Abstract
Purpose: The aim of this project is to investigate the usefulness of the absolute liver lesion ADC value and ratio of Apparent diffusion coefficient (ADC) values of a liver lesion and liver parenchyma to discriminate between a benign and malignant lesion.
Methods: Liver MRI scans performed between January 2009 and June 2015 were retrospectively analysed. Scans were performed on either a 1.5 T or 3 T MRI unit. The type of liver lesion (benign or malignant) was determined by its radiological appearance, histology result and clinical management. Lesions with undetermined diagnosis or MRI studies degraded by artifacts were excluded. Liver cysts were also excluded from the analysis. ADC value of a lesion and liver parenchyma was measured and ADCratio was calculated. The values were analysed using independent samples t-test
Results:Data set contained 39 benign lesions and 36 malignant lesions. Mean ADC value for benign lesions was 1678, and the mean value for malignant lesions was 1097 with a statistically significant difference of p < 0.001. All lesions with ADC value below 955 were malignant, while all lesions with ADC value above 1880 were benign. ADC value of 1260 was identified as the best available cut-off value for differentiating benign and malignant lesions, achieving sensitivity of 92%, specificity of 80% and an overall accuracy of 89%. The mean lesion to liver ADCratio for benign lesions was 1.3467 and for malignant lesions was 0.9038 with a statistically significant difference of p < 0.001. All lesions with ADCratio measuring <0.9 were malignant while lesions with ADCratio>1.5 were benign. ADCratio of 1.1 was identified statistically as the best available cut-off value for differentiating benign from malignant lesions, with sensitivity of 82%, specificity of 86% and an overall accuracy of 92%.
Conclusion:Our dataset indicates that lesion to background liver ADCratio is superior in discriminating between benign and malignant focal lesions compared to absolute ADC values of the hepatic lesions.
Keywords: Magnetic resonance imaging (MRI), Liver lesion, Benign, Malignant, Apparent diffusion coefficient (ADC), Apparent diffusion coefficient ratio. (ADCratio)
1. Introduction
Magnetic Resonance Imaging (MRI) has superior sensitivity and specificity in diagnosing focal liver lesions when compared to Computer Tomography (CT) and Ultrasound (US) [1]. Diffusion weighted imaging (DWI) is one of the non-contrast MRI sequences which is playing increasing role in the hepatic MRI interpretation [2,3]. It was initially utilised in neuroradiology but eventually applied into abdominal imaging [4,5]. Diffusion restriction within the tissue of interest demonstrated on DWI can be quantitatively measured by Apparent diffusion coefficient (ADC) map [1,6]. ADC is a composite coefficient that reflects both microcirculation and pure diffusion. Its measurement is influenced by water diffusion, tissue perfusion and flow. The decrease in true diffusion may be influenced by differences in tumour cellularity, architecture, and grade [7].
There has been promising evidence that ADC may be a viable tool to help discriminate benign versus malignant character of a hepatic lesion [1,6,[8], [9], [10], [11], [12], [13]]. However, there have been conflicting reports in literature regarding the usefulness of DWI in this regard. A few research studies found that ADC is not a reliable tool for such a task [4,14,15]. Even so, there are two meta- analysis which found that ADC is useful in discriminating the lesions [11,12].
Some solid hepatic benign lesions for example, focal nodular hyperplasia, hepatic adenoma and haemagioma can demonstrate similar ADC values to malignant hepatic lesions [16]. This significantly limits the reliability of ADC as a discriminating tool. Many studies conducted to assess the discriminating ability of ADC also included simple cysts [6,8,11]. As ADC value of simple cysts is usually significantly higher than solid lesions, inclusion of cysts in the category of benign lesions may have led to a significant difference in ADC values between benign and malignant lesions, leading these investigators to believe that ADC and DWI are useful tools. Unfortunately Testa et al have indicated that exclusion of simple cysts undermines ADC as an effective discriminating tool [6].
ADC cut-off values are fraught with difficulty due to wide variation in b-values used for acquisition across the literature and different institutional practices [17]. Calculation of ADC values in a particular lesion can vary with MRI equipment, scanning protocol and analysis software platform used for calculation. ADC values can also vary within the same patient in two different sets of examinations due to variation in biological parameters e.g. vascularity, membrane permeability changes. The ratio of ADC values between a lesion and the background liver can potentially negate these external factors and provide a more accurate representation of change in the diffusion with respect to normal tissue. In an earlier study, ADCratio values were found to have better sensitivity and specificity than stand-alone ADC values in the interpretation of hepatic malignancies [18].
