Abstract
In aquatic environments, phytoplankton represent a major source of reactive oxygen species (ROS) such as superoxide and hydrogen peroxide. Many phytoplankton taxa also produce extracellular ROS under optimal growth conditions in culture. However, the physiological purpose of extracellular ROS production by phytoplankton and its wider significance to ecosystem-scale trophic interactions and biogeochemistry remain unclear. Here, we review the rates, taxonomic diversity, subcellular mechanisms and functions of extracellular superoxide and hydrogen peroxide production by phytoplankton with a view towards future research directions. Model eukaryotic phytoplankton and cyanobacteria produce extracellular superoxide and hydrogen peroxide at cell-normalized rates that span several orders of magnitude, both within and between taxa. The potential ecophysiological roles of extracellular ROS production are versatile and appear to be shared among diverse phytoplankton species, including ichthyotoxicity, allelopathy, growth promotion, and iron acquisition. Whereas extracellular hydrogen peroxide likely arises from a combination of intracellular and cell surface production mechanisms, extracellular superoxide is predominantly generated by specialized systems for transplasma membrane electron transport. Future insights into the molecular-level basis of extracellular ROS production, combined with existing high-sensitivity geochemical techniques for the direct quantification of ROS dynamics, will help unveil the ecophysiological and biogeochemical significance of phytoplankton-derived ROS in natural aquatic systems.
Keywords: harmful algae, phytoplankton bloom, oxidative stress, redox homeostasis, biological interactions, monitoring, cryptic biogeochemistry
INTRODUCTION
Reactive oxygen species (ROS) include intermediates in the four-electron reduction of oxygen to water: superoxide, hydrogen peroxide and hydroxyl radical. Biological ROS production has been the subject of scientific inquiry since the discovery of the ubiquitous antioxidant enzyme, superoxide dismutase (SOD) (McCord and Fridovich, 1969). Since then, it has been well established that all oxygen-metabolizing organisms generate ROS and that this ROS production has potentially self-harmful effects. Yet more recently, awareness has been growing that biological ROS production can promote growth and survival. Extracellular ROS production regulates cell differentiation by fungi (Aguirre et al., 2005), innate immunity in seaweeds (Weinberger, 2007) and white blood cells (Babior, 1999), heterotrophic feeding by corals (Armoza-Zvuloni et al., 2016) and reproduction by sea urchins (Shapiro, 1991). Extracellular ROS production by the harmful bloom-forming phytoplankton species Chattonella marina has been implicated in its toxicity (Kim and Oda, 2010), growth (Oda et al., 1995) and iron acquisition (Garg et al., 2007; Liu et al., 2007), while many other phytoplankton generate extracellular ROS under non-stressful conditions for reasons that remain mysterious.
ROS occur naturally in the environment, as the products of both abiotic and biologically driven chemical reactions. In natural waters, ROS are present at low concentrations (10−18−10−6 mol L−1) and are short-lived (μsec–days), yet ubiquitous (Table I). Aquatic ROS profoundly shape the biogeochemical cycling of carbon, as well as toxic and nutrient metals (Siciliano et al., 2002; Pullin et al., 2004; Barbeau, 2006; Learman et al., 2011; Rose, 2012; Zinser, 2018). In oxygenated surface waters, biological production of ROS can be a substantial and dominant ROS source, especially in areas of elevated biological productivity, such as phytoplankton blooms (Rose et al., 2008b, 2010; Hansard et al., 2010; Vermilyea et al., 2010; Rusak et al., 2011; Dixon et al., 2013; Marsico et al., 2015; Cory et al., 2016). Despite prodigious extracellular ROS production by many cultivated phytoplankton species and the quantitative contribution of phytoplankton communities to aquatic ROS fluxes, the physiological significance of phytoplankton-derived ROS and the wider implications for food web dynamics and biogeochemistry are poorly understood. Here, we review the rates, mechanisms and ecophysiological roles of extracellular ROS production by phytoplankton, with a focus on marine taxa and the ROS superoxide and hydrogen peroxide. We also propose future research directions to clarify the ecosystem-scale significance of phytoplankton-derived ROS.
Table I:
Typical concentrations and lifetimes of ROS in aquatic systems
| ROS | Concentration (mol L−1) | Lifetime | |
|---|---|---|---|
| Superoxide | O2− | 10−12–10−9 | sec–min |
| Hydrogen peroxide | H2O2 | 10−9–10−6 | hours–days |
| Hydroxyl radical | OH• | 10−18–10−15 | μsec |
SURVEY OF EXTRACELLULAR ROS PRODUCTION BY PHYTOPLANKTON
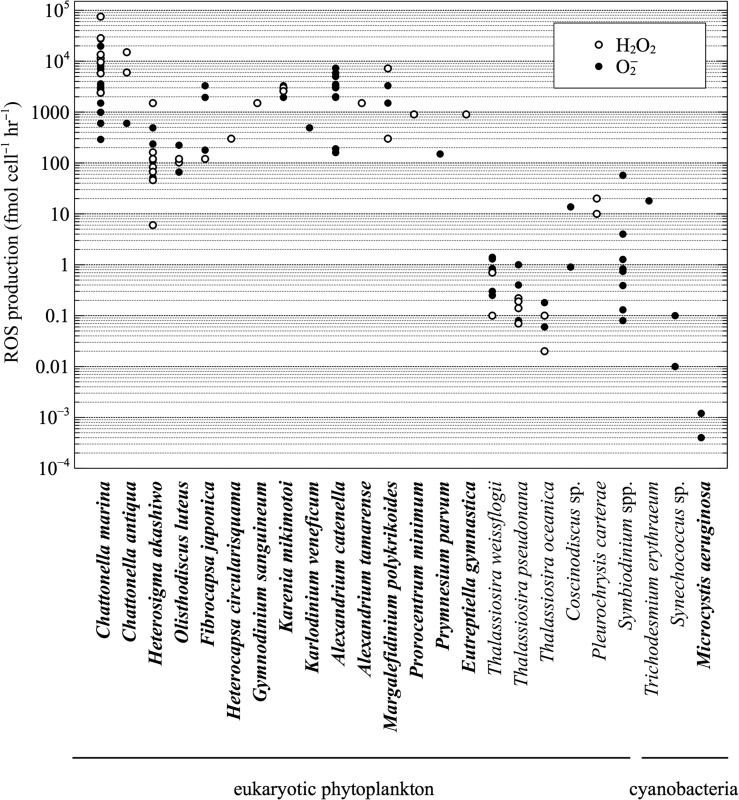
A broad diversity of phytoplankton produce extracellular ROS under optimal growth conditions in culture, including eukaryotic phytoplankton and cyanobacteria (Fig. 1; Supplementary Table S1). The major ROS superoxide (Oda et al., 1997; Marshall et al., 2005a; Portune et al., 2010; Mooney et al., 2011; Dorantes-Aranda et al., 2015; Schneider et al., 2016; Cho et al., 2017), hydrogen peroxide (Oda et al., 1997; Kim et al., 1999a; Portune et al., 2010; Schneider et al., 2016; Cho et al., 2017) and hydroxyl radical (Oda et al., 1992a; Yang et al., 1995; Cho et al., 2016) have all been examined in phytoplankton. Compared to previous studies on hydroxyl radical production, however, the literature on superoxide and hydrogen peroxide generation by phytoplankton is much more expansive. Thus, the focus here is primarily on superoxide and hydrogen peroxide.
Fig. 1.
Survey of extracellular superoxide (O2−) and hydrogen peroxide (H2O2) production rates by phytoplankton. Species known to form HABs are indicated in bold. Data and original references are provided in Supplementary Table S1.
