Abstract
Background
This study assessed the clinical value of regional ischemic preconditioning (RIP) and the role of the mitogen-activated protein kinase (MAPK) pathways in the protective mechanism of RIP in cirrhotic hepatocellular carcinoma (HCC) patients undergoing hepatectomy.
Methods
Liver resection was performed with hemi-hepatic vascular inflow occlusion (HHV) under RIP (RIP group) or with HHV alone (HHV group). Clinical data, surgical outcomes, and the levels of phosphorylated MAPKs before occlusion and 30 min after reperfusion were estimated.
Results
HHV under RIP was associated with less intraoperative blood loss (300 vs. 400 ml; P = 0.042), postoperative plasma transfused (400 vs. 800 ml; P = 0.019), and a higher level of prothrombin activity at postoperative days 3, 5, and 7 compared to HHV alone. The level of phosphorylated ERK protein was significantly increased and the levels of phosphorylated p38 and JNK proteins were significantly decreased 30 min after reperfusion compared to HHV group in the RIP group.
Conclusions
HHV under RIP may have clinical value in cirrhotic HCC patients requiring resection and the protective mechanism of RIP may be associated with changes in the protein phosphorylation level of MAPK pathways.
Electronic supplementary material
The online version of this article (10.1007/s11605-018-3960-1) contains supplementary material, which is available to authorized users.
Keywords: Hepatocellular carcinoma, Cirrhosis, Regional ischemic preconditioning, Mitogen-activated protein kinase
Introduction
Malignant liver tumors, including hepatocellular carcinoma (HCC), are treated by elective liver resection. Blood loss during liver resection is prevented by vascular occlusion, which can result in ischemia/reperfusion (IR) injury. It is generally accepted that the cirrhotic liver is particularly sensitive to IR injury.1,2
Worldwide, liver cancer is the fifth most frequently diagnosed cancer, accounting for more than 700,000 deaths per year. In 2008, almost 55% of global liver cancer cases and deaths occurred in China.3 In China and other Asian countries, more than 80% of HCC patients have underlying liver cirrhosis due to hepatitis B virus infection.4,5 Therefore, the need for effective methods to reduce the IR injury associated with vascular occlusion during liver resection in cirrhotic HCC patients has become increasingly important.
Currently, ischemic preconditioning (IP) is recommended to protect against hepatic IR injury. However, most studies supporting this approach focused on patients with benign tumors or HCC without liver cirrhosis, which are not applicable to Asian countries, and outcomes of human studies on IP during hepatectomy are controversial. Some studies have confirmed the protective effect of IP. In a randomized controlled trial, patients undergoing hemihepatectomy under IP (10 min ischemia/10 min reperfusion) had significantly lower levels of markers of hepatic injury (aspartate transferase [AST], alanine transferase [ALT]) during the immediate postoperative period than controls.6 In patients who received hepatic resection under IP, intraoperative bleeding and postoperative complications were significantly reduced compared to no IP,7 and blood transfusions.8 hepatocyte injury, and duration of surgery9 were decreased compared to alternative methods of vascular occlusion. Other studies showed that IP (10 min ischemia/10 min reperfusion) had no clinical value for postoperative liver function and reducing postoperative complications and mortality in major liver resection,10 and there were no significant differences in operative time, blood loss, hemodynamic changes, postoperative liver function, postoperative complications, and length of hospital stay in patients undergoing elective liver resection under the Pringle maneuver11,12 or complex hepatectomy under total vascular exclusion (TVE) with or without IP.13
Most surgeons use the Pringle maneuver to reduce blood loss during liver resection. However, recent reports suggest that the Pringle maneuver is associated with portal vein thrombosis, spontaneous rupture,14 IR injury15 and may accelerate tumor recurrence.16 Hemi-hepatic vascular inflow occlusion (HHV) is increasingly used as an alternative to the Pringle maneuver.17,18 HHV keeps the contralateral hepatic blood supply open, ameliorates IR injury, contributes to portal hemodynamic stability, prevents intestinal mucosal damage induced by mesenteric vascular congestion, and achieved earlier postoperative recovery of liver function compared to the Pringle maneuver in a randomized controlled trial of patients undergoing partial hepatectomy.19
Accounting for the controversy surrounding IP and the advantages of HHV, we propose the use of regional ischemic preconditioning (RIP) in cirrhotic HCC patients. This process involves occluding only 50% of the hepatic blood inflow. Data from our preliminary clinical trial indicated that HHV under RIP may be safer and achieve earlier postoperative recovery of liver function than HHV alone in HCC patients.20
The protective mechanism of RIP remains to be elucidated. The pathophysiology of hepatic IR may include a number of mechanisms that the formation of pro- and anti-inflammatory mediators, expression of adhesion molecules, and the role of oxidant stress, preventing microcirculatory disturbances by nitric oxides.21 Recent studies have identified mitogen-activated protein kinase (MAPK) as the major intracellular signal transduction system in IR injury.22 MAPKs are regulated by phosphorylation and have key roles in the control of cell proliferation, differentiation, and apoptosis.23 In mammalian cells, the MAPK cascade includes the ERK pathway, JNK pathway, and the p38 pathway. ERK is an important transcription factor, and the JNK and p38 pathways are involved in cellular responses to environmental stress and inflammatory cytokines. IR injury, heat shock, hypertonic conditions, and certain inflammatory cytokines such as tumor necrosis factor can activate the MAPK signaling pathway. MAPKs are involved in IR injury;24,25 however, the most of the previous researches were performed in cell or animal models, clinical studies in hepatic IR injury and those considering IP are limited.