The aim of this project is to investigate the usefulness of ADCratio of a solid liver lesion to liver parenchyma to discriminate between benign and malignant lesions.
2. Material and methods
Ethics approval was obtained from institutional Human Research Ethics Committee.
2.1. Data acquisition
The liver MRI scans performed between January 2009 and June 2015 were retrospectively analysed. Studies without focal liver lesions were excluded.
The type of liver lesion (benign or malignant) was determined by its radiological appearance, histology result and clinical management. Radiological appearance for benign lesions are determined by the presence of well described radiological features [19]. Apart from radiological appearance, associations were also made with patients’ clinical history of metastatic malignancy, with and without histological confirmation. Patients with liver lesions that were treated with chemotherapy were also presumed to have malignant disease. Only pre-treatment liver MRIs were assessed.
Studies without liver lesions or lesions less than 10 mm were excluded. MRI studies without a complete hepatic liver MR sequences or heavily degraded by artefact were also excluded. Simple hepatic cysts were also excluded from the analysis.
2.2. Liver MRI technical data
Patients were examined with either a Siemens Avanto 1.5 T Scanner or Phillips Ingenia 3 T superconducting MR imaging system.
The liver MR imaging protocol included transverse T2-weighted single-shot turbo spin-echo images, transverse T1-weighted gradient echo in- and opposed-phase images, axial transverse single-shot echo-planar (SS-EPI) DW images (free-breathing respiratory triggered sequence) with 3 b values covering the whole liver and transverse T2-weighted turbo spin-echo images with fat suppression. At 3 T scanner, the highest b value was 800 or 1000sec/mm2. At 1.5 T scanner, the highest b value was 1000sec/mm2.
Dynamic post contrast images were acquired following injection of an intravenous bolus injection of contrast. The contrast injection was followed by IV injection of 20 mL of saline. Various contrast media used were 0.1 mmol/kg dimeglumine gadopentetate (Magnevist; Bayer Group, Germany) or 0.025 mmol/kg gadoxetate disodium (Primovist, Bayer Group, Germany) or 0.1 mmol/kg Gadobenate disodium (Multihance, Bracco Diagnostics, Italy). The choice of contrast utilised was determined by the reporting radiologists on the day the scan was performed.
Hepatic arterial-dominant, portal venous, and equilibrium phase (10 min post contrast injection) sequences were performed. Hepatobiliary phase was performed if hepatobiliary specific contrast agent was administered. The total examination time was approximately 30 min.
2.3. Lesion analysis
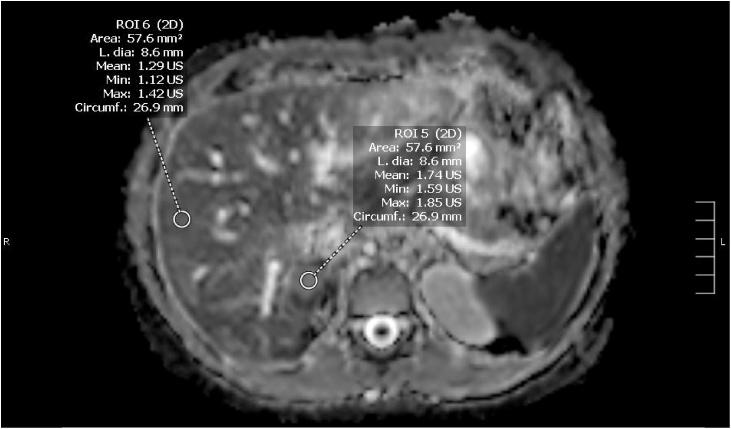
Criteria used for image quality were visual assessment of noise, motion artifacts, and image distortion. Blinded to the diagnosis, ADC value of a lesion and liver parenchyma were measured by a radiologist with 12 years’ experience interpreting liver MRIs. ADC values of each lesion and background hepatic parenchyma were measured twice and an average ADC value was obtained. The area of the region of interest (lesion/background liver) was between 50-60 mm2 (Figs. 1 and 2). Care was taken to avoid cystic necrotic appearing regions (bright on T2 weighted images and non enhancing on post contrast images) in a lesion as well as adjacent vessels. For a lesion with heterogeneous signal on ADC images, the ROIs were placed in the brightest and darkest regions within solid enhancing components of the lesion. For homogenous appearing lesions, ROIs were placed in the lesion at different axial slices. A similar process was repeated for the background liver, where the ROIs were measured in each liver lobe.