Extracellular ROS production has been quantified on a per-cell basis in at least 21 eukaryotic phytoplankton species, and the majority of these are capable of forming harmful algal blooms (HABs) (Yang et al., 1995; Kim et al., 1999a; Marshall et al., 2005a; Mooney et al., 2011; Cho et al., 2017). Raphidophytes are the most thoroughly studied HAB group in terms of the production and potential functions of extracellular ROS, especially the Chattonella species C. marina and C. antiqua (Oda et al., 1997; Marshall et al., 2003, 2005a, 2005b), as well as Heterosigma akashiwo, Olisthodiscus luteus and Fibrocapsa japonica (Oda et al., 1992b; Yang et al., 1995; Oda et al.,1997; Marshall et al., 2005a; Portune et al., 2010). Extracellular ROS production has been examined in harmful bloom-forming dinoflagellates, including Alexandrium spp. (Marshall et al., 2005a; Mooney et al., 2011; Dorantes-Aranda et al., 2015; Mardones et al., 2015; Cho et al., 2017), Margalefidinium polykrikoides (Kim et al., 1999a, 2002; Tang and Gobler, 2009b; Griffith and Gobler, 2016) and Karenia mikimotoi (Yamasaki et al., 2004; Dorantes-Aranda et al., 2015; Cho et al., 2017). Among non-HAB forming eukaryotic phytoplankton, extracellular ROS are produced by the symbiotic dinoflagellates Symbiodinium spp. (Saragosti et al., 2010; Zhang et al., 2016a), the coccolithophorid Pleurochrysis carterae (Palenik et al., 1987) and diatoms, including the genus Thalassiosira (Kustka et al., 2005; Rose et al., 2008b; Milne et al., 2009; Waring et al., 2010; Schneider et al., 2016). Extracellular superoxide production has also been quantified in at least four species of cyanobacteria (Rose et al., 2005, 2008b; Godrant et al., 2009; Fujii et al., 2011; Hansel et al., 2016).
Cell-normalized rates of extracellular ROS production vary widely among phytoplankton species (10−4–105 fmol cell−1 h−1; Fig. 1;Supplementary Table S1). The ichthyotoxic raphidophyte C. marina is capable of the highest extracellular ROS production rates, yet other HAB species can reach similar rates of ROS production, including Alexandrium catenella (Mardones et al., 2015), M. polykrikoides (Kim et al., 1999a, 2002), K. mikimotoi (Yamasaki et al., 2004; Dorantes-Aranda et al., 2015; Cho et al., 2017) and F. japonica (Dorantes-Aranda et al., 2015). Overall, HAB-forming eukaryotes generate much more extracellular ROS than other phytoplankton taxa, including the harmful freshwater cyanobacterium Microcystis aeruginosa (Fujii et al., 2011), as well as non-HAB species such as Thalassiosira spp. Cell size is a major control on the interspecific variability in extracellular ROS production (Oda et al., 1997; Marshall et al., 2005a; Diaz et al., 2013), yet when accounting for cell size, some harmful algae still generate substantially more ROS than non-harmful species (Kustka et al., 2005). Thus, to some degree, extracellular ROS production may be related to the tendency of some phytoplankton species to form HABs.
In addition to large interspecific variability, extracellular ROS production rates also vary considerably within phytoplankton species (<10 to 103-fold; Fig. 1; Supplementary Table S1). Major factors underlying this variability include growth phase (Kawano et al., 1996; Kim et al., 1999a, 2004; Skeen et al., 2004; Garg et al., 2007; Portune et al., 2010), cell density (Yang et al., 1995; Twiner and Trick, 2000; Kim et al., 2002; Marshall et al., 2005b; Dorantes-Aranda et al., 2015), cell lysis (Dorantes-Aranda et al., 2013; Mardones et al., 2015), inter-strain differences (Ishimatsu et al., 1996; Oda et al., 1997; Portune et al., 2010; Dorantes-Aranda et al., 2013; Mardones et al., 2015), irradiance (Kim et al., 1999a; Dorantes-Aranda et al., 2013), temperature (Twiner and Trick, 2000), salinity (Mardones et al., 2015) and iron availability (Twiner and Trick, 2000). Examining the effect of these parameters on extracellular ROS production by phytoplankton has helped to illuminate potential ecophysiological functions (see section Ecophysiological roles of phytoplankton-derived extracellular ROS).
SUBCELLULAR PATHWAYS OF EXTRACELLULAR ROS PRODUCTION
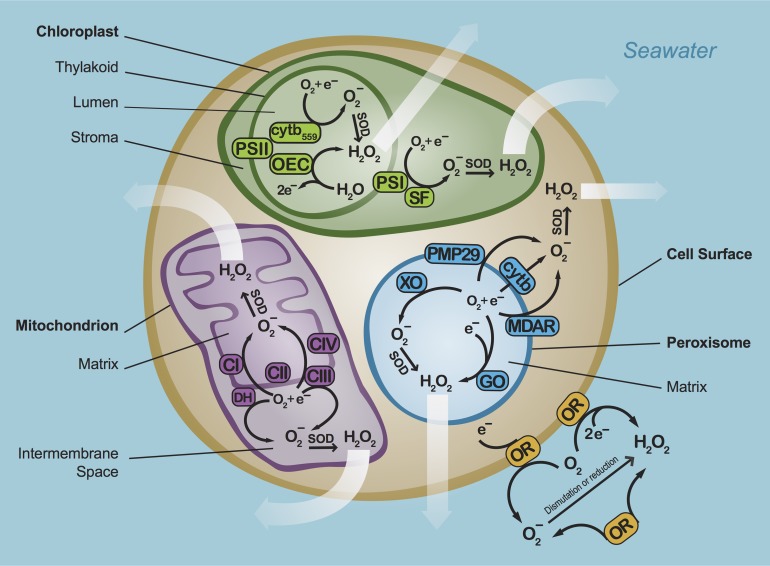
In phytoplankton, ROS production occurs at several major sites: the chloroplasts and mitochondria (or thylakoid membranes in cyanobacteria), peroxisome (eukaryotes only), cell surface and the cell-free environment (Fig. 2). The principal ROS-generating reaction at these sites is the formation of superoxide by the single-electron reduction of oxygen. In turn, rapid dismutation of superoxide by SOD is a primary mechanism for the production of hydrogen peroxide. Hydrogen peroxide can also be produced independently from superoxide through the two-electron reduction or oxidation of oxygen and water, respectively (Fig. 2).
Fig. 2.
Mechanisms of superoxide (O2−) and hydrogen peroxide (H2O2) production in phytoplankton. Chloroplast: PSI—photosystem I, PSII—photosystem II, SF—stromal factor, OEC—oxygen-evolving complex, cytb559—cytochrome b559; Mitochondria: CI—complex I, CIII—complex III, DH—NAD(P)H dehydrogenase; Peroxisomes: GO—glycolate oxidase, XO—xanthine oxidase, MDAR—monodehydroascorbate reductase, cytb—cytochrome b, PMP29—peroxisome membrane polypeptide 29; Cell surface and cell-free environment: OR—oxidoreductase. Intracellular hydrogen peroxide can diffuse within and outside of cells (white arrows), but intracellular superoxide is unlikely to escape the cell.
Intracellular ROS arise through several key processes. For example, in chloroplasts, ROS production within the stromal fluid occurs via the Mehler reaction, which is mediated by photosystem I (PSI; probably via iron-sulfur clusters) and/or with the involvement of a stromal factor (SF) such as monodehydroascorbate reductase (MDAR) (Asada, 1999, 2006). ROS production also occurs in the chloroplast lumen at several sites within photosystem II (PSII) such as the oxygen-evolving complex (OEC) and cytochrome b559 (cytb559), although ROS production in PSII is thought to be minor compared to PSI (Pospíšil, 2014). In mitochondria, intracellular production of ROS in the matrix and intermembrane space is primarily mediated by complex I (CI), complex III (CIII) and membrane-bound NAD(P)H dehydrogenases (DH) (Møller, 2001; Murphy, 2009). ROS production within the peroxisome matrix occurs via several oxidoreductases such as glycolate oxidase (GO) and xanthine oxidase (XO). On the peroxisome membrane, ROS are produced via NAD(P)H-dependent reactions mediated by MDAR, cytochrome b (cytb), and/or peroxisome membrane polypeptide 29 (PMP29) (Noctor et al., 2002; Foyer et al., 2009; del Rio and Lopez-Huertas, 2016).
