929-016-0281-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann S., Te Boekhorst V., Odenthal J., Bianchi R., van Helvert S., Ikenberg K.. et al. (2017) Hypoxia induces a HIF-1-dependent transition from collective-to-amoeboid dissemination in epithelial cancer cells. Curr. Biol. 27, 392–400 10.1016/j.cub.2016.11.057 [DOI] [PubMed] [Google Scholar]
- 13.Palazon A., Goldrath A.W., Nizet V. and Johnson R.S. (2014) HIF transcription factors, inflammation, and immunity. Immunity 41, 518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kietzmann T., Mennerich D. and Dimova E.Y. (2016) Hypoxia-inducible factors (HIFs) and phosphorylation: impact on stability, localization, and transactivity. Front. Cell Dev. Biol. 4, 11 10.3389/fcell.2016.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G.L. and Semenza G.L. (1993) Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 268, 21513–21518 [PubMed] [Google Scholar]
- 16.Wenger R.H., Stiehl D.P. and Camenisch G. (2005) Integration of oxygen signaling at the consensus HRE. Sci. STKE 2005, re12 [DOI] [PubMed] [Google Scholar]
- 17.Fang H., Hughes R., Murdoch C., Coffelt S.B., Biswas S.K., Harris A.L.. et al. (2009) Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood 114, 844–859 10.1182/blood-2008-12-195941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhandari T., Olson J., Johnson R.S. and Nizet V. (2013) HIF-1α influences myeloid cell antigen presentation and response to subcutaneous OVA vaccination. J. Mol. Med. 91, 1199–1205 10.1007/s00109-013-1052-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosseto M.C., Palma P.V.B., Covas D.T. and Giorgio S. (2010) Hypoxia modulates phenotype, inflammatory response, and leishmanial infection of human dendritic cells. APMIS 118, 108–114 10.1111/j.1600-0463.2009.02568.x [DOI] [PubMed] [Google Scholar]
- 20.Spirig R., Djafarzadeh S., Regueira T., Shaw S.G., von Garnier C., Takala J.. et al. (2010) Effects of TLR agonists on the hypoxia-regulated transcription factor HIF-1alpha and dendritic cell maturation under normoxic conditions. PLoS ONE 5, e0010983 10.1371/journal.pone.0010983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q., Liu C., Zhu F., Liu F., Zhang P., Guo C.. et al. (2010) Reoxygenation of hypoxia-differentiated dentritic cells induces Th1 and Th17 cell differentiation. Mol. Immunol. 47, 922–931 10.1016/j.molimm.2009.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jantsch J., Wiese M., Schödel J., Castiglione K., Gläsner J., Kolbe S.. et al. (2011) Toll-like receptor activation and hypoxia use distinct signaling pathways to stabilize hypoxia-inducible factor 1α (HIF1A) and result in differential HIF1A-dependent gene expression. J. Leukoc. Biol. 90, 551–562 10.1189/jlb.1210683 [DOI] [PubMed] [Google Scholar]
- 23.Lloberas N., Rama I., Llaudó I., Torras J., Cerezo G., Cassis L.. et al. (2013) Dendritic cells phenotype fitting under hypoxia or lipopolysaccharide; adenosine 5’-triphosphate-binding cassette transporters far beyond an efflux pump. Clin. Exp. Immunol. 172, 444–454 10.1111/cei.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fliesser M., Morton C.O., Bonin M., Ebel F., Hünniger K., Kurzai O.. et al. (2015) Hypoxia-inducible factor 1α modulates metabolic activity and cytokine release in anti-Aspergillus fumigatus immune responses initiated by human dendritic cells. Int. J. Med. Microbiol. 305, 865–873 10.1016/j.ijmm.2015.08.036 [DOI] [PubMed] [Google Scholar]
