Abstract
1. Factors affecting the transfer of the non-metabolized, `actively transported' sugar, 3-O-methyl-D-glucose (3MG) across the small intestinal epithelium have been examined in vascularly perfused anuran intestine. Transfer has been studied during absorption in the steady state, and also during the period of transition from one steady state to another.
2. During the steady state, the rate of absorption of 3MG from the intestinal lumen is equal to the rate of appearance in the portal venous effluent; this rate of transfer is to a small, but significant, extent directly related to the rate of arterial perfusion. With phlorizin in the intestinal lumen, transfer across the epithelium is reduced to very low rates which are independent of the rate of vascular perfusion.
3. The apparent size of the tissue pool(s) of 3MG that have to be loaded to achieve the steady state rate of transfer are less than those that unload into the vascular bed after 3MG is removed from the intestinal lumen. This `up—down asymmetry' is abolished when phlorizin is present in the intestinal lumen during the unloading phase.
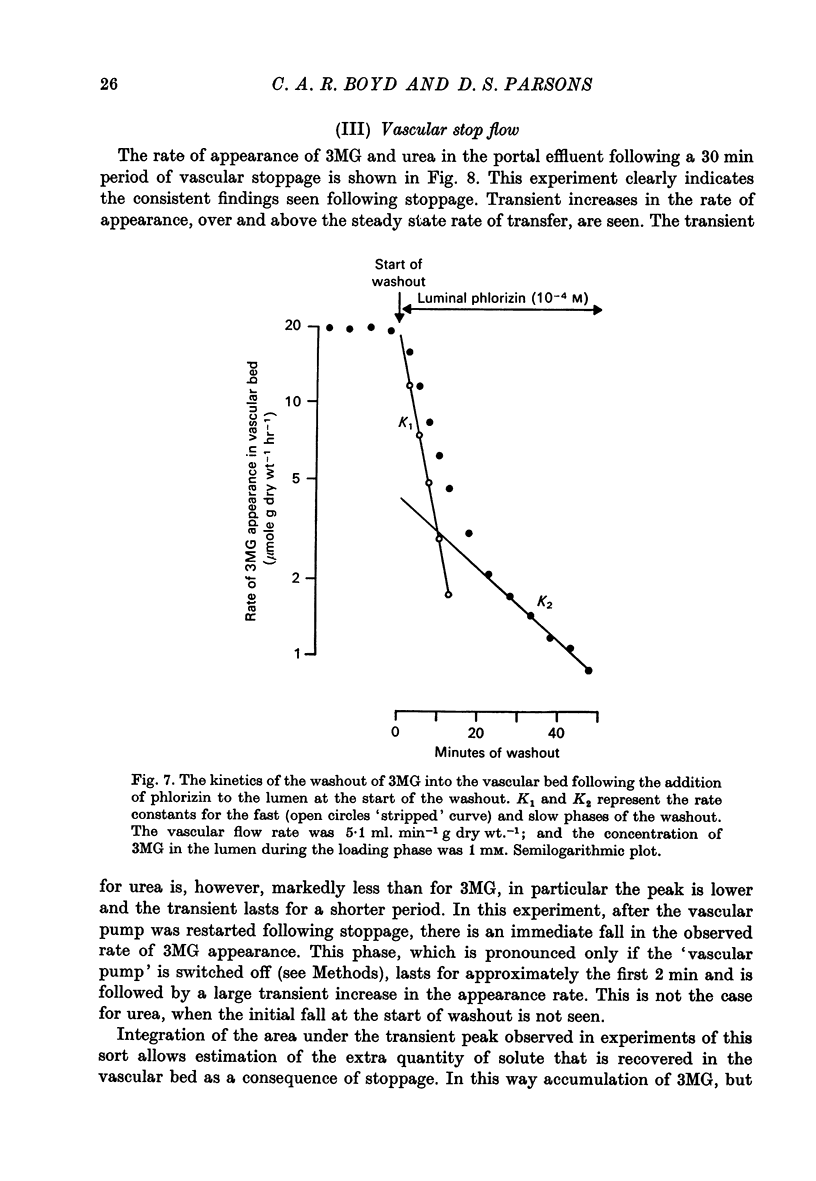
4. When 3MG is abruptly removed from the intestinal lumen after the tissue has been previously loaded with the sugar, the rate of washout into the vascular bed can be described by the sum of two exponential terms. The two terms differ in that the rate constant of the earlier `fast' term is sensitive to the rate of vascular perfusion, while the later, `slow', rate constant is insensitive to flow rate. The total quantity of 3MG that can be unloaded from the tissue into the portal venous effluent is decreased when phlorizin is present in the intestinal lumen during the unloading phase.
