Abstract
Aims:
The primary study aim was to evaluate the feasibility and acceptability of cognitive-behavioral therapy (CBT) for opioid use disorder and chronic pain. The secondary aim was to examine its preliminary efficacy.
Methods:
In a 12-week pilot randomized clinical trial, 40 methadone-maintained patients were assigned to receive weekly manualized CBT (n=21) or Methadone Drug Counseling (MDC) to approximate usual drug counseling (n=19).
Results:
Twenty of 21 patients assigned to CBT and 18 of 19 assigned to MDC completed the pilot study. Mean (SD) sessions attended were 8.4 (2.9) for CBT (out of 12 possible) and 3.8 (1.1) for MDC (out of 4 possible); mean (SD) patient satisfaction ratings (scored on1–7 Likert-type scales) were 6.6 (0.5) for CBT and 6.0 (0.4) for MDC (p<.001). The proportion of patients abstinent during the baseline and each successive 4-week interval was higher for patients assigned to CBT than for those assigned to MDC [Wald χ2 (1) = 5.47, p=.02]; time effects (p=.69) and interaction effects between treatment condition and time (p=.10) were not significant. Rates of clinically significant change from baseline to end of treatment on pain interference (42.9% vs. 42.1%, [χ2 (1, N=40)=0.002, p=0.96]) did not differ significantly for patients assigned to CBT or MDC.
Conclusions:
We found support for the feasibility, acceptability, and preliminary efficacy of cognitive-behavioral therapy relative to standard drug counseling in promoting abstinence from nonmedical opioid use among patients with opioid use disorder and chronic pain. Overall, patients exhibited improved pain outcomes, but these improvements did not differ significantly by treatment condition.
Keywords: Treatment outcome, clinical trial, opioid-related disorders, chronic pain, methadone, cognitive therapy, counseling
1. Introduction
The management of co-occurring opioid use disorder and chronic pain is challenging (Dhingra et al., 2013; Wachholtz et al., 2011). Unrelieved pain in patients with opioid use disorder is associated with medical and nonmedical use of opioids (often to self-medicate), sleep disturbance, trauma, anxiety, depression, personality disorders, and decreased dispositional optimism (Barry et al., 2009a; Barry et al., 2009b; Beitel et al., 2015; Beitel et al., 2012; Peles et al., 2009; Rosenblum et al., 2003). Chronic pain often goes untreated or undertreated among patients with both interrelated chronic medical conditions (Scimeca et al., 2000), and clinicians in different treatment settings report difficulty managing these patients and finding appropriate treatment referrals (Barry et al., 2008; Barry et al., 2010b; Beitel et al., 2017)1. Integrated care would be a major advance providing a unique opportunity to address both disorders. Methadone maintenance treatment (MMT) programs are optimal settings to test such an approach: the prevalence of chronic pain is high with estimates ranging from 37% to more than 60% (Barry et al., 2009a; Jamison et al., 2000; Rosenblum et al., 2003), and most patients entering MMT with chronic pain want integrated opioid treatment program (OTP) and pain management services (Barry et al., 2010a).
Interdisciplinary pain management involving a physician, nurse, psychologist, physical therapist, and occupational therapist has a strong evidence-base for decreasing pain-related interference in functioning and improving psychosocial outcomes (Gatchel et al., 2014), but costs (often not covered by medical insurance), shortages of clinicians with the requisite expertise, and other logistical considerations limit the implementation of this approach in MMT programs (Gatchel and Okifuji, 2006; Gordon et al., 2014). Consequently, we set out to develop a practical integrated treatment approach combining medication-assisted treatment (i.e., MMT) and a psychosocial intervention for co-occurring opioid use disorder and chronic pain that could be offered onsite at OTPs. We selected cognitive-behavioral therapy (CBT) as the psychosocial platform for the integrated treatment because of its efficacy in treating substance use disorders (Carroll and Onken, 2005; Dutra et al., 2008), chronic pain (Burns et al., 2015; Ehde et al., 2014), and associated psychiatric disorders (Barry et al., 2016; Cuijpers et al., 2016), both alone and when combined with medications. Additionally, three pilot studies (Currie et al., 2003; Ilgen et al., 2011; Morasco et al., 2016) and one randomized clinical trial (RCT) (Ilgen et al., 2016) have found support for the efficacy of CBT for co-occurring substance use disorders and chronic pain. However, no studies have examined the efficacy of MMT with CBT for co-occurring opioid use disorder and chronic pain (Eilender et al., 2016).
The primary study aims were to evaluate the feasibility (i.e., treatment completion and session attendance) and acceptability (i.e., post-session satisfaction) of CBT for opioid use disorder and chronic pain, while the secondary aim was to examine its preliminary efficacy. The current study focused on low back pain because it is the most common site of chronic pain (including among MMT patients) (Barry et al., 2009b; Institute of Medicine, 2011; Rosenblum et al., 2003), and it is one of leading reasons for healthcare visits (Institute of Medicine, 2011). We anticipated that participants assigned to CBT compared to those assigned to standard drug counseling would (a) exhibit higher rates of abstinence from nonmedical opioid use and (b) be more likely to exhibit clinically significant reductions in pain interference.
2. Methods
2.1. Patients and setting
Patients met criteria for: (a) DSM-IV-TR diagnosis of opioid dependence (American Psychiatric Association, 2000) and American Academy of Pain Medicine/American Pain Society/American Society of Addiction Medicine consensus definition of opioid addiction (Savage et al., 2001) and (b) at least six months duration of moderate-to-severe low-back pain, defined as non-specific, non-cancer in origin using American Pain Society/American College of Physicians guidelines (Chou et al., 2007). Moderate-to-severe low back pain was defined as ratings of pain at its worst in the past week ≥4 on a 0 [no pain] to 10 [worst pain imaginable] numerical rating scale. While patients could report multiple somatic sites of pain, study inclusion required low back pain to be a primary pain site. Exclusion criteria included (a) current suicide or homicide risk, (b) inability to provide informed consent because of psychiatric or cognitive impairment, (c) and contraindication to the limited physical exercise encouraged in CBT, as determined by a study physician.
