HHV-8 vIL-6 promotes productive replication in the context of reactivated lytic replication in primary effusion lymphoma (PEL) and endothelial cells and sustains latently infected PEL cell viability. Viral IL-6 is also considered to contribute significantly to HHV-8-associated pathogenesis, since vIL-6 can promote cell proliferation, cell survival, and angiogenesis that are characteristic of HHV-8-associated Kaposi’s sarcoma, PEL and multicentric Castleman’s disease (MCD), in addition to proinflammatory activities observed in MCD-like “Kaposi’s sarcoma-associated herpesvirus-induced cytokine syndrome.” We show in the present study that vIL-6 can promote productive replication and latent PEL cell viability through upregulation of the mannose-6-phosphate- and peptide hormone-interacting receptor IGF2R, which is a positive factor in HHV-8 biology via these activities. VKORC1v2-enhanced ER-associated degradation of IGF2R and vIL-6 promotion of IGF2R expression through prevention of its interaction with VKORC1v2 and consequent rescue from degradation represent newly recognized activities of VKOCR1v2 and vIL-6.
KEYWORDS: ER-associated degradation, cation-independent mannose-6-phosphate receptor, endoplasmic reticulum, human herpesvirus 8, insulin-like growth factor 2 receptor, latency, replication, viral interleukin-6, vitamin K epoxide reductase complex subunit 1 variant-2
ABSTRACT
Human herpesvirus 8 (HHV-8) viral interleukin-6 (vIL-6) localizes largely to the endoplasmic reticulum (ER) and here associates functionally with both the gp130 signal transducer and the novel ER membrane protein vitamin K epoxide reductase complex subunit 1 variant-2 (VKORC1v2). The latter interaction contributes to the viability of latently infected primary effusion lymphoma (PEL) cells and to HHV-8 productive replication, in part via promotion of ER-associated degradation (ERAD) of nascent pro-cathepsin D (pCatD) and consequent suppression of lysosome-localized proapoptotic mature CatD. Here we report that VKORC1v2 associates with insulin-like growth factor 2 receptor (IGF2R), also known as cation-independent mannose-6-phosphate receptor, which is involved in trafficking of mannose-6-phosphate-conjugated glycoproteins to lysosomes. VKORC1v2 effected reduced IGF2R expression in a manner dependent on VKORC1v2-IGF2R interaction, while vIL-6, which could inhibit VKORC1v2-IGF2R interaction, effected increased expression of IGF2R. These effects were independent of changes in IGF2R mRNA levels, indicating likely posttranslational mechanisms. In kinetic analyses involving labeling of either newly synthesized or preexisting IGF2R, vIL-6 promoted accumulation of the former while having no detectable effect on the latter. Furthermore, vIL-6 led to decreased K48-linked ubiquitination of IGF2R and suppression of ERAD proteins effected increased IGF2R expression and loss of IGF2R regulation by vIL-6. Depletion-based experiments identified IGF2R as a promoter of PEL cell viability and virus yields from lytically reactivated cultures. Our findings identify ER-transiting nascent IGF2R as an interaction partner of VKORC1v2 and target of vIL-6 regulation and IGF2R as a positive contributor to HHV-8 biology, thereby extending understanding of the mechanisms of VKORC1v2-associated vIL-6 function.
IMPORTANCE HHV-8 vIL-6 promotes productive replication in the context of reactivated lytic replication in primary effusion lymphoma (PEL) and endothelial cells and sustains latently infected PEL cell viability. Viral IL-6 is also considered to contribute significantly to HHV-8-associated pathogenesis, since vIL-6 can promote cell proliferation, cell survival, and angiogenesis that are characteristic of HHV-8-associated Kaposi’s sarcoma, PEL and multicentric Castleman’s disease (MCD), in addition to proinflammatory activities observed in MCD-like “Kaposi’s sarcoma-associated herpesvirus-induced cytokine syndrome.” We show in the present study that vIL-6 can promote productive replication and latent PEL cell viability through upregulation of the mannose-6-phosphate- and peptide hormone-interacting receptor IGF2R, which is a positive factor in HHV-8 biology via these activities. VKORC1v2-enhanced ER-associated degradation of IGF2R and vIL-6 promotion of IGF2R expression through prevention of its interaction with VKORC1v2 and consequent rescue from degradation represent newly recognized activities of VKOCR1v2 and vIL-6.
INTRODUCTION
Human herpesvirus 8 (HHV-8) viral interleukin-6 (vIL-6), unlike its cellular counterparts, is secreted very inefficiently, is retained largely in the endoplasmic reticulum (ER), and is functional in this compartment. Activities of vIL-6 are mediated via interactions with the gp130 (IL-6) signaling receptor and also the ER membrane protein vitamin K epoxide reductase complex subunit 1 variant-2 (VKORC1v2) (1–3). The former interaction promotes gp130-mediated mitogen-activated protein kinase and signal transducer and activator of transcription (STAT) signaling; the latter interaction affects protein folding through associations with calnexin cycle enzymes and also regulates ER-associated degradation (ERAD) via interactions with ERAD chaperone and translocon proteins (4, 5). Although vIL-6 is produced in abundance during productive (lytic) replication, it is also expressed at low, functional levels during latency in primary effusion lymphoma (PEL) B cells and is important for maintenance of cell viability in examined PEL cell lines (6). This activity appears to involve interactions of vIL-6 with both gp130 and VKORC1v2 (1, 2). These axes of vIL-6 function are involved also in the vIL-6-mediated promotion of HHV-8 productive replication in reactivated PEL and/or endothelial cells, with demonstrable positive contributions of gp130-activated STAT3 signaling, specifically, to virus replication (3, 7). Proviability and proreplication activities of vIL-6 via VKORC1v2 in PEL cells involve the suppression of proapoptotic cathepsin D (CatD) expression, via vIL-6/VKORC1v2-enhanced ERAD of ER-transiting pro-CatD (4, 7). Pro-folding activities of vIL-6 via VKORC1v2 and calnexin cycle protein interactions (5) could conceivably also contribute to virus latent and/or lytic biology. These activities of VKORC1v2 are the first to be described and represent novel means of vIL-6 function.
In this report we identify insulin-like growth factor 2 receptor (IGF2R; also known as cation-independent mannose-6-phosphate receptor) as a new interaction partner of VKORC1v2. IGF2R is a glycan-modified single-pass (type I) transmembrane receptor with 15 partially conserved repeat domains, one of which (repeat 11) recognizes insulin-like growth factor 2 (IGF2) and three of which (repeats 3, 5, and 9) recognize mannose-6-phosphate (M6P) moieties on glycoproteins within the secretory pathway (8–10). The receptor localizes mainly to trans-Golgi membranes, where it serves to direct M6P-containing Golgi-lumenal proteins to lysosome-destined endosomes, but cycles between intracellular and plasma membranes (11, 12). Other functions of IGF2R include roles in lysosome biogenesis, regulation of extracellular concentrations of IGF2 and leukemia inhibitory factor through uptake and degradation (13, 14), activation of transforming growth factor β (15), and intracellular uptake of exogenous lysosomal enzymes (16). It is notable that in some contexts, IGF2R functions as a tumor suppressor; for example, exogenous expression of IGF2R in SW48 colon carcinoma cells has been reported to inhibit cell growth and increase rates of apoptosis, while the opposite was true in IGF2R-depleted MCF-7 breast carcinoma cells, which were also more responsive to IGF2-induced cell growth and resistant to TGFβ-induced apoptosis (17, 18). Furthermore, loss of heterozygosity in the IGF2R gene is found frequently in a variety of cancers (19).
Here, in addition to identifying IGF2R interaction with VKORC1v2, we report that vIL-6 is able to compete for IGF2R binding to the receptor and promote IGF2R expression and that IGF2R functions to support both latent PEL cell viability and HHV-8 productive replication. Our findings extend understanding of VKORC1v2 and vIL-6 interactions and activities in HHV-8 biology.
RESULTS
VKORC1v2 interaction with IGF2R.
Preliminary experiments were undertaken in which StrepII plus Flag affinity/epitope-tagged VKORC1v2 was used for tandem affinity precipitation from transfected HEK293T cell extracts and coprecipitated proteins identified by mass spectrometry (MS), as outlined previously (5). Among the VKORC1v2-associated proteins identified was IGF2R, a largely Golgi-localized protein involved in the trafficking of mannose-6-phosphate glycan-containing proteins to lysosomes (8, 20). We tested the potential interaction between VKORC1v2 and IGF2R by a transfection-based coprecipitation assay. Chitin-binding domain (CBD)-fused VKORC1v2 was coexpressed with S-peptide-tagged IGF2R via cotransfection of the respective expression vectors (see Materials and Methods); CBD-fused VKORC1 (variant 1 [v1]), which did not interact with IGF2R in the preliminary MS studies and does not interact with vIL-6 (1), was used as a negative control. Interaction between VKORC1v2 and IGF2R was evidenced by mutual coprecipitation using either chitin beads for VKORC1v2-CBD precipitation or S-protein beads for IGF2R-S precipitation (Fig. 1A). Little or no precipitation of the respective “prey” proteins was detected using VKORC1v1-CBD (negative control) instead of VKORC1v2-CBD. These data verified the initial MS-identified interaction (direct or indirect) between VKORC1v2 and IGF2R.