The objective of the current study was to conduct a prospective non-randomized controlled trial comparing HHV under RIP with HHV alone in cirrhotic HCC patients undergoing liver resection to assess the clinical value of RIP and explore the role of the MAPK pathways in the endogenous protective mechanism of RIP in these patients.
Materials and Methods
Patients and Tissue Samples
The study protocol was approved by the Institutional Review Board at the Cancer Hospital, Chinese Academy of Medical Sciences. A total of 91 cirrhotic patients requiring resection for HCC were eligible for this study. Inclusion criteria were as follows: ≥ 18 years of age; primary HCC diagnosed by imaging and serum alpha-fetoprotein (AFP); compensated cirrhosis (Child-Pugh score grade A); tumor located in the right lobe of liver, close to the right hepatic vein, right portal vein, inferior vena cava or other important blood vessels, or not near major blood vessels but with a diameter > 5 cm; single or multiple lesions confined to three segments; laparotomy; indocyanine Green Retention rate at 15 min (ICG-R15) < 15%; and absence of serious cardiopulmonary complications. Exclusion criteria were as follows: other liver pathology or previous interventions (e.g., radiation therapy, radiofrequency therapy).
As a non-randomized cohort research, patients were assigned to a RIP group (HHV under RIP) or an HHV group (HHV alone) alternately with a ratio of 1:2. Case number was calculated by software PASS according to previous research. All patients consulted with a research nurse, who provided patients with comprehensive information about the advantages and disadvantages of the two occlusion techniques. All patients provided written informed consent before participation in the study.
Specimens of liver tissue (wedge, approximately 0.5–0.8 cm3) were obtained from subjects before occlusion and 30 min after reperfusion. Each specimen was divided into two parts. One part was immediately placed in liquid nitrogen and stored at −80 °C until Western blotting. The other part was washed three times in PBS buffer, fixed in 10% neutral formalin solution, dehydrated, embedded in paraffin, and sectioned for H&E staining and immunohistochemistry.
Surgical Procedure
All operations were performed by one board-certified hepatobiliary surgeon with a single, dedicated surgical support team. After laparotomy, the abdominal and pelvic areas were carefully explored to determine the location, size, and number of liver tumors, tumor location relative to important hepatic ducts and vessels, and resection extent. Intraoperative ultrasound was used if necessary.
In the HHV group, liver resection was performed with HHV only. An ultrasonic scalpel or electrotome with excellent hemostatic effect was used to relatively slowly dissect the liver surface. Continuous HHV was performed while dissecting deep inside the liver tissue. Vascular anastomosis silk was used to ligate or suture the cut surface of the liver when bleeding was obvious. After complete resection, intrahepatic structures (blood vessels and bile ducts) and the liver surface were repaired, and the continuous hemi-hepatic inflow occlusion was de-clamped. The natural position of the remnant liver was retained after covering the cut surface with a hemostatic sponge.
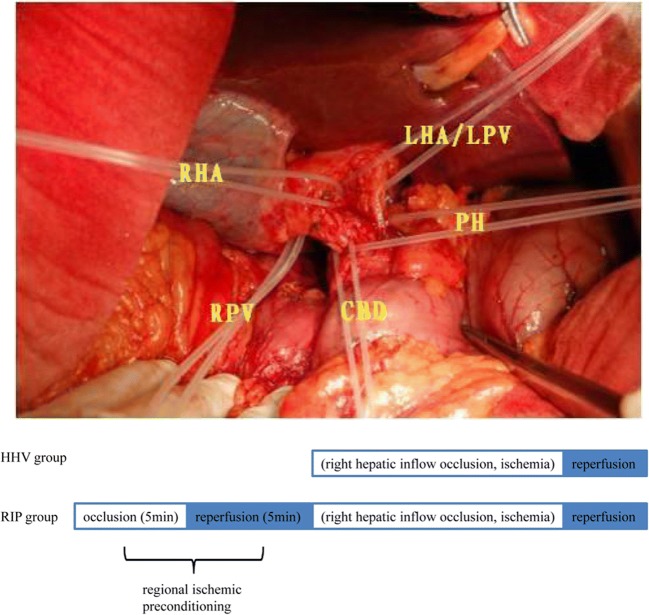
In the RIP group, liver resection was performed with HHV after a short period of RIP. According to previous studies, 5 min ischemia/5 min reperfusion is recommended for IP in the cirrhotic liver.26 The first porta hepatis was elaborately dissected to reveal the left and right branch of the hepatic artery and portal vein; a rubber band was preset on each vessel (Fig. 1). Clamping of the hemi-portal inflow was performed with the tourniquet technique using rubber tape. The right hepatic inflow was occluded by tightening the rubber band on the right branch of the hepatic artery and portal vein. Immediately after a brief period of ischemia (5 min) followed by 5 min of reperfusion (RIP), liver transection was initiated under continuous HHV as HHV group.