Patients initiated treatment at the APT Foundation, Inc. (hereafter referred to as APT), a not-for-profit community-based organization and OTP affiliated with Yale School of Medicine, and were recruited by referrals from intake coordinators and from flyers. APT did not offer additional specialized pain services at the OTP study location during the conduct of this trial. Research clinicians and physicians served as patients’ primary providers for the duration of patients’ participation in the study. Other than attending an “orientation to methadone group” and a treatment plan review at 30 and 90 days following MMT entry (as required by federal law), participants were not required to receive additional counseling services at APT for the duration of their study participation. Neither APT nor the funding agency played a role in the trial design, data analysis, or manuscript preparation. Enrollment for the study (NCT01334580) began in April 2011 and ended in July 2013. Written informed consent was obtained from all patients. The study was approved by both the APT Board of Directors and the Yale School of Medicine Human Investigation Committee (HIC# 0703002496).
2.2. Cognitive-Behavioral Therapy development and training
To develop the initial draft of the CBT manual, the PI (DTB) conducted CBT with five patients, using specific CBT sessions drawn from manualized CBT approaches for cocaine use disorder (Carroll, 1998) and chronic pain (Otis, 2005). Subsequently, the PI trained five therapists who had experience providing CBT for substance use disorders on CBT for chronic pain and opioid use disorder (See Section 2.3.5 on counseling implementation and fidelity).
2.3. Design and treatment allocation
The 16-week study consisted of a 3-week baseline prior to randomization followed by a 12-week clinical trial and a 1-week transfer to ongoing MMT at APT. Patients were recruited into the study during their first two weeks of starting MMT at APT. A 3-week study baseline was employed to ensure a stable methadone dose, after which patients were randomly assigned 1:1 to receive either 12 weeks of CBT or Methadone Drug Counseling (MDC). No patients were prescribed opioids for pain relief for the duration of the trial. (One patient had been prescribed opioids, but the provider discontinued the prescription when the patient enrolled in the study.) An investigator not involved in enrollment or determining eligibility conducted an urn randomization procedure to ensure that the groups were similar on gender (BAM). A different investigator then informed the patient of the treatment assignment (CJC).
2.3.1. Physician evaluation.
Prior to being randomized, each patient met with a study physician to confirm study eligibility, including whether patients were appropriate for physical exercise.
2.3.2. Provision of methadone.
A standard methadone dosing protocol targeting nonmedical opioid use (and not pain) was followed, irrespective of treatment allocation. Patients started on 30mg methadone daily and their dose was then increased to a target maintenance dose of 80mg-90mg daily. Any successive dose increases to address ongoing craving, withdrawal symptoms, or nonmedical opioid use required the approval of a study physician blind to patient allocation.
2.3.3. Cognitive-Behavioral Therapy.
CBT comprised twelve 30–45 minute sessions designed to provide coping skills training to effectively manage opioid use disorder and chronic pain2. Session 1 included an assessment of substance use, pain, and costs and benefits of using opioids nonmedically along with a review of the integrated focus of treatment. Sessions 2–11 were structured in three 10–15 minute phases: check-in (review of past-week methadone adherence, substance use, pain coping, and assignments) and agenda setting; coping skill acquisition; and formulation of intersession assignments (to promote practice of coping skills). The CBT modules covered in sessions 2–11 are summarized in Table 1. Covering eight modules in ten sessions allowed therapists to repeat topics based on each patient’s perceived needs, and allowed clincians sufficient time to cover module content (e.g., some therapists took two weeks to cover the relaxation training module). Session 12 involved a review of coping skills and the development of an “All-Purpose Coping Plan.” Twelve weekly individual sessions were offered since this format and duration are common in treatment trials of substance use disorders (Carroll, 1998), and we wished to allow therapists sufficient time to address the clinical needs of patients with co-existing opioid use disorder and chronic pain (Barry et al., 2016). Although the CBT intervention was designed to address chronic pain generally (and not back pain specifically), the examples used focused on low back pain (e.g., deconditioning contributing to poorly managed low back pain).
Table 1:
Psychosocial treatment modules
| Modules | CBT | MDC |
|---|---|---|
| Functional Analysis of Behavior (Identify substance use and pain flare-up relapse precursors and consequences and review future plans) |
Үes | No |
| Coping with Cravings or Pain Flare-Ups (Self-soothing, distraction, thinking through consequences, and mindfulness) |
Үes | No |
| Psychoeducation (Distinctions between acute and chronic pain, and a framework for understanding biological, psychological, and social factors that influence self-management of opioid use disorder and chronic pain) |
Үes | No |
| Exercise and Behavioral Activation (Graded, time-limited, and controlled engagement in physical activity and non-drug-related pleasurable activities) |
Үes | No |
| Relaxation Training (Diaphragmatic breathing, progressive muscular relaxation, imagery) |
Үes | No |
| Cognitive Restructuring (Identify and address thinking errors related to opioid use disorder and chronic pain) |
Үes | No |
| Resilience Training (Attenuate unhelpful attributions related to stressors and foster gratitude and accomplishment) |
Үes | No |
| Assertiveness Training (Review of different communication styles and practice assertive communication related to opioid use disorder (e.g., refusal skills) and pain (e.g., effective communication with providers)) |
Үes | No |
| Process of Recovery (Long-term process of recovery involving abstinence from illicit drugs) |
No | Үes |
| Relationships in Recovery (Identify how relationships are affected by drug use and understand elements of healthy relationships) |
No | Үes |
| Self-Help Groups and Support Systems (Understand the role of 12 steps in recovery along with the structure and format of 12-step programs) |
No | Үes |
| Coping with Shame and Guilt (Identify and develop strategies for managing shame and guilt) |
No | Yes |
CBT=cognitive-behavioral therapy. MDC=methadone drug counseling.
2.3.4. Methadone drug counseling.
MDC consisted of four 15–20-minute individual case-management sessions targeting opioid use disorder (and not pain), and was designed to approximate usual drug counseling in MMT (Chawarksi et al., 2005). MDC is a didactic, 12-step based treatment approach. In each session, the counselor reviewed a worksheet that included objectives, main points and questions pertaining to an educational topic. Session 1 comprised a review of opioid use disorder and related medical, psychiatric, substance use, employment, family, legal, or other problems. Patients were provided an informational booklet on MMT and a listing of local Narcotics Anonymous (NA) and Alcohol Anonymous (AA) self-help meetings. Counselors highlighted “methadone friendly” meetings, and recommended that patients consider attending NA or AA self-help groups, especially early in recovery from addiction. Sessions 2 and 3 began with a brief check-in regarding substance use or other problems, and counselors used clinical judgment to select from a list of educational topics summarized in Table 1. Session 4 began with a brief check-in and then the counselor reviewed patients’ plans for future abstinence, and recommended continued participation in self-help groups.