FIG 1.
Interaction between VKORC1v2 and IGF2R. (A) Coprecipitation assays were carried out using transfected HEK293T cell lysates as a source for affinity-tagged VKORC1v2 (CBD-fused, v2-CBD), or negative-control VKORC1v1-CBD (v1-CBD), and IGF2R (S-peptide-linked, IGF2R-S), which were precipitated with chitin and S-protein beads, respectively. Coprecipitated IGF2R-S and VKORC1-CBD proteins were identified by immunoblotting of the respective sedimented material. Appropriate protein expression in vector-transfected cells was confirmed by immunoblotting of cell lysates. (B) CBD-tagged VKORC1v2 variants (Δ1 to Δ5) containing various deletions in the ER-lumenal portion of the receptor, C-terminal to the transmembrane (TM) domain, were used in similar coprecipitation assays. Endogenous IGF2R coprecipitated with chitin bead-bound VKORC1v2-CBD was detected by immunoblotting. IGF2R expression in cell lysates was also checked, along with β-actin, used as a protein loading and membrane-transfer control. The arrowhead indicates the position of the VKORC1v2Δ1 band, expressed at much lower levels than the other VKORC1v2 proteins.
Follow-up experiments were undertaken to try to map the region(s) of VKORC1v2 required for its association with IGF2R. Transfection-based coprecipitation assays similar to those performed previously were used to screen a panel of VKORC1v2 variants containing various deletions within the ER-lumenal region of the membrane protein (Fig. 1B, top) (1). IGF2R immunoblotting of VKORC1v2-CBD coprecipitates was used to assess interactions of the VKORC1v2 variants with endogenous IGF2R. Data from this experiment identified the vIL-6-binding domain (vBD, residues 31 to 39), reported previously (1), as required for interaction of VKORC1v2 with IGF2R (Fig. 1B).
Competitive interactions of vIL-6 and IGF2R with VKORC1v2.
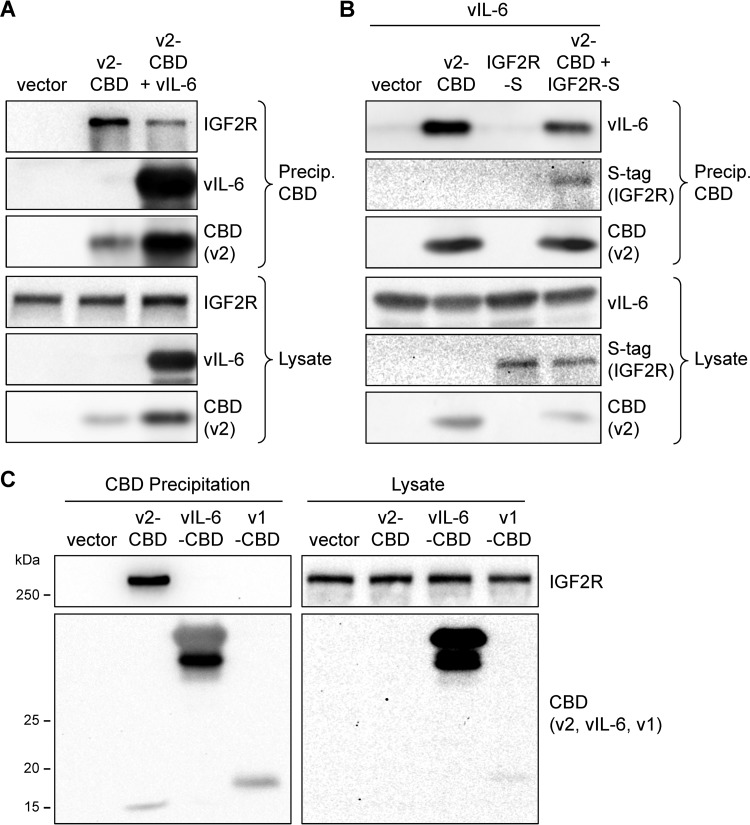
In view of the apparently coincident interactions of vIL-6 and IGF2R on VKORC1v2, coprecipitation experiments were carried out to determine whether the interactions of vIL-6 and IGF2R with VKORC1v2 were competitive. First, coprecipitation of endogenous IGF2R with VKORC1v2-CBD was assessed in the absence and presence of coexpressed vIL-6. This experiment revealed the inhibitory effect of vIL-6 on VKORC1v2-IGF2R interaction, as evidenced by diminished coprecipitation of IGF2R with VKORC1v2-CBD from transfected HEK293T cell lysates (Fig. 2A). Second, vIL-6 was coexpressed with VKORC1v2-CBD in the absence or presence of overexpressed IGF2R (S-tagged), and vIL-6 coprecipitation with chitin bead-precipitated VKORC1v2-CBD was assessed. Overexpression of IGF2R diminished VKORC1v2-associated vIL-6 (Fig. 2B). Combined, these binding data indicate that interactions of IGF2R and vIL-6 with VKORC1v2, requiring coincident residues of the receptor, are mutually exclusive. A further experiment was undertaken to examine the possibility of vIL-6 interaction with IGF2R; the viral cytokine, in contrast to VKORC1v2, could not coprecipitate the receptor (Fig. 2C).
FIG 2.
Competitive vIL-6 and IGF2R interactions with VKORC1v2. (A) VKORC1v2-CBD (v2-CBD)-based coprecipitation assays were carried out in the absence or presence of vIL-6 coexpression to assess the influence of vIL-6 on VKORC1v2-IGF2R interaction. Chitin-bead precipitates and cell lysates were analyzed by immunoblotting for detection of IGF2R (endogenous), VKORC1v2-CBD, and vIL-6. (B) A similar experiment was undertaken to explore the effect of overexpressed IGF2R (S-tagged, IGF2R-S) on vIL-6 interaction with VKORC1v2 in appropriately transfected cells. Chitin-bead precipitates and cell lysates were immunoblotted for detection of CBD (VKORC1v2), vIL-6, and S-tag (IGF2R). (C) Chitin bead-based precipitation analyses of (endogenous) IGF2R interactions with VKORC1v2-CBD (v2-CBD) and vIL-6-CBD; VKORC1v1-CBD (v1-CBD) and empty vector were transfected into parallel cultures to provide negative controls.
Regulation of IGF2R expression by VKORC1v2 and vIL-6.
We previously identified lysosomal protein cathepsin D (CatD) as being regulated (negatively) by vIL-6 via its association with VKORC1v2; suppression of CatD was found to involve interactions of VKORC1v2, vIL-6 and full-length, unprocessed pro-cathepsin D (pCatD) with the ER-associated degradation (ERAD) machinery (4). In view of this, we hypothesized that the interaction of VKORC1v2 with IGF2R might affect IGF2R expression. Examination of IGF2R levels in the presence of increasing amounts of ectopically expressed VKORC1v2 in transfected HEK293T cells revealed suppression of endogenous IGF2R expression by VKORC1v2 (Fig. 3A). Reverse transcription-quantitative PCR (RT-qPCR) of mRNA extracted from parallel cultures transfected with either empty vector or the largest amount of VKORC1v2 expression plasmid (effecting the largest decrease in IGF2R expression) determined that IGF2R mRNA levels were essentially unaffected by VKORC1v2 expression (Fig. 3B); these data indicate a posttranscriptional mechanism of IGF2R regulation by VKORC1v2. A similar experiment was carried out to test the relative effects of wild-type and vBD-deleted (ΔvBD) VKORC1v2 on IGF2R expression. Only the former was able to suppress IGF2R protein levels (Fig. 3C); therefore, IGF2R-VKORC1v2 interaction is required for VKORC1v2-mediated IGF2R suppression.
FIG 3.
