Abstract
Patients with psychosis exhibit subsyndromal depressive symptoms during the course of illness and yet the clinical correlates of these symptoms remain under-investigated. We aimed to investigate the clinical correlates of subsyndromal depression in psychosis including the extent to which they mediate commonly observed comorbid substance dependence. We developed a model of depression in a non-clinical sample recruited via Amazon’s Mechanical Turk (N = 266), and confirmed that model in a locally recruited African-American clinical sample comprising psychotic and non-psychotic individuals (N = 256). Using scores from this model we tested: the strength of relationships between depressive symptomatology and positive, negative and disorganized symptoms in a range of psychotic disorders; whether depressive symptoms were higher in individuals with affective psychoses versus schizophrenia; and if depressive symptomatology mediated the relationship between psychosis and substance dependence. Subsyndromal depressive symptomatology was significantly higher in individuals with psychosis than without psychosis, but did not significantly differ between affective and non-affective psychotic groups. Depressive symptomatology was significantly related to positive (but not negative or disorganized) psychotic symptoms, and mediated the relationship between psychosis and substance dependence. The present study underlines the importance of assessing subsyndromal depression in patients with psychosis, and generates a number of testable predictions for future work. In particular, the examination of the relationships between comorbid psychopathology, namely depression and substance abuse, may improve insight into the neurobiology of psychosis.
Introduction
Symptoms of depression are highly prevalent in schizophrenia patients, with up to 59% meeting diagnostic criteria for depression (Sands and Harrow 1999;Kessler et al. 1994;Siris and Bench 2003) and 80% experiencing at least one clinically significant depressive episode during the course of the illness (Upthegrove et al. 2017). Clinically, depressive symptomatology is associated with worse longitudinal outcomes, increased rates of suicide, and comorbid substance use disorders (Siris and Bench 2003;Sim et al. 2004;Buckley 2006). The co-occurrence of depression and psychosis may hint at some overlooked biological (and possibly etiological) overlap between these putatively distinct disorders (Buckley et al. 2009). This overlap could prove informative in the search for the neurobiological underpinnings of schizophrenia and/or MDD by identifying pairs of strongly related clinical phenotypes tonform phenotype selection in multivariate approaches for gene discovery since multivariate approaches are statistically more powerful than univariate ones (Bearden and Freimer 2006;Knowles et al. 2014a). However, little is known about depressive symptomatology across psychosis spectrum disorders (i.e. affective versus non-affective), as well as how depressive symptoms are related to specific psychotic symptoms, particularly negative symptoms.
Depressive symptoms in schizophrenia challenges the dichotomous view of psychotic disorders (Upthegrove et al. 2017). Indeed, psychiatric research in recent years has moved away from a framework of discrete diagnostic categories towards a dimensional view (Walker et al. 2002;Ketter et al. 2004;Taylor 1992;Wigman et al. 2015). For example, patients with psychotic disorders (e.g., schizophrenia, bipolar or major depressive disorder with psychotic symptoms) may be placed along the psychosis spectrum (Keyes et al. 2013;Reininghaus et al. 2016;Kotov et al. 2017a;Tamminga et al. 2013;Craddock and Owen 2007). Similarly, an extensive literature suggests that depressive symptomatology has a rich multidimensional structure (Shafer 2006;van Loo et al. 2012;Vrieze et al. 2014;Li et al. 2014). Analogously, the NIMH RDoC initiative, a committee-designed framework encapsulating various dimensional constructs, aims to organize research efforts independent of traditional diagnoses. The RDoC vision has since been updated, with a recent call to develop new data-driven dimensions of symptomatology that better reflect the true structure of behavior (Gordon 2017). Work in this vein has already begun with the introduction of the HiTOP (Hierarchical Taxonomy of Psychopathology) consortium to develop an empirically driven classification system of psychopathology (Kotov et al. 2017b). In this study, we aimed to build directly on this by 1) deriving data-driven dimensions of depressive symptomatology and 2) utilizing these dimensions to investigate the clinical correlates of depression in transdiagnostic psychosis. A key advantage of this dimensional view is that depressive symptomatology can be assessed in individuals without a formal depression diagnosis, either because their symptoms are subsyndromal, or missed due to the presence of psychosis.
One of the challenges in assessing depression in psychosis is being able to disentangle depressive and negative symptoms (Siris and Bench 2003). Moreover, sum scores from classical psychometric tools (developed primarily for depressed patients) are not refined enough to discriminate between negative and depressive symptoms (Fitzgerald et al. 2002;Allan and Martin 2009;Lako et al. 2012). However, it is possible to tease these dimensions apart using carefully constructed factor models of individual items from those tools. In the present study, we applied a bifactor latent structure (Holzinger and Swineford 1937) whereby two types of latent factors are specified: (1) a general factor reflecting commonality among all items i.e. the target dimension, here general depressive symptomatology; and (2) several orthogonal “group” factors that explain additional common variance not accounted for by the general factor, reflecting subdomains. These subdomains are sometimes referred to as “nuisance” dimensions since they arise from items that potentially interfere with measurement of the target dimension (Reise et al. 2010a;Mansolf and Reise 2017). Thus, a bifactor model distinguishes between variance attributable to general depression and to other mood-like symptomatology (e.g. negative symptoms). This approach has been utilized previously to investigate the latent structure of individual psychometric instruments in depressed individuals (Brouwer et al. 2013;Ward 2006;Vanheule et al. 2008;Al-Turkait and Ohaeri 2010), but has not previously been applied across multiple instruments or in schizophrenia patients.
Substance use is also common in psychosis spectrum disorders, with contentions that substance use dual diagnosis might be the rule rather than the exception (Buckley 2006;Regier et al. 1990;Seddon et al. 2016). Previous attempts to delineate the reasons for this co-occurrence have been hampered by a tendency to exclude participants with substance use disorders (Buckley et al. 2009). We hypothesize that increased severity of depression might increase risk for substance abuse, with one possible reason being that patients attempt to reduce the distress associated with depressive symptoms (Thoma and Daum 2013;Krystal et al. 2006). Indeed, substance use is also associated with depressive symptoms in schizophrenia (Brady et al. 1993;Kerfoot et al. 2011;Potvin et al. 2007).
We used a large (total N = 522; comprising a non-clinical sample recruited via Amazon’s Mechanical Turk (N = 266) and a locally recruited clinical sample comprising psychotic and non-psychotic individuals (N = 256)) sample with the aim of clarifying the clinical correlates of depressive symptomatology in psychosis. First, we developed a structural model of depressive symptomatology in a non-clinical sample using exploratory factor analysis, which makes no prior assumptions about the relationships between items. Next, we confirmed and extended the model by applying a bifactor structure in a clinical sample of patients with psychosis spectrum disorders and healthy controls. The bifactor model contained a general depression factor, Gd, as well as orthogonal sub-domains. We calculated factor scores for all individuals in the clinical sample and used these scores to examine whether: (a) patients with and without psychosis differed in their factors scores, (b) patients with affective psychoses exhibited greater general depression (Gd ) than those with schizophrenia; and (c) factor scores were associated with particular dimensions of psychosis (i.e., positive, negative, or disorganized symptoms). Our second aim was to investigate (d) the extent to which depressive symptomatology mediates the relationship between psychosis and substance dependence disorders.
Methods
Participants
The study contained two samples of participants. The first, referred to hereon as the MTurk sample, comprised 266 individuals (45% female; mean age = 36.74 years, sd = 12.44 years, range = 19–75 years) recruited via Amazon’s Mechanical Turk, a crowdsourcing platform that allows the hiring and paying of “workers” to complete computer-based tasks (Paolacci and Chandler 2014). Each MTurk worker was paid $1 for each completed task. On average it took a worker 20 minutes and 16 seconds to complete all questions. Amazon does not provide any published data on the average amount paid per task (also referred to as a HIT). Instead they advise requesters to ‘look at HITs similar to yours to see what the “going rate” is for HITS on Mechanical Turk’ (Amazon 2015). At the time of testing, other studies of similar length and content were paying ~$1 per HIT. Although all participants provided informed consent, the study was granted exemption by the institutional review board at Yale since no identifying information was collected from participants. This sample was not selected for any psychiatric illness, and the only inclusion criteria were that participants were at least 18 years old and lived in the USA. Due to unsatisfactory responses to some questionnaire items (see later), data from 134 additional participants were collected, but not included in any analyses.
The second sample, referred to hereon as the HH sample, comprised 256 African Americans (48% female; mean age = 39.98, sd = 13.73, range 18–70 years) recruited through outpatient clinics and community mental health facilities in the Hartford area. All HH participants provided informed consent and the study was approved by institutional review boards at Hartford Hospital and Yale University. Eighty-six participants had a psychotic disorder, including schizophrenia (N = 41) schizoaffective disorder (N = 23), psychotic bipolar disorder (N = 7), psychotic major depression (N = 4), psychosis not otherwise specified (NOS; N = 10), and brief psychotic disorder (N = 1). The remainder of the sample comprised individuals without psychosis, referred to hereon as “controls” for simplicity. Twenty-two participants had received a lifetime diagnosis of major depression, either due to a single major depressive episode (N = 6) or recurrent major depression (N = 16). Of these 22 individuals, only five had also been diagnosed with a psychotic disorder and all five had received a diagnosis of recurrent major depression. To increase the ecological validity of the sample as a whole, participants (either those with or without psychosis) were not excluded for having non-psychotic psychiatric disorders or substance dependencies (see (Mathias et al. 2017). DSM-IV diagnoses were confirmed using the Structured Clinical Interview for DSM-IV (SCID) (First et al. 2002) and a consensus process. Additional clinical characteristics of the sample are provided in the supplemental materials (Table S1). All of the subjects in the HH sample were African Americans. Like other minority groups, African Americans are underserved by psychiatric research, and we specifically chose to study them for this reason.
Assessment of depressive symptomatology
Participants from both samples completed electronic versions of the Beck Depression Inventory, version II (BDI-II) (Beck et al. 1996) and the Depression Anxiety Stress Scales (DASS) (Crawford and Henry 2003), in addition to the two SCID screener questions pertaining to major depression: “Have you ever been consistently down, depressed or hopeless, for most of the day, nearly every day, for at least two weeks?”; and “Have you ever been much less interested in most things or much less able to enjoy the things you used to enjoy most of the time over at least two weeks?”. The Mturk sample completed additional questionnaire items, asking whether they had 1) ever visited a health professional as a result of low mood, 2) received a diagnosis of major depression, and 3) received a prescription for anti-depressant medication. MTurk participants also completed two items from the BDI and two items from the DASS twice, and four simple catch items (e.g. “Select the word beginning with the letter A: [Bear, Xylophone, Apple, Zebra]”). Participants with less than perfect consistency on the four repeated questions or any incorrect responses to the catch questions were dropped (= 134 individuals). Due to a coding error, item 10 from the BDI was missing from the HH sample, and thus was dropped from analyses in both samples.