- 25.Fliesser M., Wallstein M., Kurzai O., Einsele H. and Löffler J. (2016) Hypoxia attenuates anti-Aspergillus fumigatus immune responses initiated by human dendritic cells. Mycoses 59, 503–508 10.1111/myc.12498 [DOI] [PubMed] [Google Scholar]
- 26.Qu X., Yang M.-X., Kong B.-H., Qi L., Lam Q.L.K., Yan S.. et al. (2005) Hypoxia inhibits the migratory capacity of human monocyte-derived dendritic cells. Immunol. Cell Biol. 83, 668–673 10.1111/j.1440-1711.2005.01383.x [DOI] [PubMed] [Google Scholar]
- 27.Yang M., Ma C., Liu S., Sun J., Shao Q., Gao W.. et al. (2009) Hypoxia skews dendritic cells to a T helper type 2-stimulating phenotype and promotes tumour cell migration by dendritic cell-derived osteopontin. Immunology 128, e237–49 10.1111/j.1365-2567.2008.02954.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao W., Darmanin S., Fu Q., Chen J., Cui H., Wang J.. et al. (2005) Hypoxia suppresses the production of matrix metalloproteinases and the migration of human monocyte-derived dendritic cells. Eur. J. Immunol. 35, 3468–3477 10.1002/eji.200526262 [DOI] [PubMed] [Google Scholar]
- 29.Goth S.R., Chu R.A. and Pessah I.N. (2006) Oxygen tension regulates the in vitro maturation of GM-CSF expanded murine bone marrow dendritic cells by modulating class II MHC expression. J. Immunol. Methods 308, 179–191 10.1016/j.jim.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 30.Elia A.R., Cappello P., Puppo M., Fraone T., Vanni C., Eva A.. et al. (2008) Human dendritic cells differentiated in hypoxia down-modulate antigen uptake and change their chemokine expression profile. J. Leukoc. Biol. 84, 1472–1482 10.1189/jlb.0208082 [DOI] [PubMed] [Google Scholar]
- 31.Jantsch J., Chakravortty D., Turza N., Prechtel A.T., Buchholz B., Gerlach R.G.. et al. (2008) Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J. Immunol. 180, 4697–4705 10.4049/jimmunol.180.7.4697 [DOI] [PubMed] [Google Scholar]
- 32.Mancino A., Schioppa T., Larghi P., Pasqualini F., Nebuloni M., Chen I.H.. et al. (2008) Divergent effects of hypoxia on dendritic cell functions. Blood 112, 3723–3734 10.1182/blood-2008-02-142091 [DOI] [PubMed] [Google Scholar]
- 33.Rama I., Bruene B., Torras J., Koehl R., Cruzado J.M., Bestard O.. et al. (2008) Hypoxia stimulus: An adaptive immune response during dendritic cell maturation. Kidney Int. 73, 816–825 10.1038/sj.ki.5002792 [DOI] [PubMed] [Google Scholar]
- 34.Ricciardi A., Elia A.R., Cappello P., Puppo M., Vanni C., Fardin P.. et al. (2008) Transcriptome of hypoxic immature dendritic cells: modulation of chemokine/receptor expression. Mol. Cancer Res. 6, 175–185 10.1158/1541-7786.MCR-07-0391 [DOI] [PubMed] [Google Scholar]
- 35.Zhao P., Li X., Yang M., Shao Q., Wang D., Liu S.. et al. (2008) Hypoxia suppresses the production of MMP-9 by human monocyte-derived dendritic cells and requires activation of adenosine receptor A2b via cAMP/PKA signaling pathway. Mol. Immunol. 45, 2187–2195 10.1016/j.molimm.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 36.Zhang X., Li S., Li M., Huang H., Li J. and Zhou C. (2016) Hypoxia-inducible factor-1α mediates the toll-like receptor 4 signaling pathway leading to anti-tumor effects in human hepatocellular carcinoma cells under hypoxic conditions. Oncol. Lett. 12, 1034–1040 10.3892/ol.2016.4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krawczyk C.M., Holowka T., Sun J., Blagih J., Amiel E., DeBerardinis R.J.. et al. (2010) Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115, 4742–4749 10.1182/blood-2009-10-249540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Vries I.J.M., Adema G.J., Punt C.J.a. and Figdor C.G. (2005) Phenotypical and functional characterization of clinical-grade dendritic cells. Methods Mol. Med. 109, 113–126 [DOI] [PubMed] [Google Scholar]
