To the Editor,
We have carried out four kidney transplants from genetically-modified pigs in baboons, details of three of which have been reported (1,2). In all four, at various time-intervals after renal transplantation, and on more than one occasion in three baboons, we have observed the development of features of hypovolemia and dehydration, manifest by an increase in serum creatinine (Figure 1). The baboons remained fully active, maintained a steady weight, and did not drink or urinate more or less than usual. However, when anesthetized, they were found to have a very low central venous pressure (initially <2mm Hg), a low systolic arterial blood pressure (initially <60mm Hg), and dehydrated skin (loss of turgor) and tissues.
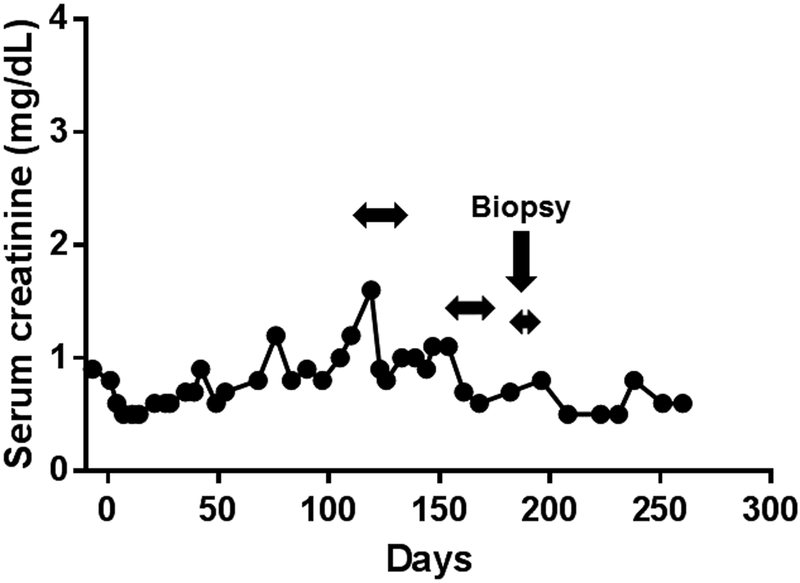
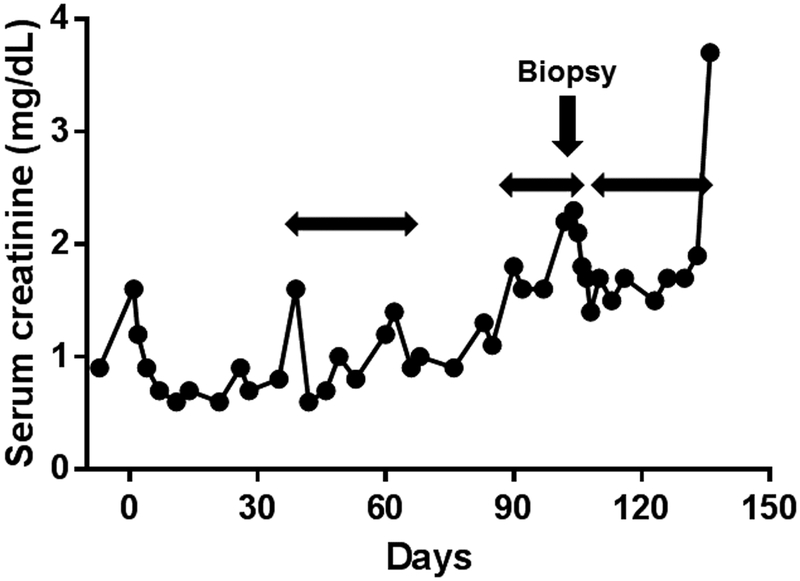
Figure 1: Changes in serum creatinine in baboons with life-supporting pig kidney grafts (with nephrectomy of the native kidneys at the time of pig kidney transplantation) in (A) B10815, (B) B17315, (C) B17615, (D) B9313.