Fig. 1.
Example of lesion and liver ROIs acquired of a benign lesion (haemangioma).
Fig. 2.
Example of lesion and liver ROIs acquired of a malignant lesion (metastasis).
2.4. Data analysis
All statistical analysis was performed using SPSS 24 (IBM). ADC values were obtained for both the neoplastic lesion and the surrounding liver parenchyma, and ADCratiowas then calculated. Levene’s test was performed to ensure equal variance between the benign and malignant groups. Student T-tests were used to analyse benign and malignant ADC values and ADCratio.
Receiver operative characteristic curves were generated to assess the ability to differentiate benign and malignant neoplasms based on ADC value and ADCratio. Cut-off values for benign and malignant neoplasms were determined for both ADC values and ADCratio. A P value of <0.05 was considered statistically significant for all statistical analyses.
Further stratification of data into 1.5 T and 3 T groups, with subsequent analysis was performed.
3. Results
A total of 188 patients had hepatic MRI from January 2009 to June 2015. 113 patients were excluded due to various reasons detailed in Table 1. The 75 patients were included. The data was comprised of 39 benign lesions (13 FNH, 25 haemangioma, 1 abscess) and 36 malignant lesions (27 HCC, 1 cholangiocarcinoma, 8 metastases). Levene’s test demonstrated equal variances between the analysed groups (Table 2).
Table 1.
Patient demographics, results and variance stratified into 1.5 T and 3 T groups.
| 1.5 T | 3 T | Total | |
|---|---|---|---|
| Total Included | 38 | 37 | 75 |
| Benign lesion | 20 | 19 | 39 |
| Malignant lesion | 18 | 18 | 36 |
| Total Excluded | 78 | 35 | 113 |
| No lesion/ simple cyst | 28 | 11 | 39 |
| Incomplete MRI sequences | 7 | 4 | 11 |
| Lesion <10 mm | 16 | 6 | 22 |
| Significant degraded by artifact | 27 | 14 | 41 |
| Total MRI performed | 116 | 72 | 188 |
Table 2.
Number of cases obtained from data collection.
| 1.5 T | 3 T | |
|---|---|---|
| Age (Mean) | 54 | 58 |
| Gender (numbers) | ||
| Male | 16 | 20 |
| Female | 20 | 19 |
| Mean ADCratio | ||
| Benign | 1.3352 | 1.3575 |
| Malignant | 0.9379 | 0.8411 |
| MediADCratio | ||
| Benign | 1.3179 | 1.3889 |
| Malignant | 0.9551 | 0.8394 |
| Variance | ||
| Benign | 0.065 | 0.089 |
| Malignant | 0.032 | 0.05 |
3.1. ADC value
Mean ADC value for benign lesions was 1678, and mean value for malignant lesions was 1097. These means were statistically significant (p < 0.001). All lesions with ADC value below 955 were malignant, while all lesions with ADC value above 1880 were benign.
ROC curve analysis demonstrated an area under the curve of 0.89. ADC value of 1260 was determined to be the best available cut-off value for differentiating benign and malignant lesions, achieving sensitivity and specificity of 92% and 80%, respectively (Fig. 4).
Fig. 4.
Receiver operating curve demonstrating optimum ADC value cut-off.
3.2. ADCratio
Mean ADCratio for benign lesions was 1.3467 and for malignant lesions was 0.9038. There was a statistically significant difference between these values (p < 0.001). All lesions with ADCratio less than 0.9 were malignant while all lesions with ADCratio greater than 1.5 were benign (Fig. 3).
Fig. 3.
Spread of all malignant and benign lesions ADC ratio in the study.
ROC curve analysis demonstrated an area under the curve of 0.92. ADCratio of 1.1 was determined to be the best available cut-off value for differentiating benign from malignant lesions, with sensitivity and specificity of 82% and 86%, respectively (Fig. 5).
Fig. 5.
ROC curve demonstrating optimum ADC ratio of 1.1 with Sens = 82% and Spec = 86%.