2.3.5. Counseling implementation and fidelity.
CBT and MDC are manualized interventions. CBT and MDC were provided by trainees with at least a master’s level of training, none of whom were licensed independent practitioners. Standard research protocols for training and supervision were followed: didactic workshops, requiring therapists to proficiently treat a training case before treating randomized patients, audiotaping sessions, use of structured clinical notes, and ongoing supervision (Rounsaville et al., 2001; Sholomskas et al., 2005; Weissman et al., 1982). During separate weekly supervision with CBT and MDC clinicians, two investigators (DTB, CJC) followed standard procedures to enhance treatment fidelity (Fiellin et al., 2013). Supervisors and therapists reviewed session tapes, identified and addressed clinicians’ performance strengths and weaknesses, and reviewed plans for the subsequent counseling session. During therapist training, CBT and MDC clinicians were instructed to deliver the counseling modules in a pre-determined order (See Table 1). Upon demonstrating proficiency, therapists were permitted to alter the module order to address patients’ presenting issues. During supervision, clinicians discussed their clinical rationale for any alterations, and supervisors monitored which modules clinicians had implemented. Therapists were encouraged to maximize patient exposure to a variety of modules or self-management skills.
2.4. Measures
2.4.1. Assessment of treatment feasibility and acceptability.
Feasibility was assessed by examining rates of treatment completion and frequency of CBT and MDC session attendance, whereas acceptability was assessed by post-session satisfaction ratings of CBT and MDC (scored on 1–7 Likert-type scales).
2.4.2. Preliminary efficacy outcomes.
The primary outcomes were rates of abstinence from nonmedical opioid use from baseline to study completion (receipt of medication through week 16 and final research assessment completed) as assessed by weekly urine toxicology testing, and clinically significant reductions in pain interference (defined as ≥ 2 points) from baseline to study completion as assessed weekly by Brief Pain Inventory (BPI) pain interference subscale (Cleeland, 1991; Cleeland and Ryan, 1994). Nonmedical opioid use was operationally defined as use of opioids other than methadone. Secondary outcomes were maximum consecutive number of weeks of abstinence from nonmedical opioid use during the 12-week RCT (as evidenced by urine samples testing negative for metabolites related to morphine or oxycodone) and rates of clinically significant reductions in pain intensity from baseline to treatment completion (≥2 points on Brief Pain Inventory pain intensity subscale) (Cleeland, 1991; Cleeland and Ryan, 1994). Abstinence rates are commonly used as treatment outcomes in substance use disorder research and clinical practice. Maximum number of consecutive weeks of abstinence provides a measure of sustained abstinence (Milby et al., 2010). Using a reduction of at least 2 points on 0–10 numerical rating scales of pain interference and intensity is a common strategy for assessing clinically significant improvement in clinical trials (Dworkin et al., 2008; Farrar et al., 2001).
2.4.3. Assessment of preliminary efficacy outcomes.
Abstinence from nonmedical opioid use was assessed via urine toxicology testing of weekly collected urine samples using a semiquantitative homogeneous enzyme immunoassay for morphine, oxycodone, and methadone (Redwood Toxicology Laboratory). Pain interference and pain intensity were measured by the Brief Pain Inventory (BPI) (Cleeland, 1991; Cleeland and Ryan, 1994). Pain interference encompassed the mean of seven items related to past-week pain-related interference in general activity, walking, work, mood, enjoyment of life, relations with others, and sleep, whereas pain intensity encompassed the mean of four items related to current pain and past-week average pain, pain at its worst, and pain at its least. BPI items and BPI pain interference as well as pain intensity subscales were scored on 0–10 numerical rating scales.
2.5. Data analysis
Pearson’s chi-square was used to compare MMT with CBT and MMT with MDC on single categorical outcomes (i.e., treatment completion) and Student’s t-tests were used to compare treatment groups on single continuous outcomes (i.e., treatment satisfaction). Analyses of preliminary efficacy occurred in multiple steps. First, the proportions of patients in each treatment condition abstinent from nonmedical opioid use were calculated for four time periods: 3-week baseline and weeks 1–4, 5–8, and 9–12 (post-randomization). Patients were classified as abstinent during the baseline period if they had three successive opioid negative tests and no positive tests and were classified as abstinent during weeks 1–4, 5–8, and 9–12 if they had four successive opioid-negative weekly urine tests and no positive tests during each 4-week period. A General Estimating Equation (GEE) logistic regression model with a robust variance estimation and autoregressive (AR1) working correlation structure with intercept was performed to evaluate the effects of treatment group over four months on the dichotomous treatment outcome of monthly opioid abstinence (Brown et al., 2008; West, 2009). Pain interference scores were calculated for five time points (first week of baseline, end of baseline/randomization, end of month 1, end of month 2, and end of month 3). Differences between the first week of baseline and end-of-month scores on pain interference were calculated: Differences ≥2 points were classified as clinically significant. Pearson’s chi-square was used to compare the proportions of patients in both treatment arms who were classified as having clinically significant reductions in pain interference. Second, maximum consecutive number of weeks of abstinence from nonmedical opioid use during the 12-week RCT was calculated for each patient; Student’s t-tests were used to compare treatment groups on this outcome. We used the same procedures outlined for pain interference to calculate rates of clinically significant reductions in pain intensity and to perform analyses comparing the proportions of patients in both treatment arms who exhibited clinically significant reductions in pain intensity. We considered p<.05 as statistically significant. All analyses were conducted using SPSS 22 (Armonk, NY: IBM Corp.).
3. Results
3.1. Demographic and clinical characteristics
As shown in the consort diagram in Figure 1, 21 patients were randomly assigned to CBT and 19 to MDC. Table 2 summarizes patients’ baseline demographic and clinical characteristics: 63% of patients were men; 85% self-identified as White; 85% were single, divorced, or separated; 23% were full-time employed; 80% completed high school; 63% identified heroin as their primary opioid used; and 65% had a history of chronic pain treatment. Mean (SD) age was 38.1 (11.3) years. Mean (SD) duration of opioid use disorder was 11.8 (9.9) years and mean (SD) duration of chronic pain was 16.0 (10.8) years.
Figure 1.