Regulation of IGF2R expression by VKORC1v2. (A) Expression of IGF2R in HEK293T cell was monitored as a function of the dose of transfected VKORC1v2-CBD (v2-CBD) expression plasmid (0.4, 0.8, and 1.2 μg) and encoded protein. IGF2R and VKORC1v2-CBD levels were assessed by immunoblotting of cell extracts; probing for β-actin provided a loading control. Quantified levels of IGF2R, relative to the level in empty vector (vec)-transfected cells (set at 1), are shown below the IGF2R blot. (B) RT-qPCR analysis of IGF2R mRNA levels in parallel cultures transfected with 1.2 μg of either empty vector (vec) or VKORC1v2-CBD (v2) expression plasmid. RNA samples were derived from duplicate cultures and each reverse-transcribed sample was analyzed in duplicate by qPCR; averages of the mean qPCR values for each biological replicate were calculated, along with the standard deviations from these average values. The average GAPDH mRNA-normalized IGF2R mRNA level in the VKORC1v2 vector-transfected cultures is shown relative to that in empty vector-transfected cells (set at 1). (C) Wild-type (WT) and vBD-mutated (ΔvBD, lacking residues 31 to 39 [1]) VKORC1v2 proteins were expressed in vector-transfected cells to determine the requirement for (vBD-dependent) VKORC1v2-IGF2R interaction for VKORC1v2 suppression of IGF2R. Representative immunoblots from one of five experiments are shown, together with quantified data from all experiments (chart). IGF2R levels were normalized to β-actin and expressed relative to levels (set at 1) in empty vector-transfected cultures. Standard deviations from average values are indicated along with Student t test P value for VKORC1v2 suppression of IGF2R.
An analogous experiment was carried out to identify any effect of vIL-6 on IGF2R expression. In contrast to the previously observed suppressive effect of vIL-6, via VKORC1v2, on CatD (7), vIL-6 expression correlated with increased levels of endogenous IGF2R (Fig. 4A). This was independent of IGF2R mRNA levels, as determined by RT-qPCR analysis of mRNA extracted from parallel transfected cultures in which the largest amount of vIL-6 vector was used (Fig. 4B). Whether this effect of vIL-6 on IGF2R levels involved VKORC1v2 was tested by comparing vIL-6 activity in genetically engineered VKORC1v2-null versus native HEK293T cells (5). Regulation of IGF2R expression by vIL-6 was not apparent in the VKORC1v2-deficient cells, whereas vIL-6 again led to elevated IGF2R levels in wild-type HEK293T cells (Fig. 4C). Transfection efficiencies (% transfected cells) were determined by cotransfection of a green fluorescent protein (GFP) expression vector and were, in fact, higher for the (nonresponsive) VKORC1v2-deficient HEK293T cultures (70%) than for the native HEK293T cultures (∼50%). Notably, expression of vIL-6 was disproportionately higher in the VKORC1v2-deficient cells, possibly indicating a vIL-6-suppressive effect of VKORC1v2; regardless of the underlying cause, however, the data demonstrated that even at levels of vIL-6 exceeding those achieved in wild-type cells, IGF2R could not be regulated by vIL-6 in the VKORC1v2-deficient cells. To control for potential VKORC1v2-independent effects on vIL-6-regulated IGF2R expression of VKORC1 gene mutation and cell line selection, wild-type VKORC1v2 (expressed from a gRNA/Cas9-resistant ORF) was reintroduced by transfection into the VKORC1v2 knockout cells. VKORC1v2 complementation restored the ability of vIL-6 to boost IGF2R levels, in the context of IGF2R suppression by exogenously added VKORC1v2 (Fig. 4D). These data are consistent with vIL-6 acting to inhibit VKORC1v2-mediated IGF2R suppression.
FIG 4.
Regulation of IGF2R expression by vIL-6. (A) Expression of endogenous IGF2R was measured in response to increasing levels of vIL-6 (expressed from 0.1 to 0.5 μg of vector per transfected culture) relative to empty vector-transfected cells (vec, 0.5 μg). Cell extracts from cultures harvested 30 h after transfection were analyzed by immunoblotting for detection of IGF2R, vIL-6, and β-actin (loading control). Levels of IGF2R (normalized to β-actin) in vIL-6-expressing cells are shown relative to the level (set at 1.0) in empty vector-transfected cells. (B) IGF2R mRNA levels from parallel cultures transfected with 0.5 μg of empty vector or vIL-6 expression plasmid were determined by RT-qPCR, as outlined in the legend to Fig. 3B. (C) Similar analyses of IGF2R protein expression were undertaken in transfected native and VKORC1v2-null (5) HEK293T cells. The levels of IGF2R in vIL-6-expressing cells relative to levels in empty vector (vec)-transfected wild-type and VKORC1 gene-mutated cells (each set at 1.0) are shown below the IGF2R blot. Relative transfection efficiencies in WT cells (50%) and VKORC1v2-null cells (70%) were determined by cotransfection of a GFP expression plasmid. (D) VKORC1v2 (StrepII+Flag-tagged, SF) was introduced exogenously into VKORC1v2-deficient HEK293T cells by transfection of a VKORC1v2 expression plasmid (altered, codon-synonymously, in gRNA target sequences and therefore Cas9-resistant [“CR”]), along with either empty vector (vec) or vIL-6 expression plasmid to verify the role of VKORC1v2 in vIL-6-mediated augmentation of IGF2R expression. IGF2R expression levels, normalized to β-actin and expressed relative to the IGF2R levels (set at 1) in empty vector-transfected cultures, are shown below the IGF2R blot.
Effects of vIL-6 on nascent, ER-localized IGF2R protein.
Considering the ER localization of VKORC1v2 and predominant localization of vIL-6 within this compartment (1, 6), we reasoned that these proteins were likely to act on nascent, ER-transiting IGF2R to mediate its regulation, for example through effects on protein folding and/or ERAD, known to be affected by vIL-6 and VKORC1v2 (4, 5). To test this, we generated a plasmid vector expressing IGF2R protein fused to HaloTag (21), allowing fluorescence pulse-labeling of the protein in transfected cells. Preexisting IGF2R-Halo protein expressed in cells cotransfected with either empty vector or vIL-6 expression plasmid was blocked with HaloTag succinimidyl ester (O4) ligand, and then this was washed out and replaced with the Halo-binding fluorescent probe, TMR (carboxytetramethylrhodamine). TMR-treated cells were harvested at different times for preparation of extracts, and polyacrylamide-gel size-fractionated labeled IGF2R-Halo was visualized by UV illumination. The results revealed that vIL-6 promoted the accumulation of newly synthesized IGF2R-Halo protein (Fig. 5A), consistent with its acting in the ER compartment. Using a converse experimental design in analogously transfected cultures, we probed preexisting IGF2R-Halo with TMR and then removed the probe, washed with fresh medium, and blocked with HaloTag-O4 (to ensure no further labeling by any remaining TMR); cells were harvested at various times thereafter. While this experiment detected clearly elevated levels of IGF2R in vIL-6-expressing cells, consistent with our previous findings, there was no detectable difference in the rate of IGF2R decay (Fig. 5B). These data, along with those of Fig. 5A, suggest that vIL-6 acts only on nascent, ER-localized IGF2R.
FIG 5.
vIL-6 regulation of nascent IGF2R. (A) Halo-tagged IGF2R was introduced into HEK292T cells via expression vector transfection, along with empty vector or vIL-6 expression plasmid. Pulse-labeling of IGF2R-Halo was achieved by addition of fluorescent Halo-ligand probe TMR to cultures 24 h posttransfection, replacing blocking ligand, HaloTag succinimidyl ester O4 (“Halo-O4”), added immediately after transfection. Parallel cultures were harvested at different times post-TMR addition, extracts were run on a polyacrylamide gel, and TMR-labeled IGF2R-Halo was visualized under UV illumination. Proteins were then transferred to nitrocellulose membrane for immunoblotting for vIL-6 (to check expression) and β-actin (normalization control). Digitally quantified levels of IGF2R-Halo/TMR fluorescence, normalized to β-actin, are shown as fold increases above background levels (at the zero time points). (B) A similar experiment was undertaken, but here TMR was added for 15 min to label all IGF2R-Halo in “unblocked” cultures, and then was removed and replaced with blocking agent (Halo-O4) to prevent any labeling of subsequently produced IGF2R-Halo. Replicate cultures were harvested at different times postlabeling to track IGF2R-Halo decay as a function of vIL-6 expression. Analyses were as outlined for panel A. The levels of TMR-labeled IGF2R, normalized to β-actin, are shown relative to levels (set at 1) expressed at 0 h in both the absence and presence of vIL-6 (ND, not determined; specific signal versus background uncertain). (C) Testing of ER-restricted, KDEL motif-tagged vIL-6 (vIL-6.K), relative to native vIL-6, for enhancement of IGF2R expression. Equal amounts of the respective vIL-6 vectors and empty vector (vec) were transfected separately into parallel HEK293T cell cultures and extracts, made 24 to 48 h posttransfection (depending on the particular experiment), and coharvested media were analyzed by immunoblotting. Proteins from media were first precipitated with trichloroacetic acid to enable concentration. Immunoblot data from one of five experiments are shown; β-actin-normalized levels of IGF2R in vIL-6- and vIL-6-KDEL-expressing cells relative to those in empty vector-transfected cells are shown in the chart, using the same presentation and analysis outlined for Fig. 3C. (D) To test exogenously added vIL-6 for potential IGF2R-regulatory activity, media from vIL-6 vector-transfected HEK293T cells or from empty vector (vec)-transfected cells were applied to naive HEK293T cultures for 24 h prior to preparation of cell extracts. Relative levels of IGF2R (shown normalized to β-actin) were determined by immunoblotting. Immunodetection of active phospho-STAT3 (pSTAT3, Y705-phosphorylated) and total STAT3 (normalization control) confirmed vIL-6-induced signaling and therefore functional levels of vIL-6. A representative of three experiments is shown.