3.3. Cutoff values
There was a statistically significant difference between ADCratio and ADC values when discriminating benign from malignant lesions using the above cutoff values (p = 0.039).
3.4. 1.5 T vs 3 T
There was no significant difference in ADCratio data between the 1.5 T and 3 T units (p = 0.80 in the benign group and p = 0.060 in the malignant group). Again, equal variances for 1.5 T and 3 T units were assumed (p = 0.073 and p = 0.413, respectively). A statistically significant difference between the mean benign and malignant ADCratiowas observed in both the 1.5 T and 3 T groups (p < 0.001 and p < 0.001, respectively).
4. Discussion
Quantitative measurements of ADC values have been shown to outperform visual inspection of ADC map in differentiating benign from malignant lesions [20]. A large number of covariates can influence ADC values. ADCratio analysis method utilised in this study is aimed to reduce heterogeneities due to these covariates where possible, while retaining easy application for daily reporting. Lesion to normal liver ADCratio is also easily calculated and can be used in daily practice without having the need to use new software or formulae. ADC value variability in hepatic tumour can vary by as much as 30% between two consecutive MRI scans due to rescan variability on the same patient cohort and same scanning parameters done 15 min apart [21,22]. In our opinion, utilising ratio values theoretically nulls potential heterogeneity caused by covariates, leaving the ratio reflecting true difference between the lesion and background liver. This method had also been used in previous study to access hepatic lesions [18]. The use of lesion: background ADCratio has also been described to assess prostate lesions on MRI [23]. ADCratio in assessment of prostate cancer was found to be superior to ADC value alone [22].
4.1. Factors influencing scanning parameters
Recent meta-analysis of the diagnostic capability of breath-hold diffusion-weighted imaging (DWI) for differentiation between malignant and benign hepatic lesions by Chen et al, showed that overall diagnostic performance was high. However, significant heterogeneity among studies was noted in their analysis. A number of covariates analysed were not found to be the source of this heterogeneity. The analysed covariates included MR scanner, scanning technique, TR, TE, maximum b factor, number of b factors used, mean tumour size and mean patient age [11].
Apart from variable scanning parameters, patient related factors also have an impact on the generated ADC images. Cirrhotic livers have been reported to have lower ADC values when compared to healthy livers [5,20,24,25]. Nonalcoholic liver disease was also noted to record lower ADC values [26]. ADC value variations are demonstrated amongst different hepatic segments where hepatic segment 2 was found to exhibit higher ADC values [4,27].
DWI Images in all our patients were acquired with respiratory trigger. Respiratory triggered image acquisition yielded better image quality and signal to noise ratio when compared to breath-hold and free breathing technique [28,29]. To our knowledge, there is none or very limited literature comparing respiratory and cardiac triggered image acquisition. Up to the present time, dual respiratory and cardiac synchronisation is conducted only in animal experiments using strong magnetic fields above 4.7 T [30,31].
There was no statistical difference between measurement of ADCratios between 1.5 T and 3 T MRI unit used in our case series. This would need to be examined by other investigators; this will be useful as the strength of MRI units varies in different institutions. To our knowledge, there is no previous study comparing the discriminating ability of ADC between images acquired on 1.5 T and 3 T MRI unit.
4.2. Variability in assessing a lesion
A single ADC value may not be able to reliably represent the entire lesion [32]. This is particularly true for larger lesions. Hence, to address intralesional ADC heterogeneity, two ROIs were drawn in the lesion to obtain an average lesion ADC value. For a lesion with heterogeneous signal on ADC images, the ROIs were placed in the brightest and darkest regions within the lesion after excluding cystic necrosis. For homogenous appearing lesions, ROIs were placed in the lesion at different axial slices. A similar process was repeated for the background liver, where the ROIs were measured in each liver lobe.