The movement of superoxide and hydrogen peroxide within the intracellular space differs strongly, such that fundamentally different processes are likely involved in the biogenic fluxes of these ROS into the environment. For example, intracellular hydrogen peroxide readily diffuses across membranes, which may be an important route for the release of biogenic hydrogen peroxide into seawater, as seen for C. marina (Kim et al., 2000a, 2007). However, as a much shorter-lived (~μs) anion at physiological pH with a limited diffusive distance (~100 s of nm), superoxide does not readily cross biological membranes (Korshunov and Imlay, 2002; Lesser, 2006). Even the complete lysis of cells under severe oxidative stress cannot release enough superoxide to account for the steady-state concentrations that have been measured in natural waters (Rose, 2012). Intracellular processes such as photosynthesis are, therefore, unlikely to be a direct source of biologically derived extracellular superoxide. Indeed, Symbiodinium spp. (Saragosti et al., 2010; Zhang et al., 2016a) and Thalassiosira spp. (Schneider et al., 2016) produce extracellular superoxide in the dark, indicating the presence of non-photosynthetic mechanisms for superoxide production. Furthermore, the photosynthetic inhibitor dichlorophenyldimethylurea (DCMU) does not alter extracellular superoxide production by C. marina and H. akashiwo (Oda et al., 1998).
Rather than originating from intracellular sources, most extracellular superoxide is likely produced directly at the cell surface. Cell surface NADPH oxidoreductases catalyze the production of extracellular superoxide in many organisms, including protozoa, seaweeds, fungi, plants, animals (Saran, 2003; Aguirre et al., 2005; Hervé et al., 2005; Bedard et al., 2007; Weinberger, 2007; Anderson et al., 2011) and the freshwater alga Chlamydomonas reinhardtii (Anderson et al., 2016). In fact, extracellular superoxide production by C. marina occurs on the cell surface through an NADPH oxidoreductase that is homologous to human neutrophil NADPH oxidase (Shimada et al., 1993; Kim et al., 2007). Light exposure stimulates extracellular superoxide production by C. marina (Dorantes-Aranda et al., 2013; Li et al., 2015), Thalassiosira spp. (Schneider et al., 2016) and Symbiodinium spp. (Saragosti et al., 2010), which suggests that photosynthesis (Kim et al., 1999a; Marshall et al., 2002) may play an indirect role in extracellular superoxide production by supplying NADPH to the cell surface NADPH oxidoreductase, as suggested previously (Saragosti et al., 2010). In addition to superoxide, extracellular hydrogen peroxide may also be directly generated at the cell surface. For example, extracellular hydrogen peroxide production by Prymnesium parvum is mediated by amino acid oxidases during metabolism of exogenous organic nitrogen sources (Palenik et al., 1988).
Extracellular ROS production can also occur in the cell-free environment. For instance, the C. marina NADPH oxidoreductase can become dislodged from the cell surface and actively generate superoxide in cell-free spent media (Kim et al., 2000a, 2007). Cell-free hydrogen peroxide production has also been documented for the model diatom Phaeodactylum tricornutum (Schneider et al., 2016), although the mechanism remains unresolved.
ECOPHYSIOLOGICAL ROLES OF PHYTOPLANKTON-DERIVED EXTRACELLULAR ROS
ROS commonly arise as metabolic byproducts, whose damaging effects on vital biomolecules such as DNA, lipids and proteins are well known (Lesser, 2006). However, ROS production can be directed through specialized pathways (Fig. 2) to participate in a variety of regulatory and signaling processes that aid in the growth and survival of the organism making the ROS (Foyer and Noctor, 2005; Lesser, 2006; Mittler et al., 2011; Scheibe and Dietz, 2012; Schmitt et al., 2014). As discussed below, extracellular ROS production by phytoplankton may modulate biological interactions such as HAB toxicity, allelopathy, grazing and viral infection, while also aiding in growth and iron acquisition. ROS may serve many of these functions in the same phytoplankton species while also sharing similar purposes across different phytoplankton taxa. For instance, even though Chattonella is the most prolific producer of extracellular ROS among phytoplankton, the role of extracellular ROS in this genus may not be unique, as outlined in the following sections.
Ichthyotoxicity of HABs
ROS-forming HABs have caused immense financial losses to aquaculture industries in Australia (Hallegraeff et al., 1998), Japan (Okaichi, 1997) and Chile (Fuentes et al., 2006; Mardones et al., 2010). ROS are involved in the noxious or toxic effects of several HAB-forming species, such as raphidophytes (Yang et al., 1995; Oda et al., 1997; Kim et al., 1999b), and the dinoflagellates M. polykrikoides (Kim et al., 1999a; Tang and Gobler, 2009b, 2010) and Alexandrium spp. (Flores et al., 2012; Mardones et al., 2015). For example, antioxidants alleviate the toxic effect of multiple HAB species on various marine organisms (Yang et al., 1995; Oda et al., 1992b, 1997; Kim et al., 1999a, 1999b; Tang and Gobler, 2009a, 2009b, 2010; Flores et al., 2012). Furthermore, fish mucus and/or surface receptor-binding lectins stimulate ROS production by several raphidophytes, suggesting a role for extracellular ROS in modulating these interactions (Tanaka et al., 1994; Nakamura et al., 1998; Oda et al., 1998; Kim et al., 1999b, 2000b; Kim and Oda, 2010).
Although ROS may be involved in some cases of HAB toxicity, the mechanism(s) are still controversial. For example, C. marina is thought to cause fish death by inducing suffocation via gill tissue damage, and ROS may be involved in gill tissue injury (Kim and Oda, 2010). The toxic role of ROS is generally thought to be indirect or synergistic with other toxins. For instance, in the case of several HAB species, ROS have been shown to stimulate the toxicity of lipid peroxidation products such as polyunsaturated fatty acids (PUFAs) (Arzul et al., 1998; Kim et al., 1999a; Jenkinson and Arzul, 2001; Marshall et al., 2003, 2005b; Mardones et al., 2015). This mode of ROS toxicity helps to explain how transient ROS molecules can exert potentially harmful effects at concentrations that are not directly cytotoxic and over spatio-temporal scales that may exceed ROS lifetimes and diffusive distances. In some cases, cell lysis and the concomitant stimulation of extracellular ROS production are thought to be an important aspect of ichthyotoxicity in fish-killing phytoplankton species (Dorantes-Aranda et al., 2013, 2015; Mardones et al., 2015).
Despite the evidence suggesting that HAB-derived ROS are harmful, chemical additions of ROS that represent concentrations expected during harmful blooms of H. akashiwo, C. marina and M. polykrikoides have been insufficient to completely account for toxic effects on fish and invertebrates (Twiner et al., 2001; Marshall et al., 2003; Woo et al., 2006; Tang and Gobler, 2009a). Such lines of evidence have been used as an argument against the potentially harmful effects of ROS during HABs.
Other biological interactions
Phytoplankton-derived extracellular ROS may shape other biological interactions, such as grazing, allelopathy, and viral infection. For example, similar lectin-receptor-binding processes have been implicated in the production of extracellular superoxide by phytoplankton (Oda et al., 1998) and the recognition and capture of phytoplankton prey by the microzooplankton species Oxyrrhis marina (Wootton et al., 2007). Thus, lectin-stimulated extracellular ROS production by phytoplankton has been proposed to play a role in grazing interactions (Martel, 2009). In fact, extracellular ROS production by Alexandrium spp. has been linked to the mortality of microzooplankton grazers (Flores et al., 2012). Extracellular ROS production by C. marina and other raphidophytes also modulates interactions with non-predatory organisms, such as the bacterium Vibrio alginolyticus, by inhibiting its growth in an antioxidant-dependent manner (Oda et al., 1992b, 1997; Kim et al., 1999b). Furthermore, viral infection of the cosmopolitan phytoplankton species Emiliania huxleyi is associated with elevated levels of intracellular ROS and extracellular hydrogen peroxide, although the mechanisms and role(s) of this ROS production are not well understood (Evans et al., 2006).
