Significance
Remyelination of the CNS is a critical process in restoring function and protecting nerve fibers from degeneration in multiple sclerosis and other demyelinating diseases. It is currently thought that myelin can only be repaired by the generation of new oligodendrocytes from progenitor cells and that remaining mature cells are unable to participate. Here we show, using unique large animal models, including a nonhuman primate, that oligodendrocytes that are partially injured can participate in myelin repair. The capacity of mature oligodendrocytes to remyelinate in demyelinating disease remains unknown, yet it provides an additional cell source for recruitment for myelin repair.
Keywords: adult oligodendrocyte, remyelination, large animal models
Abstract
Endogenous remyelination of the CNS can be robust and restore function, yet in multiple sclerosis it becomes less complete with time. Promoting remyelination is a major therapeutic goal, both to restore function and to protect axons from degeneration. Remyelination is thought to depend on oligodendrocyte progenitor cells, giving rise to nascent remyelinating oligodendrocytes. Surviving, mature oligodendrocytes are largely regarded as being uninvolved. We have examined this question using two large animal models. In the first model, there is extensive demyelination and remyelination of the CNS, yet oligodendrocytes survive, and in recovered animals there is a mix of remyelinated axons interspersed between mature, thick myelin sheaths. Using 2D and 3D light and electron microscopy, we show that many oligodendrocytes are connected to mature and remyelinated myelin sheaths, which we conclude are cells that have reextended processes to contact demyelinated axons while maintaining mature myelin internodes. In the second model in vitamin B12-deficient nonhuman primates, we demonstrate that surviving mature oligodendrocytes extend processes and ensheath demyelinated axons. These data indicate that mature oligodendrocytes can participate in remyelination.
Remyelination is the most effective and robust endogenous repair mechanism of the central nervous system (CNS) and can lead to complete remyelination of large and disseminated areas of demyelination in the CNS, with resultant functional recovery (1, 2). It has been known for some time that remyelination occurs in multiple sclerosis (MS) (3–5), although it has been reported as being limited, variable in patient populations, and more robust in early rather than late disease (6). There has been both extensive research and speculation into why this is so (2). Two detailed studies in 2006–2007 analyzed remyelination in the forebrain of MS patients with different disease course and duration (7, 8). Perhaps surprisingly, extensive—albeit variable—remyelination was seen in older patients, some of whom had long-standing MS. Although it was not known when remyelination occurred in these patients, these observations underscore the ability of even the mature brain in long-lasting MS to remyelinate or sustain earlier myelin repair. However, the lack of complete endogenous response has led to remyelination becoming a major therapeutic target, both to restore function and as the ultimate form of neuroprotection (9–13).
The origin of remyelinating oligodendrocytes in MS and in experimental models of demyelination has been the subject of considerable interest. The weight of available evidence suggests that remyelinating oligodendrocytes arise from oligodendrocyte progenitor cells (OPCs) that reside either in or adjacent to demyelinated lesions, or adult neural stem cells residing in the subependymal zone (14, 15). Experimental proof that OPCs generate remyelinating oligodendrocytes comes from many transplant studies in de- and dysmyelinating models, as well as from toxin-induced demyelinating disorders (15, 16). However, new strategies to promote remyelination should consider recruiting all cells of the oligodendrocyte lineage, including adult oligodendrocytes, if there is persuasive evidence that they are capable of participating in myelin repair.
The case against adult oligodendrocytes comes from both in vivo and in vitro data. Blakemore and coworkers (17) have shown that human oligodendrocytes transplanted into demyelinated areas of the rat spinal cord survived but were unable to remyelinate. Similarly, surviving endogenous postmitotic oligodendrocytes did not remyelinate axons in areas of demyelination created by injection of antibody to Galactocerebroside (18). In vitro studies in which select stages of the oligodendrocyte lineage were added to cultures of retinal ganglion cells reported that only perinatal and adult OPCs developed into myelinating oligodendrocytes (19). Most recently, fate-mapping experiments reported that mature oligodendrocytes took no part in the remyelination of focal, lysolecithin-induced demyelination in the mouse spinal cord (20). However, none of these data exclude the potential role of adult oligodendrocytes that lose some of their internodes following primary attack on myelin sheaths, yet remain intact and viable.
The case for the possible role of the adult oligodendrocyte in remyelination is less well documented yet requires consideration. Initially it was proposed that mature oligodendrocytes could proliferate and provide more oligodendrocytes for remyelination (21). In vitro studies by Wood and Bunge (22) suggested that cell-sorted mature oligodendrocytes were more capable of generating myelinating oligodendrocytes in cocultures than OPCs. Transplantation of similarly sorted mature oligodendrocytes, showed that they were also capable of myelinating axons in vivo (23, 24). In vitro studies of mature oligodendrocytes subjected to process disruption by various methods, have shown that these cells may demonstrate plasticity, regrow processes, and are capable of ensheathing and remyelinating axons. Oligodendrocyte processes disrupted by transection or exposure to NMDA, retracted back to the cell body but regrew within 36 h (25). Reextension or recovery of processes of mature oligodendrocytes following damage has also been shown in vivo. In a model of focal cerebral ischemia, McIver, et al. (26) showed that some oligodendrocytes survived, although with fragmented processes. Oligodendrocytes that had been prelabeled with eGFP were found 1 wk later in areas of ischemia with intact processes, suggesting that they may be able to participate in white matter repair (26). Similarly, mature oligodendrocytes at the edge of demyelinated lesions in the spinal cord, that overexpressed ERK1/2, extended processes into the lesions and remyelinated axons (27).
Despite these data, the dominant opinion remains that remyelination is dependent upon the OPC, yet it is critical to consider more mature cells of the oligodendrocyte lineage if alternative models provide the opportunity to reexamine this question. We have explored this issue in two models. The first unique model is feline irradiated food-induced demyelination (FIDID), in which demyelinated and remyelinated axons are found mixed with mature myelin sheaths (1). Mature myelin sheaths are those that have a normal g-ratio (axon diameter/axon + myelin sheath diameter) (<0.75), while remyelinated axons have thin sheaths with g-ratios of over 0.75, in contrast to demyelinated axons that lack any myelin (g-ratio 1.0). From our data, we propose that surviving adult oligodendrocytes in such lesions are primarily responsible for the robust remyelination seen. We have also examined archived spinal cord tissue from a model of vitamin B12 deficiency in a rhesus monkey in which there was extensive demyelination but oligodendrocyte survival, mimicking the neuropathology of subacute combined degeneration (28, 29). Here we found evidence of adult oligodendrocytes initiating remyelination by reensheathing axons, and in some instances producing thin myelin sheaths. These complementary models provide strong evidence that in certain pathological milieu, the adult oligodendrocyte can participate in remyelination.
Results
Scattered Demyelination and Remyelination Lead to a Mix of Remyelinated and Mature Myelin Sheaths in the Lateral and Ventral Columns but Severe Demyelination in the Dorsal Column.
Six months after beginning the irradiated diet, cats developed progressive hind leg ataxia and weakness. At this point, there was extensive demyelination and concurrent remyelination of the spinal cord in cats. In the ventral and lateral columns, scattered demyelinated and remyelinated axons were mixed with mature myelin sheaths around axons of all calibers (Fig. 1B). In contrast, in the dorsal column, large, contiguous areas of demyelination were seen (Fig. 1D). With recovery, mature myelin sheaths (defined as those with a g-ratio of 0.75 or less) remained dispersed throughout the ventral and lateral columns, both at early and complete stages of remyelination (Fig. 1C), suggesting that these myelin sheaths were unaffected by the demyelinating “insult.” Hence, their oligodendrocytes had remained intact yet close to demyelinated axons (Fig. 1). Around 40% of axons of all calibers had thin myelin sheaths, as previously reported (1). The pattern seen on recovery, which is a mixture of mature and remyelinated myelin sheaths, persisted over time (30). In one animal examined 2.5 y after termination of the irradiated diet, the pattern of mixed distribution of remyelinated and mature myelin sheaths remained in the ventral column (Fig. 1). Examination of longitudinal sections through these areas of white matter showed that there were many short internodes on thinly myelinated (remyelinated) axons (30). In contrast to the lateral and ventral column, remyelination of the dorsal column resulted in large areas of homogeneously thin myelin sheaths (Fig. 1E).