As an independent approach to assess the ER-localized regulation of IGF2R by vIL-6, we examined the ability of an ER-retained form of vIL-6, vIL-6-KDEL (6), to affect IGF2R expression. vIL-6-KDEL, which was retained almost exclusively intracellularly by virtue of the attached KDEL ER-localization signal, was fully active in enhancing IGF2R expression in transfected cells (Fig. 5C), providing strong evidence that IGF2R regulation by vIL-6 occurs in the ER (where vIL-6 and VKORC1v2 are naturally localized [1, 6]), acting on newly synthesized IGF2R protein transiting the compartment. We further tested whether extracellularly acting vIL-6 could modulate IGF2R, by applying transfected culture-derived conditioned media containing vIL-6, or control media from empty vector-transfected cultures, to fresh HEK293T cultures for 24 h. While vIL-6 was clearly active, as determined by increased Y705-phosphorylated STAT3 levels relative to those in cultures treated with control media, IGF2R levels were unaffected (Fig. 5D). These data indicate that intracellularly acting vIL-6 is essential for IGF2R regulation.
Regulation of ER-associated degradation of IGF2R by vIL-6.
To examine the involvement of ERAD in vIL-6 regulation of IGF2R, we tested the influence of vIL-6 on IGF2R ubiquitination, and specifically K48-linked polyubiquitination, which would occur if the (newly synthesized) protein is processed through ERAD. We carried out transfection-based immunoprecipitation experiments employing vectors expressing Flag-tagged wild-type (Flag-Ub), K48-only (Flag-K48), or K-null (Flag-K0) ubiquitin and cotransfected empty vector or vIL-6 expression plasmid to determine the relative levels of ubiquitinated IGF2R in response to vIL-6. We found that vIL-6 coexpression reduced the level of (Flag-immunoprecipitated) ubiquitinated IGF2R in cells overexpressing both wild-type and K48-only ubiquitin proteins (Fig. 6A), indicating suppression by vIL-6 of ERAD-processing of IGF2R. Consistent with this model, overexpression of K-null ubiquitin, abrogating ERAD-associated K48-polyubiquitination and retrotranslocation of IGF2R (22), led to loss of vIL-6 suppression of IGF2R ubiquitination.
FIG 6.
IGF2R regulation by vIL-6 via ERAD. (A) Influence of vIL-6 on IGF2R ubiquitination. HEK293T cultures were transfected with either empty vector (vec, negative control) or vIL-6 expression plasmid along with vectors expressing Flag-tagged ubiquitin proteins (wild-type [Flag-Ub], K48-only [Flag-K48], or K-null [Flag-K0]). Cells were harvested 48 h after transfection, Flag-tagged ubiquitin was immunoprecipitated from cell lysates, and precipitated, urea-washed material was immunoblotted for detection of IGF2R. Equivalent Flag-Ub precipitation between paired samples was verified via Flag immunoblotting of immunoprecipitates. Relative amounts (rel. amt.) of ubiquitinated IGF2R precipitated from lysates of empty vector-transfected cells (set at 1) and vIL-6-expressing cells are shown below the IGF2R blot. Cell lysates were probed for vIL-6 and β-actin to verify appropriate vIL-6 expression. (B) Plasmid vector-expressed shRNAs directed to mRNA of either HRD1 or gp78 ERAD E3 ligases were tested relative to control (NS) shRNA for their efficacies in HEK293T cells cotransfected with vectors expressing S-epitope-tagged HRD1 or gp78. Cell extracts were made 48 h posttransfection and analyzed by immunoblotting. (C) The involvement of ERAD in vIL-6 regulation of IGF2R was tested directly by comparing vIL-6 activity in response to shRNA-mediated depletion of HRD1 and gp78. Cell lysates from test (HRD1 and gp78) and control (NS) shRNA (sh) vector-transfected cultures cotransfected with either empty vector (vec) or vIL-6 expression plasmid were analyzed for IGF2R expression 48 h posttransfection. The relative levels of IGF2R, normalized to β-actin, are shown below the IGF2R blot.
The role of ERAD in vIL-6-mediated regulation of IGF2R was examined also by shRNA-mediated depletion of each of two critical components of the ERAD machinery, the E3 ligases HRD1 and gp78 (23, 24). Initial testing of the plasmid-expressed shRNAs was done in cells cotransfected with vector-expressed S-tagged HRD1 or gp78, confirming target protein depletion (Fig. 6B). The shRNA vectors were then used in the context of cells cotransfected with either empty vector or vIL-6 expression plasmid. Immunoblotting of cell extracts for the detection of endogenous IGF2R levels revealed increased IGF2R expression in the presence of vIL-6 in nonsilencing (NS) shRNA vector-transfected cells, but complete abrogation of this activity in the context of HRD1 or gp78 depletion (Fig. 6C). These data demonstrate the involvement of ERAD in vIL-6-mediated regulation of IGF2R. Importantly, depletion of the ERAD proteins led to vIL-6-indepenent increases in IGF2R levels, revealing the importance of ER-localized, ERAD-mediated regulation of nascent IGF2R for overall expression of the protein. Combined with data from the VKORC1v2 knockout studies (Fig. 4B and C), our results provide evidence of vIL-6-mediated rescue of IGF2R from VKORC1v2-promoted ERAD.
IGF2R regulation by vIL-6 and VKORC1v2 in the context of infected cells.
The effect of vIL-6 on IGF2R was investigated in HHV-8-infected PEL cells, TRExBCBL1-RTA (25) and JSC-1 (26), via lentiviral vector-mediated ectopic overexpression or shRNA-mediated depletion of the viral cytokine. As in the transfected HEK293T cells (Fig. 4), expression of IGF2R correlated positively with ectopic expression of vIL-6 in both PEL cell lines (Fig. 7A). Conversely, shRNA-mediated depletion of vIL-6 in these cells led to reduced IGF2R expression (Fig. 7B). Reduced IGF2R expression was also observed in the context of lytically reactivated, doxycycline-treated, TRExBCBL1-RTA cells (Fig. 7C). The same effect was seen in iSLK epithelial cells infected with HHV-8 BAC16 virus (27), where IGF2R expression was diminished in lytically reactivated cells infected with vIL-6-null HHV-8 (5) relative to levels observed in wild-type virus-infected cells, expressing vIL-6 during lytic replication (Fig. 7D). The influence of endogenous VKORC1v2 on IGF2R regulation was assessed using shRNA-mediated depletion; this led to increased IGF2R expression in both BCBL-1 (TRExBCBL1-RTA) and JSC-1 cells (Fig. 7E). Therefore, vIL-6 and VKORC1v2 enhance and suppress, respectively, the expression of IGF2R in infected cells as in transfected cells (Fig. 3 and 4).
FIG 7.