- 39.Helft J., Böttcher J., Chakravarty P., Zelenay S., Huotari J., Schraml B.U.. et al. (2015) GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c+MHCII+ macrophages and dendritic cells. Immunity 42, 1197–1211 10.1016/j.immuni.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 40.Randolph G.J., Inaba K., Robbiani D.F., Steinman R.M. and Muller W.A. (1999) Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11, 753–761 10.1016/S1074-7613(00)80149-1 [DOI] [PubMed] [Google Scholar]
- 41.Ko H.-J., Brady J.L., Ryg-Cornejo V., Hansen D.S., Vremec D., Shortman K.. et al. (2014) GM-CSF-responsive monocyte-derived dendritic cells are pivotal in Th17 pathogenesis. J. Immunol. 192, 2202–2209 10.4049/jimmunol.1302040 [DOI] [PubMed] [Google Scholar]
- 42.Xing Y., Wang R., Chen D., Mao J., Shi R., Wu Z.. et al. (2015) COX2 is involved in hypoxia-induced TNF-α expression in osteoblast. Sci. Rep. 5, 10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell J., Ciesielski C.J., Hunt A.E., Horwood N.J., Beech J.T., Hayes L.a. et al. (2004) A novel mechanism for TNF-alpha regulation by p38 MAPK: involvement of NF-kappa B with implications for therapy in rheumatoid arthritis. J. Immunol. 173, 6928–6937 10.4049/jimmunol.173.11.6928 [DOI] [PubMed] [Google Scholar]
- 44.Lee J.C., Laydon J.T., McDonnell P.C., Gallagher T.F., Kumar S., Green D.. et al. (1994) A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739–746 10.1038/372739a0 [DOI] [PubMed] [Google Scholar]
- 45.Kumar S., Jiang M.S., Adams J.L. and Lee J.C. (1999) Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 263, 825–831 10.1006/bbrc.1999.1454 [DOI] [PubMed] [Google Scholar]
- 46.Cuenda A., Rouse J., Doza Y.N., Meier R., Cohen P., Gallagher T.F.. et al. (1995) SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364, 229–233 10.1016/0014-5793(95)00357-F [DOI] [PubMed] [Google Scholar]
- 47.Waelchli R., Bollbuck B., Bruns C., Buhl T., Eder J., Feifel R.. et al. (2006) Design and preparation of 2-benzamido-pyrimidines as inhibitors of IKK. Bioorg. Med. Chem. Lett. 16, 108–112 10.1016/j.bmcl.2005.09.035 [DOI] [PubMed] [Google Scholar]
- 48.Mercurio F., Murray B.W., Shevchenko A., Bennett B.L., Young D.B., Li J.W.. et al. (1999) IkappaB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol. Cell. Biol. 19, 1526–1538 10.1128/MCB.19.2.1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snodgrass R.G., Boß M., Zezina E., Weigert A., Dehne N., Fleming I.. et al. (2016) Hypoxia potentiates palmitate-induced pro-inflammatory activation of primary human macrophages. J. Biol. Chem. 291, 413–424 10.1074/jbc.M115.686709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S.Y., Choi Y.J., Joung S.M., Lee B.H., Jung Y.S. and Lee J.Y. (2010) Hypoxic stress up-regulates the expression of Toll-like receptor 4 in macrophages via hypoxia-inducible factor. Immunology 129, 516–524 10.1111/j.1365-2567.2009.03203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raingeaud J., Gupta S., Rogers J.S., Dickens M., Han J., Ulevitch R.J.. et al. (1995) Pro-inflammatory cytokines and environmental stress cause p38 mitogen- activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270, 7420–7426 10.1074/jbc.270.13.7420 [DOI] [PubMed] [Google Scholar]