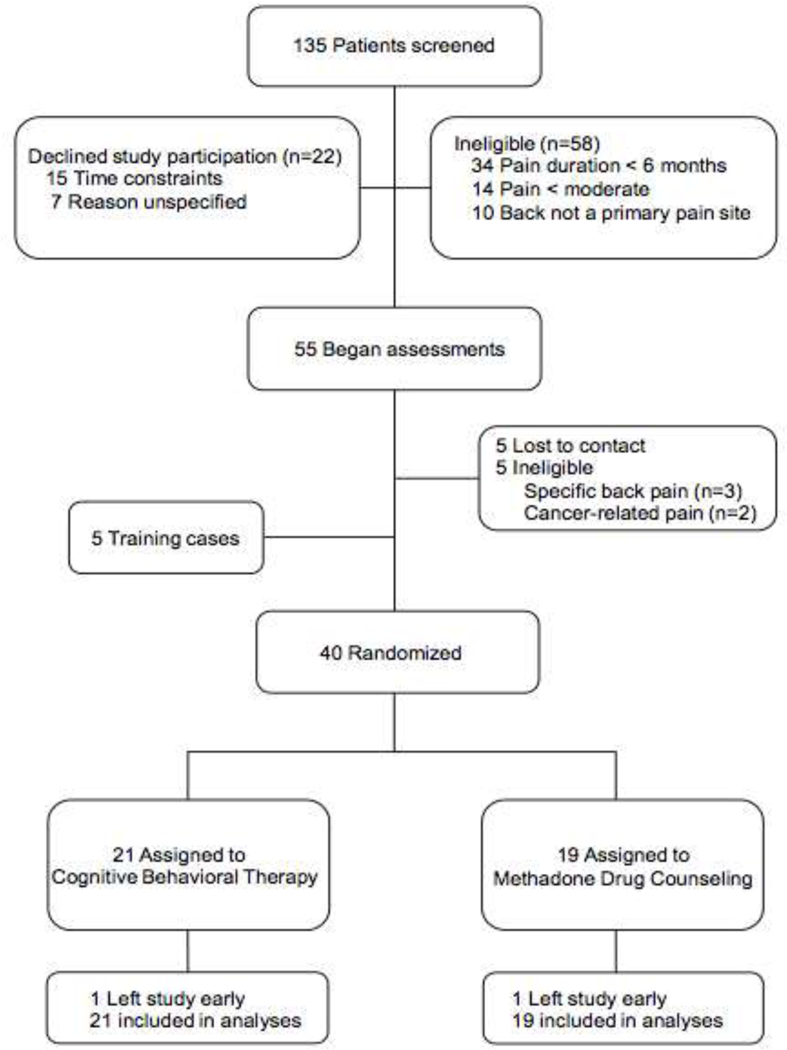
Trial profile
Table 2:
Baseline patient demographics and clinical characteristics.
| Total N=40 |
CBT N=21 |
MDC N=19 |
t (38)/ χ2 (1) |
p | |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 38.1 (11.3) |
38.4 (12.1) |
37.7 (10.6) |
0.19 | .848 |
| % Male (n) | 62.5 (25) | 66.7 (14) | 57.9 (11) | 0.33 | .567 |
| % Single/divorce/separated (n) | 85.0 (34) | 90.5 (19) | 78.9 (15) | 1.04 | .398 |
| % White (n) | 85.0 (34) | 80.9 (17) | 89.5 (17) | 0.57 | .664 |
| % High school or greater (n) | 80.0 (32) | 80.9 (17) | 78.9 (15) | 0.03 | .874 |
| % Full-time employment (n) | 22.5 (9) | 19.0 (4) | 26.3 (5) | 0.30 | .712 |
| Age of onset, mean (SD) | |||||
| Opioid use disorder | 26.2 (9.9) |
27.8 (11.3) |
24.5 (8.0) | 1.04 | .307 |
| Chronic pain | 22.1 (12.1) |
22.6 (13.0) |
21.5 (11.2) |
0.28 | .779 |
| Years opioid use disorder, mean (SD) |
11.8 (9.9) |
10.6 (8.9) |
13.2 (11.0) |
0.80 | .426 |
| Years chronic pain, mean (SD) | 16.0 (10.8) |
15.8 (12.4) |
16.2 (9.1) | 0.11 | .910 |
| % Heroin was primary opioid used (n) |
62.5 (25) | 61.9 (13) | 63.2 (12) | 0.01 | .997 |
| % Past week heroin use (n) | 42.5 (17) | 38.1 (8) | 47.4 (9) | 0.35 | .554 |
| % Past week prescription opioid use (n) |
2.5 (1) | 4.8 (1) | 0.0 (0) | N/A | N/A |
| % Prior pain treatment (n) | 65.0 (26) | 76.2 (16) | 52.6 (10) | 3.28 | .070 |
| % Prior opioid use disorder treatment (n) |
35.0 (14) | 19.0 (4) | 52.6 (10) | 4.95 | .026* |
| % Use of substances in prior 30 days (n) |
|||||
| Alcohol | 37.5 (15) | 28.6 (6) | 47.4 (9) | 1.50 | .220 |
| Cocaine | 22.5 (9) | 19.0 (4) | 26.3 (5) | 0.30 | .712 |
| Marijuana | 30.0 (12) | 28.6 (6) | 31.6 (6) | 0.04 | .836 |
| Pain intensity, mean (SD) | 5.9 (1.5) | 6.2 (1.3) | 5.7 (1.6) | 0.94 | .351 |
| Pain interference, mean (SD) | 6.2 (2.5) | 6.7 (2.2) | 5.8 (2.7) | 1.10 | .275 |
p<.05.
CBT=cognitive-behavioral therapy. MDC=methadone drug counseling
3.2. Treatment feasibility and acceptability
Treatment completion rates were high and did not differ for those assigned to CBT (95%) or MDC (95%). One patient in each arm did not complete the study; one patient violated probation and was incarcerated, while the other moved to a different state. Out of 12 anticipated CBT sessions, the mean (SD) attendance was 8.4 (2.9). Out of four anticipated MDC sessions, the mean (SD) attendance was 3.8 (1.1). The mean (SD) minutes per session of CBT and MDC were 45.0 (11.3) and 17.9 (10.3), respectively. Therapy satisfaction ratings (scored on 1–7 Likert-type scale) were high for both conditions, but higher for the CBT condition (M=6.6 (SD=0.5)) than for MDC (M=6.0 (SD=0.4)) [t (37) =3.58, p=0.001; Cohen’s d =0.66].