In order to obtain a reading reflecting true ADC value, a region of interest (ROI) of 50 – 60 mm2 was used. A study in 2013 conducted by Filipe JP and team showed that measurement above 1 cm2 can provide an accurate measurement of a homogenous lesion [4]. The authors also reported that there is no difference in measuring 1 cm2 of the lesion and the entire area of the lesion. Use of a circular ROI of 1 cm2 needs the lesion to be at least 11.28 mm in diameter, which can potentially exclude some smaller lesions. At the same time, using a very small ROI may not be representative of ADC value. A 50-60mm2 ROIs were obtained as a compromise. Use of an ROI of 50 – 60 mm2 needs the lesion diameter to be between 7.07–7.74 mm. Hence, we excluded lesions smaller than 10 mm diameter, to avoid placing the ROI outside the lesion. Also, two ROIs readings were obtained to account for intralesional heterogeneity. There has been no study in the literature that has evaluated the variation in size of ROI as a factor influencing the accuracy of ADC measurement in liver lesions. While is possible to use smaller ROI in lesions smaller than 1 cm in diameter, effectiveness of smaller ROI can be explored in future investigations.
4.3. Study methods and results yield
Some studies in the past which reported DWI and ADC values to be useful in distinguishing benign vs malignant liver lesion included simple cysts [6,8,10,16]. In daily practice, simple liver cysts are easily identified as they demonstrate distinct fluid MRI signal characteristics. They highly affect the statistical analysis as they have free water mobility and very high ADC values, thus raising ADC average on the benign lesions group and consequently the cut-off point, leading to a selection bias [6]. The key requirement in daily practice is mainly to distinguish and diagnose solid lesions. When Testa et al 2014 excluded simple cysts fom their data set, accuracy of ADC to distinguish between benign and malignant lesions reduced [6]. Other investigators have reported that solid benign lesions ADC values were similar to malignant lesions [15,16]. A study excluding simple cysts conducted by Sutherland et al. concluded that DWI is unreliable in differentiating benign from malignant lesions [14]. Despite the exclusion of simple cysts, the results of our study demonstrate statistically significant ADCratio difference between benign and malignant lesions.
We found that all lesions in our study with ADCratio measuring <0.9 were malignant while lesions with ADCratio >1.5 were benign. In theory, malignant lesions by virtue of their increased cellularity are expected to display restricted diffusion and can consequently be differentiated from less cellular, benign lesions [17]. Using pure diffusion coefficient, Wagnet et al showed that within a focal malignant lesions, the “benign” necrotic and fibrotic areas showed less diffusion restriction [33]. Despite careful exclusion of cystic necrotic regions of a lesion, our results correspondingly yield an overlap in ADCratio between benign and malignant lesions. This may be attributed to relatively less retricted diffusion non-cystic necrosis in malignant lesions.
4.4. Limitations of this study
The first is limitations inherent to retrospective analysis. The incidence of lesions in our study group may not be representative of the general population. Another limitation relates to the reference standard used and final diagnosis of the lesions. Majority of benign lesions were not biopsied and the diagnosis was established based on imaging and/or clinical management. A histological examination is an ideal gold standard. However, it can be hard to justify performing a biopsy of a lesion deemed to be benign on imaging, in view of the invasive nature and risks of a biopsy. The cases in study include pre-treatment liver MRIs hence, findings will not be directly applicable to patients who were treated with chemotherapy. ADC assessment is only applicable to solid liver lesions and not applicable to cystic metastasis. Also, our results need to be validated in larger series.
5. Conclusion
Our dataset indicates that lesion to background liver ADCratio is superior in discriminating between benign and malignant focal lesions compared to absolute ADC values of the hepatic lesions. With increasing ADCratio, there is a trend towards benignity. A cut off of ADCratio below 0.9 reflected malignancy while ADCratio above 1.5 reflected benign aetiology. These cut offs can be validated further with further studies with larger number of individual malignant and benign lesions. ADCratio did not differ between 1.5 T and 3 T MRI unit.
Declarations of interest
None.
Funding
This study did not require any funding. No funding was received.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgements
Nil.
Contributor Information
Tarun Pankaj Jain, Email: tarun.jain@act.gov.au.
Wen Ter Kan, Email: Wenter.Kan@act.gov.au.
Sean Edward, Email: Sean.Barrett@act.gov.au.
Helen Fernon, Email: ht.fernon@gmail.com.
Renuvathy Kansan Naider, Email: renu.KansanNaider@act.gov.au.