Examples of spontaneous increases in serum creatinine unrelated to to an immune response, and corrected by fluid administration, are indicated. The most obvious increase was in B10815 on post-transplant day 39. On each occasion, the rapid reduction in creatinine to normal (human) levels was associated solely with i.v. and/or s.c. infusions of normal saline. The normal range of serum creatinine in baboon, human, and pig is 0.6–0.8mg/dL, 0.5–1.4mg/dL, and 0.6–1.6mg/dL, respectively (3). Horizontal arrows indicate when fluid was administered.
(*In Figure 1A, the serum creatinine increased to 5.3 on day 78. The biopsy on day 82 was the only case out of 5 that showed any histopathological features of rejection. In Figure 1D, the final increase in serum creatinine was related to systemic infection.)
The intravenous infusion of several hundred milliliters of normal saline (approximately 100ml/kg body weight) was required to raise and maintain the venous pressure to normal levels. The intravenous infusion of normal saline was administered to the baboons under anesthesia over several hours. We discontinued administering fluid when the venous pressure and the systolic blood pressure had increased and were maintained at >5mm Hg and >80mm Hg, respectively. No features of fluid overload, e.g., pulmonary edema, were observed during or after fluid administration. The observation that the state of hypovolemia/dehydration occurred intermittently is presumably because, having corrected the volemic state of the baboon, it took some days or weeks for hypovolemia and dehydration to recur.
We subsequently maintained a state of good hydration for several days by the subcutaneous administration of normal saline (approximately 150–200ml x2–3 weekly for 1–3 weeks). In some cases, when the serum creatinine again began to increase, daily subcutaneous saline infusions alone proved sufficient to correct the situation.
We could find no obvious cause for these episodes. In no case did immune assays suggest rejection, and in some cases we were able to exclude immune-mediated injury by biopsy (Figure 2). In no case was there more than minimal or modest proteinuria, and the serum albumin remained within the normal range (baboon 3.8–4.5g/dL; human 3.4–5.0g/dL; pig 2.3–4.5g/dL) (3) without the need for albumin infusion. Unfortunately, we did not measure urinary excretion of sodium or creatinine, and nor did we collect tissue samples to identify renin granules (but we will do so in future experiments). We are not aware that any of the drugs the baboons were receiving abnegate the sense of thirst (see references 1 and 2 for details).
Figure 2: Histological appearance of a renal biopsy taken on day 103 in B9313.
The biopsy was taken at the time when the serum creatinine had increased, and rejection was considered a strong possibility (see Figure 1D). However, no histopathological features of rejection were seen, and the serum creatinine returned to the normal range within 3 days after the i.v. infusion of several hundred milliliters of normal saline.
Although we have been unable to confirm this (as we have to date been unable to locate any laboratory that will measure renin for us in baboons or pigs), we suggest that abnormality of renin function may be a contributory factor. Renin (produced by the kidney [4]) cleaves angiotensinogen (produced by the liver) to angiotensin I, which is further cleaved (primarily in the lungs) to angiotensin II, which, partly through its effect on the release of aldosterone, regulates body fluid volume and potassium balance. Renin responds to (i) a fall in blood pressure (that may be associated with a reduced blood volume), (ii) a decrease in sodium load, and/or (iii) decreased sympathetic nervous activity. (We found no evidence of a decrease in sodium load.)
Human angiotensinogen can only be cleaved by human renin and not by other mammalian renin (5). However, non-primate mammalian angiotensinogen is a satisfactory substrate for both nonhuman primate and human renin, although a more optimum enzymatic reaction is found with homologous reactants (6). Pig renin is therefore unable to cleave human angiotensinogen (7,8), which might result in a reduced ability for vasoconstriction in the baboon recipient and to an impaired ability to retain fluid in the body. The episodes of hypovolemia/dehydration we have documented might be associated with an abnormality in, or an absence of, renin function.