Assessment of psychotic symptoms
Additionally, the HH sample (but not the MTurk sample) completed the Lifetime Dimensions of Psychosis Scale (LDPS) (Levinson et al. 2002). The LDPS captures information pertaining to dimensions of psychosis including (but not limited to) positive, negative and disorganized symptoms. This instrument allows the creation of a multidimensional profile for each individual in terms of their psychotic characteristics (Levinson et al. 2002).
Exploratory Factor Analysis: Developing a Factor Model of Depressive Symptomatology
We subjected the MTurk data to an exploratory factor analysis (EFA) in order to derive a factor solution that would later be fit to the HH data. All exploratory analyses were conducted in R (R Core Team 2017). First, the data were checked for errors and Cronbach’s α was calculated for each questionnaire using the psych package (Revelle 2017). Nonparametric correlation matrices (Kendall’s τ-b) were used to inspect the data for collinearity. Parallel analysis with 1000 iterations (using the nFactors (Raiche 2010) package) was used to choose the number of factors. We then conducted an EFA on the data with the suggested number of factors and promax rotation (using the FactoMineR package (Le et al. 2008)). Previous studies recommend that factor solutions of similar complexity as ours utilize a minimum sample of 50–300 (MacCallum et al. 1999;Jung and Lee 2011). Thus, the MTurk sample (266 individuals) should have been large enough for the solution we found.
Confirmatory Factor Analysis: Confirming the Factor Model of Depressive Symptomatology
Using confirmatory factor analysis (CFA), we fitted the factor solution from the EFA model to the HH data via Mplus (Muthén and Muthén 2011). Three CFA models were fitted to the HH data: a model with correlated factors, a hierarchical model with a general depression factor (Gd), and a bifactor model. Goodness of fit was evaluated for each of the models using several indices, including χ2, Comparative Fit Index (CFI), Tucker Lewis Index (TLI), Root Mean Square Error of Approximation (RMSEA), and the Weighted Root Mean Square Residual (WRMR). Ordinarily, a significant χ2 value is suggestive of poor model fit; however, given our large sample size, less weight was attributed to this index (Kline 2005). For RMSEA, a value below 0.05 is considered excellent fit, while CFI and TLI values of >0.90 are indicative of a reasonably good fit (Hu and Bentler 1999). For WRMR a value that is close to 1 indicates a good fit. For CFA, power analysis can be used to determine the sample size required so that models with poor fit can be reliably rejected. This is done by manipulating the sample size so that the upper limit of a confidence interval for the population RMSEA, given the hypothesized RMSEA value, is less than what is operationally defined as an ill-fitting model (Maxwell et al. 2008). Given a three-factor model where α = 0.05, df = 1481, sample size = 128, null RMSEA = 0.05 and alternate RMSEA = 0.08, we had 100% power to be able to reject ill-fitting models (Preacher and Coffman 2006).
Confirmatory Factor Analysis: Deriving Factor Scores
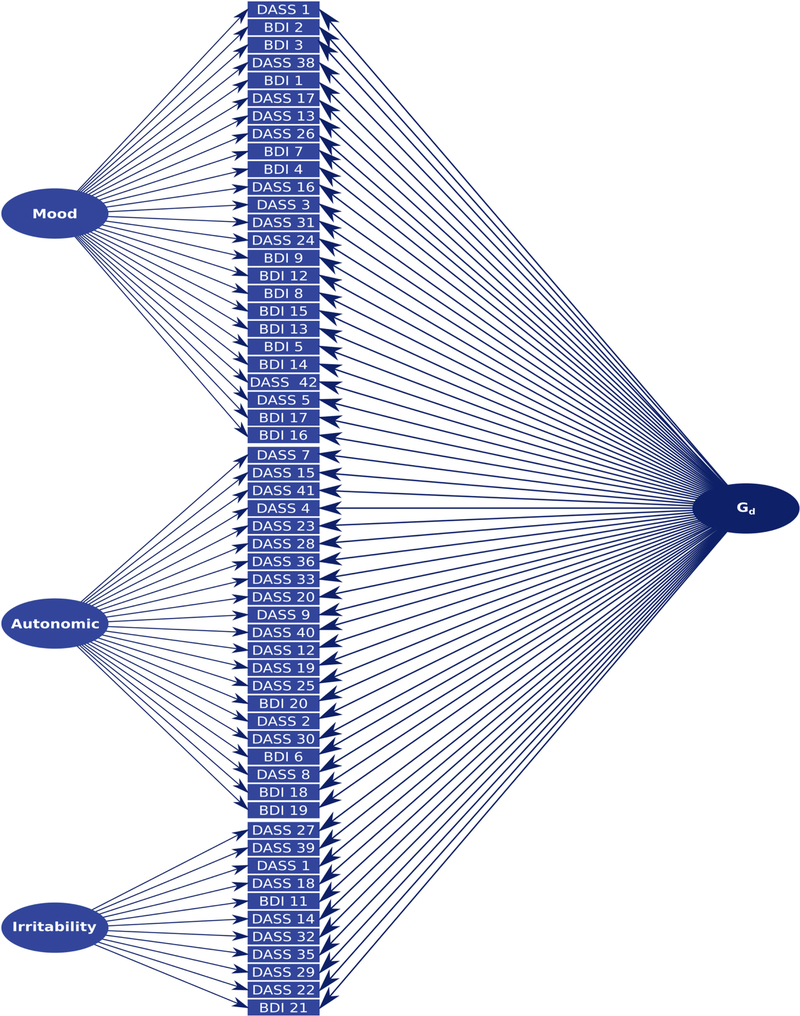
Factor scores were calculated from the bifactor model for each participant in the HH sample and saved for subsequent analysis. To be consistent with our previous work (Knowles et al. 2014b;Knowles et al. 2015;Knowles et al. 2012;Knowles et al. 2015), factor scores were subjected to rank-based inverse normalization prior to any further analysis. In this bifactor model, all items load on the Gd factor, representing individual differences in general depressive symptomatology, and also load on factors representing individual symptom dimensions (Mood, Autonomic, and Irritability), which are not correlated with the Gd factor. Thus, this model allows us to quantify individuals’ general depressive symptomatology, as well as specific groups of symptoms that are independent of general depression (Holzinger and Swineford 1937;Reise et al. 2010a;Knowles et al. 2014b).
Differences in Depressive Symptomatology in Psychosis Versus Non-Psychosis
All analyses pertaining to investigating differences in depressive symptomatology in psychosis versus non-psychosis as well as the mediation analyses were conducted in the HH sample. First, we used Wald t-tests to investigate whether scores on the general depression factor or specific factors differed between individuals with and without psychosis, and whether scores differed between those individuals with affective (schizoaffective, psychotic major depressive and bipolar disorders) and non-affective (schizophrenia) psychosis. Then, using multiple linear regressions, we explored the relationships between depression and specific psychotic symptoms. The regression models contained up to three independent variables, which reflected the three categories of psychotic symptoms—positive, negative or disorganized—from the LDPS (Levinson et al. 2002). These variables were coded such that 0 denoted absence and 1 denoted presence of any symptom from the corresponding category. Each regression model had scores on the Gd factor as the dependent variable. Bootstrapped 95% confidence intervals around the coefficients were computed using the boot package (Canty and Ripley 2017;Davison and Hinkley 2017). A robust version of the final regression model was also fitted using the MASS package (Venables and Ripley 2002) and can be viewed in the supplemental material.
Mediation Analysis: Mediating Role of Gd on the Relationship Between Psychosis and Substance Dependence
We performed mediation analysis in MPlus (Muthén and Muthén 2011) to determine whether the association between substance dependence and psychosis was mediated by Gd factor scores. We constructed four models. The first posited a direct relationship between the independent variable, psychosis (0 indicating absence and 1 indicating presence of any psychotic disorder), and the dependent variable, substance dependence. To create the dependent variable, we collapsed three substance-dependence diagnoses (alcohol, cannabis, and cocaine) into a single variable, within 0 denoting absence of any substance diagnosis and 1 denoting presence of at least one substance diagnosis. In the second model, the relationship between psychosis and substance dependence was ‘partially’ mediated by scores on the general depression factor. In the third model, the relationship was ‘fully’ mediated, also referred to as maximum evidence for mediation. The fourth model was a multi-outcome partial mediation model and represents a post-hoc analysis wherein the three substance dependence disorders were treated as separate dependent variables.
Since the dependent variables in the mediation models were always categorical, we used a robust weighted least squares estimator (i.e. weighted least squares means and variance adjusted WLSMV). This estimator is the optimal choice for modeling categorical data since it does not assume that variables are normally distributed (Brown 2006). We also used bootstrapping to obtain confidence intervals around the mediated effect (with 5000 resampling iterations) to determine whether it differed significantly from zero. Bootstrapping is a powerful non-parametric re-sampling method that, when applied over a large number of samples, yields more accurate confidence intervals than typical approaches (Kenny 2016). In Mplus, under WLSMV, any variable denoted as being categorical is represented by an assumed underlying normal latent distribution i.e. as a continuous variable. We specified only Substance Dependence (Y) as being categorical and not Psychosis (X) because, as is standard in regression analysis the scale of the IV does not affect model estimation. Numerous options for standardization are available in Mplus including scaling on X and/or Y (StdX, StdXY, StdY). In our model, where X was dichotomous but Y was treated as continuous it made sense to scale regression coefficients only by Y, because standardization using a dichotomous variable does not make sense i.e. a standard deviation change in a dichotomous variable is not meaningful. Therefore the regression coefficients presented in the paper are scaled by Y (StdY) (Li 2016;Muthén et al. 2016).
To control for multiple testing, the false discovery rate (FDR) was set at 5% (Benjamini and Yekutieli 2001) for all main effects including all tests aimed at testing differences in depressive symptomatology in psychosis versus non psychosis and mediation analyses.
Results
Descriptive Statistics of BDI and DASS Scores
Table 1 presents means and standard deviations of scores on the BDI and DASS subscales in both samples, as well as clinical characteristics of the HH sample. Inspection of the distributions of scores on the BDI and DASS subscales (Figure S1) reveals that they were somewhat negatively skewed in both the MTurk and HH samples, which is typical of likert-scale data. Internal consistency was excellent: in the MTurk sample, Cronbach’s α was 0.93 for the BDI and 0.97 for the DASS; for the HH sample, Cronbach’s α was 0.93 for the BDI and 0.97 for the DASS. Item-total correlations in the MTurk sample (BDI rτ range = 0.04–0.79; DASS rτ range = 0.17–0.91) and in the HH sample (BDI rτ range = 0.00–0.67; DASS rτ range = 0.05–0.77) indicated good discrimination for all items. In terms of convergent validity, the total scores for the BDI and the DASS correlated significantly in both the MTurk sample (rτ = 0.89, pFDR = 3.31×10−89) and the HH sample (rτ = 0.84, pFDR = 3.75×10−69). Inspection of the correlations between all BDI/DASS items (Figure S2) reveals that the patterns of correlations were largely similar across the two samples.