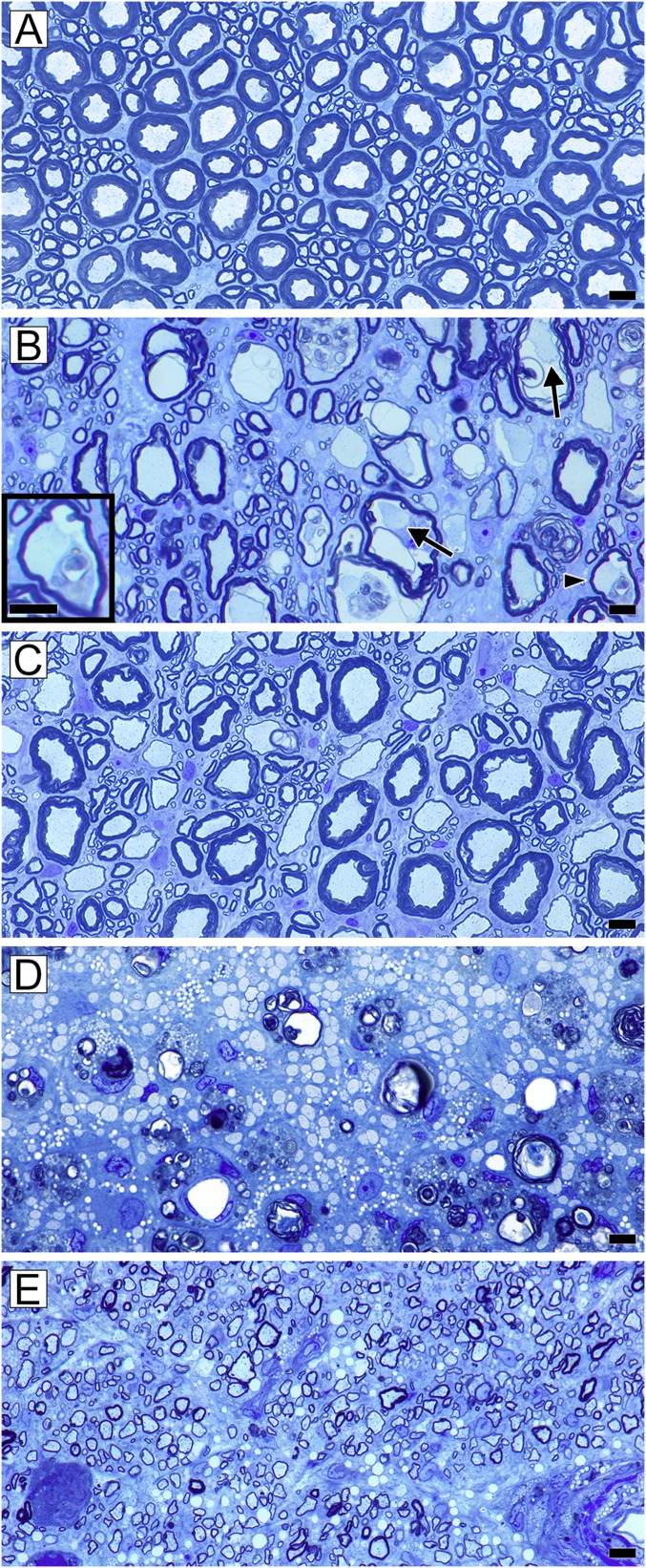
Fig. 1.
Mature myelin sheaths are seen in ventral columns at all stages of the disease and are mixed with demyelinated and remyelinated axons while the dorsal column initially has large areas of demyelination. (A) Normal mature white matter in the feline spinal cord ventral column. There is a mix of large, medium, and small diameter axons with appropriately thick myelin sheaths. (B) At the onset of disease in the ventral column, many axons become vacuolated, yet in each the axon remains intact (arrows). As myelin breaks down, intact axons can still be seen (arrowhead and high power shown in Inset). At this stage, occasional demyelinated and remyelinated axons are present. (C) In the ventral column of the recovered animal, there is a mix of remyelinated and mature myelin sheaths around axons of all diameters. (D) In the subpial area of the dorsal column of the same animal in B, there is complete demyelination with myelin debris. The axons are much smaller than in B as they are part of the fasciculus gracilis. (E) In the dorsal column of the same animal shown in C, all of the axons have thin remyelinated sheaths, different from the mosaic shown in C. (Scale bars, 20 µm.)
Examination for Cell Death in Acute or Chronic Disease.
Apoptotic cells, if present, can be readily detected in 1-μm plastic sections (31) but despite examining multiple sections at all levels of the spinal cord, optic nerves and brain from 14 cats, few or no pyknotic nuclei were seen at any stage of the disease. Similarly, cells at the early stage of apoptosis with condensed chromatin (31), or pyknotic cells, were rarely seen on ultrastructural analyses during acute disease. To confirm these observations, we used the TUNEL assay on spinal cord tissue from four cats during acute disease. In one affected cat, no TUNEL+ cells were observed. In the other three affected cats, only 3 to 12 TUNEL+ cells were counted in the whole spinal cord cross-section (SI Appendix, Fig. S1 C and D). Several of the TUNEL+ cells found in the affected cat tissue were within myelin vacuoles (SI Appendix, Fig. S1D), suggesting they were macrophages (SI Appendix, Fig. S1D). The tissue sections from cats on the normal diet did not contain any TUNEL+ cells (SI Appendix, Fig. S1A), while all positive control tissue sections showed many brown, darkly stained nuclei throughout the section (SI Appendix, Fig. S1B).
Oligodendrocytes Are Seen to Contact and Ensheath both Remyelinated Axons and Those with Mature Myelin Sheaths.
The lateral and ventral columns of four cats that showed a mix of remyelinated axons and mature myelin sheaths were examined in detail to determine whether oligodendrocytes could be seen to extend processes to both thick and thin myelin sheaths. In the animal where remyelination was complete 2 y after recovery (Fig. 1), the lack of space in the neuropil between myelinated axons made this difficult to visualize. However, in the two other cats, which were examined 2–3 mo after cessation of the irradiated diet, we found numerous examples where an oligodendrocyte cell body was seen to extend processes to both mature myelin sheaths and thinly remyelinated axons, and there was direct continuity between the oligodendrocyte membrane and the outer lamella of the myelin sheath (Fig. 2 A–F). These relationships were independent of axon size: that is, the mature/thin myelin sheaths arising from a single oligodendrocyte were seen around both large and small diameter axons. Many of these glial units were surrounded by astrocyte processes, with no other adjacent glial cell nuclei, suggesting their potential isolation from other oligodendrocytes. Confirmation that individual myelin sheaths were either mature or new (remyelinated) was demonstrated by measuring the g-ratios in these oligodendrocyte/axon groups (Fig. 2).