Growth
The production of extracellular ROS by a broad diversity of phytoplankton under optimal growth conditions (Fig. 1) suggests that ROS may serve a role in the baseline physiology of these microorganisms. In particular, ROS production may have important consequences for cellular physiology, viability and growth. For example, the removal of superoxide and hydrogen peroxide through the addition of exogenous SOD and catalase, respectively, inhibits the growth of C. marina and changes its cell morphology from spindle to round-shaped (Oda et al., 1995). This morphological shift is also observed in C. antiqua when superoxide is removed via oxidation by an electrode (Tanaka et al., 1992). These results suggest that extracellular ROS play an essential role in the vitality and survival of Chattonella spp. Hansel et al. (2016) recently summarized several lines of evidence suggesting a role for extracellular superoxide production in growth regulation by a number of different microbial species. For example, extracellular superoxide is an autocrine growth promoter in other microorganisms such as Saccharomyces cerevisiae, Escherichia coli and Salmonella typhimurium. In these microorganisms, the transition to stationary phase requires a decrease in superoxide concentrations, which is accomplished by cell surface SODs (Saran, 2003; Buetler et al., 2004). Observations demonstrating that biomass-normalized extracellular superoxide production by C. marina is highest in exponential phase and lower in stationary phase (Oda et al., 1995; Kawano et al., 1996; Garg et al., 2007) are consistent with the positive relationship between superoxide and growth. Similar growth phase-dependent trends in superoxide production have been observed for other raphidophyte species such as C. antiqua and H. akashiwo (Skeen et al., 2004; Portune et al., 2010), as well as the dinoflagellate M. polykrikoides (Kim et al., 1999a).
Many HAB species modulate cell-normalized ROS production rates in an inverse relationship with cell density (Yang et al., 1995; Twiner and Trick, 2000; Kim et al., 2002; Marshall et al., 2005b), consistent with a potential signaling role for ROS, as recently proposed for the marine cyanobacterium Trichodesmium spp. (Hansel et al., 2016). In fact, diluted C. marina cultures up-regulate superoxide production rates within timescales of one hour, suggesting that superoxide may act as a dynamic signal to relay information on bloom density (Marshall et al., 2005b), which could potentially be related to growth regulation, as discussed above. ROS signaling does not necessarily imply the production of superoxide by one cell and the detection of that same superoxide anion by another cell, however. Such a process may be unlikely, given the typical lifetime of superoxide in natural waters (Table I). Rather, in the absence of other ROS scavengers, extracellular superoxide produced by one cell may react with cell surface constituents, such as thiols (Winterbourn and Hampton, 2008) and/or lipids (Saran, 2003) on the surface of the same cell, thus generating an endogenous redox signaling cascade.
Iron acquisition
Besides a potential role as an autocrine growth signal, extracellular ROS production may promote the growth of phytoplankton by more indirect, alternative means via metal nutrient acquisition. For example, superoxide is a potent oxidant and reductant of iron. Under some environmental conditions, extracellular superoxide can increase the bioavailability of iron, especially when this micronutrient is growth-limiting (Rose, 2012). In fact, extracellular superoxide production has been proposed as a strategy for iron acquisition by Lyngbya majuscula (Rose et al., 2005), T. erythraeum (Roe and Barbeau, 2014), M. aeruginosa (Fujii et al., 2011) and C. marina (Garg et al., 2007; Liu et al., 2007), although superoxide had no effect on iron uptake by Thalassiosira spp. (Kustka et al., 2005) or Chlorella kessleri (Middlemiss et al., 2001). Ultimately, the ability of superoxide to facilitate iron acquisition depends on prevailing biogeochemical conditions, which dictate the effect of this ROS on the steady-state concentrations of biologically labile mononuclear inorganic complexes of iron (II) and iron (III) (Rose, 2012). The reader is referred to Rose (2012) for a detailed review on the potential role of extracellular superoxide in microbial iron acquisition.
FUTURE RESEARCH DIRECTIONS
In aquatic environments, ROS concentrations can be low or undetectable due to rapid reactions with carbon and metals. ROS therefore “invisibly” drive major transformations of key elemental cycles via cryptic biogeochemistry (Hansel et al., 2015). Similarly, we suggest that ROS may play a cryptic role in biological interactions. For example, previous work has revealed that antioxidants can alleviate the toxic effects of ROS-producing HABs (Oda et al., 1992b, Yang et al., 1995; Oda et al., 1,997; Kim et al., 1999a, 1999b; Tang and Gobler, 2009a, 2009b, 2010; Flores et al., 2012), yet representative HAB-derived ROS concentrations are insufficient to induce toxicity (Twiner et al., 2001; Marshall et al., 2003; Woo et al., 2006; Tang and Gobler, 2009a). However, organisms may experience higher doses of ROS than suggested by steady-state ROS concentrations, depending on the underlying kinetics and pathways of ROS production and degradation. For example, ROS concentrations represent the balance of ROS production and decay. Low concentrations of ROS may, therefore, disguise rapid production rates, if decay rates are also high. Depending on the identity and efficiency of ROS-degrading constituents (e.g. PUFAs), high ROS production rates by natural HABs could potentially be toxic without necessarily leading to elevated concentrations of ROS in the surrounding environment.
In order to test this “cryptic interactions” hypothesis, ROS fluxes and concentrations should be assessed together, particularly in natural systems. For example, the majority of phytoplankton-ROS research has been conducted using controlled laboratory experiments with model cultures. Yet much remains to be discovered about phytoplankton-driven ROS dynamics in natural aquatic environments. In fact, the scarcity of ROS measurements during natural HABs makes it difficult to assess whether ROS levels reach toxicity thresholds during these events. In addition to cryptic toxicity, the potential (cryptic) role of phytoplankton-derived extracellular ROS in other biological interactions such as grazing, allelopathy and viral infection should be considered. By potentially mediating biological interactions within and across trophic levels in these ways, ROS may modify food web dynamics and shape aquatic ecosystem health and large-scale biogeochemical cycling via pathways that remain to be discovered.
The inverse dependence of extracellular ROS production rates on phytoplankton cell density and the potential role of extracellular ROS in phytoplankton growth suggest that elevated ROS concentrations and production rates could be expected in aquatic systems leading up to phytoplankton blooms. For instance, in Lake Erie, total hydrogen peroxide concentrations (attributed to biological production) peaked approximately 2 weeks before the appearance of Microcystis spp. blooms and the occurrence of maximum microcystin levels during summer 2014 and 2015 (Cory et al., 2016). We suggest that a possible link between phytoplankton bloom formation and elevated biological ROS production could be taxonomically widespread, which has implications for the evaluation and development of ROS-based strategies for predicting algal blooms. In contrast to cryptic cycling, this bloom prediction hypothesis suggests that biological ROS production may peak before a phytoplankton bloom, leading to ROS concentrations and production rates, far from being hidden or invisible, which can be used as a bloom-forecasting index. Given the short lifetimes of ROS (Table I), such a prediction tool could be responsive to ecosystem variables over relatively short timescales, and thus provide a high temporal-resolution indicator (~days to weeks) of potential interest to natural resource managers.