Table 1.
Depression and BDI/DASS characteristics of the MTurk (N = 266) and HH (N = 256) samples.
| MTurk | HH | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample Characteristics | Median | Mean | SD | Range | Median | Mean | SD | Range |
| Age | 34.00 | 36.74 | 12.44 | 19–75 | 39 | 39.98 | 13.73 | 18–70 |
| BDI Total | 3 | 7.52 | 9.44 | 0–41 | 5 | 8.08 | 9.35 | 0–44 |
| DASS Total | 1 | 6.25 | 9.56 | 0–42 | 9.5 | 18.21 | 20.58 | 0–94 |
| GAF Score | 72 | 70.26 | 11.64 | 40–92 | ||||
| N | % | N | % | |||||
| Female | 119 | 44.74 | 123 | 48.05 | ||||
| SCID Screener MDD (a) | 78 | 29.32 | 139 | 54.30 | ||||
| SCID Screener MDD (b) | 78 | 29.32 | 112 | 43.75 | ||||
|
Visited professional for low mood? |
58 | 21.80 | ||||||
| Received a diagnosis of MDD | 40 | 15.04 | 22 | 8.59 | ||||
|
Received Rx for anti- depressants? |
37 | 13.91 | ||||||
| Single-episode major depression | 6 | 2.34 | ||||||
| Recurrent major depression | 16 | 6.25 | ||||||
Developing the Factor Model in the MTurk Sample
Parallel analysis of the BDI and the DASS in the MTurk sample indicated that a three-factor solution should be selected (Figure S3). An EFA with a specified three-factor solution exhibited a number of items with factor loadings >1 due to collinearity (DASS items: 10, 34, and 37). These problematic items were excluded and the factor analysis was performed again. The second EFA accounted for 51% of the total variance. Table 2 presents the factor solution. The first factor consisted mostly of items relating to affect, with references to “getting upset”, feeling “hopeless” and “blue”, as well as references to cognition and anhedonia. Therefore, we termed the first factor Mood. The second factor consisted of items related to autonomic aspects of depression and anxiety: feelings of “shakiness”, “faintness”, “breathing difficulty”, and “nervous tension”; as well as alterations in appetite and energy levels. We termed this factor Autonomic. The third factor consisted of items relating to irritability: for example, being “irritable”, “touchy”, “impatient”, and “agitated”. We termed this factor Irritability. Correlations between factors were high, but did not indicate complete overlap (Table 2).
Table 2.
Factor loadings and variance explained for the 3-factor promax rotated model.
| Factor Loading | ||||||
|---|---|---|---|---|---|---|
| Measure | No. | Item | 1. Mood |
2. Autonomic |
3. Irritability |
|
| BDI | 2 | I feel my future is hopeless and will only get worse |
0.97 | −0.17 | −0.08 | |
| DASS | 21 | I found myself getting upset by quite trivial things | 0.96 | 0.01 | −0.17 | |
| BDI | 3 | I feel like I am a total failure as a person | 0.91 | −0.05 | −0.09 | |
| DASS | 38 | I felt that life was meaningless | 0.91 | −0.05 | −0.10 | |
| BDI | 1 | I am so sad or unhappy that I can’t stand it | 0.88 | 0.08 | −0.15 | |
| DASS | 17 | I felt that I wasn’t worth much as a person | 0.87 | 0.06 | −0.08 | |
| DASS | 13 | I felt sad and depressed | 0.87 | 0.04 | 0.01 | |
| DASS | 26 | I felt down-hearted and blue | 0.82 | 0.07 | 0.05 | |
| BDI | 4 | I can’t get any pleasure from the things I used to enjoy |
0.77 | 0.00 | 0.12 | |
| BDI | 7 | I dislike myself | 0.74 | −0.08 | 0.11 | |
| DASS | 16 | I felt that I had lost interest in just about everything |
0.74 | 0.11 | 0.06 | |
| DASS | 3 | I couldn’t seem to experience any positivefeeling at all |
0.73 | 0.10 | 0.02 | |
| DASS | 13 | I was unable to be enthusiastic about anything | 0.71 | −0.02 | 0.15 | |
| DASS | 24 | I couldn’t seem to get any enjoyment out of the things I did |
0.71 | 0.02 | 0.18 | |
| BDI | 9 | I would kill myself if I had the chance | 0.69 | 0.17 | −0.20 | |
| BDI | 12 | It’s hard to get interested in anything | 0.65 | −0.09 | 0.26 | |
| BDI | 8 | I blame myself for everything bad that happens | 0.61 | −0.03 | 0.18 | |
| BDI | 15 | I don’t have enough energy to do anything | 0.59 | 0.22 | 0.04 | |
| BDI | 13 | I have trouble making any decisions | 0.53 | 0.18 | 0.06 | |
| BDI | 5 | I feel guilty all of the time | 0.51 | 0.03 | −0.03 | |
| BDI | 14 | I feel utterly worthless | 0.44 | 0.19 | 0.03 | |
| DASS | 42 | I found it difficult to work up the initiative to do things |
0.43 | 0.28 | 0.17 | |
| BDI | 10 | I don’t cry any more than usual | 0.43 | −0.04 | 0.13 | |
| DASS | 5 | I just couldn’t seem to get going | 0.39 | 0.23 | 0.26 | |
| BDI | 17 | I am irritable all the time | 0.39 | 0.14 | 0.23 | |
| BDI | 16 | I wake up 1–2 hours early/I sleep most of the day | 0.29 | 0.16 | 0.17 | |
| DASS | 15 | I had a feeling of faintness | −0.18 | 0.92 | −0.14 | |
| DASS | 7 | I had a feeling of shakiness | −0.23 | 0.91 | −0.04 | |
| DASS | 28 | I felt I was close to panic | 0.06 | 0.79 | −0.08 | |
| DASS | 36 | I felt terrified | 0.09 | 0.72 | −0.15 | |
| DASS | 41 | I experienced trembling | −0.16 | 0.71 | 0.06 | |
| DASS | 23 | I had difficulty swallowing | 0.04 | 0.70 | −0.22 | |
| DASS | 4 | I experienced breathing difficulty | 0.15 | 0.69 | −0.23 | |
| DASS | 33 | I was in a state of nervous tension | −0.13 | 0.68 | 0.30 | |
| DASS | 9 | I found myself in situations that made me so anxious I was… |
0.03 | 0.65 | 0.16 | |
| DASS | 40 | I was worried about situations in which I might panic |
0.17 | 0.62 | −0.02 | |
| DASS | 20 | I felt scared without any good reason | 0.06 | 0.61 | 0.06 | |
| DASS | 12 | I felt that I was using a lot of nervous energy | −0.04 | 0.61 | 0.24 | |
| DASS | 19 | I perspired noticeably in the absence of high temperatures |
0.00 | 0.53 | 0.04 | |
| DASS | 30 | I feared that I would be “thrown” by some trivial task |
0.15 | 0.49 | 0.09 | |
| DASS | 2 | I was aware of dryness of my mouth | −0.10 | 0.48 | 0.14 | |
| DASS | 25 | I was aware of the action of me heart | 0.11 | 0.48 | −0.01 | |
| DASS | 22 | I found it hard to wind down | −0.02 | 0.41 | 0.43 | |
| BDI | 20 | I am too tired or fatigued to do most of the things I used to |
0.31 | 0.46 | 0.00 | |
| DASS | 8 | I found it difficult to relax | 0.08 | 0.40 | 0.38 | |
| BDI | 6 | I feel I am being punished | 0.26 | 0.42 | −0.09 | |
| BDI | 18 | I have no appetite at all/I crave food all the time | 0.16 | 0.33 | −0.09 | |
| BDI | 19 | I find I can’t concentrate on anything | 0.12 | 0.22 | −0.09 | |
| DASS | 27 | I found that I was very irritable | −0.07 | −0.04 | 0.94 | |
| DASS | 39 | I found myself getting agitated | −0.08 | 0.04 | 0.92 | |
| DASS | 1 | I found myself getting upset by quite trivial things | −0.03 | −0.03 | 0.88 | |
| DASS | 18 | I felt that I was rather touchy | −0.15 | 0.09 | 0.87 | |
| DASS | 11 | I found myself getting upset rather easily | 0.04 | −0.01 | 0.85 | |
| DASS | 6 | I tended to over-react to situations | −0.05 | −0.07 | 0.84 | |
| BDI | 11 | I am so restless or agitated that I have to keep moving or… |
0.23 | −0.18 | 0.76 | |
| DASS | 14 | I found myself getting impatient when I was delayed… |
−0.14 | 0.09 | 0.74 | |
| DASS | 32 | I found it difficult to tolerate interruptions | 0.14 | 0.07 | 0.64 | |
| DASS | 35 | I was intolerant of anything that kept me from getting on… |
0.23 | −0.04 | 0.56 | |
| DASS | 29 | I found it hard to calm down after something upset me |
−0.11 | 0.47 | 0.50 | |
| BDI | 21 | I have lost interest in sex completely | 0.24 | −0.06 | 0.32 | |
| Cumulative Variance Explained: | 22.50% | 37.30% | 50.80% | |||
| Factor Correlations in the EFA model: | 1 | 1 | ||||
| 2 | 0.70 | 1 | ||||
| 3 | −0.74 | −0.67 | 1 | |||
Confirming the Factor Model in the HH Sample
A confirmatory factor model with three correlated factors (Mood, Autonomic, and Irritability) was fitted to the HH sample. The model was a good fit to the data: χ2 (= 2125.531649, p<0.001) was significant, but likely due to the large sample size. Nevertheless, other fit indices were excellent (RMSEA = 0.034 (95%CI = 0.029–0.038), p = 1.00; CFI = 0.98; TLI = 0.98, WRMR = 1.02). In the HH sample, forcing the factors to be orthogonal (without the inclusion of a general factor) resulted in a significant reduction in model fit (χ2 = 17423.111652, p <0.00001, χ2 = 15298.113, p<0.001; RMSEA = 0.19 (95%CI = 0.19–0.20), p <0.001; CFI = 0.27; TLI = 0.24, WRMR = 7.31). In the interests of calculating a general index of depressive symptomatology (see below), we fitted an additional three-factor hierarchical model (Figure S4), which is mathematically equivalent to the correlated three-factor model and consequently was also an excellent fit to the data (see fit indices above). Finally, we fitted a bifactor model (Figure 1) where individual symptom dimensions (Mood, Autonomic and Irritability) were forced to be orthogonal from the general factor. The bifactor model, like the correlated factors and hierarchical models, was an excellent fit to the data (χ2 = 1960.541593, p <0.001; RMSEA = 0.030 (95%CI = 0.025–0.034), p = 1.00; CFI = 0.98; TLI = 0.98, WRMR = 0.91), and RMSEA indicated slightly improved fit over the hierarchical model. Inspection of the loadings under this model (Table 3) show that all items loaded significantly onto the Gd factor. Some items no longer loaded significantly on their original factors under the correlated factor model; however, these alterations are not sufficient to change the interpretation of the factors (the distributions of the factor scores are shown in Figure S5).