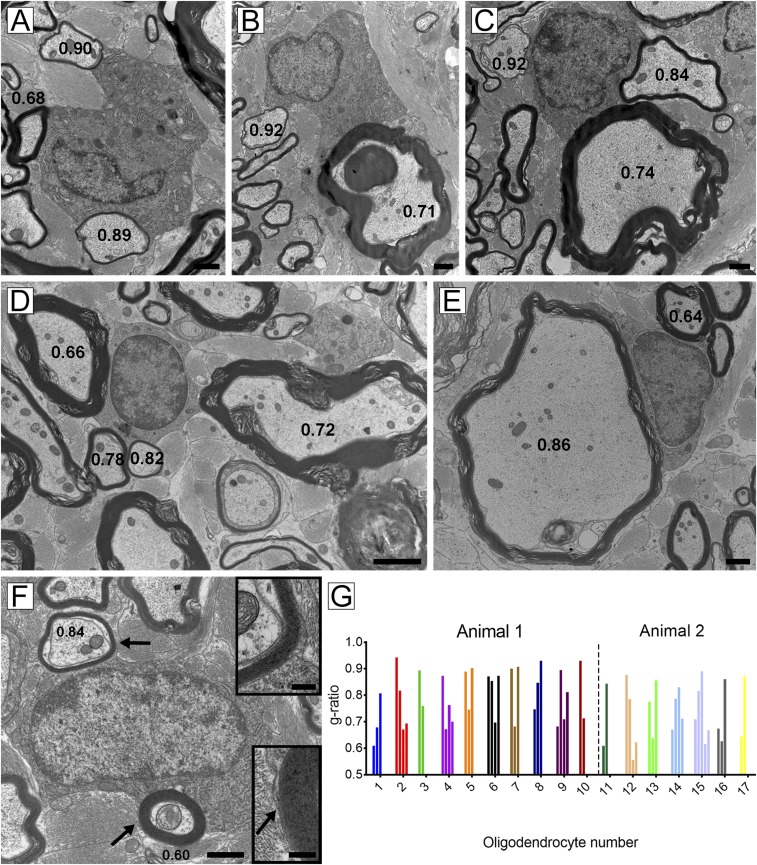
Fig. 2.
Oligodendrocytes have cytoplasmic connections to axons of variable diameter with thin or thick myelin sheaths. These five oligodendrocytes have cytoplasmic connections, seen on high power, contacting axons that have mature sheaths (g-ratios < 0.75) and thin myelin (remyelinated) sheaths (g-ratio > 0.75). Remyelinated myelin sheaths were seen around small diameter (A–C and E) or large diameter (D) axons. (F) Definitive cytoplasmic connection is seen in an oligodendrocyte that myelinates a mature myelin sheath (g-ratio 0.60) and remyelinated axon (g-ratio 0.84). Insets confirm the sheaths are derived from this cell (arrows). (G) Histogram detailing the oligodendrocyte/myelin sheath connections of 17 oligodendrocytes derived from two animals. The g-ratios were measured for each myelin sheath with which they had connections. The variability in each demonstrates that many mature oligodendrocytes have participated in remyelination. [Scale bars: 1 µm (A, C, E, and F), 2 µm (D), and 200 nm (F, Inset).]
The oligodendrocyte nuclei and cytoplasm within these glial “units” were variable in appearance. Those in Fig. 2 A and B, had copious cytoplasm and organelles, suggesting that active remyelination had occurred recently compared with those in Fig. 2 C–F, where the cells looked more mature. To illustrate the frequency of oligodendrocytes connected to both thick and thin myelin sheaths, we identified an additional 13 oligodendrocytes from two animals that had clear cytoplasmic association with each cell, and measured the g-ratios of fibers, as demonstrated in Fig. 2G. Each oligodendrocyte was connected to mature and thin myelin sheaths that had g-ratios ranging from 0.6 to 0.92, confirming that many oligodendrocytes had maintained mature myelin internodes while remyelinating adjacent demyelinated axons.
Serial EM Imaging of Oligodendrocyte Ensheathment of Mature and Thin Myelin Sheaths.
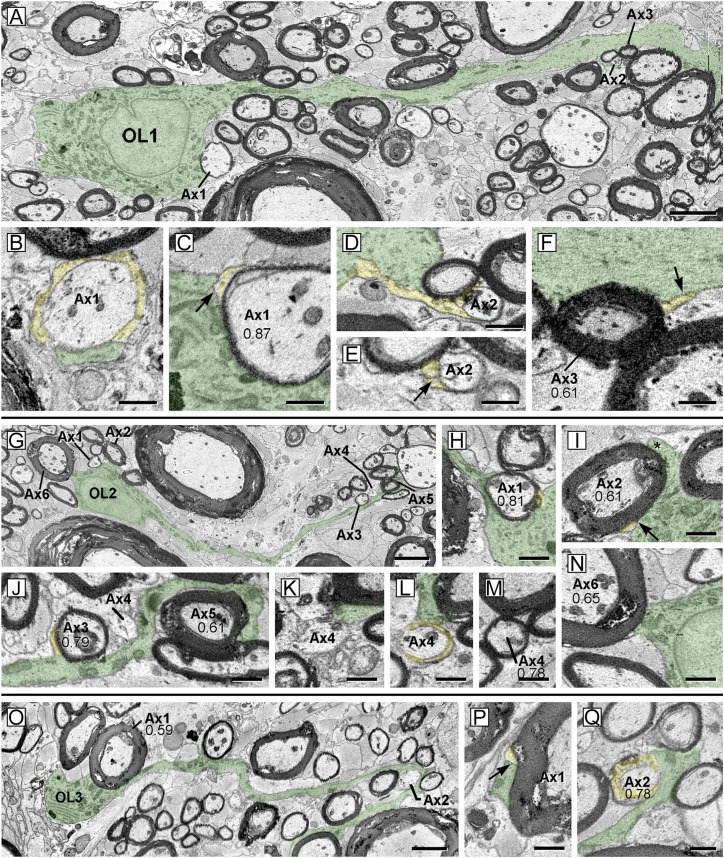
The cellular connections underpinning remyelination were investigated using automated serial electron microscopy (EM) imaging. In eight image sets, 44 partial or complete oligodendrocytes were identified, including 6 with well-defined connections to both mature and relatively thin myelin. Three examples are shown in Fig. 3 A–F (OL1), Fig. 3 G–N (OL2), and Fig. 3 O–Q (OL3). Characteristic of some mature oligodendrocytes, OL1 (Fig. 3 A–F, overlaid in green) featured a prominent trunk-like process that was rich in microtubules and extended for at least 75 µm. Adjacent to the cell body, OL1 surrounded a short internode (Fig. 3 A–C, Ax1) that consisted of four to five myelin wraps and was only 44-µm long. Reconstruction of serial images confirmed direct OL1 cell body contact with the axon (Fig. 3B, green) and that OL1 contributed to paranodal loops (Fig. 3B, spiral loops in yellow). Somatic plasma membrane also formed the outer membrane of the mesaxon (Fig. 3C, arrow), which is the most lateral component of the spiral myelin membrane. A mature fiber (Fig. 3A, Ax3) was also connected at the cell soma (detail not shown). The OL1 trunk process gave rise to a fine cytoplasmic process (Fig. 3 D and E, yellow) that connected with a small axon (Fig. 3 A and D–F, Ax2) and extended a single-layer myelin wrap for 5 µm along the axon surface. This single wrap featured a mesaxon (Fig. 3E, arrow), partial compaction, and had a g-ratio > 0.90, indicating that it was an early stage of remyelination. A nearby mature myelin internode (g-ratio 0.61) also connected with the main process (Fig. 3 A and F, Ax3; arrow, mesaxon, yellow outer tongue process). It should be noted that oligodendrocyte processes connecting with short, thin, newly formed internodes contained abundant cytoplasm, whereas connections to mature (low g-ratio) internodes were typically narrow with condensed cytoplasm. Because these latter were more difficult to follow with confidence, it is likely many connections to mature fibers, such as those surrounding OL1 (Fig. 3A), were overlooked. OL2 (Fig. 3G) lay close to OL1, and also surrounded both thin (Fig. 3 G and H, Ax1) and thick internodes (Fig. 3 G and I, Ax2) around the margins of the cell body. On one thick fiber (Fig. 3I, Ax2), an oligodendrocyte process (Fig. 3I, asterisk and yellow), extended around the myelin circumference to form the outer mesaxon (Fig. 3I, arrow). Distal to the cell body, the primary process (green) contacted several axons (Fig. 3J, Ax3–5). Digital reslicing (Fig. 3J) demonstrated contacts with a high g-ratio internode (Fig. 3J, Ax3), the node of another (Fig. 3J, Ax4), and a low g-ratio fiber (Fig. 3J, Ax5). In serial images of Ax4 (Fig. 3 K–M), a branch of the nodal OL2 process was followed into paranodal processes (Fig. 3L, yellow) of a thin internodal myelin (Fig. 3M, Ax4). Contact between OL2 and a 5-µm diameter axon (Fig. 3 G and N, Ax6; g-ratio 0.65) suggested possible connection to that internode also. OL3 (Fig. 3O, green) encircled a large myelinated fiber (Fig. 3O, Ax1) (7 µm axon, g-ratio 0.59) and formed a mesaxon (Fig. 3P) as the outermost tongue process (Fig. 3P, green). OL3 also extended a process for 70 μm to associate with a small axon (Fig. 3 O and Q, Ax2), where it encircled the axolemma at the node (Fig. 3Q, yellow, paranodal loops) and formed the myelin internode. Note that both oligodendrocytes 2 and 3 had other branches that also myelinated additional thin and thick myelin internodes.