Regulation of IGF2R by vIL-6 and VKORC1v2 in the context of infection. (A) IGF2R expression as a function of lentiviral vector-mediated vIL-6 overexpression in BCBL-1 (TRExBCBL1-RTA) and JSC-1 PEL cells was determined by immunoblotting of cell extracts made 48 h after lentiviral vector transduction. Lentivirus with no inserted ORF (vec) was used for transduction of parallel cultures to allow comparisons. Levels of IGF2R expression relative to those in empty vector-transduced cells (set at 1) and normalized to β-actin are shown. (B) Similar experiments were carried out to investigate the effect of vIL-6 depletion on IGF2R levels in the PEL cell lines. vIL-6 mRNA-directed shRNA or nonsilencing (NS) control shRNA was transduced via lentiviral vector infection. The relative levels of IGF2R expression, calculated as outlined in panel A, are shown. (C) The same approach was used in TRExBCBL1-RTA cells to investigate the effect of vIL-6 depletion on IGF2R expression in the context of lytic replication, induced by doxycycline (Dox) treatment (+Dox). Cells were harvested 48 h postinduction (lytic) or mock treatment (latent). Latent and lytic cell extracts were run on the same gel; the dotted lines indicate deletion of a blank lane. Levels of IGF2R expression in vIL-6-depleted cells relative to those (set at 1) in NS shRNA-transduced cells are shown (values are β-actin-normalized). (D) Expression of IGF2R in latently infected and lytically reactivated (doxycycline- and sodium butyrate-treated) iSLK cells infected with wild-type (WT) or vIL-6-null (vIL-6X) BAC16-derived HHV-8. Cells were harvested 48 h postinduction for preparation of cell extracts for immunoblotting. The LANA immunoblot confirms equivalent latent viral loads in cultures infected with identical infectious doses of the wild-type and mutated viruses. Relative IGF2R expression levels, normalized to β-actin, between cells infected with wild-type virus (set at 1) and vIL-6-null virus are shown below the IGF2R blot. (E) The role of endogenously expressed VKORC1v2 on IGF2R expression was tested in BCBL-1 (TRExBCBL1-RTA) and JSC-1 cells using lentivirally delivered VKORC1v2 (v2) mRNA-targeting shRNA or control NS shRNA. Cells were harvested 4 days (BCBL-1) or 5 days (JSC-1) postransduction for immunoblot analysis of IGF2R expression; the values shown represent β-actin-normalized IGF2R expression levels in VKORC1v2-depleted cells relative to controls (set at 1) for each cell type. RT-PCR was applied to mRNA extracted from samples of the same cultures to verify shRNA-mediated depletion of VKORC1v2 mRNA; VKORC1v1 (v1) mRNA (lacking the shRNA target site) was unaffected. RT-PCR for detection of GAPDH mRNA provided a normalization control.
Influence of IGF2R on PEL cell viability and HHV-8 productive replication.
To test the biological significance of IGF2R in HHV-8 biology, we first investigated the effect of its depletion on PEL cell growth and viability. Lentiviral vectors expressing each of two IGF2R mRNA-directed shRNAs, along with NS control shRNA, were used to infect BCBL-1 (TRExBCBL1-RTA) and JSC-1 cells to yield transduction efficiencies of >90%, as determined by lentiviral vector-coexpressed GFP fluorescence. After 2 to 3 days of rest, viable (trypan blue-excluding) cell densities of the cultures were normalized and monitored for a further 3 days. On the last day, cells were analyzed by annexin V-Cy3 and Hoechst 33342 staining to determine the frequency of apoptotic cells. The data from these analyses revealed that IGF2R depletion effected substantial reductions of cell viability and overall culture growth for both PEL cell lines tested (Fig. 8A and B). Therefore, IGF2R is important for sustained latent cell viability.
FIG 8.
Role of IGF2R in HHV-8 biology. (A) and (B) Effect of IGF2R on latent PEL cell growth. BCBL-1 (TRExBCBL1-RTA) (A) and JSC-1 (B) cultures were transduced at high efficiency (>90%) with (GFP+) lentiviral vectors expressing either of two shRNAs (designated sh3 and sh4) directed to IGF2R mRNA or nonsilencing (NS) shRNA (control). After 3 days, trypan blue-excluding, viable-cell densities determined by hemocytometric counting were normalized (day 0) and then monitored for a further 3 days. On day 3, culture samples were analyzed by annexin V-Cy3 staining and nuclear counterstaining with Hoechst 33342 to determine rates of apoptosis. Data were collected from multiple, random fields from each culture (>400 cells viewed per culture). Average values from triplicate cultures are shown for the growth and apoptosis analyses (top and bottom charts, respectively); error bars show standard deviations from these averages. P values from Student t test (paired, two-tailed) are indicated. The immunoblots (bottom) show effective depletion of IGF2R by each of the IGF2R-specific shRNAs relative to the NS shRNA control; samples were derived from pooled terminal BCBL-1 and JSC-1 cultures in each treatment group. (C) Effect of IGFR2 depletion on HHV-8 productive replication. TRExBCBL1-RTA cells were induced into lytic replication by treatment with doxycycline (see Materials and Methods) and after 4 days, culture media were harvested for assessment of released viral titers, as determined by latency-associated nuclear antigen (LANA) immunofluorescence staining of inoculated iSLK cells (infectious assay) or qPCR-measured copy numbers of DNase I-resistant (encapsidated) viral genomes (virion DNA assay), using established methods (3, 28). Data were derived from duplicate cultures; error bars show standard deviations from the plotted mean values. Student t test-derived P values are indicated. Cell lysates from terminal cultures were immunoblotted to confirm IGF2R depletion.
We next tested the influence of IGF2R on HHV-8 productive replication, using the same approach of IGF2R depletion coupled with lytic reactivation from TRExBCBL1-RTA cells, which induce efficiently upon treatment with doxycycline (25) to give readily measurable virus yields. Infectious virus and encapsidated viral DNA titers in media derived from NS shRNA- versus IGF2R shRNA-transduced cultures 4 days after lytic induction were determined by LANA staining of inoculated iSLK cells and qPCR quantification of DNase I-resistant viral DNA, as reported previously (3, 28). Data from this experiment (Fig. 8C) identified a positive role of IGF2R in HHV-8 productive replication in these cells.
DISCUSSION
Activities of HHV-8 vIL-6 in both latency (in PEL cells) and productive replication (in PEL and endothelial cells) have been reported (3, 6, 7). vIL-6 acts through gp130 signaling and VKORC1v2-mediated pathways to effect proviability and proreplication functions in these infected cell types. Two identified mechanisms of VKORC1v2-associated activities of vIL-6 are the suppression of proapoptotic CatD expression through promotion of ER-associated proteasomal degradation of precursor, ER-transiting pCatD and enhancement of protein folding via VKORC1v2 and vIL-6 interactions with calnexin cycle components (4, 5). Here, we report that IGF2R interacts with VKORC1v2 and that IGF2R expression is regulated through ER-associated activities of vIL-6. While VKORC1v2 suppresses IGF2R protein levels, in a binding-dependent manner, vIL-6 increases IGF2R expression, and specifically leads to increased kinetics of accumulation of newly synthesized IGF2R protein passaging through the ER. This effect of vIL-6 is essentially the opposite of its influence on pCatD. The different outcomes may be the result of distinct sites of interaction on VKORC1v2 of IGF2R and pCatD; the former, specifically, requires for interaction the same residues of VKORC1v2 that bind vIL-6, resulting in competition by vIL-6 for IGF2R binding, directly or via other proteins, to VKORC1v2. In contrast, vIL-6, VKORC1v2, and pCatD can cocomplex and, indeed, vIL-6 promotes the interaction of pCatD with VKORC1v2 (7). We hypothesize that competition versus cocomplexing may, in part at least, determine the fate of VKORC1v2-interacting proteins in relation to their regulation by vIL-6 via the receptor, competition enabling removal of VKORC1v2-interacting IGF2R from the prodegradation activities of VKORC1v2 through ERAD protein interactions (4). We have provided evidence here that VKORC1v2-IGF2R interaction, mediated via VKORC1v2 “vBD” residues 31 to 39, is necessary for VKORC1v2 suppression of IGF2R. However, while we consider it likely that direct pro-ERAD functions of VKORC1v2 are involved in IGF2R regulation by the receptor and vIL-6, it is also possible that vIL-6 may promote IGF2R folding, through VKORC1v2 and vIL-6 interactions with calnexin cycle proteins (5), resulting a higher proportion of correctly folded nascent IGF2R and thereby overall rescue of IGF2R from ERAD. The ER-localized effect of vIL-6 specifically on newly synthesized IGF2R (Fig. 5) is consistent with each of these models. That ERAD is involved in IGF2R regulation by vIL-6 can be deduced from the suppression of IGF2R ubiquitination, and specifically K48-linked polyubiquitination, by vIL-6 (Fig. 6A) and from the inhibition of vIL-6 regulation of IGF2R by depletion of ERAD translocon-associated E3 ligases HRD1 and gp78 (Fig. 6C). Together, the mechanistic data presented here provide evidence that vIL-6 inhibits VKORC1v2-promoted proteasomal degradation of (likely misfolded) nascent IGF2R through vIL-6-VKORC1v2 interaction-effected competitive release of IGF2R from VKORC1v2 association.