Fig. 1.
Schematic of the bifactor model (for loadings see Table 3).
Table 3.
Factor loadings for the bifactor model.
| Factor Loading | ||||||
|---|---|---|---|---|---|---|
| Measure | No. | Item | Mood | Autonomic | Irritability | Gd |
| BDI | 2 | I feel my future is hopeless and… | 0.45**** | 0.61**** | ||
| DASS | 21 | I found myself getting upset by… | 0.26**** | 0.86**** | ||
| BDI | 3 | I feel like I am a total failure as… | 0.44**** | 0.65**** | ||
| DASS | 38 | I felt that life was meaningless | 0.26*** | 0.81**** | ||
| BDI | 1 | I am so sad or unhappy that… | 0.19** | 0.79**** | ||
| DASS | 17 | I felt that I wasn’t worth… | 0.38**** | 0.81**** | ||
| DASS | 13 | I felt sad and depressed | 0.17**** | 0.87**** | ||
| DASS | 26 | I felt down-hearted and blue | 0.17**** | 0.85**** | ||
| BDI | 4 | I can’t get any pleasure from… | 0.34**** | 0.70**** | ||
| BDI | 7 | I dislike myself | 0.51**** | 0.68**** | ||
| DASS | 16 | I felt that I had lost interest… | 0.20**** | 0.87**** | ||
| DASS | 3 | I couldn’t seem to experience… | 0.24**** | 0.73**** | ||
| DASS | 31 | I was unable to be enthusiastic… | 0.24**** | 0.84**** | ||
| DASS | 24 | I couldn’t seem to get any… | 0.22**** | 0.84**** | ||
| BDI | 9 | I would kill myself if… | 0.19 | 0.68**** | ||
| BDI | 12 | It’s hard to get interested… | 0.41**** | 0.82**** | ||
| BDI | 8 | I blame myself for everything… | 0.37**** | 0.64**** | ||
| BDI | 15 | I don’t have enough energy… | 0.34**** | 0.76**** | ||
| BDI | 13 | I have trouble making any… | 0.30**** | 0.75**** | ||
| BDI | 5 | I feel guilty all of the time | 0.23**** | 0.67**** | ||
| BDI | 14 | I feel utterly worthless | 0.49**** | 0.72**** | ||
| DASS | 42 | I found it difficult to work… | 0.28**** | 0.82**** | ||
| DASS | 5 | I just couldn’t seem to get… | 0.19*** | 0.78**** | ||
| BDI | 17 | I am irritable all the time | −0.04 | 0.79**** | ||
| BDI | 16 | I wake up 1–2 hours early… | 0.23**** | 0.68**** | ||
| DASS | 15 | I had a feeling of faintness | 0.42**** | 0.62**** | ||
| DASS | 7 | I had a feeling of shakiness | 0.46**** | 0.67**** | ||
| DASS | 28 | I felt I was close to panic | 0.32**** | 0.81**** | ||
| DASS | 36 | I felt terrified | 0.25*** | 0.83**** | ||
| DASS | 41 | I experienced trembling | 0.42**** | 0.70**** | ||
| DASS | 23 | I had difficulty swallowing | 0.47**** | 0.64**** | ||
| DASS | 4 | I experienced breathing difficulty | 0.52**** | 0.58**** | ||
| DASS | 33 | I was in a state of nervous tension | 0.19*** | 0.88**** | ||
| DASS | 9 | I found myself in situations that… | 0.27**** | 0.83**** | ||
| DASS | 40 | I was worried about situations… | 0.27**** | 0.83**** | ||
| DASS | 20 | I felt scared without any good… | 0.23** | 0.80**** | ||
| DASS | 12 | I felt that I was using a lot of… | 0.18* | 0.76**** | ||
| DASS | 19 | I perspired noticeably in the… | 0.19 | 0.59**** | ||
| DASS | 30 | I feared that I would be “thrown”… | 0.19** | 0.74**** | ||
| DASS | 2 | I was aware of dryness of… | 0.34**** | 0.38**** | ||
| DASS | 25 | I was aware of the action… | 0.40**** | 0.59**** | ||
| DASS | 22 | I found it hard to wind down | −0.02 | 0.72**** | ||
| BDI | 20 | I am too tired or fatigued to… | −0.12 | 0.81**** | ||
| DASS | 8 | I found it difficult to relax | −0.02 | 0.82**** | ||
| BDI | 6 | I feel I am being punished | −0.16 | 0.74**** | ||
| BDI | 18 | I have no appetite at all… | −0.02 | 0.67**** | ||
| BDI | 19 | I find I can’t concentrate on… | −0.09 | 0.82**** | ||
| DASS | 27 | I found that I was very irritable | 0.25**** | 0.89**** | ||
| DASS | 39 | I found myself getting agitated | 0.28**** | 0.83**** | ||
| DASS | 1 | I found myself getting upset… | 0.15* | 0.77**** | ||
| DASS | 18 | I felt that I was rather touchy | 0.36**** | 0.68**** | ||
| DASS | 11 | I found myself getting upset… | 0.27**** | 0.84**** | ||
| DASS | 6 | I tended to over-react to… | 0.20* | 0.75**** | ||
| BDI | 11 | I am so restless or agitated… | −0.04 | 0.74**** | ||
| DASS | 14 | I found myself getting impatient… | 0.22**** | 0.75**** | ||
| DASS | 32 | I found it difficult to tolerate… | 0.46**** | 0.77**** | ||
| DASS | 35 | I was intolerant of anything… | 0.51**** | 0.77**** | ||
| DASS | 29 | I found it hard to calm down… | 0.23**** | 0.81**** | ||
| BDI | 21 | I have lost interest in sex … | −0.28** | 0.61**** | ||
p<0.05
p<0.01
p<0.001
p<0.0001
Is Psychosis Related to General Depression (Gd) Score?
On average, scores on the general depression factor from the bifactor model, Gd, were higher in individuals with psychosis (Figure S6-A), (M = 0.74) than without psychosis (M = −0.36). This difference was significant (t(159.78) = −9.81, pFDR = 9.22×10−17), and the effect was large (r = 0.61).
Is There a Difference in General Depression Scores Between Affective and Non-Affective Psychoses?
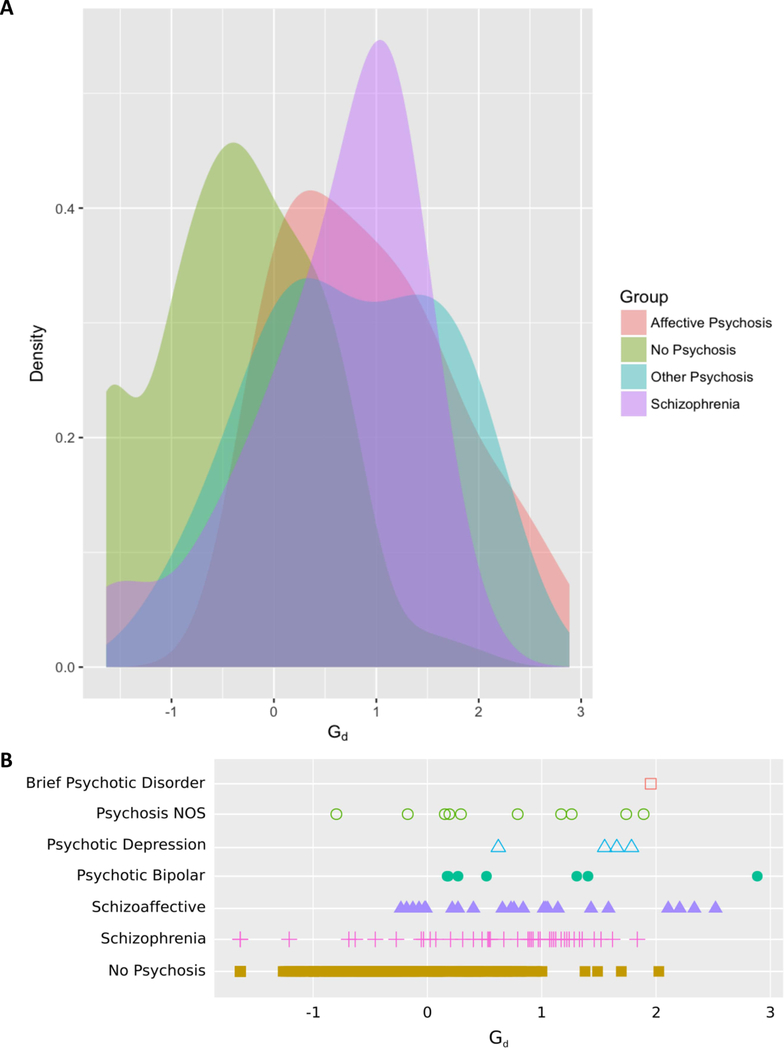
Figure 2A shows a kernel density plot of scores on the Gd factor by group and Figure 2B a stripplot of Gd scores split by diagnosis, inspection of these figures indicates a large degree of overlap between disparate psychotic disorders in terms of severity of depressive symptomatology. Scores on the Gd factor were slightly higher in the affective psychosis (M = 0.56) than the non-affective psychosis (M = 0.35) group but the difference was not significant (t(71.12) = −1.79, pFDR = 0.48).
Fig. 2.
Density plot (A) and strip plot (B) of scores on the general depression (Gd) factor across psychotic diagnoses.
Is the General Depression (Gd) Score Related to Specific Aspects of Psychosis?