3.3. Nonmedical opioid use
The proportions of patients who exhibited abstinence from nonmedical opioid use by time and treatment condition are shown in Figure 2. Across the baseline and three successive 4week periods following randomization, the proportions of patients abstinent from non-medical opioid use were higher for patients assigned to CBT than for those assigned to MDC [Wald χ2 (1)=5.47, p=.019]; the proportions of patients abstinent were not significantly affected by time (p =.69) or by the interaction between counseling condition and time (p=.10). The mean (SD) number of maximum consecutive weeks of abstinence from nonmedical opioid use was numerically higher for patients assigned to MMT with CBT than for patients assigned to MMT with MDC: 6.1 (4.2) and 3.9 (3.3), respectively, [t (38)=1.831, p=0.06]3. Findings from weekly self-report measures of substance use (not reported in this paper) were consistent with urine toxicology data.
Figure 2.
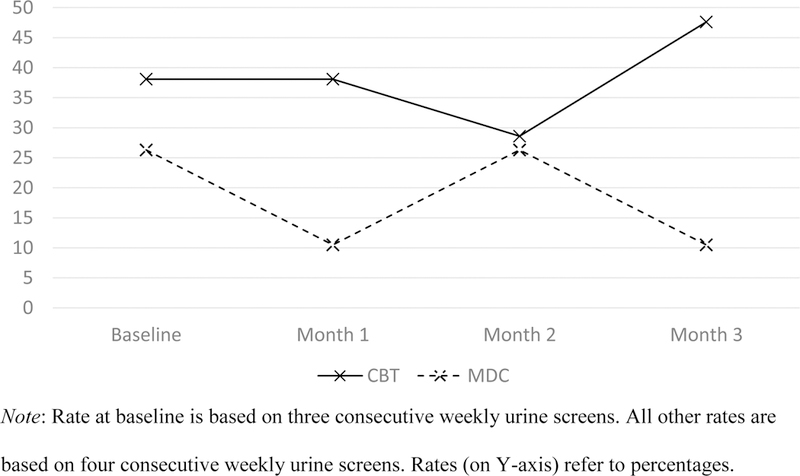
Rates of abstinence from nonmedical opioid use over time
3.4. Pain interference and intensity
Mean pain interference and pain intensity scores by time and treatment condition are summarized in Figure 3. For patients assigned to MMT with CBT or MMT with MDC, rates of clinically significant (≥ 2 point) change from baseline to end of treatment on pain interference (42.9% vs. 42.1%, [χ2 (1, N=40)=0.002, p=0.962]) and pain intensity (14.3% vs. 15.8%, [χ2 (1, N=40)=0.018, p=0.894]) did not differ significantly. Additional analyses examining weekly pain scores over the course of the study yielded no significant treatment group or treatment group by time effects on pain interference (p’s=.27 and .72) or intensity (p’s=.25 and .88).
Figure 3.
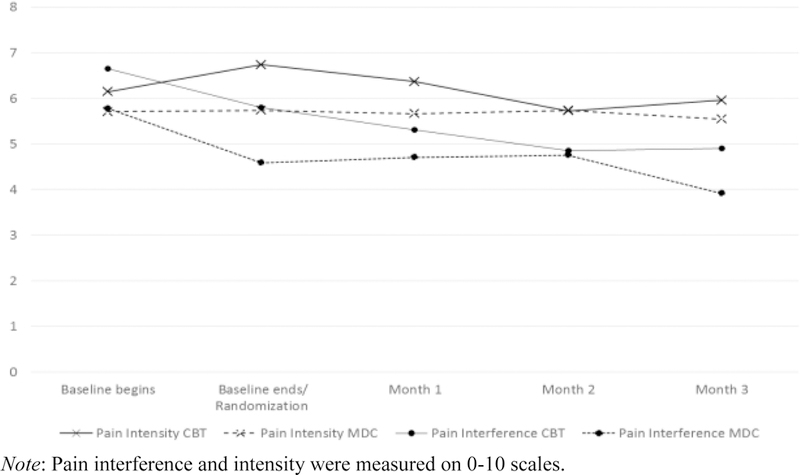
Pain interference and intensity over time
4. Discussion
This study is one of the first to examine the use of manualized CBT for patients receiving MMT with opioid use disorder and chronic pain. Two main findings emerged. First, CBT was found to be feasible and acceptable. Second, preliminary support was found for the efficacy of MMT with CBT (compared to MMT with MDC) for reducing nonmedical opioid use.
4.1. Feasibility, acceptability, and preliminary efficacy of CBT
High rates of treatment completion and therapy session attendance demonstrate the feasibility of providing MMT with CBT to patients with opioid use disorder and chronic pain. The almost-daily clinic attendance requirements during the first 90 days of MMT may have contributed to the demonstrated feasibility of CBT for opioid use disorder and chronic pain. Although both counseling approaches were associated with high satisfaction, participants assigned to CBT reported higher satisfaction than those assigned to MDC, suggesting that CBT may be associated with particularly high patient acceptability, despite requiring substantially more frequent and longer participation in therapy sessions.
Participants assigned to MMT with CBT (compared to MMT with MDC) exhibited higher rates of abstinence from nonmedical opioid use from baseline to treatment completion. Patients assigned to MMT with CBT also had numerically more consecutive weeks of abstinence over the 12 weeks of counseling than those assigned to MMT with MDC. Study findings supporting the preliminary efficacy of CBT in attenuating nonmedical opioid use expand upon those reported in prior studies regarding the efficacy of CBT in addressing substance use disorders and chronic pain (Currie et al., 2003; Ilgen et al., 2016; Ilgen et al., 2011; Morasco et al., 2016). The current study, along with the Ilgen et al. study (2016), used a control group; the others did not. It is noteworthy that these prior CBT studies were conducted either in settings that offered abstinence-based treatment for non-opioid substance-related disorders (Currie et al., 2003; Ilgen et al., 2016; Ilgen et al., 2011) or in a primary care clinic with individuals who were not necessarily involved in substance use disorder treatment (Morasco et al., 2016). In contrast, the current study is the first to examine a pain- and addiction-focused CBT intervention among patients with opioid use disorder on MMT. Medication-assisted treatments with methadone (opioid agonist), buprenorphine (partial opioid agonist), or injectable naltrexone are evidencebased interventions for opioid use disorder and comprise an important public health strategy in confronting the current opioid crisis (Murthy, 2016).