With regard to the possible biological significance of IGF2R regulation by vIL-6, we have demonstrated empirically through depletion and deletion analyses that vIL-6 positively regulates IGF2R expression in the contexts of latently infected PEL cells and lytically infected PEL and iSLK cells and that IGF2R is required for sustained viability of latently infected PEL cells (JSC-1 and BCBL-1) and for efficient HHV-8 productive replication in this cell type. There are several reported functions of IGF2R. These include the trafficking of M6P-containing glycoproteins from the Golgi to the lysosome, capture and lysosomal localization and/or degradation of exogenous lysosomal proteins and signaling ligands, and signal transduction via binding of particular ligands, including IGF2 and proliferin, to IGF2R (reviewed in reference 8). In view of our previous work on VKORC1v2 and vIL-6 regulation of pCatD, a lysosomal protein and interactor of IGF2R, we were particularly interested in the potential for VKORC1v2 and vIL-6 to regulate this lysosomal trafficking receptor when we identified it as an interactor of VKORC1v2. However, counter to our initial hypothesis that vIL-6 might, through cointeraction, destabilize IGF2R via ERAD, in turn effecting reduced levels of CatD and other “stress-related” lysosomal enzymes, the opposite was found to be the case. Indeed, as already stated, we found that IGF2R enhances both latent PEL cell viability and HHV-8 productive replication. Therefore, the promotion of IGF2R expression by vIL-6 represents a “proviral” function. At present, we do not know the underlying mechanistic basis of IGF2R activity in the context of PEL infection. It is possible that unnaturally low or high levels of CatD and other lysosomal proteins in addition to dramatically altered lysosomal function are detrimental to PEL cell viability and productive replication; this would explain both the positive effects of CatD depletion and negative effects of IGF2R depletion on PEL cell and virus biology. Of note is that intracellular retention of cathepsin B mediated via HHV-8-induced IGF2R is critical for postconfluent growth of latently infected endothelial cells (29); similar mechanisms may be operative in other cell types and contribute to cell growth and virus replication. However, considering the varied activities of IGF2R and its divergent effects in different cells (e.g., tumor suppressor versus progrowth activities [18, 19, 30, 31]), it is premature to speculate on the relevant pathways of latent and lytic IGF2R activities that we have detected in PEL cells and that may occur in other cell types; identification of these mechanisms will require extensive additional work.
In summary, we have identified here a new VKORC1v2-associated protein, IGF2R, a new mechanism of vIL-6 function via disruption of this interaction, and the significance of IGF2R to HHV-8 latent and lytic biology. These findings extend significantly our understanding of VKORC1v2 and vIL-6 functions in the context of HHV-8 infection.
MATERIALS AND METHODS
Plasmids and oligonucleotides.
Previously reported pSG5- and pCEBZ-based vectors expressing chitin-binding domain (CBD) fusions of vIL-6, VKORC1v1, and full-length and deletion variants (Δ1-5) of VKORC1v2 (1, 5) were used in this study. Also used here and previously reported were the vIL-6 expression vectors pcDNA3.1(+)-vIL6-twin-StrepII (vIL-6-TS) (7), pSG5.S-vIL-6 (32), pSG5.S-vIL-6-KDEL (6), and pRZ1.1-RFP-P2A-vIL-6 (7), as well as lentiviral vectors expressing nonsilencing (NS; control) shRNA or shRNAs directed to vIL-6, VKORC1v2, or HRD1 mRNA (1, 4, 6). HHV-8 BAC16 recombinant virus HHV-8.vIL-6.TTG (vIL-6 knockout) was recently published (33). A plasmid (pCEBZ-hsp-StrepII-IGF2R-S) expressing IGF2R fused N-terminally with the signal peptide sequence of hIL-6 and the StrepII tag and C-terminally with the S peptide tag was generated by cloning of the PCR-amplified coding sequences of the mature (signal sequence-cleaved) form of IGF2R between the NheI and EcoRI sites of the vector pCEBZ-hSP-StrepII (11). StrepII-IGF2R-encoding sequences were excised from pCEBZ-hsp-StrepII-IGF2R-S using SbfI and AgeI restriction digestion and inserted between the corresponding sites of the Halo tag vector pHTC HaloTag CMV-neo (Promega, G7711) to generate pHTC-StrepII-IGF2R. A pCEBZ-based, lentiviral vector for expression of a CBD-fused vBD-deleted version of VKORC1v2 was generated by cloning of PCR-amplified VKORC1v2Δ3 open reading frame (ORF) sequences between the AgeI and BamHI sites of pCEBZ-RFP-CBD (9), replacing red fluorescent protein (RFP) coding sequences. An equivalent expression vector containing CRISPR-resistant (CR) VKORC1v2 coding sequences was generated by cloning of guide-RNA (gRNA) target site-mutated VKORC1v2 ORF sequences between the AgeI and BamHI sites of pCEBZ-RFP-CBD. The gRNA target site gcggctcgctctttgcctgacgg (5) was mutated, codon-synonymously, to gcgActTgcActCtgTctCacAg (capitals indicate mutations) using overlapping PCR to generate the mutated sequences in the context of the complete VKORC1v2 ORF. Plasmids encoding Flag-tagged wild-type, K48-only, and lysine-knockout ubiquitin were made by PCR amplification of the respective ORF sequences from plasmids pRK-HA-Ubiquitin-WT, -K48, and -K0 (Addgene, plasmid numbers 17608, 17605, and 17603; deposited by T. Dawson), using a Flag-encoding and SbfI restriction site-containing primer directed to 5′ ubiquitin ORF sequences and a reverse primer complementary to 3′ ORF sequences and specifying an MluI restriction site. The derived PCR fragments were cloned between SbfI and MluI sites of a pCEBZ vector derivative (pSGCR) containing an EGFP expression cassette inserted upstream of the cytomegalovirus promoter. For IGF2R depletion, sequences specifying IGF2R mRNA-directed shRNAs were cloned into the lentiviral vector pYNC352 using BamHI and MluI restriction sites; the target sequences were GGAAGCTGTTGATACCAAA (shRNA 3) and GCGATACCTCTCAAGTCAA (shRNA 4). Sequences specifying gp78 mRNA-directed shRNA, containing the target sequence TGCACACCTTGGCTTTCAT, were cloned between the two BsmBI sites of the lentiviral vector psEF1a-mCherry-H1-BB (4). The primers used for IGF2R-specific RT-PCR were 5′-TAGATATCGGCCGGGTAGCA-3′ (forward, ORF positions 5313 to 5332) and 5′-CTTAGGCGTTCCCATGCTCA-3′ (reverse, ORF positions 5476 to 5457); the primers were designed based on the GenBank database sequence NM000876. Primers directed to VKORC1 mRNA (identifying alternatively spliced and terminating transcripts) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA (RT-PCR normalization control) were described previously (1, 7).
Cell culture, transfection, and virus production.
HEK293T and iSLK cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics (penicillin and streptomycin; Corning, catalog no. 30-002-CI). TRExBCBL1-RTA and JSC-1 cells were grown in RPMI 1640 medium supplemented with 10% FBS and antibiotics. HEK293T cells (40% to 60% confluence) were transfected with polyplex formed by plasmid DNA and cationic polymer linear polyethylenimine (Polysciences, catalog no. 593215) at 16 to 24 h postseeding. For lentivirus production, HEK293T cells in T-75 flasks and at ∼80% confluence were cotransfected with 12 μg of the lentiviral vector, 9 μg of psPAX2, and 3 μg of pMD2.G (Addgene, catalog no. 12260 and 12259, respectively; deposited by D. Trono), encoding HIV gag-pol and VSV glycoprotein G packaging and envelope proteins. After 6 to 8 h of transfection, the culture medium was replaced with fresh medium containing 10% FBS. After 2 days of culture, virus-containing medium was collected and concentrated by ultracentrifugation in an SW-32 Ti rotor at 25,000 rpm for 2 h at 4°C. The supernatant was discarded, and 5 ml of RPMI 1640 medium supplemented with 10% FBS was added to the viral pellets and left at 4°C overnight; aliquots of resuspended virus were stored at –80°C. For generation of BAC16 virus, BAC16 genomic DNA was transfected into iSLK cells with FuGENE 6 transfection reagent (Promega, catalog no. E2693) and BAC16-containing cells (GFP positive) were selected by treatment of cultures with hygromycin B (2.3 mM) (Invitrogen, catalog no. 10687010) for 3 weeks. Productive replication was induced by the addition of doxycycline (1.9 nM [1 μg/ml]) and sodium butyrate (1 mM); virus-containing media were harvested at 3 and 5 days after lytic induction, and these media were combined. Virus was concentrated by ultracentrifugation in an SW-32 Ti rotor at 25,000 rpm for 2 h at 4°C. The supernatant was discarded, and the virus pellet was resuspended in 5 ml of DMEM containing 10% FBS; aliquots of virus stock were stored at –80°C. Equivalent titers of lentiviruses (test and controls) and BAC16 viruses (wild type and mutated) were used experimentally to provide “normalized” cultures for phenotypic analyses.