Multiple linear regression was used to predict Gd scores from the presence or absence of positive, negative and disorganized symptoms, as defined using the LDPS. Together, these three categorical predictors explained 33% of the variance in Gd score (R2 = 0.33, F(6, 249) = 20.58, pFDR = 3.34×10−18), but only positive symptoms significantly predicted scores on the Gd factor (βpositive = 1.06, pFDR = 6.04×10−12, 95%CI = 0.77–1.34). Neither negative (βnegative = 0.05, pFDR = 1.00, 95%CI = −0.32–0.41) nor disorganized (βdisorganized = 0.10, pFDR = 1.00, 95%CI = −0.22–0.45) symptoms were significant predictors of Gd scores in this model. A robust version of the same regression model yielded the same results (see Supplemental Material). When negative and disorganized symptoms were dropped from the model, positive symptoms alone explained 31% of the variance (R2 = 0.31, F(1, 254) = 113.50, p = 3.77×10−22; βpositive = 1.12, p = = 3.77×10−22, 95%CI = 0.89–1.32). The exclusion of negative and disorganized symptoms did not lead to a significant reduction in the variance explained (F(2,252) = 0.22, p = 0.80). In conclusion, positive psychotic symptoms—but not negative or disorganized symptoms—were significantly associated with greater scores of general depressive symptomatology.
The inclusion of antipsychotic and antidepressant medication status into the above regression model did not change the results (R2 = 0.31, F(3, 252) = 38.19, p = 2.22×10−20; βpositive = 1.09, p = 9.79×10−12, 95%CI = 0.79–1.39). Neither antipsychotic (βantipsychotic = −0.06, p = 0.73, 95%CI = −0.40–0.32) nor antidepressant (βantidepressant = −0.18, p = 0.25, 95%CI = −0.18–0.51) status was a significant predictor of Gd scores. The psychosis and non-psychosis groups did not differ in terms of the distribution of sex (χ2 = 0.051, p = 0.83) or age (t(187.91) = −0.06, p = 0.95). The Gd score did not correlate significantly with either age (r = 0.06, p = 0.37) or sex (r = 0.10, p = 0.13).
Are Mood, Autonomic or Irritability Scores Related to Psychosis?
Of the three symptom domains unrelated to Gd, only Autonomic symptoms varied as a function of psychosis (Figure S6B-D). Autonomic symptom scores were higher in individuals with psychosis (M = 0.28) than without psychosis (M = −0.14), with a small r = 0.25, but statistically significant difference t(144.74) = −3.06, pFDR = 0.02). Neither Mood (t(129.28) = −1.20, pFDR = 1.00, r = 0.10) nor Irritability (t(129.37) = −1.04, pFDR = 1.00, r = 0.09) symptom scores significantly varied as a function of psychosis. Correlation analyses indicated that Negative psychotic symptoms were significantly correlated with Mood symptoms (rτ = 0.23, pFDR = 2.84×10−03), and that Positive and Disorganized symptoms were both significantly correlated with Autonomic symptoms (rτ = 0.19, pFDR = 0.02 and rτ = 0.20, pFDR = 0.01 respectively). In all cases, however, these effects were small (see Table S2 for all correlations between all psychotic and depression symptoms).
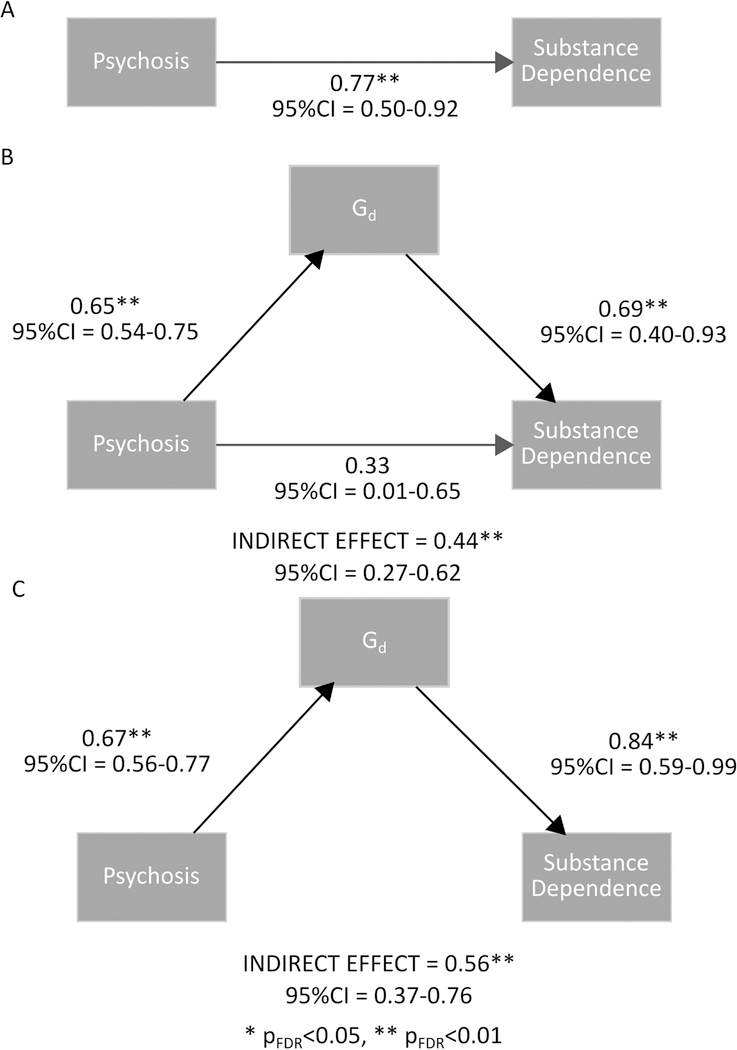
Does General Depression (Gd) Mediate Substance Dependence in Psychosis?
Substance dependence was significantly more frequent in the psychosis group than the control group, 70% of people with psychosis had a substance dependence diagnosis versus 41% of controls (OR = 3.47, pFDR = 1.18×10−04), and was significantly and positive correlated with Gd score (rpb =0.36, pFDR = 6.06×10−08). Figure 3 summarizes the results of the mediation analysis. A direct model (Figure 3A) indicated a strong effect of psychosis on substance dependence. A model with partial mediation (Figure 3B) indicated a significant indirect effect of psychosis on substance dependence via Gd, while the direct effect was reduced and non-significant. A model with full mediation (Figure 3C) supported an indirect effect of psychosis on substance dependence via Gd. These results suggest a strong partial, if not full, mediation of the relationship between psychosis and substance dependence by Gd i.e. general depressive symptomatology. The inclusion of antipsychotic and antidepressant medication in the model did not alter the results (Figure S7).
Fig. 3.
Direct (A), partial mediation (B) and full mediation (C) models of psychosis, general depression (Gd) and substance dependence.
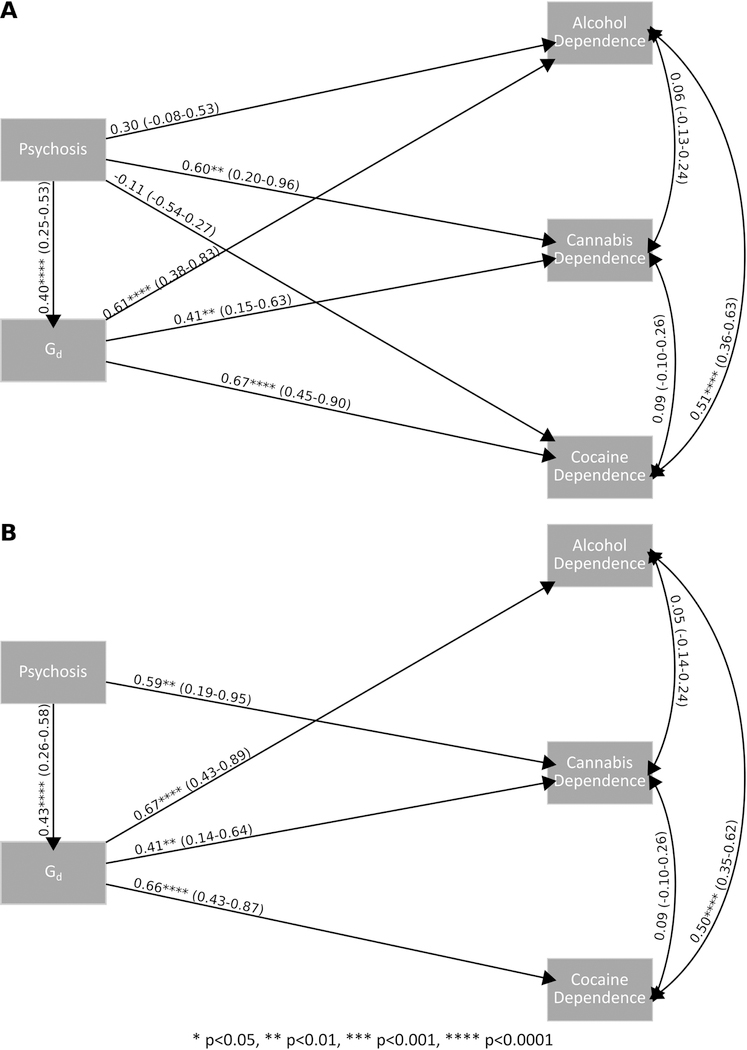
Post-hoc mediation analysis was conducted to investigate the potential mediation of any specific type of substance dependence by Gd in psychosis, a multi-outcome model with three outcomes including alcohol, cannabis and cocaine dependence was fit to the data. Figure 4A shows a partial mediation model with bootstrapped standard errors where all direct and indirect paths were allowed to vary freely. Direct effects of psychosis on alcohol (β = 0.30 p = 0.18, 95%CI = −0.08–0.67) and cocaine (β = −0.11 p = 0.65, 95%CI = −0.54–0.27) dependence were not significant, while the direct effect of psychosis on cannabis was significant (β = 0.60 p = 0.01, 95%CI = 0.20–0.96). Indirect effects were significant for alcohol (β = 0.25 p = 1.00×10−03, 95%CI = 0.14–0.39), cannabis (β = 0.16 p = 0.02, 95%CI = 0.06–0.30), and cocaine (β = 0.27 p = 1.00×10−03, 95%CI = 0.16–0.23). Figure 4B shows the same model with non-significant direct paths (psychosis on alcohol and cocaine dependence) dropped. In this model, all direct (for psychosis on cannabis dependence: β = 0.56 p = 8.00×10−03, 95%CI = 0.19–0.95) and indirect paths were significant (for alcohol dependence: β = 0.29 p = 4.00×10−03, 95%CI = 0.15–0.44; for cannabis dependence: β = 0.17 p = 0.03, 95%CI = 0.06–0.33; and for cocaine dependence: β = 0.28 p = 1.00×10−03, 95%CI = 0.16–0.45). These findings support the hypothesis that Gd partially mediates the relationship between psychosis and alcohol, cannabis and cocaine dependence.
Fig. 4.
Multi-outcome mediation models for alcohol, cannabis, and cocaine dependence where (A) all paths are included and (B) non-significant paths are dropped.