Fig. 1.
Treatment schemes. The first porta hepatis was elaborately dissected to reveal the left and right branch of the hepatic artery and portal vein; a rubber band was preset on each vessel. The right hepatic inflow was occluded by tightening the rubber band on the right branch of the hepatic artery (RHA) and portal vein (RPV); the occluded right hepatic inflow was restored by loosening the rubber band. Methodology of regional ischemic preconditioning is based on right hepatic inflow occlusion. RHA, right hepatic artery; RPV, right portal vein; PH, porta hepatis; CB, common bile duct; LHA/LPV, left hepatic artery/left portal vein
Postoperative complications were evaluated according to clinical practice and published literature.27–29
Immunohistochemistry
Immunohistochemistry was performed as previously described.30 Primary antibodies were used including ERK (1:100, Cell Signaling Technology), phosphorylated ERK (1:100, Cell Signaling Technology), p38 (1:100, Cell Signaling Technology), phosphorylated p38 (1:100, Cell Signaling Technology), JNK (1:50, Santa Cruz Biotechnology), and phosphorylated JNK (1:50, Cell Signaling Technology). Sections were counterstained with Harris’ hematoxylin. Slides were scored according to staining intensity and the proportion of positive cells. Staining intensity was determined according to the staining characteristics and was scored as follows: no staining, 0 (None); light yellow, 1 (Weak); brown yellow, 2 (Moderate); brown, 3 (Strong). Five fields were randomly selected at a high magnification and 100 cells were counted in each field in which the proportion of positive cells was determined and scored as follows: 1 = 0 to 10%; 2 = 11–25%; 3 = 26–50%; 4 = > 50%. The total score was the multiplication of both scores: 0, negative (−), 1 to 4, weakly positive [+]; 5–8, moderately positive [++]; 9–12, strongly positive [+++]. All the sections were scored independently by two experienced pathologists.
Western Blot Analysis
Western blot analysis was performed with the use of conventional protocols as described previously31 using liver homogenates and the above-mentioned antibodies. All the antibodies (including GAPDH (Proteintech)) were used at 1:1000 dilution. Densitometry was performed using ImageQuant LAS 4000 mini (GEHealthcare). Data was analyzed with Image J software.
Statistical Analysis
Statistical analysis was performed using SPSS software version 16.0 (SPSS, Inc., Chicago, IL). Continuous data are expressed as mean ± standard deviation and were compared using an independent Student’s t test or Wilcoxon rank sum test. Categorical data were compared using the χ2 test or Fisher’s exact probability test. P < 0.05 was considered statistically significant.
Results
Study Subjects
A total of 91 patients were eligible for inclusion in this study. Of these, 15 patients did not meet the inclusion criteria, and 4 patients refused to participate; therefore, 72 patients were included in the analyses. A total of 23 patients chose to be included in the RIP group and 49 patients chose to be included in the HHV group. Demographic and clinical characteristics of the study subjects are shown in Table 1. Mean age was 55.4 years (range, 31–72 years) and mean tumor diameter was 4.4 cm (1.0–14.5 cm). Thirty-four tumors were > 5 cm in diameter and 41 tumors were located close to large vessels. There were no significant differences in demographic and clinical characteristics between the two groups.
Table 1.