Fig. 3.
SBC–EM images of three oligodendrocytes from cat ventral spinal cord during recovery, illustrating connection to developing and mature myelin internodes. Numbers throughout indicate g-ratios. (A) Oligodendrocyte (OL1 green) digitally resliced to show the cell body, principal process, and connected myelin on axons (Ax1–Ax3). (B and C) Two levels of Ax1. One process (B, green) at paranodal loops (B, yellow) was followed directly to cell body as the outermost cytoplasmic compartment of the outer tongue process. (C) At mid-cell body level (12 μm deeper than B), the plasma membrane of the cell body (green) forms the outer mesaxon (arrow) against the inner aspect of the outer tongue process (yellow). Myelin is thickest here (g-ratio 0.87). (D and E) Thin process (yellow) extends from trunk and encircles an axon (Ax2). Digital reslice image (D) shows continuity of process to axon, and in E, process (yellow) encircles the axon once and forms the mesaxon (arrow). (F) Trunk process (green) also forms mesaxon (arrow) with the outer tongue (yellow) of low g-ratio internode (Ax3). (G) Oligodendrocyte (OL2 green) digitally resliced to follow one major process and connecting axons (Ax1–Ax6). (H and I) The somatic membrane surrounds a small fiber (H; Ax1, axon) and thick myelin (I; axon Ax2). The process indicated by an asterisk encircles the internode perimeter to become an inner aspect of the outer tongue (yellow) at the mesaxon (yellow). (J) Digital reslice image following process of OL2 (green) which forms high g-ratio internode (Ax3, axon; yellow, outer tongue), contacts the surface of the adjacent axon (Ax4) and encircles a thick myelin internode (Ax5, axon). (K–M) Three planes of axon (Ax4). At the node (K), OL2 process (green) contacts axolemma, to become paranodal loops (L, yellow), and produce the thin internode (M, thickest myelin). (N) The somatic membrane also contacts surface of thicker myelin internode (Ax6, axon), suggesting possible connection. (O) Oligodendrocyte (OL3, green) digitally resliced as for G. The process encircles large axon (Ax1) and myelin, and forms a mesaxon (P, arrow) at the outer tongue (yellow). Another process extends to surround the nodal axon (Q, Ax2) and form paranodal loops (yellow) and myelin. OL over oligodendrocyte nucleus in A and G. [Scale bars: 1 µm (B–F, H–N, P, and Q) and 5 µm (A, G, and O).]
Demyelination and Remyelination in a Nonhuman Primate Model.
As previously reported, 3 y of vitamin B12 deprivation led to the development of large areas of demyelination in the spinal cord, with marked distension of the extracellular space allowing the visualization of individual oligodendrocytes and their processes (28, 29) (Fig. 4). At the edge of these lesions, some oligodendrocytes were noted to have connections only to mature myelin sheaths (Fig. 4B) or were adjacent to scattered demyelinated axons (Fig. 4C). In the core of these lesions there was extensive myelin vacuolation leading to widespread demyelination (Fig. 4 D and E). In response to the myelin damage and demyelination, oligodendrocytes both adjacent to and within the lesion demonstrated a wide array of responses (Fig. 5). These oligodendrocytes were frequently associated with mature myelin sheaths and had processes that were contacting, ensheathing, and in some instances, remyelinating demyelinated axons (Fig. 5). Remyelination was not as frequent as in FIDID, yet both thinly remyelinated axons and those with short internodes were seen (Fig. 5). Ultrastructural analysis confirmed that oligodendrocytes that had associated mature myelin sheaths also had demyelinated axons embedded within their cytoplasm, as the first step toward remyelination (Fig. 6).
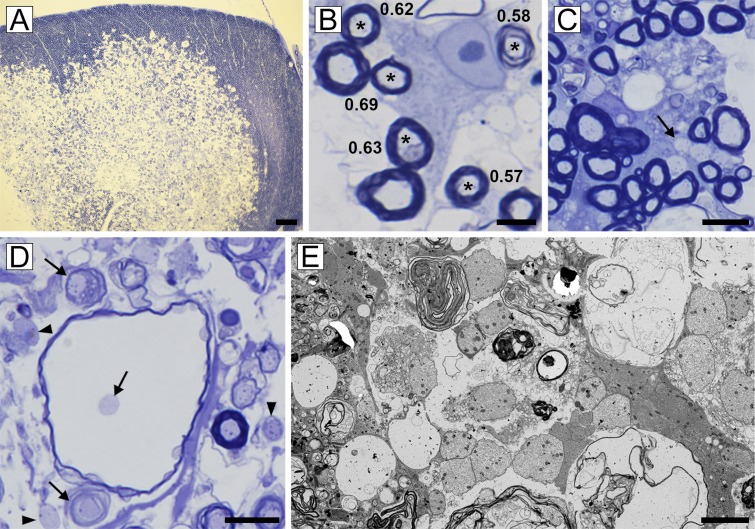
Fig. 4.