- 52.Mole D.R., Blancher C., Copley R.R., Pollard P.J., Gleadle J.M., Ragoussis J.. et al. (2009) Genome-wide association of hypoxia-inducible factor (HIF)-1 and HIF-2 DNA Binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 284, 16767–16775 10.1074/jbc.M901790200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lo R. and Matthews J. (2012) High-resolution genome-wide mapping of AHR and ARNT binding sites by ChIP-Seq. Toxicol. Sci. 130, 349–361 10.1093/toxsci/kfs253 [DOI] [PubMed] [Google Scholar]
- 54.Messeguer X., Escudero R., Farré D., Núñez O., Martínez J. and Albà M.M. (2002) PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 18, 333–334 10.1093/bioinformatics/18.2.333 [DOI] [PubMed] [Google Scholar]
- 55.Farré D., Roset R., Huerta M., Adsuara J.E., Roselló L., Albà M.M.. et al. (2003) Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 31, 3651–3653 10.1093/nar/gkg605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu Y., Green N., Gavrin L.K., Janz K., Kaila N., Li H.-Q.. et al. (2006) Inhibition of Tpl2 kinase and TNFalpha production with quinoline-3-carbonitriles for the treatment of rheumatoid arthritis. Bioorg. Med. Chem. Lett. 16, 6067–6072 10.1016/j.bmcl.2006.08.102 [DOI] [PubMed] [Google Scholar]
- 57.Dumitru C.D., Ceci J.D., Tsatsanis C., Kontoyiannis D., Stamatakis K., Lin J.H.. et al. (2000) TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103, 1071–1083 10.1016/S0092-8674(00)00210-5 [DOI] [PubMed] [Google Scholar]
- 58.Acuff N.V, Li X., Elmore J., Rada B. and Watford W.T. (2017) Tpl2 promotes neutrophil trafficking, oxidative burst, and bacterial killing. J. Leukoc. Biol. 101, 1325–1333 10.1189/jlb.3A0316-146R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salmeron A., Ahmad T.B., Carlile G.W., Pappin D., Narsimhan R.P. and Ley S.C. (1996) Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 15, 817–826 10.1002/j.1460-2075.1996.tb00417.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aronov A.M., Baker C., Bemis G.W., Cao J., Chen G., Ford P.J.. et al. (2007) Flipped out: Structure-guided design of selective pyrazolylpyrrole ERK inhibitors. J. Med. Chem. 50, 1280–1287 10.1021/jm061381f [DOI] [PubMed] [Google Scholar]
- 61.O’Neill L. A.J. and Pearce E.J. (2016) Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 213, 15–23 10.1084/jem.20151570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G.. et al. (2013) Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 10.1038/nature11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chandel N.S., Trzyna W.C., McClintock D.S. and Schumacker P.T. (2000) Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J. Immunol. 165, 1013–1021 10.4049/jimmunol.165.2.1013 [DOI] [PubMed] [Google Scholar]
- 64.Jantsch J. and Schödel J. (2015) Hypoxia and hypoxia-inducible factors in myeloid cell-driven host defense and tissue homeostasis. Immunobiology 220, 305–314 10.1016/j.imbio.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 65.Kuschel A., Simon P. and Tug S. (2012) Functional regulation of HIF-1α under normoxia–is there more than post-translational regulation? J. Cell. Physiol. 227, 514–524 10.1002/jcp.22798 [DOI] [PubMed] [Google Scholar]