Clinicopathological characteristics of the patients
| Characteristics | RIP group (n = 23) | HHV group (n = 49) | P value |
|---|---|---|---|
| Age (years, mean ± SD) | 56.5 ± 10.3 | 55.0 ± 8.9 | 0.535 |
| Sex (male/female, n) | 19/4 | 36/3 | 0.556 |
| Hepatitis B surface antigen serology positive (n) | 16 | 38 | 0.466 |
| Hepatitis C surface antigen serology positive (n) | 3 | 3 | 0.322 |
| Cirrhosis (n) | 0.994 | ||
| Mild/moderate | 15 | 32 | |
| Severe | 8 | 17 | |
| Size of tumor (cm, median and range) | 4.5 (2.0–14.5) | 4.4 (1.0–9.5) | 0.184 |
| Number of tumor (n) | 0.429 | ||
| Single | 18 | 42 | |
| Multiple | 5 | 7 | |
| Vascular involvement (n) | 0.939 | ||
| RHV | 5 | 10 | |
| RPV | 2 | 1 | |
| MHV | 1 | 2 | |
| MHV and RPV | 1 | 2 | |
| RHV and RPV | 1 | 3 | |
| MHV and RHV | 2 | 1 | |
| IVC and RHV | 1 | 2 | |
| Adhesion to the third hepatic hilum | 1 | 3 | |
| IVC and RHV and RPV | 1 | 2 |
Vascular involvement was defined as tumor close to venous wall less than 1 cm or resectable attachment of HCC onto the venous wall
RIP regional ischemic preconditioning, HHV hemi-hepatic vascular inflow occlusion, RHV right hepatic vein, RPV right portal vein, MHV middle hepatic vein, IVC inferior vena cava
Intraoperative and Postoperative Outcomes
Intraoperative and postoperative outcomes are shown in Table 2. There were no significant differences in operative procedure, liver resection volume, incisal margin, operative time, continuous regional ischemia time, and number of intraoperative blood transfusions between the two groups.
Table 2.
Operative outcomes
| Characteristics | RIP group (n = 23) | HHV group (n = 49) | P value |
|---|---|---|---|
| Operative procedure (major/minor)a | 10/13 | 21/28 | 0.960 |
| Resection volumes (cm3) | 288 | 264 | 0.740 |
| Incisal margin (n) | 0.445 | ||
| 0 cm | 13 | 20 | |
| 0–2 cm | 2 | 7 | |
| ≥ 2 cm | 8 | 22 | |
| Intraoperative bile duct damage (n) | 1 | 4 | 1.000 |
| Duration of surgery (min, mean ± SD) | 222.4 ± 69.4 | 236.4 ± 60.2 | 0.383 |
| Continuous regional ischemia time (min) | 21(10–58) | 18(10–70) | 0.272 |
| Intraoperative bleeding (ml, median and range) | 300 (100–1100) | 400 (100–1500) | 0.042 |
| Total intraoperative blood transfused (ml, median and range) | 900 (600–1200) | 1200(400–2400) | 0.445 |
| Intraoperative transfusions (cases) | 2 | 7 | 0.504 |
| Postoperative plasma transfused (ml, median and range) | 400 (200–400) | 800 (400–2800) | 0.019 |
| Postoperative RBC transfused (cases) | 0 | 4 | 0.299 |
| Postoperative plasma transfused (cases) | 4 | 17 | 0.132 |
| ALT recovering within 1 week (n) | 8 | 1 | < 0.001 |
| Postoperative hospital stay (n, median and range) | 9 (2–17) | 9 (6–49) | 0.932 |
| Complications (n) | |||
| Hepatic dysfunction | 1 | 5 | 0.657 |
| Mass ascites | 1 | 8 | 0.260 |
| Postoperative infection | 2 | 10 | 0.214 |
| Pleural effusion | 0 | 3 | 0.547 |
| Pulmonary and cardiac dysfunction | 0 | 5 | 0.170 |
| Bile leak | 0 | 0 | – |
| Intestinal ventilation time | 4 (2–6) | 4 (2–6) | 0.311 |
Italic emphasis: P value is less than 0.05
RIP regional ischemic preconditioning, HHV hemi-hepatic vascular inflow occlusion, ALT alanine aminotransferase, RBC red blood cell
Operative procedurea: Minor resection: no more than one segment resection;Major resection: two or three segments resection (more than one segment and less than hemihepatectomy).
There was less intraoperative blood loss (300 vs. 400 ml; P = 0.042) and postoperative plasma transfused (400 vs. 800 ml; P = 0.019), in the RIP group compared to the HHV group. There were no deaths or biliary fistulas in either group at postoperative 30 days. There were no significant differences in postoperative length of hospital stay, postoperative infection, pleural effusion, and cardiac and pulmonary dysfunction between the two groups.
Liver Function Tests After Liver Resection
Outcomes of liver function tests after liver resection are shown in Table 2 and Fig. 2. PTA was significantly higher on postoperative days 3, 5, and 7 in the RIP group compared to the HHV group (P < 0.05) and ALT returned to normal levels within 1 week in significantly more patients in the RIP group than the HHV group (8 vs. 1; P < 0.001). There were no differences in postoperative AST, ALT, ALB, or TBIL levels between the two groups in each detection point (P > 0.05).
Fig. 2.