The efficacy of extensive psychosocial interventions in improving nonmedical opioid use and treatment retention outcomes during opioid agonist treatment for a heterogeneous population of patients with opioid use disorder (but not specifically with co-occurring chronic pain) has been called into question in recent systematic reviews (Amato et al., 2011; Carroll and Weiss, 2017; Dugosh et al., 2016). However, the results of this study suggest that a particular psychotherapy, CBT, may be specifically efficacious for a certain sub-population of patients with opioid use disorder and chronic pain, whose treatment is complicated by interactions between nonmedical opioid use, chronic pain, and psychopathology (Barry et al., 2016). This view is consistent with findings from a recent secondary analysis of an earlier study examining the relative efficacy of adding addiction-focused CBT to medication management for opioid use disorder (i.e., buprenorphine/naloxone along with brief physician counseling); CBT improved outcomes with medication management for the subset of patients with nonmedical prescription opioid use, for whom chronic pain is particularly prevalent and problematic (Moore et al., 2016).
Clinically significant reductions in pain interference were observed for 42.5% of patients overall (and clinically significant reductions in pain intensity were observed for 15.0% of participants overall) but did not differ significantly between patients assigned to CBT or MDC. It is noteworthy that patients assigned to CBT (compared to MDC) experienced similar reductions in pain interference and pain intensity while achieving significantly greater abstinence from nonmedical opioid use. These findings suggest that the association between chronic pain and nonmedical opioid use is complex. The relative analgesic and hyperalgesic effects of initiating and maintaining patients with opioid use disorder and chronic pain on MMT is currently unclear (Arout et al., 2015; Wachholtz et al., 2015). This situation is further complicated by findings that MMT patients with chronic pain report nonmedical opioid use to alleviate pain (Barry et al., 2009b), allowing for the possibility that the higher rates of nonmedical opioid use among patients assigned to MDC (compared to CBT) may be related to attempts to alleviate pain. However, the findings of reductions in both nonmedical opioid use and in pain interference and intensity among patients receiving MMT and CBT may be reassuring and encourage greater adherence to treatment recommendations. This is particularly relevant among patients who may fear an exacerbation of pain if they discontinue nonmedical opioid use. It should also be noted that abstinence from nonmedical opioid use allows patients with opioid use disorder and chronic pain to try out additional conventional pain interventions.
As noted previously, this is only the second study to employ randomization to a comparison group in evaluating CBT for patients with substance use disorder and chronic pain. It is unclear whether significant pre-post treatment differences in pain reduction documented in prior single-cell studies would have demonstrated efficacy relative to a comparison treatment (Currie et al., 2003; Ilgen et al., 2011; Morasco et al., 2016). Future studies could evaluate whether the inclusion of additional pain-focused interventions with CBT improves pain outcomes. Increased engagement in exercise and behavioral activation may be good initial targets, considering findings that for patients receiving MMT chronic pain is associated with decreased likelihood of meeting public health guidelines for physical activity, and the important role that deconditioning plays in perpetuating chronic back pain (Beitel et al., 2016; Qaseem et al., 2017). Findings from recent studies suggest that mindfulness-based interventions might also be useful to explore (Garland et al., 2017; Smallwood et al., 2016).
4.2. Limitations
This study had limitations. The relatively small sample size limits power to detect significant differences associated with important but small effect sizes. Because the study was designed as a pilot, the level of statistical significance was not corrected to adjust for multiple comparisons. The findings regarding significant between-group differences in the proportions of patients abstinent from nonmedical opioid use during the study should be interpreted cautiously because of the observed (but not statistically significant) differences during the 3-week baseline. The study was conducted in patients with chronic low back pain, and the study findings may not generalize to other pain sites or conditions. Restricting eligibility to patients with low back pain had the advantage, however, of reducing heterogeneity and enhancing the ability to generalize from the study findings to the population of patients with the same condition. Nonmedical opioid use during the study period may have produced analgesia and complicates the assessment of counseling effects on pain interference and pain intensity. MDC was designed to approximate usual drug counseling and not as a time-and-attention control; patients assigned to CBT received more therapy than those assigned to MDC. A more rigorous test of the efficacy of CBT will necessitate a randomized controlled trial involving a time-and-attention control condition that matches CBT on session frequency and duration. Receipt of offsite pain treatment services during the study was not assessed; however, this concern is somewhat mitigated by the findings that addiction treatment providers have difficulty obtaining pain management referrals for patients receiving opioid agonist treatment (Barry et al., 2008; Beitel et al., 2017). The current study examined the relative efficacy of CBT and MDC over three months; the long-term effects of CBT for co-occurring opioid use disorder and chronic pain are unclear and merit further research attention (Carroll et al., 2009).
4.3. Conclusions
This is one of the first studies to examine MMT with CBT for opioid use disorder and chronic pain. Effective pain management in OTP settings has proven elusive (Scimeca et al., 2000), and is hampered by the absence of an integrated evidence-based treatment for opioid use disorder and chronic pain (Wachholtz et al., 2011). The findings of this study suggest that MMT combined with CBT is feasible, acceptable, and—compared to standard drug counseling—may be associated with greater reductions in nonmedical opioid use. Additional research on the efficacy of CBT for opioid use disorder and chronic pain is warranted. Findings from prior studies indicate that drug counselors are interested in receiving training on psychosocial interventions for chronic pain and view CBT as a credible treatment approach (Barry et al., 2008; Beitel et al., 2017). Future research should also examine long-term treatment outcomes and training for front-line drug counselors on this approach.
Supplementary Material
Highlights.
Evaluated cognitive-behavioral therapy (CBT) for opioid use disorder and chronic pain
CBT was found to be feasible and acceptable
We found preliminary support for efficacy of CBT in reducing nonmedical opioid use
Acknowledgments
This research was supported by funding from the National Institute on Drug Abuse to Dr. Barry (K23 DA024050), Dr. Schottenfeld (K24 DA000445, R01DA024695) and Dr. Moore (K01 DA022398). Dr. Kerns was supported by the Veterans Health Administration Health Services Research and Development Service Center of Innovation (CIN 13-407). The sponsors had no role in the study design; data collection, analysis or interpretation; the writing of the manuscript; or in the decision to submit the paper for publication. We would also like to acknowledge the support and encouragement of the late Dr. Bruce Rounsaville for this research. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the funding agencies or affiliated institutions, including the APT Foundation, Department of Veterans Affairs, or the United States government.
Role of the Funding Source
The sponsors had no role in the study design; the collection, analysis and interpretation of data; the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the positions or policies of their respective institutions.