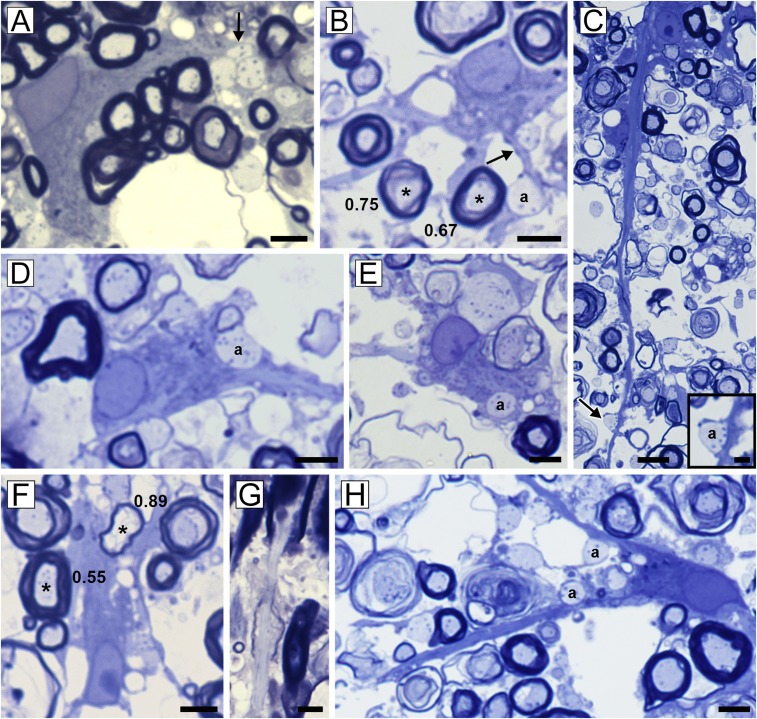
Vitamin B12 deficiency in the rhesus macaque is primarily a demyelinating disease. (A) Focal area of myelin vacuolation in the dorsal (posterior) column with pronounced increase in the intracellular space. (B) At the edge of the lesion adult oligodendrocytes myelinating normal-thickness myelin sheaths (*) were seen (g-ratios noted). (C) Scattered demyelinated axons were also seen at the edge (arrow). (D) Within the core of the lesion, most myelin sheaths were vacuolated but axons were intact (arrows). Demyelinated axons were present in the neuropil (arrowheads). (E) These were confirmed on EM. [Scale bars: 4 µm (E), 10 µm (B), 20 µm (C and D), and 200 µm (A).]
Fig. 5.
Oligodendrocytes respond to demyelination by contacting naked axons and engulfing them in their cytoplasm, resulting in restricted remyelination. In this montage, a range of the early and late interactions of mature oligodendrocytes with demyelinated axons is seen. Early contact with demyelinated axons is seen in A–F and H, with fine processes (arrows) beginning to contact and surround axons (denoted by “a”). The adult oligodendrocyte in B is connected to two axons with mature myelin sheaths (*, g-ratios 0.75 and 0.67) and has a process connected to a demyelinated axon (a). Demyelinated axons can be seen indented within the oligodendrocyte cytoplasm (D) and within oligodendrocytes that have both mature and remyelinated sheaths (E). A mature oligodendrocyte myelinates both thin (*, g = 0.89) and thick (*, g = 0.55) sheaths (F). Remyelination is confirmed by the presence of short, thin internodes (G). [Scale bars: 5 µm (C, Inset), 10 µm (A, B, and D–H), and 20 µm (C).]
Fig. 6.
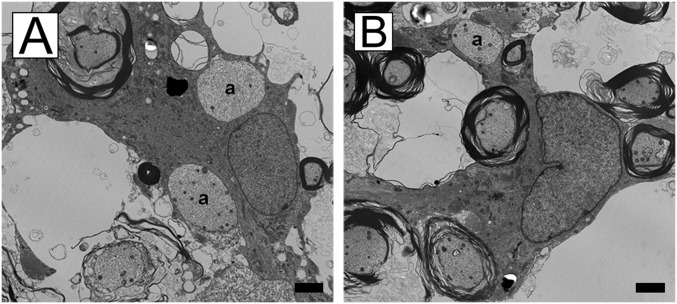
EM confirms the oligodendrocyte/demyelinated axon interaction. (A and B) Two examples of oligodendrocytes connected to mature myelin sheaths that have initiated early ensheathment of demyelinated axons (denoted by “a”). (Scale bars, 2 µm.)
Discussion
We present compelling evidence in two models of demyelination that adult oligodendrocytes can play a role in remyelination in the spinal cord. This conclusion is based on 2D and 3D analyses of oligodendrocytes with cytoplasmic connections to both thick, mature myelin sheaths and thin remyelinated sheaths. Our interpretation of this finding is that such oligodendrocytes had survived the myelin breakdown, maintaining one or more of their original internodes while reextending processes to remyelinate adjacent demyelinated axons (Fig. 7). However, it is critical to consider whether there are alternative explanations of oligodendrocytes that are connected to asymmetrically thick and thin myelin sheaths. One potential explanation is that this finding results from asynchronous development of myelin sheaths during remyelination by newly generated oligodendrocytes. However, in development myelination of the ventral column of the cat spinal cord (the same species and area examined in this study) has been analyzed in detail by Remahl and Hildebrand (32). Using serial sectioning EM, the authors concluded that myelin sheaths associated with single oligodendrocytes had similar myelin sheath thickness. Whether this is true in the adult CNS should be considered, given the growing interest and description of plasticity in mature white matter (33, 34). It is clear that myelin remodeling occurs in the adult brain, perhaps associated with ongoing oligodendrogenesis. However, in both rodents (35) and humans (36), the data suggest that there is little turnover of oligodendrocytes; hence, myelin remodeling would result from preexisting oligodendrocytes (37). Such myelin sheath changes would likely not be comparable to the large-scale remyelination seen in FIDID and other demyelinating diseases. In FIDID we have shown that the mosaic pattern of myelin sheaths persists for over 2 y with no long-term remodeling (30).
Fig. 7.
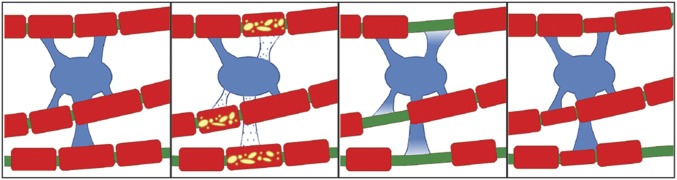
Cartoon illustrating the proposed response of mature oligodendrocytes to adjacent demyelinated internodes. There is partial loss of internodes of this oligodendrocytes territory and retraction of processes. This is quickly followed by process reextension and remyelination, resulting in thin, short internodes, while the axon maintains its original myelin sheaths.
The alternative hypothesis that asynchronous myelin sheath thickness is a feature seen on remyelination but not in development should also be considered. However, review of the remyelinating pattern in a range of species following demyelination in toxic diseases—including cuprizone, lysolecithin and ethidium bromide (38–44), multiple sclerosis (45, 46), and viral demyelinating disease (47, 48)—shows the generation of uniformly thin myelin sheaths. In addition, transplantation of neonatal and adult glial cells from different species into de- or dysmyelinated lesions, also results in large, confluent areas of thinly myelinated axons (49–53). Hence we conclude that in the model presented here, the most likely interpretation of an oligodendrocyte connected to mature and remyelinated myelin sheaths is that it is a mature oligodendrocyte that has participated in remyelination.
While studies of several models of remyelination have concluded that mature oligodendrocyte are incapable of remyelination (2), it is important to note that the two models presented here are different from the in vitro and in vivo models used previously to define the OPC as the primary, if not the sole, origin of remyelinating oligodendrocytes in many experimental systems. Therefore, it may be that the lesion type dictates the cellular response. In large yet focal areas of demyelination in which there is marked oligodendrocyte loss, such as in some MS plaques, remaining or adjacent OPCs may be the only or primary source of remyelinating oligodendrocytes. In lesions, such as seen in FIDID or vitamin B12 deficiency, where demyelinated axons are scattered among persistent mature myelinated axons and there is little or no oligodendrocyte death, surviving mature oligodendrocytes may be recruited to take part in myelin repair.