Affinity precipitations, immunoprecipitations, and Western blotting.
For coprecipitation assays involving chitin-bead or S-protein-bead affinity precipitation of CBD- or S-tagged proteins, HEK293T cells were cotransfected with appropriate expression plasmids or empty-vector controls. At 48 h posttransfection, the cells were harvested and lysed in 0.2% NP-40 lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, and 0.2% NP-40) containing protease inhibitor cocktail (Sigma, catalog no. P8340). The cells were incubated for 1 h at 4°C and the lysates were clarified by centrifugation at 16,000 × g for 10 min at 4°C. CBD- and S-tagged proteins were precipitated from the supernatants by incubation at 4°C overnight with chitin resin (New England Biolabs, catalog no. S6651) or S-protein agarose (Novagen, catalog no. 69704), respectively. Precipitates were washed three to five times with 0.05% NP-40 wash buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 0.05% NP-40). Coprecipitated proteins, released from beads by boiling in denaturing buffer, were identified by immunoblotting. For immunoprecipitation of Flag-ubiquitin-conjugated proteins (to examine IGF2R ubiquitination), Flag-ubiquitin expression vector-transfected cells were incubated for 1 h at 4°C in 0.2% NP-40 lysis buffer, and samples were then sonicated. Anti-Flag magnetic beads (Sigma, catalog no. M8823) were used for magnetic capture of Flag-ubiquitin. Washing included a step using TBS (50 mM Tris-HCl [pH 7.5], 150 mM NaCl) containing 1 M urea following three to five washes with TBS containing 0.05% NP-40. For immunoblotting, membranes were blocked in 5% nonfat milk in TBS-T (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% Tween 20) for 1 h at room temperature, followed by incubation for at least overnight at 4°C with primary antibody. Membranes were washed three times (5 min for each wash) in TBS-T prior to the addition of secondary antibody and, following incubation for 1 h at room temperature, washed three times in TBS-T. The protein bands were visualized using Amersham ECL Western blotting detection reagent (GE Healthcare, catalog no. RPN2106).
Antibodies.
Primary antibodies used in this study were directed to S-peptide (Abcam, catalog no. ab184223), CBD (New England BioLabs, catalog. no. E8034S), IGF2R (Abcam, catalog no. ab124767), β-actin (Sigma, catalog no. A5316), Flag (Sigma, catalog no. F1804), LANA (Advanced Biotechnologies, catalog no. 13-210-100), pSTAT3 (Cell Signaling Technology, catalog no. 9131S), and STAT3 (Santa Cruz, catalog no. sc-482). Rabbit polyclonal antiserum to vIL-6 was described previously (32). The following secondary antibodies were used: horseradish peroxidase-linked anti-mouse IgG (GE Healthcare, catalog no. NA931), anti-rabbit IgG (GE Healthcare, catalog no. NA934), and anti-rat IgG (Invitrogen, catalog no. 31470); Cy3-conjugated anti-rat IgG (Invitrogen, catalog no. A10522).
RT-PCR.
RNA was isolated from transfected HEK293T cells using TRIzol reagent (Invitrogen, catalog no. 15596026) according to the manufacturer’s protocol. Reverse transcription (RT) of DNase I-treated (DNA-free) RNA samples was carried out using Superscript III reverse transcriptase (Invitrogen, catalog no. 56575) in the presence of 0.5 mM deoxynucleoside triphosphates and 2.5 μM random hexamer deoxyoligonucleotides in RT buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2). First-strand cDNA was subjected to quantitative-PCR (qPCR) using Power SYBR green PCR master mix (Thermo Fisher Scientific, catalog no. 4367659), as described previously (34), using IGF2R- or GAPDH-specific primers (see above). Reactions were run on an Eppendorf RealPlex2 machine and relative abundances of target cDNA calculated from the threshold cycle (CT) values. Abundances of VKORC1v2 mRNA versus VKORC1v1 and GAPDH mRNA were undertaken by semiquantitative RT-PCR using appropriate primer pairs (see above) and agarose gel-based analysis of the derived products.
HaloTag-based kinetic analysis of IGF2R.
To assess the effects of vIL-6 on accumulation of newly synthesized IGF2R, HEK293T cultures transfected with IGF2R-Halo expression vector were treated with 50 μM HaloTag succinimidyl ester (O4) ligand (Promega, catalog no. P6751) to “mask” subsequent HaloTag labeling of preexisting IGF2R-Halo; HaloTag amine O4 was applied immediately after transfection (6 h after DNA addition to cultures). After 24 h, medium containing the blocking ligand was removed, cell monolayers were washed three times with phosphate-buffered saline (PBS), and medium containing 50 nM HaloTag carboxytetramethylrhodamine (TMR) ligand (Promega, catalog no. G8252) (21) was added to label IGF2R-Halo. Parallel cultures were harvested at different times postlabeling for generation of cell lysates, samples of which were loaded onto a denaturing polyacrylamide gel; fluorescently labeled (TMR-bound) IGF2R-Halo was visualized under UV (Bio-Rad molecular imager gel Doc XR+). Similar methods were used to assess the influence of vIL-6 on preexisting IGF2R-Halo, but here, the blocking agent (HaloTag-O4) was applied after TMR treatment; the ligand (50 nM final concentration) was added for 5 min to replicate cultures 24 h posttransfection, followed by the discarding of TMR-containing media, PBS washing of cell monolayers, and addition of medium containing HaloTag-O4 (50 mM). Cultures were harvested at different times for generation of cell lysates, which were analyzed as before for detection of IGF2R-Halo.
Cell growth and apoptosis assays.
The influence of IGF2R on PEL cell growth and survival was assessed in TRExBCBL1-RTA and JSC-1 cells by monitoring viable-cell densities over time and assessment of % annexin V-Cy3 staining of cells in cultures transduced with either nonsilencing (NS) or IGF2R mRNA-directed shRNAs (see above). Transduction of shRNAs was achieved via lentiviral vector (GFP-expressing) infection of cultures to achieve a transduction rate of ∼90%, as assessed by GFP fluorescence, as outlined previously (6). After 2 to 3 days of rest, viable (trypan blue-excluding) cell densities in NS shRNA- versus IGF2R shRNA-transduced cultures were normalized, and cell densities in replicate cultures were monitored by hemocytometry daily for 3 days. Cell samples from the last day were analyzed by staining with annexin V-Cy3 (BioVision, catalog no. 1002-200) staining and nuclear counterstaining with Hoechst 33342 to determine the percentage of apoptotic cells in each culture. For annexin V-Cy3 staining, cells were washed with annexin V binding buffer (10 mM HEPES [pH 7.4], 140 mM NaCl, and 2.5 mM CaCl2), and annexin V-Cy3 reagent was added for 5 min at a dilution of 1:500 in this buffer in the dark. Cells were then washed again in binding buffer by centrifugation pelleting and resuspension (twice) prior to fixing in PBS containing 4% paraformaldehyde (PFA) and nuclear staining with Hoechst 33342 (1 μg/ml). Finally, cells were washed in PBS prior to final resuspension in PBS containing 4% PFA and transfer to a microscope slide; after desiccation, cells were overlaid with 90% glycerol and a coverslip for visualization of Cy3 (annexin V, red) fluorescence and Hoechst-counterstained (blue) nuclei by UV microscopy.
HHV-8 reactivation and replication assays.
TRExBCBL1-RTA cells, transduced with either NS or IGF2R shRNA, were normalized for cell density 2 to 3 days posttransduction and treated with doxycycline (1 μg/ml) for 24 h to induce lytic reactivation. Cells were then placed in fresh media, and, after 4 days, virus-containing media were harvested for determinations of virus yields. Infectious virus and encapsidated viral genome titers were determined from duplicate cultures essentially as described previously (33), using LANA-based immunofluorescence staining of medium-inoculated HHV-8-naive iSLK cells and qPCR-based quantitation of viral DNA present in medium samples pretreated with DNase I to remove unencapsidated DNA. For the LANA immunofluorescence assay, four random fields (>300 cells) were viewed by UV microscopy for calculations of the percentage of LANA+ (Cy3-staining) cells among the total population of cells, counterstained with Hoechst 33342 for visualization of nuclei. For reactivation of HHV-8 from BAC16-infected iSLK cells, cultures were treated with doxycycline (1 μg/ml) and sodium butyrate (1 mM).
ACKNOWLEDGMENT
This study was supported by NIH grant CA76445 to J.N.