ROS measurements can be technically complex, especially in systems with high organic matter and metal loading. However, future research on phytoplankton-derived ROS is tractable in a range of environments, given currently available geochemical tools for the high sensitivity detection of ROS concentrations and dynamics in complex natural systems (Rose et al., 2008a; Godrant et al., 2009; Heller and Croot, 2010; Yamaguchi et al., 2010; Miller et al., 2011; Burns et al., 2012; Marsico et al., 2015; King et al., 2016; Roe et al., 2016; Zhang et al., 2016b). For a review of ROS quantification methods and technological advancements, the reader is referred to papers by Bartosz (2006), Soh (2006) and Burns et al. (2012). In addition to direct ROS measurements, future advances in the understanding of subcellular ROS production mechanisms will lead to new insights on phytoplankton-derived ROS. A variety of methods have been utilized to characterize the molecular basis of ROS production in microorganisms, including gene knockouts (Anderson et al., 2016), as well as chemical activity assays combined with peptide fingerprinting (Andeer et al., 2015) and immunoblotting and immunofluorescence (Kim et al., 2000a). Tracking molecular targets for ROS production in the field could provide critical information on natural ROS dynamics, especially if ROS cycling is rapid and difficult to detect by direct geochemical means.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Dee King for her assistance with literature searches and reference indexing and Lee Ann DeLeo for her help with the design and preparation of graphics for this paper.
FUNDING
This work was supported by a Junior Faculty Seed Grant from the University of Georgia Research Foundation (J.M.D.) and a National Science Foundation Graduate Research Fellowship (S.P.).
REFERENCES
- Aguirre J., Rios-Momberg M., Hewitt D. and Hansberg W. (2005) Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol., 13, 111–118. [DOI] [PubMed] [Google Scholar]
- Andeer P. F., Learman D. R., McIlvin M., Dunn J. A. and Hansel C. M. (2015) Extracellular haem peroxidases mediate Mn(II) oxidation in a marine Roseobacter bacterium via superoxide production. Environ. Microbiol., 17, 3925–3936. [DOI] [PubMed] [Google Scholar]
- Anderson A., Bothwell J. H., Laohavisit A., Smith A. G. and Davies J. M. (2011) NOX or not? Evidence for algal NADPH oxidases. Trends Plant Sci., 16, 580–581. [DOI] [PubMed] [Google Scholar]
- Anderson A., Laohavisit A., Blaby I. K., Bombelli P., Howe C. J., Merchant S. S., Davies J. M. and Smith A. G. (2016) Exploiting algal NADPH oxidase for biophotovoltaic energy. Plant Biotechnol. J., 14, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armoza-Zvuloni R., Schneider A., Sher D. and Shaked Y. (2016) Rapid hydrogen peroxide release from the coral Stylophora pistillata during feeding and in response to chemical and physical stimuli. Sci. Rep., 6, 21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzul G., Bodennec G., Gentien P., Bornens P. and Crassous M.-P. (1998) The effect of dissolved oxygen on the haemolytic property of Gymnodinium ichthyotoxins In Reguera B., Blanco J., Fernandez M. L. and Wyatt T. (eds), Harmful Algae. Xunta de Calicia and Intergovernmental Oceanographic Commision of UNESCO, Vigo, Spain, pp. 611–614. [Google Scholar]
- Asada K. (1999) The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol., 50, 601–639. [DOI] [PubMed] [Google Scholar]
- Asada K. (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol., 141, 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. (1999) NADPH oxidase: an update. Blood, 93, 1464–1476. [PubMed] [Google Scholar]
- Barbeau K. (2006) Photochemistry of organic iron(III) complexing ligands in oceanic systems. Photochem. Photobiol., 82, 1505–1516. [DOI] [PubMed] [Google Scholar]
- Bartosz G. (2006) Use of spectroscopic probes for detection of reactive oxygen species. Clin. Chim. Acta, 368, 53–76. [DOI] [PubMed] [Google Scholar]
- Bedard K., Lardy B. and Krause K. H. (2007) NOX family NADPH oxidases: not just in mammals. Biochimie, 89, 1107–1112. [DOI] [PubMed] [Google Scholar]
- Buetler T. M., Krauskopf A. and Ruegg U. T. (2004) Role of superoxide as a signaling molecule. News Physiol. Sci., 19, 120–123. [DOI] [PubMed] [Google Scholar]
- Burns J. M., Cooper W. J., Ferry J. L., King D. W., DiMento B. P., McNeill K., Miller C. J., Miller W. L. et al. (2012) Methods for reactive oxygen species (ROS) detection in aqueous environments. Aquat. Sci., 74, 683–734. [Google Scholar]
- Cho K., Kasaoka T., Ueno M., Basti L., Yamasaki Y., Kim D. and Oda T. (2017) Haemolytic activity and reactive oxygen species production of four harmful algal bloom species. Eur. J. Phycol., 52, 311–319. [Google Scholar]
- Cho K., Sakamoto J., Noda T., Nishiguchi T., Ueno M., Yamasaki Y., Yagi M., Kim D. et al. (2016) Comparative studies on the fish-killing activities of Chattonella marina isolated in 1985 and Chattonella antiqua isolated in 2010, and their possible toxic factors. Biosci. Biotechnol. Biochem., 80, 811–817. [DOI] [PubMed] [Google Scholar]
- Cory R. M., Davis T. W., Dick G. J., Johengen T., Denef V. J., Berry M. A., Page S. E., Watson S. B. et al. (2016) Seasonal dynamics in dissolved organic matter, hydrogen peroxide, and cyanobacterial blooms in Lake Erie. Front. Mar. Sci., 3, 54. [Google Scholar]
- del Rio L. A. and Lopez-Huertas E. (2016) ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol., 57, 1364–1376. [DOI] [PubMed] [Google Scholar]
- Diaz J. M., Hansel C. M., Voelker B. M., Mendes C. M., Andeer P. F. and Zhang T. (2013) Widespread production of extracellular superoxide by heterotrophic bacteria. Science, 340, 1223–1226. [DOI] [PubMed] [Google Scholar]
- Dixon T. C., Vermilyea A. W., Scott D. T. and Voelker B. M. (2013) Hydrogen peroxide dynamics in an agricultural headwater stream: evidence for significant nonphotochemical production. Limnol. Oceanogr., 58, 2133–2144. [Google Scholar]
- Dorantes-Aranda J. J., Nichols P. D., Waite T. D. and Hallegraeff G. M. (2013) Strain variability in fatty acid composition of Chattonella marina (Raphidophyceae) and its relation to differing ichthyotoxicity toward rainbow trout gill cells. J. Phycol., 49, 427–438. [DOI] [PubMed] [Google Scholar]
- Dorantes-Aranda J. J., Seger A., Mardones J. I., Nichols P. D. and Hallegraeff G. M. (2015) Progress in understanding algal bloom-mediated fish kills: the role of superoxide radicals, phycotoxins and fatty acids. PLoS One, 10, e0133549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C., Malin G., Mills G. P. and Wilson W. H. (2006) Viral infection of Emiliania huxleyi (Prymnesiophyceae) leads to elevated production of reactive oxygen species. J. Phycol., 42, 1040–1047. [Google Scholar]
- Flores H. S., Wikfors G. H. and Dam H. G. (2012) Reactive oxygen species are linked to the toxicity of the dinoflagellate Alexandrium spp. to protists. Aquat. Microb. Ecol., 66, 199–209. [Google Scholar]
- Foyer C. H., Bloom A. J., Queval G. and Noctor G. (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu. Rev. Plant Biol., 60, 455–484. [DOI] [PubMed] [Google Scholar]
- Foyer C. H. and Noctor G. (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell, 17, 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes C., Clément A. and Aguilera A. (2006) Summer Alexandrium catenella bloom and the impact on fish farming, in the XI Aysén region. In Chile12th International Conference on Harmful Algae. International Society for the Study of Harmful Algae (ISSHA), Copenhagen, Denmark.