Poorly managed pain is also associated with worse treatment outcomes among patients with alcohol use disorder (Witkiewitz et al., 2015)
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
References
- Amato L, Minozzi S, Davoli M, Vecchi S, 2011. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst. Rev CD004147. doi: 101002/14651858CD004147pub4. [DOI] [PubMed]
- American Psychiatric Association, 2000. Diagnostic and statistical manual of mental disorders American Psychiatric Association, Washington, DC. [Google Scholar]
- Arout CA, Edens E, Petrakis IL, Sofuoglu M, 2015. Targeting opioid-induced hyperalgesia in clinical treatment: Neurobiological considerations. CNS Drugs 29, 465–486. [DOI] [PubMed] [Google Scholar]
- Barry DT, Beitel M, Cutter CJ, Joshi D, Falcioni J, Schottenfeld RS, 2010a. Conventional and nonconventional pain treatment utilization among opioid dependent individuals with pain seeking methadone maintenance treatment: A needs assessment study. J. Addict. Med 4, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Beitel M, Garnet B, Joshi D, Rosenblum A, Schottenfeld RS, 2009a. Relations among psychopathology, substance use, and physical pain experiences in methadone-maintained patients. J. Clin. Psychiatry 70, 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Beitel M, Joshi D, Schottenfeld RS, 2009b. Pain and substance-related pain reduction behaviors among opioid dependent individuals seeking methadone maintenance treatment. Am. J. Addict 18, 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Bernard MJ, Beitel M, Moore BA, Kerns RD, Schottenfeld RS, 2008. Counselors’ experiences treating methadone-maintained patients with chronic pain: A needs assessment study. J. Addict. Med 2, 108–111. [DOI] [PubMed] [Google Scholar]
- Barry DT, Cutter CJ, Beitel M, Kerns RD, Liong C, Schottenfeld RS, 2016. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J. Clin. Psychiatry 77, 1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Irwin KS, Jones ES, Becker WC, Tetrault JM, Sullivan LE, Hansen H, O’Connor PG, Schottenfeld RS, Fiellin DA, 2010b. Opioids, chronic pain, and addiction in primary care. J. Pain 11, 1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel M, Oberleitner L, Kahn M, Kerns RD, Liong C, Madden LM, Ginn J, Barry DT, 2017. Drug counselor responses to patients’ pain reports: A qualitative investigation of barriers and facilitators to treating patients with chronic pain in methadone maintenance treatment. Pain Med 18, 2152–2161. [DOI] [PubMed] [Google Scholar]
- Beitel M, Peters S, Savant JD, Cutter CJ, Cecero JJ, Barry DT, 2015. The psychometric properties of the Iowa Personality Disorder Screen in methadonemaintained patients: An initial investigation. J. Pers. Disord 29, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel M, Savant JD, Cutter CJ, Peters S, Belisle N, Barry DT, 2012. Psychopathology and pain correlates of dispositional optimism in methadone-maintained patients. Am. J. Addict 21, S56–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel M, Stults‐Kolehmainen M, Cutter CJ, Schottenfeld RS, Eggert K, Madden LM, Kerns RD, Liong C, Ginn J, Barry DT, 2016. Physical activity, psychiatric distress, and interest in exercise group participation among individuals seeking methadone maintenance treatment with and without chronic pain. Am. J. Addict 25, 125–131. [DOI] [PubMed] [Google Scholar]
- Brown CH, Wang W, Kellam SG, Muthen BO, Petras H, Toyinbo P, Poduska J, Ialongo N, Wyman PA, Chamberlain P, Sloboda Z, MacKinnon DP, Windham A, Prevention S, Methodology G, 2008. Methods for testing theory and evaluating impact in randomized field trials: Intent-to-treat analyses for integrating the perspectives of person, place, and time. Drug Alcohol Depend 95 Suppl. 1, S74–S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J, Nielson WR, Jensen MP, Heapy A, Czlapinski R, Kerns RD, 2015. Specific and general therapeutic mechanisms in cognitive-behavioral treatment for chronic pain. J. Consult. Clin. Psychol 83, 1–11. [DOI] [PubMed] [Google Scholar]
- Carroll KM, 1998. Therapy manuals for drug addiction, manual 1: A cognitive-behavioral approach: Treating cocaine addiction. 98–4308 National Institute on Drug Abuse, Rockville, MD. [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Rounsaville BJ, 2009. Enduring effects of a computer-assisted training program for cognitive behavioral therapy: A 6-month follow-up of CBT4CBT. Drug Alcohol Depend 100, 178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Onken LS, 2005. Behavioral therapies for drug abuse. Am. J. Psychiatry 162, 1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Weiss RD, 2017. The role of behavioral interventions in buprenorphine maintenance treatment: A review. Am. J. Psychiatry 174, 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarksi M, Barry DT, Schottenfeld RS, 2005. Methadone Drug Counseling (MDC) Manual, Unpublished. Yale University School of Medicine. [Google Scholar]
- Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr., Shekelle P, Owens DK, Clinical Efficacy Assessment Subcommittee of the American College of Physicians, American College of Physicians, American Pain Society Low Back Pain Guidelines Panel, 2007. Diagnosis and treatment of low back pain: A joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann. Intern. Med 147, 478–491. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, 1991. Pain assessment in cancer. In: Osaba D (Ed.), Effect of cancer on quality of life CRC Press, Boca Raton, FL: pp. 293–305. [Google Scholar]
- Cleeland CS, Ryan KM, 1994. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad. Med. Singapore 23, 129–138. [PubMed] [Google Scholar]
- Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Huibers MJ, 2016. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry 15, 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SR, Hodgins DC, Crabtree A, Jacobi J, Armstrong S, 2003. Outcome from integrated pain management treatment for recovering substance abusers. J. Pain 4, 91100. [DOI] [PubMed] [Google Scholar]
- Dhingra L, Masson C, Perlman DC, Seewald RM, Katz J, McKnight C, Homel P, Wald E, Jordan AE, Young C, Portenoy RK, 2013. Epidemiology of pain among outpatients in methadone maintenance treatment programs. Drug Alcohol Depend 128, 161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D, 2016. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. J. Addict. Med 10 91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW, 2008. A meta-analytic review of psychosocial interventions for substance use disorders. Am. J. Psychiatry 165, 179–187. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, 2008. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain 9, 105–121. [DOI] [PubMed] [Google Scholar]