- 66.Nizet V. and Johnson R.S. (2009) Interdependence of hypoxic and innate immune responses. Nat. Rev. Immunol. 9, 609–617 10.1038/nri2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mi Z., Rapisarda A., Taylor L., Brooks A., Creighton-Gutteridge M., Melillo G.. et al. (2008) Synergystic induction of HIF-1alpha transcriptional activity by hypoxia and lipopolysaccharide in macrophages. Cell Cycle 7, 232–241 10.4161/cc.7.2.5193 [DOI] [PubMed] [Google Scholar]
- 68.Albina J.E., Mastrofrancesco B., Vessella J.a, Louis C.a, Henry W.L. and Reichner J.S. (2001) HIF-1 expression in healing wounds: HIF-1alpha induction in primary inflammatory cells by TNF-alpha. Am. J. Physiol. Cell Physiol. 281, C1971–C1977 10.1152/ajpcell.2001.281.6.C1971 [DOI] [PubMed] [Google Scholar]
- 69.Conrad P.W., Rust R.T., Han J., Millhorn D.E. and Beitner-Johnson D. (1999) Selective Activation of p38α and p38γ by hypoxia. J. Biol. Chem. 274, 23570–23576 10.1074/jbc.274.33.23570 [DOI] [PubMed] [Google Scholar]
- 70.Senger K., Pham V.C., Varfolomeev E., Hackney J.a., Corzo C.a., Collier J.. et al. (2017) The kinase TPL2 activates ERK and p38 signaling to promote neutrophilic inflammation. Sci. Signal. 10, eaah4273 10.1126/scisignal.aah4273 [DOI] [PubMed] [Google Scholar]
- 71.Pattison M.J., Mitchell O., Flynn H.R., Chen C.-S., Yang H.-T., Ben-Addi H.. et al. (2016) TLR and TNF-R1 activation of the MKK3/MKK6-p38α axis in macrophages is mediated by TPL-2 kinase. Biochem. J. 473, 2845–2861 10.1042/BCJ20160502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soria-Castro I., Krzyzanowska A., Pelaéz M.L., Regadera J., Ferrer G., Montoliu L.. et al. (2010) Cot/tpl2 (MAP3K8) mediates myeloperoxidase activity and hypernociception following peripheral inflammation. J. Biol. Chem. 285, 33805–33815 10.1074/jbc.M110.169409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu L., Pathak P.S. and Fukumura D. (2004) Hypoxia-induced activation of p38 mitogen-activated protein kinase and phosphatidylinositol 3’-kinase signaling pathways contributes to expression of interleukin 8 in human ovarian carcinoma cells. Clin. Cancer Res. 10, 701–707 10.1158/1078-0432.CCR-0953-03 [DOI] [PubMed] [Google Scholar]
- 74.Wu M.-Z., Chen S.-F., Nieh S., Benner C., Ger L.-P., Jan C.-I.. et al. (2015) Hypoxia drives breast tumor malignancy through a TET-TNFα-p38-MAPK signaling axis. Cancer Res. 75, 3912–3924 10.1158/0008-5472.CAN-14-3208 [DOI] [PubMed] [Google Scholar]
- 75.Henze A.-T. and Mazzone M. (2016) The impact of hypoxia on tumor-associated macrophages. J. Clin. Invest. 126, 3672–3679 10.1172/JCI84427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ceci J.D., Patriotis C.P., Tsatsanis C., Makris A.M., Kovatch R., Swing D.A.. et al. (1997) Tpl-2 is an oncogenic kinase that is activated by carboxy-terminal truncation. Genes Dev. 11, 688–700 10.1101/gad.11.6.688 [DOI] [PubMed] [Google Scholar]
- 77.Lee H.W., Joo K.M., Lim J.E., Cho H.J., Cho H.J., Park M.C.. et al. (2013) Tpl2 kinase impacts tumor growth and metastasis of clear cell renal cell carcinoma. Mol. Cancer Res. 11, 1375–1386 10.1158/1541-7786.MCR-13-0101-T [DOI] [PubMed] [Google Scholar]