Discussion
The co-occurrence of subsyndromal depression with psychosis is well established, but its clinical correlates, specifically in relation to features of the psychotic experience and comorbid substance use disorders, remain unclear. Moreover, the hierarchical nature of psychiatric diagnoses, where schizophrenia takes precedence over depression, means that depressive symptoms are often overlooked in psychotic patients. Consequently, clinicians are unlikely to give a dual diagnosis of schizophrenia and depressive disorder (Buckley et al. 2009). This failure to fully appreciate the widespread nature of depressive symptomatology in psychosis may have serious consequences for both clinical practice and research. We developed and validated a multidimensional bifactor model of depressive symptomatology in two independent samples. First, an exploratory factor model was developed in a Mechanical Turk sample collected online. This model was then confirmed and extended in a sample of locally recruited psychotic cases and healthy controls. Using the dimensions derived from this model we showed that subsyndromal depressive symptomatology is: (a) significantly higher in individuals with psychosis than healthy controls, but (b) not significantly different in individuals with non-affective and affective psychosis, and (c) significantly related to positive, but not negative or disorganized, symptoms of psychosis. Finally, (d) our mediation analysis indicates that we have maximum evidence for mediation of the relationship between psychosis and substance dependence by general depressive symptomatology.
Our final model of depression is shown in Figure 1. This bifactor model includes both a general factor encapsulating all items, in addition to a set of orthogonal, or independent, factors that capture subgroups of items. Herein lies the major strength of bifactor models, in that they capture multidimensionality in the data, while simultaneously retaining a single construct of interest (Reise et al. 2010b). The general factor here, Gd, represents overall depressive symptomatology as indexed by all items from the BDI and the DASS. Meanwhile, the independent factors represent symptomatology relating to Mood, Autonomic and Irritability symptoms, and capture variance that is not accounted for by Gd. In line with previous findings that the majority of schizophrenia patients experience depression at some point during the illness (Buckley et al. 2009;Tandon et al. 2009;Majadas et al. 2012), we found that scores on the Gd factor were significantly higher in individuals with psychosis. However, our results also extend previous work by demonstrating that subsyndromal depressive symptoms are present across the spectrum of psychotic disorders, but also that depression severity is similar in non-affective and affective psychoses. Given the potential ramifications of depression on functional outcome (Siris and Bench 2003;Sim et al. 2004;Buckley 2006), our findings highlight the importance of thorough investigations of depression across the psychosis spectrum since failure to identify and treat depression in these patients may seriously hinder efforts to manage their illnesses.
Psychosis is characterized by positive and negative symptoms (Liddle 1987;Andreasen 1995). Positive, in this context, refers to symptoms of perceptual aberration, including hallucinations and delusions. Negative symptoms, on the other hand, refer to a deficit, or reduction of behaviors. One of the major challenges in investigating depression in psychosis is its potential overlap with negative symptoms, that is they can be difficult to distinguish from eachother (Siris et al. 1988). However, we found that scores on the Gd factor were most strongly associated with positive, not negative, symptoms. In addition, both positive and disorganized symptoms correlated significantly with Autonomic symptoms relating to panic. Conversely, only negative symptoms exhibited a significant correlation with the Mood subdomain, which represents mood symptoms independent of general depression. Thus, our findings suggest that it is possible to successfully disentangle variance relating to depressive symptomatology versus negative symptoms. The association of depression with positive and not negative symptoms also fits within the previous literature on this topic. Previous work suggests that there exists an independent depressive dimension in the structure of psychosis (Upthegrove et al. 2010) where anhedonia is common to negative symptoms and depression but other core depressive symptoms are distinct (Upthegrove et al. 2017).
Under a hierarchical model of diagnoses with psychosis at the pinnacle, lower-level affective type disorders are neglected (Freeman and Garety 2003). Our results challenge this approach by providing evidence that subsyndromal depression may arise as a consequence of, or even contribute to, the heart of the psychotic experience: positive symptoms. This ties in with research using ecological momentary assessment (EMA), where affective experience in schizophrenia patients is repeatedly sampled throughout their daily lives and shows that patients, as compared to controls, consistently report more negative and less positive emotion (Cho et al. 2017). Indeed abundant evidence that affective disorders are significantly correlated with positive symptoms in psychosis supports our finding (Hartley et al. 2013;Foulds and Bedford 1975). Moreover, a relationship between depressive and positive symptoms is consistent with an affective model of psychosis where emotional changes and concomitant increases in arousal are instigated by the onset of anomalous perceptual experiences and contribute to the typically negative connotations of hallucinations and delusions. Thus, a vicious cycle of psychosis and non-psychotic affective psychopathology (historically referred to as neurosis) ensues where psychosis begets emotional distress, which, in turn, colors the nature of the positive symptoms, increasing distress and so on (Freeman and Garety 2003;Garety et al. 2001;Freeman et al. 2001). Indeed, retrospective studies suggest that peak depression coincides with peak psychosis (Hafner et al. 1999). Another school of thought is that depression in psychosis is a “smoking gun” for a history of childhood trauma (Upthegrove et al. 2017;Birchwood et al. 2005). Future work might address this potential using the same methods as that in the present study, where the relationship between trauma and psychosis might be mediated by depressive symptomatology or vice versa.
This study has some potential limitations. First, is its cross-sectional design, which cannot answer questions about the time sequence of affective disturbance in relation to the advent and worsening of psychotic symptoms. Future work, particularly utilizing advances in technology enabling symptoms to be assessed on a day-to-day or even hour-to-hour basis, might address this (Gibbons et al. 2012), this would be in line with the work using an EMA, or experience sampling method (ESM) approach (Cho et al. 2017;Oorschot et al. 2009), which utilizes technology to collect participant experiences as they occur. Second, some might view is its use of online crowdsourcing platform MTurk as a weakness of the study. However, it is a strength that both non-clinical and clinical samples were used for the identification and validation of the depression model. In addition this study demonstrates the potential utility of quickly and easily gathered data via crowdsourcing for the purposes of research in psychiatry (Buhrmester et al. 2011). In particular the design of the MTurk survey, which included a number of catch and consistency items, demonstrates one way in which datasets that might be considered unreliable that are collected online can be cleaned to yield reliability in line with that seen in a locally recruited sample. There are the potential issues of residual confounding and reverse causality that may affect our mediation models, where it is possible that the direction of effect is opposite to that stated in the paper (i.e. from depression via substance dependence to psychosis), this is a problem that is common to cross-sectional research. Future work, particularly of a longitudinal design, might assess the role of confounding factors not assessed in the present study and the timeline of events where it is possible that substance dependence might precede psychosis, a relationship that could conceivably be mediated by affective factors. Moreover, future work might attempt to capture a larger sample with a greater number of psychotic individuals, particularly with affective psychoses, as the present work is somewhat limited in terms of sample size. In addition, while we examined psychosis transdiagnostically, we made a distinction between those with and without psychosis, rather than utilizing a fully dimensional view of psychosis. Future work might address this by attempting to generate and replicate a model of depressive and psychotic symptoms within the same factor model. Moreover, it would be of benefit to treat substance dependence in a similarly dimensional fashion, future work might also incorporate scales that enable substance dependence to be modeled as such. Finally, the HH subjects were African American, like many other minority groups African Americans are underserved by psychiatric research, and we specifically chose to study them for this reason. However, this does raise the issue that, by focusing on one group, we have limited the generalizability of our findings. We would not expect the structure of depressive symptomatology to differ across ethnic groups however this might be addressed in future work.
Comorbidity of substance use disorders in psychosis is associated with relapse, as well as poor functional outcomes, such as homelessness, unemployment, treatment non-compliance, and suicidality (Potvin et al. 2007;Negrete 2003). Substance use is also associated with depression, both in psychotic (Brady et al. 1993;Kerfoot et al. 2011;Krausz et al. 1996;Scheller-Gilkey et al. 2002) and healthy individuals (Davis et al. 2008;Hasin et al. 2002). In psychotic individuals, discontinuation of substance use is associated with concomitant reductions in positive symptoms and depression (Mullin et al. 2012) and yet the relationships between substance use, depression (Swendsen and Merikangas 2000), and psychosis (Buckley et al. 2009), remain poorly understood. Increased substance use in schizophrenia has been posited as an attempt to self-medicate, however paradoxically substance use typically leads to a worsening of both the symptoms and course of the illness (Khantzian 1997). The present study, in line with previous research, suggests that substance use might be linked to severity of depression. Mediation analyses showed that Gd mediated the relationship between psychosis and substance dependence, and to varying degrees this was true for cocaine, alcohol, and cannabis dependence (Figure 4). Therefore, one possible explanation for increased substance use in psychosis is that cocaine alcohol use, and possibly to a lesser extent cannabis use, are taken to relieve distress associated with depression, but this is a tentative interpretation that requires further investigation (Thoma and Daum 2013;Khantzian 1985). Mediation analysis has drawbacks, primarily because it is, in essence, a correlational approach and consequently cannot establish causality. Therefore future studies should attempt to further tease apart these relationships, ideally studies of a longitudinal design, which might be used to establish temporal links between the diagnoses.
Prescription of antidepressants to schizophrenia patients is increasingly common (Mao and Zhang 2015) and the current findings underline the need for this practice to continue. While recent meta-analytic results suggest that the beneficial effects of adjunctive antidepressant treatment for depressive symptoms in schizophrenia are small (Helfer et al. 2016), treatment effects may be masked by relatively crude assessment of depressive symptomatology. Modeling symptoms at the item-level (as opposed to using sum scores) might reveal larger beneficial effects.
In sum, our findings highlight the importance of assessing subsyndromal depressive symptomatology in individuals with psychosis, especially in relation to positive symptoms. Assessing depressive symptomatology in psychosis will yield more clinically accurate and refined phenotypes, which could be of particular use in the field of genetics where the co-occurrence of psychosis and depression may hint at a shared etiology and could be exploited under a multivariate model to reveal shared biological underpinnings. Similarly, in neuroimaging studies, high levels of subsyndromal depression could mask or exacerbate effects. Finally, this study adds to a growing literature stressing the need for a multidimensional approach to psychiatry, which goes beyond the traditional, hierarchical and dichotomous framework in which psychosis takes precedence over all other disorders.
Supplementary Material
Acknowledgement
This work is supported by a grant from the National Institute of Mental Health (NIMH), grant number R01MH106324-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI: The authors have no conflicts of interest to declare.