References
- 1.Caraiani C., Chiorean L., Fenesan D.I. Diffusion weighted magnetic resonance imaging for the classification of focal liver lesions as benign or malignant. J. Gastrointest. Liver Dis. 2015;9:309–317. doi: 10.15403/jgld.2014.1121.243.cca. [DOI] [PubMed] [Google Scholar]
- 2.Galea N., Cantisani V., Taouli B. Liver lesion detection and characterization: role of diffusion-weighted imaging. J. Magn. Reson. Imaging. 2013;37:1260–1276. doi: 10.1002/jmri.23947. [DOI] [PubMed] [Google Scholar]
- 3.Koh D.M., Collins D.J. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am. J. Roentgenol. 2007;188:1622–1635. doi: 10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 4.Filipe J.P., Curvo-Semedo L., Casalta-Lopes J., Marques M.C., Caseiro-Alves F. Diffusion-weighted imaging of the liver: usefulness of ADC values in the differential diagnosis of focal lesions and effect of ROI methods on ADC measurements. MAGMA. 2013;26:303–312. doi: 10.1007/s10334-012-0348-1. [DOI] [PubMed] [Google Scholar]
- 5.Müller M.F., Prasad P., Siewert B., Nissenbaum M.A., Raptopoulos V., Edelman R.R. Abdominal diffusion mapping with use of a whole-body echo-planar system. Radiology. 1994;190:475–478. doi: 10.1148/radiology.190.2.8284402. [DOI] [PubMed] [Google Scholar]
- 6.Testa M., Chojniak R., Sene L. Is DWI/ADC a useful tool in the characterization of focal hepatic lesions suspected of malignancy?". PLoS One. 2014;9 doi: 10.1371/journal.pone.0101944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taouli Bachir. Diffusion weighted MR imaging for liver characterization: a critical look. Radiology. 2012;262:378–380. doi: 10.1148/radiol.11112417. [DOI] [PubMed] [Google Scholar]
- 8.Tokgoz O., Unlu E., Unal I. Diagnostic value of diffusion weighted MRI and ADC in differential diagnosis of cavernous hemangioma of the liver. Afr. Health Sci. 2016;16:227–233. doi: 10.4314/ahs.v16i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namimoto T., Nakagawa M., Kizaki Y. Characterization of liver tumors by diffusion-weighted imaging: comparison of diagnostic performance using the mean and minimum apparent diffusion coefficient. J. Comput. Assist. Tomogr. 2015;39:453–461. doi: 10.1097/RCT.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 10.Parsai A., Zerizer I., Roche O., Gkoutzios P., Miquel M.E. Assessment of diffusion-weighted imaging for characterizing focal liver lesions. Clin. Imaging. 2015;39:278–284. doi: 10.1016/j.clinimag.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z.G., Xu L., Zhang S.W., Huang Y., Pan R.H. Lesion discrimination with breath-hold hepatic diffusion-weighted imaging: a meta-analysis. World J. Gastroenterol. 2015;21:1621–1627. doi: 10.3748/wjg.v21.i5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Chen Z., Wang J. Differential diagnosis between malignant and benign hepatic tumors using Apparent diffusion coefficient on 1.5-T MR imaging: a meta analysis. Eur. J. Radiol. 2012;21:484–490. doi: 10.1016/j.ejrad.2010.12.069. [DOI] [PubMed] [Google Scholar]
- 13.Xia D., Jing J., Shen H., Wu J. Value of diffusion-weighted magnetic resonance images for discrimination of focal benign and malignant hepatic lesions: a meta-analysis. J. Magn. Reson. Imaging. 2010;32:130–137. doi: 10.1002/jmri.22211. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland T., Steele E., van Tonder F., Yap K. Solid focal liver lesion characterisation with Apparent diffusion coefficient ratios. J. Med. Imaging Radiat. Oncol. 2014;58:32–37. doi: 10.1111/1754-9485.12087. [DOI] [PubMed] [Google Scholar]
- 15.Sandrasegaran K., Akisik F.M., Lin C., Tahir B., Rajan J., Aisen A.M. The value of diffusion-weighted imaging in characterizing focal liver masses. Acad. Radiol. 2009;16:1208–1214. doi: 10.1016/j.acra.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Miller F.H., Hammond N., Siddiqi A.J. Utility of diffusion-weighted MRI in distinguishing benign and malignant hepatic lesions. J. Magn. Reson. Imaging. 2010;32:138–147. doi: 10.1002/jmri.22235. [DOI] [PubMed] [Google Scholar]