The critical difference between the models we describe here and the diverse in vitro and in vivo studies on the cellular basis of remyelination is that the adult oligodendrocytes are intact and viable as they retained an intact internode. Transplantation of mature oligodendrocytes into the demyelinated spinal cord (54) or plating them in cocultures (19) could challenge oligodendrocytes to reinitiate the entire temporal sequence of events that occur in development. Such cells may have to de-differentiate, divide, then express the genes required to generate the myelin sheath in the correct temporal sequence. The study of Makinodan et al. (25) showed no evidence of division of oligodendrocytes in which their processes were damaged, even after the addition of PDGF, hence the reextension of processes did not require the cell to divide or de-differentiate. Other experimental studies that have argued against the mature oligodendrocytes used strategies, such as irradiation of the CNS or transplantation that could have a negative effect on the remyelinating ability of these cells (20).
The strongest data supporting the limited functional capacity of mature oligodendrocytes as sources of myelin repair comes from studies of myelination of the zebrafish spinal cord (55). Using live imaging, they showed that oligodendrocytes have a very brief window of time (5 h) during development in which to generate myelin sheaths and do so in a brief, synchronous fashion. Similar developmental limitations were seen in mammalian coculture systems (19). In the studies reported herein, however, the myelin breakdown, macrophage infiltration, and associated cytokine expression will create a very different environment from that seen during development. Similarly, surviving oligodendrocytes abut demyelinated axons and therefore could enhance their remyelinating potential.
What are the differences between the models reported here and those commonly used in studies on remyelination, which may be relevant to the discussion on the cellular origin of myelin repair? Cuprizone, which is currently the de facto model of demyelination/remyelination, is very different as the toxin kills adult oligodendrocytes (56). Hence, remyelination after cessation of cuprizone treatment requires the generation of new oligodendrocytes from OPCs. Remyelinated axons have uniformly thin myelin sheaths (40, 57) that persist for over 6 mo (58, 59), resembling that seen in the dorsal column of cats with FIDID (Fig. 1E). In FIDID and also in B12 deficiency in the monkey and humans (60), there is no oligodendrocyte death. Importantly, at least in FIDID in the lateral and ventral columns of the spinal cord, remyelination occurs concurrently with demyelination; hence, it would seem unlikely that there is time for OPCs to divide, differentiate, and reensheath axons. In contrast, surviving oligodendrocytes that are scattered throughout the demyelinated milieu may respond promptly with process extension (Fig. 7), as occurs in vitro within 4 h of process damage (25). Whether there is organelle redistribution in such processes that promote the generation and transport of myelin proteins or their RNAs, including Golgi and microtubules, will require further investigation.
Therefore, if the adult oligodendrocyte might be a player in remyelination, does this have significance in MS and other human myelin disorders? We have proposed here that surviving adult oligodendrocytes are attached to at least a single internode to be viable. While oligodendrocyte death is a significant finding in MS at different stages of the disease, surviving mature oligodendrocytes, although rare in chronic lesions (61), can be present and occasionally in large numbers (62–64). MS plaques most often consist of large, contiguous areas of demyelinated axons; hence, surviving oligodendrocytes in the middle of such plaques may be incapable of process reextension, as they have no intact internodes. Although OPCs may be the only cell capable of generating remyelinating oligodendrocytes in such lesions, it has previously been suggested that surviving, mature oligodendrocytes may be involved (62). Additionally, at the periphery of plaques, intact oligodendrocytes may have lost some internodes but not all, and may extend processes into the plaque. In FIDID, the extent of demyelination varies depending on the anatomical site: that is, in the lateral and ventral columns of the spinal cord, it is patchy, whereas in the dorsal column and optic nerve it is much more generalized. In the optic nerve, which can totally demyelinate, remyelination can be complete and thus OPCs may be the cell or origin of remyelinating oligodendrocytes in this structure and in the dorsal column, leading to predominately thin myelin sheaths (Fig. 1E), in contrast to the rest of the spinal cord (Fig. 1C). Therefore, in the same disorder, the OPC and mature oligodendrocyte may play complimentary, yet CNS site-specific roles in myelin repair.
The observations of mature oligodendrocytes in the primate model initiating the process of reensheathment and myelination of demyelinated axons provides strong support that this may occur in MS lesions. In the rhesus macaque lesions, identification of single oligodendrocytes and their processes is aided by the increase in the extracellular space in lesions (29). This model mimics subacute combined degeneration (SCD) that results from vitamin B12 deficiency (60, 65). The myelopathy that develops in SCD may be the “purest” demyelinating disease of humans, and neurological and radiological recovery on B12 treatment, likely occurs from remyelination, possibly resulting from persisting, mature oligodendrocytes. The data presented here demonstrate that surviving adult oligodendrocytes have the propensity to interact with demyelinated axons, initiate the wrapping and embedding of axons in oligodendrocyte cytoplasm, and in some instances, remyelinate these axons. While remyelination by adult oligodendrocytes may be less robust than in FIDID, the lack of vitamin B12 will undoubtedly diminish the function of the oligodendrocyte. Indeed, deprivation of B12 in the human brain during development results in severe myelination delay which, like SCD, can respond to B12 therapy with restoration of function of preexisting oligodendrocytes with myelin repair (66).
Materials and Methods
All cats were handled and treated according to the guidelines of the Research Animal Resources Center and the Animal Care and Use Committee at the University of Wisconsin–Madison. The disorder (FIDID) was produced by feeding cats dried food irradiated at 45–55 kGy (Sterigenics Radiation Facility) (1). Cats developed progressive hind limb ataxia and paresis after 5–6 mo on this diet. Return to a normal diet led to a slow recovery in all cats but not those that had developed urinary incontinence. The cervical and thoracic spinal cord was collected from cats with FIDID both during active disease and partial or full recovery, 2–3 mo or 2 y after removal from the irradiated diet. Cats were perfused with a modified Karnovsky fixative and segments of the spinal cord processed and embedded in plastic for light microscopy, transmission electron microscopy (TEM), and serial blockface scanning–EM (SBF–SEM). One-micrometer sections were used to identify areas of remyelination and thin sections then cut for TEM. TEM was performed on a Philips CM120 transmission electron microscope. Images were captured with a BioSprint 12 series digital camera using AMT Image Capture Engine V700. The g-ratios were measured on electron micrographs of myelin sheaths that were seen to be adjacent to oligodendrocyte nuclei. Mature myelin sheaths were defined as those with a g-ratio of 0.75 or less.
TUNEL Assay.
TUNEL assay was performed to detect glial cell apoptosis. Following fixation, cervical spinal cord tissue from four affected cats and one normal cat were embedded in paraffin. Paraffin blocks were then sectioned (4-µm thickness), deparaffinized, and rehydrated by the University of Wisconsin–Madison School of Veterinary Medicine Histology Laboratory. Two cervical spinal cord sections were used per treatment group (positive control, negative control, and experimental TUNEL group) per animal, except for one affected cat for which there was only a negative and experimental treatment with one section/treatment group. Slides were stored in PBS overnight and then treated with the DeadEnd Colorimetric TUNEL Assay kit (Promega) according to the manufacturer’s protocol. Stained sections were examined using a Nikon Eclipse E800 microscope equipped with a Nikon Digital Sight DS-Ri1 camera and NIS-Elements Documentation software to obtain images.
Rhesus Monkey Vitamin B12 Deprivation.
Spinal cord tissue from a rhesus macaque that was part of an experimental group of animals that had been fed a vitamin B12-deficient diet for over 3 y, and which had developed visual loss and lower limb weakness and ataxia, was examined by light and EM (28, 29) (these studies were performed prior to University Animal Care Committee oversight). The spinal cord tissue was removed from a monkey perfused with aldehyde fixative after being killed. Tissue was sectioned for light microscopy and EM as above.
SBF–EM Imaging and Analysis of Spinal Cord Sections from Cats with FIDID.