References
- Allan R, Martin CR, 2009. Can the Hospital Anxiety and Depression Scale be used in patients with schizophrenia? J.Eval.Clin.Pract 15 (1) 134–141. [DOI] [PubMed] [Google Scholar]
- Al-Turkait FA, Ohaeri JU, 2010. Dimensional and hierarchical models of depression using the Beck Depression Inventory-II in an Arab college student sample. BMC Psychiatry 10 60–244X-10–60. [DOI] [PMC free article] [PubMed]
- Amazon, 2015. amazon mechanical turk (beta) Requester Best Practices Guide 2018. (09/24).
- Andreasen NC, 1995. Symptoms, signs, and diagnosis of schizophrenia. Lancet 346 (8973) 477–481. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Freimer NB, 2006. Endophenotypes for psychiatric disorders: ready for primetime? Trends Genet 22 (6) 306–313. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for the Beck Depression Inventory-II, Psychological Corporation, San Antonio, TX. [Google Scholar]
- Benjamini Y, Yekutieli D, 2001. The control of the false discovery rate in multiple testing under dependency. Annals of statistics 29 (4) 1165–1188. [Google Scholar]
- Birchwood M, Iqbal Z, Upthegrove R, 2005. Psychological pathways to depression in schizophrenia: studies in acute psychosis, post psychotic depression and auditory hallucinations. Eur.Arch.Psychiatry Clin.Neurosci 255 (3) 202–212. [DOI] [PubMed] [Google Scholar]
- Brady KT, Killeen T, Jarrell P, 1993. Depression in alcoholic schizophrenic patients. Am.J.Psychiatry 150 (8) 1255–1256. [DOI] [PubMed] [Google Scholar]
- Brouwer D, Meijer RR, Zevalkink J, 2013. On the factor structure of the Beck Depression Inventory-II: G is the key. Psychol.Assess 25 (1) 136–145. [DOI] [PubMed] [Google Scholar]
- Brown TA, 2006. Confirmatory factor analysis for applied research, Guilford Press, New York. [Google Scholar]
- Buckley PF, 2006. Prevalence and consequences of the dual diagnosis of substance abuse and severe mental illness. J.Clin.Psychiatry 67 Suppl 7 5–9. [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ, 2009. Psychiatric comorbidities and schizophrenia. Schizophr.Bull 35 (2) 383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrmester M, Kwang T, Gosling SD, 2011. Amazon’s Mechanical Turk: A New Source of Inexpensive, Yet High-Quality, Data? Perspect. Psychol.Sci 6 (1) 3–5. [DOI] [PubMed] [Google Scholar]
- Canty A, Ripley B, 2017. boot: Bootstrap R (S-Plus) Functions. R package version 1.3–19
- Cho H, Gonzalez R, Lavaysse LM, Pence S, Fulford D, Gard DE, 2017. Do people with schizophrenia experience more negative emotion and less positive emotion in their daily lives? A meta-analysis of experience sampling studies. Schizophr.Res 183 49–55. [DOI] [PubMed]
- Craddock N, Owen MJ, 2007. Rethinking psychosis: the disadvantages of a dichotomous classification now outweigh the advantages. World Psychiatry 6 (2) 84–91. [PMC free article] [PubMed] [Google Scholar]
- Crawford JR, Henry JD, 2003. The Depression Anxiety Stress Scales (DASS): normative data and latent structure in a large non-clinical sample. Br.J.Clin.Psychol 42 (Pt 2) 111–131. [DOI] [PubMed] [Google Scholar]
- Davis L, Uezato A, Newell JM, Frazier E, 2008. Major depression and comorbid substance use disorders. Curr.Opin.Psychiatry 21 (1) 14–18. [DOI] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV, 2017. Bootstrap Methods and Their Applications,. Cambridge University Press, Cambridge. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, New York State Psychiatric Institute. [Google Scholar]
- Fitzgerald PB, Rolfe TJ, Brewer K, Filia K, Collins J, Filia S, Adams A, de Castella A, Davey P, Kulkarni J, 2002. Depressive, positive, negative and parkinsonian symptomsin schizophrenia. Aust.N.Z.J.Psychiatry 36 (3) 340–346. [DOI] [PubMed] [Google Scholar]
- Foulds GA, Bedford A, 1975. Hierarchy of classes of personal illness. Psychol.Med 5 (2) 181–192. [DOI] [PubMed] [Google Scholar]
- Freeman D, Garety PA, 2003. Connecting neurosis and psychosis: the direct influence of emotion on delusions and hallucinations. Behav.Res.Ther 41 (8) 923–947. [DOI] [PubMed] [Google Scholar]
- Freeman D, Garety PA, Kuipers E, 2001. Persecutory delusions: developing the understanding of belief maintenance and emotional distress. Psychol.Med 31 (7) 1293–1306. [DOI] [PubMed] [Google Scholar]
- Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE, 2001. A cognitive model of the positive symptoms of psychosis. Psychol.Med 31 (2) 189–195. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Weiss DJ, Pilkonis PA, Frank E, Moore T, Kim JB, Kupfer DJ, 2012. Development of a computerized adaptive test for depression. Arch.Gen.Psychiatry 69 (11) 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J, 2017. RDoC: Outcomes to Causes and Back 2017 (08/07). [Google Scholar]
- Hafner H, Loffler W, Maurer K, Hambrecht M, an der Heiden W, 1999. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr.Scand 100 (2) 105–118. [DOI] [PubMed] [Google Scholar]
- Hartley S, Barrowclough C, Haddock G, 2013. Anxiety and depression in psychosis: a systematic review of associations with positive psychotic symptoms. Acta Psychiatr.Scand 128 (5) 327–346. [DOI] [PubMed] [Google Scholar]
- Hasin D, Liu X, Nunes E, McCloud S, Samet S, Endicott J, 2002. Effects of major depression on remission and relapse of substance dependence. Arch.Gen.Psychiatry 59 (4) 375–380. [DOI] [PubMed] [Google Scholar]
- Helfer B, Samara MT, Huhn M, Klupp E, Leucht C, Zhu Y, Engel RR, Leucht S, 2016. Efficacy and Safety of Antidepressants Added to Antipsychotics for Schizophrenia: A Systematic Review and Meta-Analysis. Am.J.Psychiatry 173 (9) 876–886. [DOI] [PubMed] [Google Scholar]
- Holzinger KJ, Swineford S, 1937. The Bi-factor method. Psychometrika 47 51–54. [Google Scholar]
- Hu L, Bentler PM, 1999. Cutoff criteria for fit indices in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling (6) 1–55.
- Jung S, Lee S, 2011. Exploratory factor analysis for small samples. Behav.Res.Methods 43 (3) 701–709. [DOI] [PubMed] [Google Scholar]
- Kenny DA, 2016. Mediation 2017 (07/13). [Google Scholar]
- Kerfoot KE, Rosenheck RA, Petrakis IL, Swartz MS, Keefe RS, McEvoy JP, Stroup TS, CATIE Investigators, 2011. Substance use and schizophrenia: adverse correlates in the CATIE study sample. Schizophr.Res 132 (2–3) 177–182. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS, 1994. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch.Gen.Psychiatry 51 (1) 8–19. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Wang PW, Becker OV, Nowakowska C, Yang Y, 2004. Psychotic bipolar disorders: dimensionally similar to or categorically different from schizophrenia? J.Psychiatr.Res 38 (1) 47–61. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Eaton NR, Krueger RF, Skodol AE, Wall MM, Grant B, Siever LJ, Hasin DS, 2013. Thought disorder in the meta-structure of psychopathology. Psychol.Med 43 (8) 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ, 1997. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv.Rev.Psychiatry 4 (5) 231–244. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ, 1985. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am.J.Psychiatry 142 (11) 1259–1264. [DOI] [PubMed] [Google Scholar]
- Kline RB, 2005. Multi-Sample SEM, in: Anonymous (Eds.) The Guilford Press, pp. 289–311. [Google Scholar]
- Knowles EEM, McKay DR, Kent JWJ, Sprooten E, Carless MA, Curran JE, de Almeida MA, Dyer TD, Goring HHH, Olvera RL, Duggirala R, Fox PT, Almasy L, Blangero J, Glahn DC, 2014a. Pleiotropic Locus for Emotion Recognition and Amygdala Volume Identified Using Univariate and Bivariate Linkage. American Journal of Psychiatry [DOI] [PMC free article] [PubMed]
- Knowles EE, Carless MA, de Almeida MA, Curran JE, McKay DR, Sprooten E, Dyer TD, Goring HH, Olvera R, Fox P, Almasy L, Duggirala R, Kent JW Jr, Blangero J, Glahn DC, 2014b. Genome-wide significant localization for working and spatial memory: Identifying genes for psychosis using models of cognition. Am.J.Med.Genet.B.Neuropsychiatr.Genet 165 (1) 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles EE, Kent JW Jr, McKay DR, Sprooten E, Mathias SR, Curran JE, Carless MA, de Almeida MA, Harald HH, Dyer TD, Olvera RL, Fox PT, Duggirala R, Almasy L, Blangero J, Glahn DC, 2015. Genome-wide linkage on chromosome 10q26 for a dimensional scale of major depression. J.Affect.Disord 191 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles EE, Weiser M, David AS, Dickinson D, Glahn D, Gold J, Davidson M, Reichenberg A, 2012. Dedifferentiation and substitute strategy: deconstructing the processing-speed impairment in schizophrenia. Schizophr.Res 142 (1–3) 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles EE, Weiser M, David AS, Glahn DC, Davidson M, Reichenberg A, 2015. The Puzzle of Processing Speed, Memory, and Executive Function Impairments in Schizophrenia: Fitting the Pieces Together. Biol.Psychiatry [DOI] [PMC free article] [PubMed]
- Kotov R, Fochtmann L, Li K, Tanenberg-Karant M, Constantino EA, Rubinstein J, Perlman G, Velthorst E, Fett AJ, Carlson G, Bromet EJ, 2017a. Declining Clinical Course of Psychotic Disorders Over the Two Decades Following First Hospitalization: Evidence From the Suffolk County Mental Health Project. Am.J.Psychiatry 174 (11) 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, Brown TA, Carpenter WT, Caspi A, Clark LA, Eaton NR, Forbes MK, Forbush KT, Goldberg D, Hasin D, Hyman SE, Ivanova MY, Lynam DR, Markon K, Miller JD, Moffitt TE, Morey LC, Mullins-Sweatt SN, Ormel J, Patrick CJ, Regier DA, Rescorla L, Ruggero CJ, Samuel DB, Sellbom M, Simms LJ, Skodol AE, Slade T, South SC, Tackett JL, Waldman ID, Waszczuk MA, Widiger TA, Wright AGC, Zimmerman M, 2017b. The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J.Abnorm.Psychol 126 (4) 454–477. [DOI] [PubMed] [Google Scholar]