- 17.Culverwell A., Sheridan M., Guthrie J. Diffusion weighted MRI of the liver - Interpretative pearls and pitfalls. Clin. Radiol. 2013;68:406–414. doi: 10.1016/j.crad.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Colagrande S., Regini F., Pasquinelli F. Focal liver lesion classification and characterization in noncirrhotic liver: a prospective comparison of diffusion-weighted magnetic resonance-related parameters. J. Comput. Assist. Tomogr. 2013;37:560–567. doi: 10.1097/RCT.10.1097/RCT.0b013e3182951fe9. [DOI] [PubMed] [Google Scholar]
- 19.Albiin N. MRI of focal liver lesions. Curr. Med. Imaging Rev. 2012;8:107–116. doi: 10.2174/157340512800672216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girometti R., Del Pin M., Pullini S. Accuracy of visual analysis vs. Apparent diffusion coefficient quantification in differentiating solid benign and malignant focal liver lesions with diffusion-weighted imaging. Radiol. Med. 2013;118:343–355. doi: 10.1007/s11547-012-0873-z. [DOI] [PubMed] [Google Scholar]
- 21.Park S.H. DWI at MR Enterography for evaluating bowel inflammation in crohn disease. AJR Am. J. Roentgenol. 2016;207:40–48. doi: 10.2214/AJR.15.15862. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.Y., Lee S.S., Byun J.H. Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion-weighted MR imaging. Radiology. 2010;255:815–823. doi: 10.1148/radiol.10091706. [DOI] [PubMed] [Google Scholar]
- 23.Lebovici A., Sfrangeu S., Feier D. Evaluation of the normal-to-diseased Apparent diffusion coefficient ratio as an indicator of prostate cancer aggressiveness. BMC Med. Imaging. 2014;14 doi: 10.1186/1471-2342-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim T., Murakami T., Takahashi S., Hori M., Tsuda K., Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am. J. Roentgenol. 1999;173:393–398. doi: 10.2214/ajr.173.2.10430143. [DOI] [PubMed] [Google Scholar]
- 25.Girometti R., Furlan A., Esposito G. Relevance of b-values in evaluating liver fibrosis: a study in healthy and cirrhotic subjects using two single-shot spin-echo echo-planar diffusion-weighted sequences. J. Magn. Reson. Imaging. 2008;28:411–419. doi: 10.1002/jmri.21461. [DOI] [PubMed] [Google Scholar]
- 26.Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29:1797–1810. doi: 10.1148/rg.296095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruegel M., Holzapfel K., Gaa J. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur. Radiol. 2007;18:477–485. doi: 10.1007/s00330-007-0785-9. [DOI] [PubMed] [Google Scholar]
- 28.Kandpal H., Sharma R., Madhusudhan K.S., Kapoor K.S. Respiratory-triggered versus breath-hold diffusion-weighted MRI of liver lesions: comparison of image quality and Apparent diffusion coefficient values. AJR. 2009;192:915–922. doi: 10.2214/AJR.08.1260. [DOI] [PubMed] [Google Scholar]
- 29.Kartalis N., Loizou L., Edsborg N., Segersvärd R., Albiin N. Optimising diffusion-weighted MR imaging for demonstrating pancreatic cancer: a comparison of respiratory-triggered, free-breathing and breath-hold techniques. Eur. Radiol. 2012;22:2186–2192. doi: 10.1007/s00330-012-2469-3. [DOI] [PubMed] [Google Scholar]
- 30.Baboi L., Milot L., Lartizien C. Synchronisation strategies in T2-weighted MR imaging for detection of liver lesions: application on a nude mouse model. Biomed. Imaging Intervent. J. 2007;3:1–9. doi: 10.2349/biij.3.4.e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rengle A., Baboi L., Saint-Jalmes H., Sablong R., Beuf O. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2007. Optical cardiac and respiratory device for synchronized MRI on small animal; pp. 2046–2049. [DOI] [PubMed] [Google Scholar]
- 32.Schmid-Tannwald C., Dahi F., Jiang Y. DW-MRI of liver lesions: can a single ADC-value represent the entire lesion? Clin. Radiol. 2014;69:492–498. doi: 10.1016/j.crad.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Wagnet M. Diffusion weighted MR Imaging for the regional characterization of liver tumours. Radiology. 2012;264:464–472. doi: 10.1148/radiol.12111530. [DOI] [PubMed] [Google Scholar]