Tissues for SBF–SEM were fixed as above in modified Karnovsky fixative and stained and imaged by Renovo Neural Inc. Briefly, the samples were washed, stained with 1% tannic acid for 30 min and then incubated successively with osmium ferricyanide, tetracarbohydrazide, osmium tetroxide, uranyl acetate, and lead aspartate (67, 68). Tissues were dehydrated and embedded in Epon-812 resin (Procure; Electron Microscopy Sciences).
Samples were imaged using a Zeiss Sigma VP scanning EM equipped with a Gatan 3View in-chamber ultramicrotome stage with low-kilovolt backscattered electron detectors optimized for 3View systems. Stacks of digital images were acquired at 7–8 nm per pixel resolution (x,y) at 2.25 kV, using 80-nm steps; these are standard settings that approximate TEM-based resulting serial image stacks contained 300–900 images (∼120 × 100 µm, 16,000 × 12,800 pixels each, 100–180 GB per set). For analysis, images were scaled, substacks generated, and aligned using ImageJ software with FIJI plug-in suite. In some cases, cytoplasmic process continuity was illustrated by digitally reslicing the stack such that the visible plane followed the centerline of the process. Three-dimensional models were generated by manual tracing using Reconstruct software (69).
Supplementary Material
Acknowledgments
We thank Dr. Dimitri Agamanolis for his generous donation of tissue blocks from his previous studies; Dr. Joshua Mayer for creating the cartoon used in Fig. 7; Emily Benson and Diandra Starks (Renovo) for 3D electron microscopy imaging and tracing expertise; Drs. Jayshree Samanta and John Svaren for their comments on this manuscript; and Amin Sherafat for useful discussion. These studies were supported by National Multiple Sclerosis Society Grant RG-1501-02876.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808064115/-/DCSupplemental.
References
- 1.Duncan ID, Brower A, Kondo Y, Curlee JF, Jr, Schultz RD. Extensive remyelination of the CNS leads to functional recovery. Proc Natl Acad Sci USA. 2009;106:6832–6836, and correction (2009) 106:12208. doi: 10.1073/pnas.0812500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franklin RJM, Ffrench-Constant C. Regenerating CNS myelin—From mechanisms to experimental medicines. Nat Rev Neurosci. 2017;18:753–769. doi: 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- 3.Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- 4.Raine CS, Wu E. Multiple sclerosis: Remyelination in acute lesions. J Neuropathol Exp Neurol. 1993;52:199–204. [PubMed] [Google Scholar]
- 5.Lassmann H, Brück W, Lucchinetti C, Rodriguez M. Remyelination in multiple sclerosis. Mult Scler. 1997;3:133–136. doi: 10.1177/135245859700300213. [DOI] [PubMed] [Google Scholar]
- 6.Goldschmidt T, Antel J, König FB, Brück W, Kuhlmann T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72:1914–1921. doi: 10.1212/WNL.0b013e3181a8260a. [DOI] [PubMed] [Google Scholar]
- 7.Patrikios P, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- 8.Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33:277–287. doi: 10.1111/j.1365-2990.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 9.Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- 10.Mei F, et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife. 2016;5:e18246. doi: 10.7554/eLife.18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz V, et al. Acutely damaged axons are remyelinated in multiple sclerosis and experimental models of demyelination. Glia. 2017;65:1350–1360. doi: 10.1002/glia.23167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornek B, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: A comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verden D, Macklin WB. Neuroprotection by central nervous system remyelination: Molecular, cellular, and functional considerations. J Neurosci Res. 2016;94:1411–1420. doi: 10.1002/jnr.23923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin RJ, Gallo V. The translational biology of remyelination: Past, present, and future. Glia. 2014;62:1905–1915. doi: 10.1002/glia.22622. [DOI] [PubMed] [Google Scholar]
- 15.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 16.Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- 17.Targett MP, et al. Failure to achieve remyelination of demyelinated rat axons following transplantation of glial cells obtained from the adult human brain. Neuropathol Appl Neurobiol. 1996;22:199–206. [PubMed] [Google Scholar]
- 18.Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Watkins TA, Emery B, Mulinyawe S, Barres BA. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 2008;60:555–569. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford AH, et al. Pre-existing mature oligodendrocytes do not contribute to remyelination following toxin-induced spinal cord demyelination. Am J Pathol. 2016;186:511–516. doi: 10.1016/j.ajpath.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwin SK, Bakker DA. Can oligodendrocytes attached to myelin proliferate? J Neurosci. 1988;8:1239–1244. doi: 10.1523/JNEUROSCI.08-04-01239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood PM, Bunge RP. The origin of remyelinating cells in the adult central nervous system: The role of the mature oligodendrocyte. Glia. 1991;4:225–232. doi: 10.1002/glia.440040214. [DOI] [PubMed] [Google Scholar]
- 23.Warrington AE, Barbarese E, Pfeiffer SE. Differential myelinogenic capacity of specific developmental stages of the oligodendrocyte lineage upon transplantation into hypomyelinating hosts. J Neurosci Res. 1993;34:1–13. doi: 10.1002/jnr.490340102. [DOI] [PubMed] [Google Scholar]
- 24.Duncan ID, Paino C, Archer DR, Wood PM. Functional capacities of transplanted cell-sorted adult oligodendrocytes. Dev Neurosci. 1992;14:114–122. doi: 10.1159/000111655. [DOI] [PubMed] [Google Scholar]
- 25.Makinodan M, et al. Oligodendrocyte plasticity with an intact cell body in vitro. PLoS One. 2013;8:e66124. doi: 10.1371/journal.pone.0066124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIver SR, et al. Oligodendrocyte degeneration and recovery after focal cerebral ischemia. Neuroscience. 2010;169:1364–1375. doi: 10.1016/j.neuroscience.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeffries MA, et al. ERK1/2 activation in preexisting oligodendrocytes of adult mice drives new myelin synthesis and enhanced CNS function. J Neurosci. 2016;36:9186–9200. doi: 10.1523/JNEUROSCI.1444-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agamanolis DP, et al. Neuropathology of experimental vitamin B12 deficiency in monkeys. Neurology. 1976;26:905–914. doi: 10.1212/wnl.26.10.905. [DOI] [PubMed] [Google Scholar]
- 29.Agamanolis DP, et al. An ultrastructural study of subacute combined degeneration of the spinal cord in vitamin B12-deficient rhesus monkeys. J Neuropathol Exp Neurol. 1978;37:273–299. doi: 10.1097/00005072-197805000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Duncan ID, Marik RL, Broman AT, Heidari M. Thin myelin sheaths as the hallmark of remyelination persist over time and preserve axon function. Proc Natl Acad Sci USA. 2017;114:E9685–E9691. doi: 10.1073/pnas.1714183114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipsitz D, Goetz BD, Duncan ID. Apoptotic glial cell death and kinetics in the spinal cord of the myelin-deficient rat. J Neurosci Res. 1998;51:497–507. doi: 10.1002/(SICI)1097-4547(19980215)51:4<497::AID-JNR9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Remahl S, Hilderbrand C. Relation between axons and oligodendroglial cells during initial myelination. I. The glial unit. J Neurocytol. 1990;19:313–328. doi: 10.1007/BF01188401. [DOI] [PubMed] [Google Scholar]