Liver function tests after liver resection with (blue line, n = 23) versus without (red line, n = 49) RIP. There were no significant differences between the RIP and HHV groups except for prothrombin activity at postoperative days 3, 5, and 7 (P < 0.05). D0, preoperative day; D1, postoperative day 1; D3, postoperative day 3; D5, postoperative day 5; D7, postoperative day 7; ALT, alanine transaminase; AST, aspartate transaminase; TBIL, total bilirubin; ALB, albumin; PTA, prothrombin activity
In both groups, AST and ALT levels increased to a maximum and ALB levels decreased to a minimum on postoperative day 1; TBIL levels increased to a maximum on postoperative day 3. The trend in changes in AST, ALT, ALB, and TBIL levels was similar in both groups and not significantly different. PTA decreased on postoperative day 1 and then rose; there was a significant difference in PTA between the two groups on postoperative day 5 (RIP, 89%; HHV, 72%; P < 0.001) (Fig. 2).
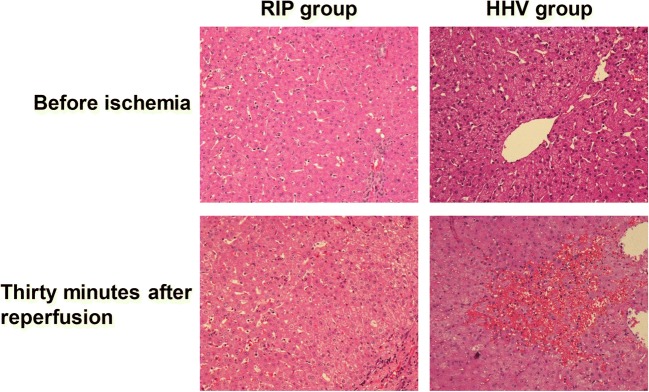
Histopathology
There were no histopathological differences between the two groups before liver resection. Thirty minutes after reperfusion, in the HHV group, there were enlarged hepatocytes, centrolobular venous dilation and congestion, sinusoidal congestion, an obvious inflammatory cell infiltration, and incomplete liver tissue. In the RIP group, there was reduced structural damage compared to the HHV group, with partial ballooning degeneration of hepatocytes, no sheet cell necrosis, and less sinusoidal congestion and inflammatory cell infiltration (Fig. 3).
Fig. 3.
Liver morphology change before vascular occlusion and 30 min after reperfusion. The RIP group showed less hepatocyte injury and inflammatory cell infiltration than the HHV group. ×200. HHV, hemi-hepatic vascular inflow occlusion; RIP, regional ischemic preconditioning
pJNK, pp38, and pERK Proteins Levels in the RIP Group and HHV Group
As shown in supplementary Figure 1, there were no significant differences in total JNK, p38, and ERK protein levels between the two groups. However, phosphorylated JNK and phosphorylated p38 protein levels were decreased in the RIP group and increased in the HHV group 30 min after reperfusion compared to before occlusion (P < 0.05) (Fig. 4a, b). The level of phosphorylated ERK protein was increased in the RIP group and decreased in the HHV group 30 min after reperfusion compared to before occlusion. Thirty minutes after reperfusion, the level of phosphorylated ERK protein was significantly higher in the RIP group compared to the HHV group (P < 0.05) (Fig. 4c).
Fig. 4.
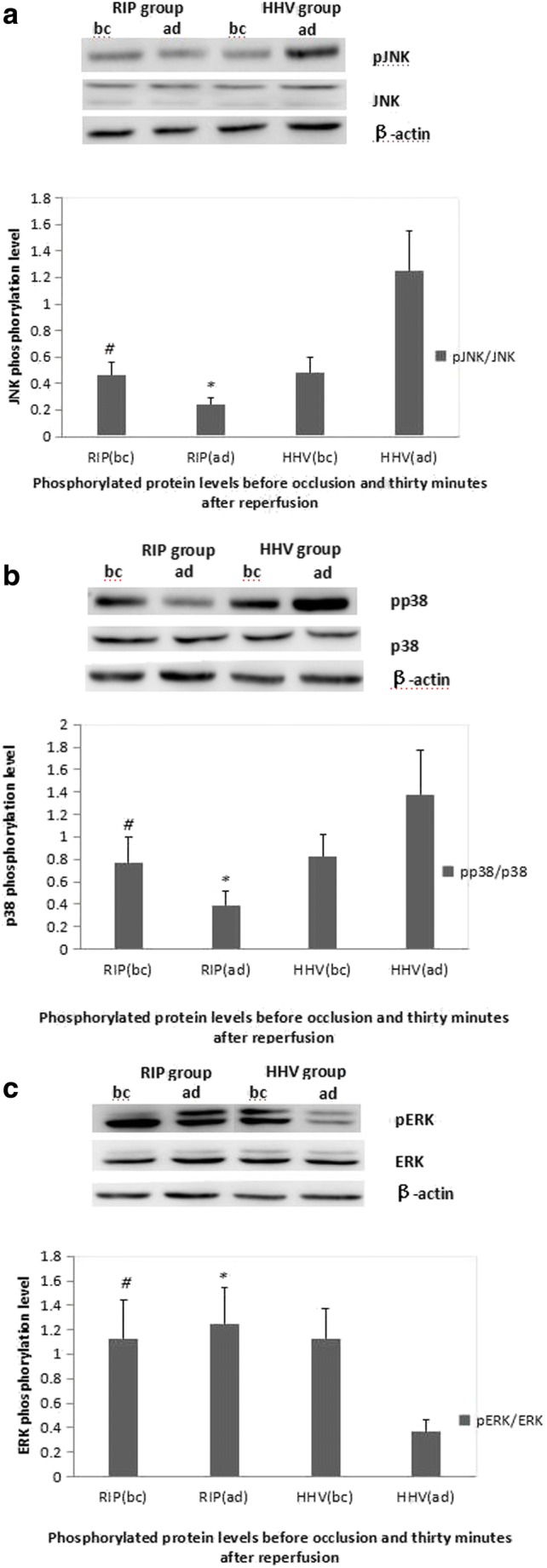
Protein phosphorylation levels before vascular occlusion and 30 min after reperfusion. a JNK phosphorylation level; b p38 phosphorylation level; c ERK phosphorylation level. bc, before; ad, after; HHV, hemi-hepatic vascular inflow occlusion; RIP, regional ischemic preconditioning; p, phosphorylated. The level of pERK was increased and the levels of pJNK and pp38 were decreased in the RIP group 30 min after reperfusion compared to the HHV group. # compared to HHV group before ischemia P > 0.05, *compared to HHV group after occlusion P < 0.05