- Fujii M., Dang T. C., Rose A. L., Omura T. and Waite T. D. (2011) Effect of light on iron uptake by the freshwater cyanobacterium Microcystis aeruginosa. Environ. Sci. Technol., 45, 1391–1398. [DOI] [PubMed] [Google Scholar]
- Garg S., Rose A. L., Godrant A. and Waite T. D. (2007) Iron uptake by the ichthyotoxic Chattonella marina (Raphidophyceae): impact of superoxide generation. J. Phycol., 43, 978–991. [Google Scholar]
- Godrant A., Rose A. L., Sarthou G. and Waite T. D. (2009) New method for the determination of extracellular production of superoxide by marine phytoplankton using the chemiluminescence probes MCLA and red-CLA. Limnol. Oceanogr. Methods, 7, 682–692. [Google Scholar]
- Griffith A. W. and Gobler C. J. (2016) Temperature controls the toxicity of the ichthyotoxic dinoflagellate Cochlodinium polykrikoides. Mar. Ecol. Prog. Ser., 545, 63–76. [Google Scholar]
- Hallegraeff G. M., Munday B. L., Baden D. G. and Whitney P. L. (1998) Chattonella marina raphidophyte bloom associated with mortality of cultured bluefin tuna (Thunnus maccoyii) in South Australia In Reguera B., Blanco J., Fernandez M. L. and Wyatt T. (eds), Harmful Algae. Galicia and IOC of UNESCO, Spain, pp. 93–96. [Google Scholar]
- Hansard S. P., Vermilyea A. W. and Voelker B. M. (2010) Measurements of superoxide radical concentration and decay kinetics in the Gulf of Alaska. Deep Sea Res. Part I Oceanogr. Res. Pap., 57, 1111–1119. [Google Scholar]
- Hansel C. M., Buchwald C., Diaz J. M., Ossolinski J. E., Dyhrman S. T. and Van Mooy B. A. S. (2016) Dynamics of extracellular superoxide production by Trichodesmium colonies from the Sargasso Sea. Limnol. Oceanogr., 61, 1188–1200. [Google Scholar]
- Hansel C. M., Ferdelman T. G. and Tebo B. M. (2015) Cryptic cross-linkages among biogeochemical cycles: novel insights from reactive intermediates. Elements, 11, 409–414. [Google Scholar]
- Heller M. I. and Croot P. L. (2010) Application of a superoxide (O2-) thermal source (SOTS-1) for the determination and calibration of O2- fluxes in seawater. Anal. Chim. Acta, 667, 1–13. [DOI] [PubMed] [Google Scholar]
- Hervé C., Tonon T., Collén J., Corre E. and Boyen C. (2005) NADPH oxidases in Eukaryotes: red algae provide new hints! Curr. Genet., 49, 190–204. [DOI] [PubMed] [Google Scholar]
- Ishimatsu A., Oda T., Yoshida M. and Ozaki M. (1996) Oxygen radicals are probably involved in the mortality of yellowtail by Chattonella marina. Fisher. Sci., 62, 836–837. [Google Scholar]
- Jenkinson I. R. and Arzul G. (2001) Mitigation by cysteine compounds of rheotoxicity, cytotoxicity and fish mortality caused by the dinoflagellates, Gymnodinium mikimotoi and G. cf. maguelonnense In Hallegraeff G., Bolch C. J. S., Blackburn S. I. and Lewis R. (eds), Harmful Algal Blooms 2000. UNESCO, Paris, pp. 461–464. [Google Scholar]
- Kawano I., Oda T., Ishimatsu A. and Muramatsu T. (1996) Inhibitory effect of the iron chelator Desferrioxamine (Desferal) on the generation of activated oxygen species by Chattonella marina. Mar. Biol., 126, 765–771. [Google Scholar]
- Kim C. S., Lee S. G., Lee C. K., Kim H. G. and Jung J. (1999. a) Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. J. Plankton Res., 21, 2105–2115. [Google Scholar]
- Kim D., Nakamura A., Okamoto T., Komatsu N., Oda T., Iida T., Ishimatsu A. and Muramatsu T. (2000. a) Mechanism of superoxide anion generation in the toxic red tide phytoplankton Chattonella marina: possible involvement of NAD(P)H oxidase. Biochim. Biophys. Acta, 1524, 220–227. [DOI] [PubMed] [Google Scholar]
- Kim D., Nakamura A., Okamoto T., Komatsu N., Oda T., Ishimatsu A. and Muramatsu T. (1999. b) Toxic potential of the raphidophyte Olisthodiscus luteus: mediation by reactive oxygen species. J. Plankton Res., 21, 1017–1027. [Google Scholar]
- Kim D., Nakashima T., Matsuyama Y., Niwano Y., Yamaguchi K. and Oda T. (2007) Presence of the distinct systems responsible for superoxide anion and hydrogen peroxide generation in red tide phytoplankton Chattonella marina and Chattonella ovata. J. Plankton Res., 29, 241–247. [Google Scholar]
- Kim D. and Oda T. (2010) Possible factors responsible for the fish-killing mechanisms of the red tide phytoplankton, Chattonella marina and Cochlodinium polykrikoides In Ishimatsu A. and Lie H.-J. (eds), Coastal Environmental and Ecosystem Issues of the East China Sea. TERRAPUB and Nagasaki University, Tokyo, Japan, pp. 245–268. [Google Scholar]
- Kim D., Oda T., Ishimatsu A. and Muramatsu T. (2000. b) Galacturonic-acid-induced increase of superoxide production in red tide phytoplankton Chattonella marina and Heterosigma akashiwo. Biosci. Biotechnol. Biochem., 64, 911–914. [DOI] [PubMed] [Google Scholar]
- Kim D., Oda T., Muramatsu T., Kim D., Matsuyama Y. and Honjo T. (2002) Possible factors responsible for the toxicity of Cochlodinium polykrikoides, a red tide phytoplankton. Comp. Biochem. Physiol. C Toxicol. Pharmacol., 132, 415–423. [DOI] [PubMed] [Google Scholar]
- Kim D., Watanabe M., Nakayasu Y. and Kohata K. (2004) Production of superoxide anion and hydrogen peroxide associated with cell growth of Chattonella antiqua. Aquat. Microb. Ecol., 35, 57–64. [Google Scholar]
- King D. W., Berger E., Helm Z., Irish E. and Mopper K. (2016) Measurement of antioxidant activity toward superoxide in natural waters. Front. Mar. Sci., 3, 217. [Google Scholar]
- Korshunov S. S. and Imlay J. A. (2002) A potential role for periplasmic superoxide dismutase in blocking the penetration of external superoxide into the cytosol of Gram-negative bacteria. Mol. Microbiol., 43, 95–106. [DOI] [PubMed] [Google Scholar]
- Kustka A. B., Shaked Y., Milligan A. J., King D. W. and Morel F. M. M. (2005) Extracellular production of superoxide by marine diatoms: Contrasting effects on iron redox chemistry and bioavailability. Limnol. Oceanogr., 50, 1172–1180. [Google Scholar]
- Learman D. R., Voelker B. M., Vazquez-Rodriguez A. I. and Hansel C. M. (2011) Formation of manganese oxides by bacterially generated superoxide. Nat. Geosci., 4, 95–98. [Google Scholar]
- Lesser M. P. (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu. Rev. Physiol., 68, 253–278. [DOI] [PubMed] [Google Scholar]
- Li X., Liu T., Wang K. and Waite T. D. (2015) Light-induced extracellular electron transport by the marine raphidophyte Chattonella marina. Environ. Sci. Technol., 49, 1392–1399. [DOI] [PubMed] [Google Scholar]
- Liu W., Au D. W. T., Anderson D. M., Lam P. K. S. and Wu R. S. S. (2007) Effects of nutrients, salinity, pH and light: dark cycle on the production of reactive oxygen species in the alga Chattonella marina. J. Exp. Mar. Bio. Ecol., 346, 76–86. [Google Scholar]
- Mardones J. I., Clément A., Rojas X. and Aparicio C. (2010) Alexandrium catenella during 2009 in Chilean waters, and recent expansion to coastal ocean. Harmful Algae, 41, 8–9. [Google Scholar]
- Mardones J. I., Dorantes-Aranda J. J., Nichols P. D. and Hallegraeff G. M. (2015) Fish gill damage by the dinoflagellate Alexandrium catenella from Chilean fjords: synergistic action of ROS and PUFA. Harmful Algae, 49, 40–49. [Google Scholar]