- Ehde DM, Dillworth TM, Turner JA, 2014. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. Am. Psychol 69, 153–166. [DOI] [PubMed] [Google Scholar]
- Eilender P, Ketchen B, Maremmani I, Saenger M, Fareed A, 2016. Treatment approaches for patients with opioid use disorder and chronic noncancer pain: A literature review. Addict. Disord. Their Treat 15, 85–98. [Google Scholar]
- Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM, 2001. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94, 149–158. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Barry DT, Sullivan LE, Cutter CJ, Moore BA, O’Connor PG, Schottenfeld RS, 2013. A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine. Am. J. Med 126, 74 e11–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Bryan CJ, Finan PH, Thomas EA, Priddy SE, Riquino MR, Howard MO, 2017. Pain, hedonic regulation, and opioid misuse: Modulation of momentary experience by Mindfulness-Oriented Recovery Enhancement in opioid-treated chronic pain patients. Drug Alcohol Depend 173, S65–S72. [DOI] [PubMed] [Google Scholar]
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B, 2014. Interdisciplinary chronic pain management: Past, present, and future. Am. Psychol 69, 119. [DOI] [PubMed] [Google Scholar]
- Gatchel RJ, Okifuji A, 2006. Evidence-based scientific data documenting the treatment and cost-effectiveness of comprehensive pain programs for chronic nonmalignant pain. J. Pain 7, 779–793. [DOI] [PubMed] [Google Scholar]
- Gordon RM, Corcoran JR, Bartley-Daniele P, Sklenar D, Sutton PR, Cartwright F, 2014. A transdisciplinary team approach to pain management in inpatient health care settings. Pain Manag. Nurs 15, 426–435. [DOI] [PubMed] [Google Scholar]
- Ilgen MA, Bohnert AS, Chermack S, Conran C, Jannausch M, Trafton J, Blow FC, 2016. A randomized trial of a pain management intervention for adults receiving substance use disorder treatment. Addiction 111, 1385–1393. [DOI] [PubMed] [Google Scholar]
- Ilgen MA, Haas E, Czyz E, Webster L, Sorrell JT, Chermack S, 2011. Treating chronic pain in Veterans presenting to an addictions treatment program. Cogn. Behav. Pract 18, 149–160. [Google Scholar]
- Institute of Medicine, 2011. Relieving pain in America: A blueprint for transforming prevention, care, education and research The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Jamison RN, Kauffman J, Katz NP, 2000. Characteristics of methadone maintenance patients with chronic pain. J. Pain Symptom Manage 19, 53–62. [DOI] [PubMed] [Google Scholar]
- Milby JB, Schumacher JE, Wallace D, Vuchinich R, Mennemeyer ST, Kertesz SG, 2010. Effects of sustained abstinence among treated substance-abusing homeless persons on housing and employment. Am. J. Public Health 100, 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Cutter CJ, Buono FD, Barry DT, Fiellin LE, O’Connor PG, Schottenfeld RS, 2016. Cognitive Behavioral Therapy improves treatment outcomes for prescription opioid users in primary care buprenorphine treatment. J. Subst. Abuse Treat 71, 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasco BJ, Greaves DW, Lovejoy TI, Turk DC, Dobscha SK, Hauser P, 2016. Development and preliminary evaluation of an integrated Cognitive-Behavior Treatment for chronic pain and substance use disorder in patients with the Hepatitis C Virus. Pain Med 17, 2280–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VH, 2016. Ending the opioid epidemic—A call to action. N. Engl. J. Med 375, 2413–2415. doi: 10.1056/NEJMp1612578. [DOI] [PubMed] [Google Scholar]
- Otis JD, 2005. Cognitive behavioral therapy for the treatment of chronic pain: Therapist’s manual. Pain Management Program, VA Boston Healthcare System
- Peles E, Schreiber S, Adelson M, 2009. Documented poor sleep among methadonemaintained patients is associated with chronic pain and benzodiazepine abuse, but not with methadone dose. Eur. Neuropsychopharmacol 19, 581–588. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Wilt TJ, McLean RM, Forciea M, Clinical Guidelines Committee of the American College of Physicians, 2017. Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the American College of Physicians. Ann. Intern. Med 166, 514–530. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK, 2003. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA 289, 2370–2378. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Carroll KM, Onken LS, 2001. A stage model of behavioral therapies research: Getting started and moving on from stage I. Clin. Psychol 8, 133–142. [Google Scholar]
- Savage S, Covington E, Heit HA, Hunt J, Joranson D, Schnoll S, 2001. Definitions related to the use of opioids for the treatment of pain: A consensus document from the American Academy of Pain Medicine, the American Pain Society, and the American Society of Addiction Medicine. WMJ 100, 28–29.11579797 [Google Scholar]
- Scimeca MM, Savage SR, Portenoy R, Lowinson J, 2000. Treatment of pain in methadone-maintained patients. Mt. Sinai J. Med 67, 412–422. [PubMed] [Google Scholar]
- Sholomskas DE, Syracuse-Siewert G, Rounsaville BJ, Ball SA, Nuro KF, Carroll KM, 2005. We don’t train in vain: A dissemination trial of three strategies of training clinicians in cognitive-behavioral therapy. J. Consult. Clin. Psychol 73, 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood RF, Potter JS, Robin DA, 2016. Neurophysiological mechanisms in acceptance and commitment therapy in opioid-addicted patients with chronic pain. Psychiatry Res. Neuroimag 250, 12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachholtz A, Foster S, Cheatle M, 2015. Psychophysiology of pain and opioid use: Implications for managing pain in patients with an opioid use disorder. Drug Alcohol Depend 146, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachholtz A, Gonzalez G, Boyer E, Naqvi Z, Rosenbaum C, Ziedonis D, 2011. Intersection of chronic pain treatment and opioid analgesic misuse: Causes, treatments, and policy strategies. Subst. Abuse 2, 145–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Rounsaville BJ, Chevron E, 1982. Training psychotherapists to participate in psychotherapy outcome studies. Am. J. Psychiatry 139, 1442–1446. [DOI] [PubMed] [Google Scholar]
- West BT, 2009. Analyzing longitudinal data with the linear mixed models procedure in SPSS. Eval. Health Prof 32, 207–228. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Vowles KE, McCallion E, Frohe T, Kirouac M, Maisto SA, 2015. Pain as a predictor of heavy drinking and any drinking lapses in the COMBINE study and the UK Alcohol Treatment Trial. Addiction 110, 1262–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