Baboons with pig kidney grafts would appear to be unaware that they are becoming relatively dehydrated (as their intake of fluid does not appear to equate with their clinical need, even though they do not produce excessive urine output). Mice in which the gene for renin has been deleted tend to have an increased serum creatinine level and low arterial blood pressure (9). Plasma renin levels in humans (0.75–4.49 ng/ml/h) (10), pigs (1.7–2.9 ng/ml/h) (11), and baboons (male: 2.4–5.5 ng/ml/h; female: 5.4–14.0 ng/ml/h) (12) have been reported in the literature, but these data alone may not indicate what is happening in primate recipients of pig kidney grafts.
If our hypothesis that episodes of a rise in serum creatinine are subsequent to a relative state of hypovolemia/dehydration in the recipient of a pig kidney graft, this would have implications for future patients with pig kidney grafts. These patients may be unaware that they are becoming volume-depleted, and this may be associated with a reduced blood flow through the kidney graft, with resulting impaired renal function. They will need to ensure that their fluid intake is maintained at a high level, whether they feel thirsty or not. They may not be able to rely on a reduced urine output to indicate they are becoming volume-depleted or dehydrated.
If we are correct, the ultimate solution may be to genetically-engineer the organ-source pig to produce human renin. Knockout of the gene for pig renin would not be advised as this may well affect the health of the pig. Whether the production of human renin by the pig kidneys will impact the volemic state of the pig (before the organs are used for transplantation) remains uncertain. Further observations and studies are clearly indicated.
Acknowledgements
Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959.
Footnotes
Conflict of interest
No author declares a conflict of interest.
References
- 1.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation 2015;22:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24. doi: 10.1111/xen.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekser B, Bianchi J, Ball S, et al. Comparison of hematologic, biochemical, and coagulation parameters in α1,3-galactosyltransferase gene-knockout pigs, wild-type pigs, and four primate species. Xenotransplantation. 2012;19:342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 1990;70:1067–1116. [DOI] [PubMed] [Google Scholar]
- 5.Boyce NW, Holdsworth SR. Direct antiGBM antibody induced alterations in glomerular permselectivity. Kidney Int. 1986;30:666–672. [DOI] [PubMed] [Google Scholar]
- 6.Boesken WH, Schmidt M, Jontofsohn R, Heinze V. Proteinuria as diagnostic marker after human kidney transplantation. Proc Eur Dial Transplant Assoc. 1975;11:333–342. [PubMed] [Google Scholar]
- 7.Sen S, Hirawawa K, Smeby RR, Bumpus FM. Measurement of plasma renin substrate using homologous and heterologous renin. Am J Physiol 1971;221:1476–1480. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Liang TC. Substrate specificity of porcine renin: P1’, P1, and P3 residues of renin substrates are crucial for activity. Biochemistry. 1994;33:14636–14641. [DOI] [PubMed] [Google Scholar]
- 9.Adams DJ, Head GA, Markus MA, et al. Renin enhancer is critical for control of renin gene expression and cardiovascular function. J Bio Chem 2006;281:31753–31761. [DOI] [PubMed] [Google Scholar]
- 10.Alderman MH, Ooi WL, Cohen H, Madhavan S, Sealey JE, Laragh JH. Plasma renin activity: a risk factor for myocardial infarction in hypertensive patients. Am J Hypertens. 1997;10:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Li D, Wang Q, Zhang Y, et al. A novel swine model of spontaneous hypertension with sympathetic hyperactivity responds well to renal denervation. Am J Hypertens. 2016;29:63–72. [DOI] [PubMed] [Google Scholar]
- 12.Harewood WJ, Gillin A, Mohamed S, et al. Cyclical changes in the renin-angiotensin-aldosterone system during the menstrual cycle of the baboon (Papio hamadryas). J Med Primatol. 1996; 25:267–271. [DOI] [PubMed] [Google Scholar]