- Marshall J. A., de Salas M., Oda T. and Hallegraeff G. (2005. a) Superoxide production by marine microalgae: I. Survey of 37 species from 6 classes. Mar. Biol., 147, 533–540. [Google Scholar]
- Marshall J. A., Hovenden M., Oda T. and Hallegraeff G. M. (2002) Photosynthesis does influence superoxide production in the ichthyotoxic alga Chattonella marina (Raphidophyceae). J. Plankton Res., 24, 1231–1236. [Google Scholar]
- Marshall J. A., Nichols P. D., Hamilton B., Lewis R. J. and Hallegraeff G. M. (2003) Ichthyotoxicity of Chattonella marina (Raphidophyceae) to damselfish (Acanthochromis polycanthus): the synergistic role of reactive oxygen species and free fatty acids. Harmful Algae, 2, 273–281. [Google Scholar]
- Marshall J. A., Ross T., Pyecroft S. and Hallegraeff G. (2005. b) Superoxide production by marine microalgae: II. towards understanding ecological consequences and possible functions. Mar. Biol., 147, 541–549. [Google Scholar]
- Marsico R. M., Schneider R. J., Voelker B. M., Zhang T., Diaz J. M., Hansel C. M. and Ushijima S. (2015) Spatial and temporal variability of widespread dark production and decay of hydrogen peroxide in freshwater. Aquat. Sci., 77, 523–533. [Google Scholar]
- Martel C. M. (2009) Conceptual bases for prey biorecognition and feeding selectivity in the microplanktonic marine phagotroph Oxyrrhis marina. Microb. Ecol., 57, 589–597. [DOI] [PubMed] [Google Scholar]
- McCord J. M. and Fridovich I. (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem., 244, 6049–6055. [PubMed] [Google Scholar]
- Middlemiss J. K., Anderson A. M., Stratilo C. W. and Weger H. G. (2001) Oxygen consumption associated with ferric reductase activity and iron uptake by iron-limited cells of Chlorella kessleri (Chlorophyceae). J. Phycol., 37, 393–399. [Google Scholar]
- Miller C. J., Rose A. L. and Waite T. D. (2011) Phthalhydrazide chemiluminescence method for determination of hydroxyl radical production: modifications and adaptations for use in natural systems. Anal. Chem., 83, 261–268. [DOI] [PubMed] [Google Scholar]
- Milne A., Davey M. S., Worsfold P. J., Achterberg E. P. and Taylor A. R. (2009) Real-time detection of reactive oxygen species generation by marine phytoplankton using flow injection-chemiluminescence. Limnol. Oceanogr. Methods, 7, 706–715. [Google Scholar]
- Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V. B., Vandepoele K., Gollery M., Shulaev V. et al. (2011) ROS signaling: the new wave? Trends Plant Sci., 16, 300–309. [DOI] [PubMed] [Google Scholar]
- Møller I. M. (2001) Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol., 52, 561–591. [DOI] [PubMed] [Google Scholar]
- Mooney B. D., Dorantes-Aranda J. J., Place A. R. and Hallegraeff G. M. (2011) Ichthyotoxicity of gymnodinioid dinoflagellates: PUFA and superoxide effects in sheepshead minnow larvae and rainbow trout gill cells. Mar. Ecol. Prog. Ser., 426, 213–224. [Google Scholar]
- Murphy M. P. (2009) How mitochondria produce reactive oxygen species. Biochem. J., 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A., Okamoto T., Komatsu N., Ooka S., Oda T., Ishimatsu A. and Muramatsu T. (1998) Fish mucus stimulates the generation of superoxide anion by Chattonella marina and Heterosigma akashiwo. Fisher. Sci., 64, 866–869. [Google Scholar]
- Noctor G., Veljovic-Jovanovic S., Driscoll S., Novitskaya L. and Foyer C. H. (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann. Bot. (Lond.), 89, 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T., Akaike T., Sato K., Ishimatsu A., Takeshita S., Muramatsu T. and Maeda H. (1992. a) Hydroxyl radical generation by red tide algae. Arch. Biochem. Biophys., 294, 38–43. [DOI] [PubMed] [Google Scholar]
- Oda T., Ishimatsu A., Shimada M., Takeshita S. and Muramatsu T. (1992. b) Oxygen-radical-mediated toxic effects of the red tide flagellate Chattonella marina on Vibrio alginolyticus. Mar. Biol., 112, 505–509. [Google Scholar]
- Oda T., Moritomi J., Kawano I., Hamaguchi S., Ishimatsu A. and Muramatsu T. (1995) Catalase-induced and superoxide dismutase-induced morphological changes and growth inhibition in the red tide phytoplankton Chattonella marina. Biosci. Biotechnol. Biochem., 59, 2044–2048. [Google Scholar]
- Oda T., Nakamura A., Okamoto T., Ishimatsu A. and Muramatsu T. (1998) Lectin-induced enhancement of superoxide anion production by red tide phytoplankton. Mar. Biol., 131, 383–390. [Google Scholar]
- Oda T., Nakamura A., Shikayama M., Kawano I., Ishimatsu A. and Muramatsu T. (1997) Generation of reactive oxygen species by raphidophycean phytoplankton. Biosci. Biotechnol. Biochem., 61, 1658–1662. [DOI] [PubMed] [Google Scholar]
- Okaichi T. (1997) Red tides in the Seto Inland Sea In Okaichi T. and Yanagi T. (eds), Sustainable Development in the Seto Inland Sea, Japan—from the Viewpoint of Fisheries. Terra Scientific Publishing Co., Tokyo, pp. 251–304. [Google Scholar]
- Palenik B., Kieber D. J. and Morel F. M. M. (1988) Dissolved organic nitrogen use by phytoplankton: the role of cell-surface enzymes. Biol. Oceanogr., 6, 347–354. [Google Scholar]
- Palenik B., Zafiriou O. C. and Morel F. M. M. (1987) Hydrogen peroxide production by a marine phytoplankter. Limnol. Oceanogr., 32, 1365–1369. [Google Scholar]
- Portune K. J., Cary S. C. and Warner M. E. (2010) Antioxidant enzyme response and reactive oxygen species production in marine raphidophytes. J. Phycol., 46, 1161–1171. [Google Scholar]
- Pospíšil P. (2014) The role of metals in production and scavenging of reactive oxygen species in photosystem II. Plant Cell Physiol., 55, 1224–1232. [DOI] [PubMed] [Google Scholar]
- Pullin M. J., Bertilsson S., Goldstone J. V. and Voelker B. M. (2004) Effects of sunlight and hydroxyl radical on dissolved organic matter: Bacterial growth efficiency and production of carboxylic acids and other substrates. Limnol. Oceanogr., 49, 2011–2022. [Google Scholar]
- Roe K. L. and Barbeau K. A. (2014) Uptake mechanisms for inorganic iron and ferric citrate in Trichodesmium erythraeum IMS101. Metallomics, 6, 2042–2051. [DOI] [PubMed] [Google Scholar]
- Roe K. L., Schneider R. J., Hansel C. M. and Voelker B. M. (2016) Measurement of dark, particle-generated superoxide and hydrogen peroxide production and decay in the subtropical and temperate North Pacific Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap., 107, 59–69. [Google Scholar]
- Rose A. L. (2012) The influence of extracellular superoxide on iron redox chemistry and bioavailability to aquatic microorganisms. Front. Microbiol., 3, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A. L., Godrant A., Furnas M. and Waite T. D. (2010) Dynamics of nonphotochemical superoxide production and decay in the Great Barrier Reef lagoon. Limnol. Oceanogr., 55, 1521–1536. [Google Scholar]
- Rose A. L., Moffett J. W. and Waite T. D. (2008. a) Determination of superoxide in seawater using 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo[1,2-a]pyrazin-3(7H)-one chemiluminescence. Anal. Chem., 80, 1215–1227. [DOI] [PubMed] [Google Scholar]