- Krausz M, Mass R, Haasen C, Gross J, 1996. Psychopathology in patients with schizophrenia and substance abuse: a comparative clinical study. Psychopathology 29 (2) 95–103. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Gallinat J, Driesen N, Abi-Dargham A, Petrakis I, Heinz A, Pearlson G, 2006. The vulnerability to alcohol and substance abuse in individuals diagnosed with schizophrenia. Neurotox Res 10 (3–4) 235–252. [DOI] [PubMed] [Google Scholar]
- Lako IM, Bruggeman R, Knegtering H, Wiersma D, Schoevers RA, Slooff CJ, Taxis K, 2012. A systematic review of instruments to measure depressive symptoms in patients with schizophrenia. J.Affect.Disord 140 (1) 38–47. [DOI] [PubMed] [Google Scholar]
- Le S, Josse J, Husson F, 2008. FactoMineR: An R Package for Multivariate Analysis. Journal of Statistical Software 25 (1) 1–18. [Google Scholar]
- Levinson DF, Mowry BJ, Escamilla MA, Faraone SV, 2002. The Lifetime Dimensions of Psychosis Scale (LDPS): description and interrater reliability. Schizophr.Bull 28 (4) 683–695. [DOI] [PubMed] [Google Scholar]
- Li CH, 2016. Confirmatory factor analysis with ordinal data: Comparing robust maximum likelihood and diagonally weighted least squares. Behav.Res.Methods 48 (3) 936–949. [DOI] [PubMed] [Google Scholar]
- Li Y, Aggen S, Shi S, Gao J, Li Y, Tao M, Zhang K, Wang X, Gao C, Yang L, Liu Y, Li K, Shi J, Wang G, Liu L, Zhang J, Du B, Jiang G, Shen J, Zhang Z, Liang W, Sun J, Hu J, Liu T, Wang X, Miao G, Meng H, Li Y, Hu C, Li Y, Huang G, Li G, Ha B, Deng H, Mei Q, Zhong H, Gao S, Sang H, Zhang Y, Fang X, Yu F, Yang D, Liu T, Chen Y, Hong X, Wu W, Chen G, Cai M, Song Y, Pan J, Dong J, Pan R, Zhang W, Shen Z, Liu Z, Gu D, Wang X, Liu X, Zhang Q, Flint J, Kendler KS, 2014. The structure of the symptoms of major depression: exploratory and confirmatory factor analysis in depressed Han Chinese women. Psychol.Med 44 (7) 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, 1987. The symptoms of chronic schizophrenia. A re-examination of the positive-negative dichotomy. Br.J.Psychiatry 151 145–151. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Widaman KF, Zhang S, Hong S, 1999. Sample size in factor analysis. Psychological Methods 4 84–99. [Google Scholar]
- Majadas S, Olivares J, Galan J, Diez T, 2012. Prevalence of depression and its relationship with other clinical characteristics in a sample of patients with stable schizophrenia. Compr.Psychiatry 53 (2) 145–151. [DOI] [PubMed] [Google Scholar]
- Mansolf M, Reise S, 2017. When and why the second-order and bifactor models are distinguishable. Intelligence 61 120–129. [Google Scholar]
- Mao Y, Zhang M, 2015. Augmentaion with antidepressants in schizophrenia treatment: benefit or risk. Neuropsychiatric Disease and Treatment 11 701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias SR, Knowles EEM, Barrett J, Beetham T, Leach O, Buccheri S, Aberizk K, Blangero J, Poldrack RA, Glahn DC, 2017. Deficits in visual working-memory capacity and general cognition in African Americans with psychosis. Schizophr. Res [DOI] [PMC free article] [PubMed]
- Maxwell SE, Kelley K, Rausch JR, 2008. Sample size planning for statistical power and accuracy in parameter estimation. Annu.Rev.Psychol 59 537–563. [DOI] [PubMed] [Google Scholar]
- Mullin K, Gupta P, Compton MT, Nielssen O, Harris A, Large M, 2012. Does giving up substance use work for patients with psychosis? A systematic meta-analysis. Aust.N.Z.J.Psychiatry 46 (9) 826–839. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Muthén LK, Asparouhov T, 2016. Mediation Analysis, in: Anonymous (Eds.), Regression And Mediation Analysis using Mplus Muthén and Muthén, Los Angeles. [Google Scholar]
- Muthén LK, Muthén BO, 2011. Mplus User’s Guide Sixth Edition ed. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Negrete JC, 2003. Clinical aspects of substance abuse in persons with schizophrenia. Can.J.Psychiatry 48 (1) 14–21. [DOI] [PubMed] [Google Scholar]
- Oorschot M, Kwapil T, Delespaul P, Myin-Germeys I, 2009. Momentary assessment research in psychosis. Psychol.Assess 21 (4) 498–505. [DOI] [PubMed] [Google Scholar]
- Paolacci G, Chandler J, 2014. Inside the Turk: Understanding Mechanical Turk as a Participant Pool. Current Directions in Psychological Science 23 (3) 184–188. [Google Scholar]
- Potvin S, Sepehry AA, Stip E, 2007. Meta-analysis of depressive symptoms in dual-diagnosis schizophrenia. Aust.N.Z.J.Psychiatry 41 (10) 792–799. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Coffman DL, 2006. Computing power and minimum sample size for RMSEA
- R Core Team, 2017. R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Raiche G, 2010. nFactors: an R package for parallel analysis and non graphical solutions to the Cattell scree test. R package version 2.3.3
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK, 1990. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 264 (19) 2511–2518. [PubMed] [Google Scholar]
- Reininghaus U, Bohnke JR, Hosang G, Farmer A, Burns T, McGuffin P, Bentall RP, 2016. Evaluation of the validity and utility of a transdiagnostic psychosis dimension encompassing schizophrenia and bipolar disorder. Br.J.Psychiatry 209 (2) 107–113. [DOI] [PubMed] [Google Scholar]
- Reise SP, Moore TM, Haviland MG, 2010a. Bifactor models and rotations: exploring the extent to which multidimensional data yield univocal scale scores. J.Pers.Assess 92 (6) 544–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reise SP, Moore TM, Haviland MG, 2010b. Bifactor models and rotations: exploring the extent to which multidimensional data yield univocal scale scores. J.Pers.Assess 92 (6) 544–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelle W, 2017. psych: Procedures for Personality and Psychological Research
- Sands JR, Harrow M, 1999. Depression during the longitudinal course of schizophrenia. Schizophr.Bull 25 (1) 157–171. [DOI] [PubMed] [Google Scholar]
- Scheller-Gilkey G, Thomas SM, Woolwine BJ, Miller AH, 2002. Increased early life stress and depressive symptoms in patients with comorbid substance abuse and schizophrenia. Schizophr.Bull 28 (2) 223–231. [DOI] [PubMed] [Google Scholar]
- Seddon JL, Birchwood M, Copello A, Everard L, Jones PB, Fowler D, Amos T, Freemantle N, Sharma V, Marshall M, Singh SP, 2016. Cannabis Use Is Associated With Increased Psychotic Symptoms and Poorer Psychosocial Functioning in First-Episode Psychosis: A Report From the UK National EDEN Study. Schizophr.Bull 42 (3) 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer AB, 2006. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J.Clin.Psychol 62 (1) 123–146. [DOI] [PubMed] [Google Scholar]
- Sim K, Mahendran R, Siris SG, Heckers S, Chong SA, 2004. Subjective quality of life in first episode schizophrenia spectrum disorders with comorbid depression. Psychiatry Res 129 (2) 141–147. [DOI] [PubMed] [Google Scholar]
- Siris S, Bench C, 2003. Depression and schizophrenia, in: Hirsch S, Weinberger D (Eds.), Schizophrenia Blackwell, Oxford, UK, pp. 142–167. [Google Scholar]
- Siris SG, Adan F, Cohen M, Mandeli J, Aronson A, Casey E, 1988. Postpsychotic depression and negative symptoms: an investigation of syndromal overlap. Am.J.Psychiatry 145 (12) 1532–1537. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR, 2000. The comorbidity of depression and substance use disorders. Clin.Psychol.Rev 20 (2) 173–189. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA, 2013. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am.J.Psychiatry 170 (11) 1263–1274. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS, 2009. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr.Res 110 (1–3) 1–23. [DOI] [PubMed] [Google Scholar]
- Taylor MA, 1992. Are schizophrenia and affective disorder related? A selective literature review. Am.J.Psychiatry 149 (1) 22–32. [DOI] [PubMed] [Google Scholar]
- Thoma P, Daum I, 2013. Comorbid substance use disorder in schizophrenia: a selective overview of neurobiological and cognitive underpinnings. Psychiatry Clin.Neurosci 67 (6) 367–383. [DOI] [PubMed] [Google Scholar]
- Upthegrove R, Birchwood M, Ross K, Brunett K, McCollum R, Jones L, 2010. The evolution of depression and suicidality in first episode psychosis. Acta Psychiatr.Scand 122 (3) 211–218. [DOI] [PubMed] [Google Scholar]
- Upthegrove R, Marwaha S, Birchwood M, 2017. Depression and Schizophrenia: Cause, Consequence, or Trans-diagnostic Issue? Schizophr.Bull 43 (2) 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loo HM, de Jonge P, Romeijn JW, Kessler RC, Schoevers RA, 2012. Data-driven subtypes of major depressive disorder: a systematic review. BMC Med 10 156–7015-10–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheule S, Desmet M, Groenvynck H, Rosseel Y, Fontaine J, 2008. The factor structure of the Beck Depression Inventory-II: an evaluation. Assessment 15 (2) 177–187. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD, 2002. Modern Applied Statistics with S Fourth Edition ed. Springer, New York. [Google Scholar]
- Vrieze E, Demyttenaere K, Bruffaerts R, Hermans D, Pizzagalli DA, Sienaert P, Hompes T, de Boer P, Schmidt M, Claes S, 2014. Dimensions in major depressive disorder and their relevance for treatment outcome. J.Affect.Disord 155 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Curtis V, Murray RM, 2002. Schizophrenia and bipolar disorder: similarities in pathogenic mechanisms but differences in neurodevelopment. Int. Clin. Psychopharmacol 17 Suppl 3 S11–9. [PubMed] [Google Scholar]
- Ward LC, 2006. Comparison of factor structure models for the Beck Depression Inventory--II. Psychol.Assess 18 (1) 81–88. [DOI] [PubMed] [Google Scholar]
- Wigman JT, van Os J, Borsboom D, Wardenaar KJ, Epskamp S, Klippel A, MERGE, Viechtbauer W, Myin-Germeys I, Wichers M, 2015. Exploring the underlying structure of mental disorders: cross-diagnostic differences and similarities from a network perspective using both a top-down and a bottom-up approach. Psychol.Med 45 (11) 2375–2387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.