Immunohistochemistry staining showed the similar results. In all tissues, phosphorylated ERK and phosphorylated p38 proteins were localized to the sinusoidal endothelial cells, and phosphorylated JNK protein was localized in the hepatocytes. Before occlusion, there were no significant differences in the levels of phosphorylated JNK, phosphorylated p38, and phosphorylated ERK proteins between the two groups (data not shown). Thirty minutes after reperfusion, the levels of phosphorylated JNK and phosphorylated p38 proteins were significantly lower in the RIP group than the HHV group, while the level of phosphorylated ERK protein was significantly higher in the RIP group (P < 0.001; Table 3) (Supplementary Fig. 1).
Table 3.
Phosphorylated protein levels before occlusion and 30 min after reperfusion
| Group | + (cases) | ++ (cases) | +++ (cases) | P value* | |
|---|---|---|---|---|---|
| Before occlusion | |||||
| pJNK | HHV | 27 | 17 | 5 | |
| RIP | 12 | 9 | 2 | 0.929 | |
| pp38 | HHV | 42 | 7 | 0 | |
| RIP | 20 | 3 | 0 | 1.000 | |
| pERK | HHV | 12 | 28 | 9 | |
| RIP | 9 | 8 | 6 | 0.206 | |
| Thirty minutes after reperfusion | |||||
| pJNK | HHV | 2 | 31 | 16 | |
| RIP | 14 | 5 | 4 | < 0.001 | |
| pp38 | HHV | 13 | 34 | 2 | |
| RIP | 18 | 5 | 0 | < 0.001 | |
| pERK | HHV | 25 | 21 | 3 | |
| RIP | 3 | 8 | 12 | < 0.001 | |
HHV hemi-hepatic vascular inflow occlusion, RIP regional ischemic preconditioning, pJNK phosphorylated JNK, pp38 phosphorylated p38, pERK phosphorylated ERK
*Two-sided Person’s χ2 test or Fisher’s exact test
Discussion
This prospective non-randomized controlled trial studied the effect of HHV under RIP on cirrhotic HCC patients undergoing liver resection. HHV under RIP was associated with less intraoperative blood loss and less postoperative plasma transfused compared to HHV alone.
These findings suggest that RIP may reduce intraoperative blood loss in cirrhotic HCC patients undergoing liver resection. However, the results should be interpreted with caution as their clinical significance remains to be elucidated. We attribute the reduced intraoperative blood loss in the RIP group compared to the HHV group to the higher intraoperative PTA observed in the RIP group. However, this supposition requires confirmation through dynamic studies that monitor intraoperative PTA in HHV under RIP and HHV alone. In our cohort study, there was no significant difference between the two groups in body mass index (BMI) and resection volumes, respectively, so we speculated that they may be not the main cause for intraoperative bleeding. However, four more TACE-treated patients enrolled in the HHV group compare to RIP group because of unclear reason. Previous study have showed that patients underwent preoperative TACE had more intraoperative blood loss because of perihepatic organs adhesion.32 This may be one of the main reasons for blood loss.