- Rose A. L., Salmon T. P., Lukondeh T., Neilan B. A. and Waite T. D. (2005) Use of superoxide as an electron shuttle for iron acquisition by the marine cyanobacterium Lyngbya majuscula. Environ. Sci. Technol., 39, 3708–3715. [DOI] [PubMed] [Google Scholar]
- Rose A. L., Webb E. A., Waite T. D. and Moffett J. W. (2008. b) Measurement and implications of nonphotochemically generated superoxide in the equatorial Pacific Ocean. Environ. Sci. Technol., 42, 2387–2393. [DOI] [PubMed] [Google Scholar]
- Rusak S. A., Peake B. M., Richard L. E., Nodder S. D. and Cooper W. J. (2011) Distributions of hydrogen peroxide and superoxide in seawater east of New Zealand. Mar. Chem., 127, 155–169. [Google Scholar]
- Saragosti E., Tchernov D., Katsir A. and Shaked Y. (2010) Extracellular production and degradation of superoxide in the coral Stylophora pistillata and cultured Symbiodinium. PLoS One, 5, e12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saran M. (2003) To what end does nature produce superoxide? NADPH oxidase as an autocrine modifier of membrane phospholipids generating paracrine lipid messengers. Free Radic. Res., 37, 1045–1059. [DOI] [PubMed] [Google Scholar]
- Scheibe R. and Dietz K. J. (2012) Reduction-oxidation network for flexible adjustment of cellular metabolism in photoautotrophic cells. Plant Cell Environ., 35, 202–216. [DOI] [PubMed] [Google Scholar]
- Schmitt F. J., Renger G., Friedrich T., Kreslavski V. D., Zharmukhamedov S. K., Los D. A., Kuznetsov V. V. and Allakhverdiev S. I. (2014) Reactive oxygen species: Re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochim. Biophys. Acta, 1837, 835–848. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Roe K. L., Hansel C. M. and Voelker B. M. (2016) Species-level variability in extracellular production rates of reactive oxygen species by diatoms. Front. Chem., 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. M. (1991) The control of oxidant stress at fertilization. Science, 252, 533–536. [DOI] [PubMed] [Google Scholar]
- Shimada M., Kawamoto S., Nakatsuka Y. and Watanabe M. (1993) Localization of superoxide anion in the red tide alga Chattonella antiqua. J. Histochem. Cytochem., 41, 507–511. [DOI] [PubMed] [Google Scholar]
- Siciliano S. D., O’Driscoll N. J. and Lean D. R. S. (2002) Microbial reduction and oxidation of mercury in freshwater lakes. Environ. Sci. Technol., 36, 3064–3068. [DOI] [PubMed] [Google Scholar]
- Skeen A. R., Tomas C. R. and Cooper W. J. (2004) The production of hydrogen peroxide by Heterosigma akashiwo under varying N:P ratios In Steidinger K. A., Landsberg J. H., Tomas C. R. and Vargo G. A. (eds), Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO, St. Petersburg, Florida, pp. 77–79. [Google Scholar]
- Soh N. (2006) Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal. Bioanal. Chem., 386, 532–543. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Muto Y. and Shimada M. (1994) Generation of superoxide anion radicals by the marine phytoplankton organism, Chattonella antiqua. J. Plankton Res., 16, 161–169. [Google Scholar]
- Tanaka S., Yoshimatsu S. and Shimada M. (1992) Generation of superoxide anions by Chattonella antiqua: possible causes for fish death caused by ‘Red Tide’. Experientia, 48, 888–890. [Google Scholar]
- Tang Y. Z. and Gobler C. J. (2009. a) Characterization of the toxicity of Cochlodinium polykrikoides isolates from Northeast US estuaries to finfish and shellfish. Harmful Algae, 8, 454–462. [Google Scholar]
- Tang Y. Z. and Gobler C. J. (2009. b) Cochlodinium polykrikoides blooms and clonal isolates from the northwest Atlantic coast cause rapid mortality in larvae of multiple bivalve species. Mar. Biol., 156, 2601–2611. [Google Scholar]
- Tang Y. Z. and Gobler C. J. (2010) Allelopathic effects of Cochlodinium polykrikoides isolates and blooms from the estuaries of Long Island, New York, on co-occurring phytoplankton. Mar. Ecol. Prog. Ser., 406, 19–31. [Google Scholar]
- Twiner M. J., Dixon S. J. and Trick C. G. (2001) Toxic effects of Heterosigma akashiwo do not appear to be mediated by hydrogen peroxide. Limnol. Oceanogr., 46, 1400–1405. [Google Scholar]
- Twiner M. J. and Trick C. G. (2000) Possible physiological mechanisms for production of hydrogen peroxide by the ichthyotoxic flagellate Heterosigma akashiwo. J. Plankton Res., 22, 1961–1975. [Google Scholar]
- Vermilyea A. W., Dixon T. C. and Voelker B. M. (2010) Use of H218O2 to measure absolute rates of dark H2O2 production in freshwater systems. Environ. Sci. Technol., 44, 3066–3072. [DOI] [PubMed] [Google Scholar]
- Waring J., Klenell M., Bechtold U., Underwood G. J. C. and Baker N. R. (2010) Light-induced responses of oxygen photoreduction, reactive oxygen species production and scavenging in two diatom species. J. Phycol., 46, 1206–1217. [Google Scholar]
- Weinberger F. (2007) Pathogen-induced defense and innate immunity in macroalgae. Biol. Bull., 213, 290–302. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. and Hampton M. B. (2008) Thiol chemistry and specificity in redox signaling. Free Radical Biol. Med., 45, 549–561. [DOI] [PubMed] [Google Scholar]
- Woo S. P. S., Liu W. H., Au D. W. T., Anderson D. M. and Wu R. S. S. (2006) Antioxidant responses and lipid peroxidation in gills and erythrocytes of fish (Rhabdosarga sarba) upon exposure to Chattonella marina and hydrogen peroxide: Implications on the cause of fish kills. J. Exp. Mar. Bio. Ecol., 336, 230–241. [Google Scholar]
- Wootton E. C., Zubkov M. V., Jones D. H., Jones R. H., Martel C. M., Thornton C. A. and Roberts E. C. (2007) Biochemical prey recognition by planktonic protozoa. Environ. Microbiol., 9, 216–222. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Kishikawa N., Ohyama K., Ohba Y., Kohno M., Masuda T., Takadate A., Nakashima K. et al. (2010) Evaluation of chemiluminescence reagents for selective detection of reactive oxygen species. Anal. Chim. Acta, 665, 74–78. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y., Kim D. I., Matsuyama Y., Oda T. and Honjo T. (2004) Production of superoxide anion and hydrogen peroxide by the red tide dinoflagellate Karenia mikimotoi. J. Biosci. Bioeng., 97, 212–215. [DOI] [PubMed] [Google Scholar]
- Yang C. Z., Albright L. J. and Yousif A. N. (1995) Oxygen-radical-mediated effects of the toxic phytoplankter Heterosigma carterae on juvenile rainbow trout Oncorhynchus mykiss. Dis. Aquat. Organ., 23, 101–108. [Google Scholar]
- Zhang T., Diaz J. M., Brighi C., Parsons R. J., McNally S., Apprill A. and Hansel C. M. (2016. a) Dark production of extracellular superoxide by the coral Porites astreoides and representative symbionts. Front. Mar. Sci., 3, 232. [Google Scholar]
- Zhang T., Hansel C. M., Voelker B. M. and Lamborg C. H. (2016. b) Extensive dark biological production of reactive oxygen species in brackish and freshwater ponds. Environ. Sci. Technol., 50, 2983–2993. [DOI] [PubMed] [Google Scholar]
- Zinser E. R. (2018) The microbial contribution to reactive oxygen species dynamics in marine ecosystems. Environ. Microbiol. Rep., Accepted article, doi:10.1111/1758-2229.12626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.