5. Absorption from the lumen of the anuran intestine continues while the mesenteric circulation is interrupted. An estimate of the concentration of 3MG during the period of vascular stoppage can be made from the quantity recovered in the portal venous effluent when vascular perfusion is reinstituted (`vascular stop-flow'). The extent of the accumulation depends upon the duration of the vascular stoppage and the presence of Na ions in the intestinal lumen is essential for accumulation to occur.
6. The findings are discussed in relation to the transfer of 3MG between various possible compartments in the tissue during absorption. Evidence is presented that a re-uptake of previously absorbed 3MG may occur across the brush border membrane. Such a recycling of 3MG across the epithelium implies that the apparent unidirectional fluxes measured across the epithelium between the bulk phase of the lumen and the blood may underestimate the size of fluxes across the epithelium at the cellular level.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN B., ZERAHN K. METHOD FOR NON-DESTRUCTIVE DETERMINATION OF THE SODIUM TRANSPORT POOL IN FROG SKIN WITH RADIOSODIUM. Acta Physiol Scand. 1963 Dec;59:319–329. doi: 10.1111/j.1748-1716.1963.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Boyd C. A., Cheeseman C. I., Parsons D. S. Amino acid movements across the wall of anuran small intestine perfused through the vascular bed. J Physiol. 1975 Sep;250(2):409–429. doi: 10.1113/jphysiol.1975.sp011062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C. A., Cheeseman C. I., Parsons D. S. Effects of sodium on solute transport between compartments in intestinal mucosal epithelium. Nature. 1975 Aug 28;256(5520):747–749. doi: 10.1038/256747a0. [DOI] [PubMed] [Google Scholar]
- Boyd C. A., Parsons D. S. Effects of substitution of Na on intestinal epithelial transport investigated by intermitten vascular perfusion [proceedings]. J Physiol. 1977 Mar;266(1):55P–56P. [PubMed] [Google Scholar]
- Boyd C. A., Parsons D. S. Proceedings: Movement of sugars between compartments of vascularly perfused intestine. J Physiol. 1976 Jun;258(1):12P–13P. [PubMed] [Google Scholar]
- Fuchs W., Gebhardt U., Lindemann B. Delayed voltage responses to fast changes of (Na) 0 at the outer surface of frog skin epithelium. Biomembranes. 1972;3:483–498. doi: 10.1007/978-1-4684-0961-1_33. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J Physiol. 1960 Sep;153:370–385. doi: 10.1113/jphysiol.1960.sp006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J. A Na+-independent, phloretin-sensitive monosaccharide transport system in isolated intestinal epithelial cells. J Membr Biol. 1975 Aug 11;23(1):57–76. doi: 10.1007/BF01870244. [DOI] [PubMed] [Google Scholar]
- Parsons D. S., Prichard J. S. A preparation of perfused small intestine for the study of absorption in amphibia. J Physiol. 1968 Sep;198(2):405–434. doi: 10.1113/jphysiol.1968.sp008614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M. Glucose transport in the kidney. Biochim Biophys Acta. 1976 Dec 14;457(3-4):303–351. doi: 10.1016/0304-4157(76)90003-4. [DOI] [PubMed] [Google Scholar]
- VOMHOF D. W., TUCKER T. C. THE SEPARATION OF SIMPLE SUGARS BY CELLULOSE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1965 Feb;17:300–306. doi: 10.1016/s0021-9673(00)99872-8. [DOI] [PubMed] [Google Scholar]
- White J. F. Intracellular potassium activities in Amphiuma small intestine. Am J Physiol. 1976 Oct;231(4):1214–1219. doi: 10.1152/ajplegacy.1976.231.4.1214. [DOI] [PubMed] [Google Scholar]
- Wilczyńska B. The dimensions of the mucosa and the structure of the alimentary canal in Rana temporaria L. and Rana arvalis Nilss. Bull Acad Pol Sci Biol. 1975;23(5):329–338. [PubMed] [Google Scholar]