We determined the need for postoperative plasma transfusions according to intraoperative exudation in the cirrhotic liver, volume of postoperative blood-tinged fluid, and in particular postoperative PTA (representing coagulation function), in order to determine the amounts of coagulation factors and plasma proteins to replace. There was no significant difference between the two groups in PTA on postoperative day 1, but PTA was significantly lower on postoperative days 3, 5, and 7 in the HHV group compared to the RIP group, which showed that the recovery of PTA in the HHV group was slower than that of the RIP group. This suggests that RIP may have some protective effect on coagulation function, causing patients in the RIP group to have less intraoperative exudation and less postoperative plasma transfused. More importantly, it should be noted that our current study included only patients with HCC and liver cirrhosis. Over 50% of tumors were located near the right or middle hepatic vein, right portal vein, the third porta of the liver, or the inferior vena cava. These were considered high-risk patients who were likely to experience postoperative liver failure. To the authors’ knowledge, the current study is the first to investigate the effects of RIP in cirrhotic HCC patients undergoing liver resection. The outcome of the current study is in accordance with our preliminary study of 54 HCC patients who underwent hepatectomy under HHV with or without RIP20 and a randomized controlled trial of 61 patients who received hepatic resection under the Pringle maneuver with or without IP.7 In these studies, IP significantly reduced intraoperative bleeding7,20 and postoperative complications7 and promoted early recovery of liver function20 compared to no IP.
For cirrhotic patients, continuous portal triad clamping (Pringle maneuver) exceeding 30 min may cause irreversible injury. Previously, we have shown that the maximum ischemic tolerance time of the liver in HCC patients is 70 min.20 This study showed the same maximum tolerance time. Total ischemic time in this study group ranging from 10 to 70 min was determined by difficulty of surgical procedure instead of a fixed time period. There was no significant difference of mean ischemia time between the two groups. But, the changes of molecular and histopathology profile were truly observed in all the patients even the least ischemic time was only 10 min. Although the degree of changes of molecular and histopathology profile theoretically correlated with ischemic time, the detail of the correlation was not the target we focused, this could be an exploratory goals in future. We propose that HHV under RIP can prolong the ischemic tolerance time of the liver and therefore has potential to increase the duration of surgery. This is especially important in patients with severe disease, such as those with cirrhosis or tumors located close to intrahepatic structures. Furthermore, we found that HHV under RIP was associated with faster postoperative recovery of transaminase levels and therefore liver function, and less intraoperative liver tissue damage compared to HHV alone.
The current study showed that the operative time, length of hospital stay, and frequency of postoperative complications were not different between the RIP and HHV groups, indicating that the safety of HHV was not compromised by RIP. These data are in accordance with a prospective randomized controlled study33 that used the Pringle maneuver under IP.
IP can mobilize endogenous protective mechanisms and enhance liver tissue tolerance of ischemia. MAPK cascade pathways are involved in cellular responses to extracellular stimuli.34 MAPK is a highly conserved family of protein kinases that is regulated by phosphorylation and includes JNK, p38, and ERK. JNK regulates cell viability, apoptosis, and cell proliferation. In the current study, 30 min after reperfusion, the levels of phosphorylated JNK protein and phosphorylated p38 protein were significantly lower in liver tissue from the RIP group compared to the HHV group. These findings are in accordance with a previous study that showed JNK activity was decreased in rat liver after IP, and hepatic injury was alleviated. This suggests that JNK activation may be associated with IR injury.35 The role of p38 in tissue damage is controversial. In rats,36 p38 activation was associated with increased levels of F-actin in liver cells, changes in the cytoskeleton, and liver failure. ERK is associated with cell proliferation, transformation, and differentiation.37 After reperfusion, the level of phosphorylated ERK protein in liver tissue of the RIP group was significantly higher than the HHV group. Studies on ischemia in the myocardium propose that a “RISK path” (reperfusion injury salvage kinases) that includes ERK1/2, PI3K/AKT, PKC, and PKG has a protective effect against IP.38 Zhang et al.39 reported IP could reduce ischemia/reperfusion-induced cell apoptosis by activating the ERK signaling pathway and inhibiting Rho-kinase activity. Importantly, MAPK pathways are part of a phosphorelay system.40 During IP, direct feedback between the ERK and p38 and JNK and p38 pathways is important.41–43 p38 and JNK pathways are activated within a few minutes after reperfusion, inducing cell apoptosis and necrosis.44 Application of a p38/JNK activator elevates transaminase levels and increases liver necrosis, while a p38/JNK inhibitor may reduce hepatic IR injury.45
This study is associated with several limitations. First, patients were not randomly assigned to RIP or HHV; this may result in patient selection and confounding bias. However, demographic and clinical characteristics of the two groups were not significantly different. Second, evidence suggests that the protective effect of IP is age-specific. IP is particularly effective in young patients (< 60 years of age) requiring a prolonged period of inflow occlusion, and is possibly related to preservation of ATP content in liver tissue.33 In the current study, patients were not stratified by age due to a small sample size. Third, recovery of transaminase and molecular profiles seems different according to the use of ischemic preconditioning. However, there was no difference in short-term clinical outcomes between the two groups. The current results suggest that preconditioning may have protective effect for the liver from ischemic injury. However, it remains difficult to determine the necessity of this maneuver in actual clinical settings.