REFERENCES
- 1.Chen D, Cousins E, Sandford G, Nicholas J. 2012. Human herpesvirus 8 viral interleukin-6 interacts with splice variant 2 of vitamin K epoxide reductase complex subunit 1. J Virol 86:1577–1588. doi: 10.1128/JVI.05782-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cousins E, Nicholas J. 2013. Role of human herpesvirus 8 interleukin-6-activated gp130 signal transducer in primary effusion lymphoma cell growth and viability. J Virol 87:10816–10827. doi: 10.1128/JVI.02047-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cousins E, Gao Y, Sandford G, Nicholas J. 2014. Human herpesvirus 8 viral interleukin-6 signaling through gp130 promotes virus replication in primary effusion lymphoma and endothelial cells. J Virol 88:12167–12172. doi: 10.1128/JVI.01751-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D, Nicholas J. 2015. Promotion of endoplasmic reticulum-associated degradation of procathepsin D by human herpesvirus 8-encoded viral interleukin-6. J Virol 89:7979–7990. doi: 10.1128/JVI.00375-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen D, Xiang Q, Nicholas J. 2017. Human herpesvirus 8 interleukin-6 interacts with calnexin cycle components and promotes protein folding. J Virol 91:e00965-17. doi: 10.1128/JVI.00965-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen D, Sandford G, Nicholas J. 2009. Intracellular signaling mechanisms and activities of human herpesvirus 8 interleukin-6. J Virol 83:722–733. doi: 10.1128/JVI.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen D, Gao Y, Nicholas J. 2014. Human herpesvirus 8 interleukin-6 contributes to primary effusion lymphoma cell viability via suppression of proapoptotic cathepsin D, a cointeraction partner of vitamin K epoxide reductase complex subunit 1 variant 2. J Virol 88:1025–1038. doi: 10.1128/JVI.02830-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Shewy HM, Luttrell LM. 2009. Insulin-like growth factor-2/mannose-6 phosphate receptors. Vitam Horm 80:667–697. doi: 10.1016/S0083-6729(08)00624-9. [DOI] [PubMed] [Google Scholar]
- 9.Reddy ST, Chai W, Childs RA, Page JD, Feizi T, Dahms NM. 2004. Identification of a low affinity mannose 6-phosphate-binding site in domain 5 of the cation-independent mannose 6-phosphate receptor. J Biol Chem 279:38658–38667. doi: 10.1074/jbc.M407474200. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt B, Kiecke-Siemsen C, Waheed A, Braulke T, von Figura K. 1995. Localization of the insulin-like growth factor II binding site to amino acids 1508-1566 in repeat 11 of the mannose 6-phosphate/insulin-like growth factor II receptor. J Biol Chem 270:14975–14982. doi: 10.1074/jbc.270.25.14975. [DOI] [PubMed] [Google Scholar]
- 11.Hille-Rehfeld A. 1995. Mannose 6-phosphate receptors in sorting and transport of lysosomal enzymes. Biochim Biophys Acta 1241:177–194. doi: 10.1016/0304-4157(95)00004-B. [DOI] [PubMed] [Google Scholar]
- 12.Boker C, von Figura K, Hille-Rehfeld A. 1997. The carboxy-terminal peptides of 46 kDa and 300 kDa mannose 6-phosphate receptors share partial sequence homology and contain information for sorting in the early endosomal pathway. J Cell Sci 110:1023–1032. [DOI] [PubMed] [Google Scholar]
- 13.Blanchard F, Duplomb L, Raher S, Vusio P, Hoflack B, Jacques Y, Godard A. 1999. Mannose 6-phosphate/insulin-like growth factor II receptor mediates internalization and degradation of leukemia inhibitory factor but not signal transduction. J Biol Chem 274:24685–24693. doi: 10.1074/jbc.274.35.24685. [DOI] [PubMed] [Google Scholar]
- 14.Oka Y, Rozek LM, Czech MP. 1985. Direct demonstration of rapid insulin-like growth factor II receptor internalization and recycling in rat adipocytes. Insulin stimulates 125I-insulin-like growth factor II degradation by modulating the IGF-II receptor recycling process. J Biol Chem 260:9435–9442. [PubMed] [Google Scholar]
- 15.Dennis PA, Rifkin DB. 1991. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A 88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braulke T, Tippmer S, Chao HJ, von Figura K. 1990. Insulin-like growth factors I and II stimulate endocytosis but do not affect sorting of lysosomal enzymes in human fibroblasts. J Biol Chem 265:6650–6655. [PubMed] [Google Scholar]
- 17.Chen Z, Ge Y, Landman N, Kang JX. 2002. Decreased expression of the mannose 6-phosphate/insulin-like growth factor-II receptor promotes growth of human breast cancer cells. BMC Cancer 2:18. doi: 10.1186/1471-2407-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souza RF, Wang S, Thakar M, Smolinski KN, Yin J, Zou TT, Kong D, Abraham JM, Toretsky JA, Meltzer SJ. 1999. Expression of the wild-type insulin-like growth factor II receptor gene suppresses growth and causes death in colorectal carcinoma cells. Oncogene 18:4063–4068. doi: 10.1038/sj.onc.1202768. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Kleiner I, Gall Troselj K. 2010. Mannose-6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2R) in carcinogenesis. Cancer Lett 289:11–22. doi: 10.1016/j.canlet.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Braulke T, Bonifacino JS. 2009. Sorting of lysosomal proteins. Biochim Biophys Acta 1793:605–614. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV. 2008. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol 3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 22.Lemus L, Goder V. 2014. Regulation of endoplasmic reticulum-associated protein degradation (ERAD) by ubiquitin. Cells 3:824–847. doi: 10.3390/cells3030824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Rapoport TA. 2018. Mechanistic insights into ER-associated protein degradation. Curr Opin Cell Biol 53:22–28. doi: 10.1016/j.ceb.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T, Xu Y, Liu Y, Ye Y. 2015. gp78 functions downstream of Hrd1 to promote degradation of misfolded proteins of the endoplasmic reticulum. Mol Biol Cell 26:4438–4450. doi: 10.1091/mbc.E15-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura H, Lu M, Gwack Y, Souvlis J, Zeichner SL, Jung JU. 2003. Global changes in Kaposi’s sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J Virol 77:4205–4220. doi: 10.1128/JVI.77.7.4205-4220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannon JS, Ciufo D, Hawkins AL, Griffin CA, Borowitz MJ, Hayward GS, Ambinder RF. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi’s sarcoma herpesvirus-containing supernatant. J Virol 74:10187–10193. doi: 10.1128/JVI.74.21.10187-10193.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brulois KF, Chang H, Lee AS, Ensser A, Wong LY, Toth Z, Lee SH, Lee HR, Myoung J, Ganem D, Oh TK, Kim JF, Gao SJ, Jung JU. 2012. Construction and manipulation of a new Kaposi’s sarcoma-associated herpesvirus bacterial artificial chromosome clone. J Virol 86:9708–9720. doi: 10.1128/JVI.01019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi YB, Sandford G, Nicholas J. 2012. Human herpesvirus 8 interferon regulatory factor-mediated BH3-only protein inhibition via Bid BH3-B mimicry. PLoS Pathog 8:e1002748. doi: 10.1371/journal.ppat.1002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose PP, Bogyo M, Moses AV, Fruh K. 2007. Insulin-like growth factor II receptor-mediated intracellular retention of cathepsin B is essential for transformation of endothelial cells by Kaposi’s sarcoma-associated herpesvirus. J Virol 81:8050–8062. doi: 10.1128/JVI.00249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou JM, Lian WS, Qiu MK, Dai YX, Dong Q, Shen J, Dong P, Wang XF, Liu YB, Quan ZW, Fei ZW. 2014. Knockdown of IGF2R suppresses proliferation and induces apoptosis in hemangioma cells in vitro and in vivo. Int J Oncol 45:1241–1249. doi: 10.3892/ijo.2014.2512. [DOI] [PubMed] [Google Scholar]
- 31.Geng XR, Yang G, Li M, Song JP, Liu ZQ, Qiu S, Liu Z, Yang PC. 2014. Insulin-like growth factor-2 enhances functions of antigen (Ag)-specific regulatory B cells. J Biol Chem 289:17941–17950. doi: 10.1074/jbc.M113.515262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan X, Wang H, Nicholas J. 1999. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J Virol 73:8268–8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang Q, Ju H, Li Q, Mei SC, Chen D, Choi YB, Nicholas J. 2018. Human herpesvirus 8 interferon regulatory factors 1 and 3 mediate replication and latency activities via interactions with USP7 deubiquitinase. J Virol 92:e02003-17. doi: 10.1128/JVI.02003-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi YB, Nicholas J. 2010. Bim nuclear translocation and inactivation by viral interferon regulatory factor. PLoS Pathog 6:e1001031. doi: 10.1371/journal.ppat.1001031. [DOI] [PMC free article] [PubMed] [Google Scholar]