- 78.Gkirtzimanaki K., Gkouskou K.K., Oleksiewicz U., Nikolaidis G., Vyrla D., Liontos M.. et al. (2013) TPL2 kinase is a suppressor of lung carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 110, E1470–E1479 10.1073/pnas.1215938110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koliaraki V., Roulis M. and Kollias G. (2012) Tpl2 regulates intestinal myofibroblast HGF release to suppress colitis-associated tumorigenesis. J. Clin. Invest. 122, 4231–4242 10.1172/JCI63917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Decicco-Skinner K.L., Trovato E.L., Simmons J.K., Lepage P.K. and Wiest J.S. (2011) Loss of tumor progression locus 2 (tpl2) enhances tumorigenesis and inflammation in two-stage skin carcinogenesis. Oncogene 30, 389–397 10.1038/onc.2010.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao Y., Jin J., Chang M., Nakaya M., Hu H., Zou Q.. et al. (2014) TPL2 mediates autoimmune inflammation through activation of the TAK1 axis of IL-17 signaling. J. Exp. Med. 211, 1689–1702 10.1084/jem.20132640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsui-Hasumi A., Sato Y., Uto-Konomi A., Yamashita S., Uehori J., Yoshimura A.. et al. (2017) E3 ubiquitin ligases SIAH1/2 regulates hypoxia-inducible factor 1(HIF1)-mediated TH17 cell differentiation. Int. Immunol. 29, 133–143 10.1093/intimm/dxx014 [DOI] [PubMed] [Google Scholar]
- 83.Li X., Acuff N.V., Peeks A.R., Kirkland R., Wyatt K.D., Nagy T.. et al. (2016) Tumor Progression Locus 2 (Tpl2) activates the mammalian target of rapamycin (mTOR) pathway, inhibits forkhead box P3 (FoxP3) Expression, and limits regulatory T cell (Treg) immunosuppressive functions. J. Biol. Chem. 291, 16802–16815 10.1074/jbc.M116.718783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melagraki G., Leonis G., Ntougkos E., Rinotas V., Papaneophytou C., Mavromoustakos T.. et al. (2018) Current status and future prospects of small–molecule protein–protein interaction (PPI) inhibitors of tumor necrosis factor (TNF) and receptor activator of NF-κB ligand (RANKL). Curr. Top. Med. Chem. 18, 661–673 10.2174/1568026618666180607084430 [DOI] [PubMed] [Google Scholar]
- 85.van Hal T.W., van Bon L. and Radstake T.R.D.J. (2011) A system out of breath: how hypoxia possibly contributes to the pathogenesis of systemic sclerosis. Int. J. Rheumatol. 2011, 824972 10.1155/2011/824972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ihn H., Yamane K. and Tamaki K. (2005) Increased phosphorylation and activation of mitogen-activated protein kinase p38 in scleroderma fibroblasts. J. Invest. Dermatol. 125, 247–255 10.1111/j.0022-202X.2005.23766.x [DOI] [PubMed] [Google Scholar]
- 87.Sanchez A., Tripathy D., Yin X., Desobry K., Martinez J., Riley J.. et al. (2012) p38 MAPK: A mediator of hypoxia-induced cerebrovascular inflammation. J. Alzheimers Dis. 32, 587–597 10.3233/JAD-2012-120829 [DOI] [PubMed] [Google Scholar]
- 88.Mielke L.A., Elkins K.L., Wei L., Starr R., Tsichlis P.N., O’Shea J.J.. et al. (2009) Tumor progression locus 2 (Map3k8) is critical for host defense against Listeria monocytogenes and IL-1 production. J. Immunol. 183, 7984–7993 10.4049/jimmunol.0901336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sugimoto K., Ohata M., Miyoshi J., Ishizaki H., Tsuboi N., Masuda A.. et al. (2004) A serine/threonine kinase, Cot/Tpl2, modulates bacterial DNA-induced IL-12 production and Th cell differentiation. J. Clin. Invest. 114, 857–866 10.1172/JCI20014 [DOI] [PMC free article] [PubMed] [Google Scholar]