- 33.Fields RD. A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat Rev Neurosci. 2015;16:756–767. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang KJ, Redmond SA, Chan JR. Remodeling myelination: Implications for mechanisms of neural plasticity. Nat Neurosci. 2016;19:190–197. doi: 10.1038/nn.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tripathi RB, et al. Remarkable stability of myelinating oligodendrocytes in mice. Cell Rep. 2017;21:316–323. doi: 10.1016/j.celrep.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeung MS, et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Sampaio-Baptista C, Johansen-Berg H. White matter plasticity in the adult brain. Neuron. 2017;96:1239–1251. doi: 10.1016/j.neuron.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeffery ND, Blakemore WF. Remyelination of mouse spinal cord axons demyelinated by local injection of lysolecithin. J Neurocytol. 1995;24:775–781. doi: 10.1007/BF01191213. [DOI] [PubMed] [Google Scholar]
- 39.Blakemore WF. Invasion of Schwann cells into the spinal cord of the rat following local injections of lysolecithin. Neuropathol Appl Neurobiol. 1976;2:21–39. [Google Scholar]
- 40.Ludwin SK. Central nervous system demyelination and remyelination in the mouse: An ultrastructural study of cuprizone toxicity. Lab Invest. 1978;39:597–612. [PubMed] [Google Scholar]
- 41.Franklin RJM, Crang AJ, Blakemore WF. Transplanted type-1 astrocytes facilitate repair of demyelinating lesions by host oligodendrocytes in adult rat spinal cord. J Neurocytol. 1991;20:420–430. doi: 10.1007/BF01355538. [DOI] [PubMed] [Google Scholar]
- 42.Blakemore WF. Observations on remyelination in the rabbit spinal cord following demyelination induced by lysolecithin. Neuropathol Appl Neurobiol. 1978;4:47–59. doi: 10.1111/j.1365-2990.1978.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 43.Caprariello AV, et al. Apoptosis of oligodendrocytes during early development delays myelination and impairs subsequent responses to demyelination. J Neurosci. 2015;35:14031–14041. doi: 10.1523/JNEUROSCI.1706-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Syed YA, et al. Antibody-mediated neutralization of myelin-associated EphrinB3 accelerates CNS remyelination. Acta Neuropathol. 2016;131:281–298. doi: 10.1007/s00401-015-1521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frohman EM, Racke MK, Raine CS. Multiple sclerosis—The plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 46.Raine CS, Scheinberg LC. On the immunopathology of plaque development and repair in multiple sclerosis. J Neuroimmunol. 1988;20:189–201. doi: 10.1016/0165-5728(88)90160-9. [DOI] [PubMed] [Google Scholar]
- 47.Miller DJ, Rivera-Quiñones C, Njenga MK, Leibowitz J, Rodriguez M. Spontaneous CNS remyelination in beta 2 microglobulin-deficient mice following virus-induced demyelination. J Neurosci. 1995;15:8345–8352. doi: 10.1523/JNEUROSCI.15-12-08345.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller DJ, Asakura K, Rodriguez M. Central nervous system remyelination clinical application of basic neuroscience principles. Brain Pathol. 1996;6:331–344. doi: 10.1111/j.1750-3639.1996.tb00859.x. [DOI] [PubMed] [Google Scholar]
- 49.Franklin RJM, Bayley SA, Blakemore WF. Transplanted CG4 cells (an oligodendrocyte progenitor cell line) survive, migrate, and contribute to repair of areas of demyelination in X-irradiated and damaged spinal cord but not in normal spinal cord. Exp Neurol. 1996;137:263–276. doi: 10.1006/exnr.1996.0025. [DOI] [PubMed] [Google Scholar]
- 50.Crang AJ, Gilson JM, Li WW, Blakemore WF. The remyelinating potential and in vitro differentiation of MOG-expressing oligodendrocyte precursors isolated from the adult rat CNS. Eur J Neurosci. 2004;20:1445–1460. doi: 10.1111/j.1460-9568.2004.03606.x. [DOI] [PubMed] [Google Scholar]
- 51.Crang AJ, Blakemore WF. Remyelination of demyelinated rat axons by transplanted mouse oligodendrocytes. Glia. 1991;4:305–313. doi: 10.1002/glia.440040308. [DOI] [PubMed] [Google Scholar]
- 52.Franklin RJM, Blakemore WF. Transplanting oligodendrocyte progenitors into the adult CNS. J Anat. 1997;190:23–33. doi: 10.1046/j.1469-7580.1997.19010023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith PM, Blakemore WF. Porcine neural progenitors require commitment to the oligodendrocyte lineage prior to transplantation in order to achieve significant remyelination of demyelinated lesions in the adult CNS. Eur J Neurosci. 2000;12:2414–2424. doi: 10.1046/j.1460-9568.2000.00137.x. [DOI] [PubMed] [Google Scholar]
- 54.Blakemore WF, Keirstead HS. The origin of remyelinating cells in the central nervous system. J Neuroimmunol. 1999;98:69–76. doi: 10.1016/s0165-5728(99)00083-1. [DOI] [PubMed] [Google Scholar]
- 55.Czopka T, Ffrench-Constant C, Lyons DA. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev Cell. 2013;25:599–609. doi: 10.1016/j.devcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kipp M, Clarner T, Dang J, Copray S, Beyer C. The cuprizone animal model: New insights into an old story. Acta Neuropathol. 2009;118:723–736. doi: 10.1007/s00401-009-0591-3. [DOI] [PubMed] [Google Scholar]
- 57.Blakemore WF. Remyelination of the superior cerebellar peduncle in the mouse following demyelination induced by feeding cuprizone. J Neurol Sci. 1973;20:73–83. doi: 10.1016/0022-510x(73)90119-6. [DOI] [PubMed] [Google Scholar]
- 58.Blakemore WF. Pattern of remyelination in the CNS. Nature. 1974;249:577–578. doi: 10.1038/249577a0. [DOI] [PubMed] [Google Scholar]
- 59.Ludwin SK, Maitland M. Long-term remyelination fails to reconstitute normal thickness of central myelin sheaths. J Neurol Sci. 1984;64:193–198. doi: 10.1016/0022-510x(84)90037-6. [DOI] [PubMed] [Google Scholar]
- 60.Pant SS, Asbury AK, Richardson EP., Jr The myelopathy of pernicious anemia. A neuropathological reappraisal. Acta Neurol Scand. 1968;44:7–36. [PubMed] [Google Scholar]
- 61.Stadelmann C, Wegner C, Brück W. Inflammation, demyelination, and degeneration—Recent insights from MS pathology. Biochim Biophys Acta. 2011;1812:275–282. doi: 10.1016/j.bbadis.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Ozawa K, et al. Patterns of oligodendroglia pathology in multiple sclerosis. Brain. 1994;117:1311–1322. doi: 10.1093/brain/117.6.1311. [DOI] [PubMed] [Google Scholar]
- 63.Kuhlmann T, et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758, and correction (2009) 132:1118. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- 64.Cui QL, et al. Sublethal oligodendrocyte injury: A reversible condition in multiple sclerosis? Ann Neurol. 2017;81:811–824. doi: 10.1002/ana.24944. [DOI] [PubMed] [Google Scholar]
- 65.Harper C, Butterworth R. Nutritional and metabolic disorders. In: Graham DI, Lantos PL, editors. Greenfield’s Neuropathology. 6th Ed. Vol 1. Arnold; New York: 1996. pp. 601–655. [Google Scholar]
- 66.Lövblad K, et al. Retardation of myelination due to dietary vitamin B12 deficiency: Cranial MRI findings. Pediatr Radiol. 1997;27:155–158. doi: 10.1007/s002470050090. [DOI] [PubMed] [Google Scholar]
- 67.Deerinck T, et al. Enhancing serial block-face scanning electron microscopy to enable high resolution 3-D nanohistology of cells and tissues. Microsc Microanal. 2010;16:1138–1139. [Google Scholar]
- 68.Mukherjee K, et al. Analysis of brain mitochondria using serial block-face scanning electron microscopy. J Vis Exp. 2016:e54214. doi: 10.3791/54214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiala JC. Reconstruct: A free editor for serial section microscopy. J Microsc. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.