Conclusions
Our study indicates that HHV under RIP may reduce intraoperative IR injury, improve coagulation. and increase the rate of recovery of postoperative liver function compared with HHV alone in HCC patients with cirrhosis requiring resection. The protective mechanism of RIP may be associated with changes in the protein phosphorylation level of MAPK pathways.
Electronic Supplementary Material
Representative cases of immunohistochemistry staining for pJNK, pp38, and pERK in RIP group and HHV group are shown. Magnification: ×100. (PNG 819 kb)
Acknowledgments
Thanks are due to Chao Bi, Yang Zhang, Liang Cui, Zheng Wang, Honglin Guo, Ting Xiao, Zhen Dai, Shengkai Huang, and Yue Xu for their kind assistance with the experiments and for their valuable comments and discussion.
Abbreviations
- AFP
Alpha-fetoprotein
- ALB
Albumin
- ALT
Alanine transferase
- AST
Aspartate transferase
- DAB
3,3′-Diaminobenzidine tetrachloride
- HCC
Hepatocellular carcinoma
- H&E
Hematoxylin eosin staining
- HHV
Hemi-hepatic vascular inflow occlusion
- IR
Ischemia/reperfusion
- IP
Ischemic preconditioning
- MAPK
Mitogen-activated protein kinase
- PTA
Prothrombin activity
- RIP
Regional ischemic preconditioning
- TBIL
Total bilirubin
- TVE
Total vascular exclusion
Author Contributions
Conception of the work: Liming Wang, Jianxiong Wu, Xiang Zhou
Data acquisition: All authors
Data analysis: Liming Wang, Li Feng, Weiqi Rong
Data interpretation: All authors
Manuscript drafting and final approval: All authors
Funding
This work was supported by PUMC Youth Fund/Fundamental Research Funds for the Central Universities (3332016031) and Young Physicians research Funds of Association of Health Exchanges and Cooperation across the Taiwan Straits (ZF-ZQ-A01).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liming Wang, Li Feng and Weiqi Rong contributed equally to this work.
Contributor Information
Liming Wang, Phone: +18911597091, Email: stewen_wang@sina.com.
Xiang Zhou, Phone: +8618910612829, Email: zhou.xiang@yeah.net.
Jianxiong Wu, Phone: +8615811140261, Email: dr.wujx@hotmail.com.
References
- 1.Torzilli G, Makuuchi M, Inoue K, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134(9):984–992. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 2.Isozaki H, Okajima K, Kobayashi M, et al. Experimental study of liver injury after partial hepatectomy with intermittent or continuous hepatic vascular occlusion. Differences in tolerance to ischemia between normal and cirrhotic livers. Eur Surg Res. 1995;27(5):313–322. doi: 10.1159/000129415. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240(4):698–708. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makuuchi M, Mori T, Gunven P, et al. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet. 1987;164(2):155–158. [PubMed] [Google Scholar]
- 6.Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232(2):155–162. doi: 10.1097/00000658-200008000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heizmann O, Loehe F, Volk A, Schauer RJ. Ischemic preconditioning improves postoperative outcome after liver resections: a randomized controlled study. Eur J Med Res. 2008;13(2):79–86. [PubMed] [Google Scholar]
- 8.Gurusamy KS, Kumar Y, Pamecha V, et al., Ischaemic pre-conditioning for elective liver resections performed under vascular occlusion, Cochrane Database Syst Rev. 2009; (1):CD007629. [DOI] [PubMed]
- 9.Zhu Y, Dong J, Wang WL, et al. Ischemic preconditioning versus intermittent clamping of portal triad in liver resection: a meta-analysis of randomized controlled trials. Hepatol Res. 2014;44(8):878–887. doi: 10.1111/hepr.12193. [DOI] [PubMed] [Google Scholar]
- 10.Azoulay D, Lucidi V, Andreani P, et al. Ischemic preconditioning for major liver resection under vascular exclusion of the liver preserving the caval flow: a randomized prospective study. J Am Coll Surg. 2006;202(2):203–211. doi: 10.1016/j.jamcollsurg.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Gurusamy KS, Kumar Y, Sharma D, Davidson BR. Methods of vascular occlusion for elective liver resections. Cochrane Database Syst Rev. 2007;4:CD006409. doi: 10.1002/14651858.CD006409.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Ye B, Zhao H, Hou H, et al. Ischemic preconditioning provides no additive clinical value in liver resection of cirrhotic and non-cirrhotic patients under portal triad clamping: a prospective randomized controlled trial. Clin Res Hepatol Gastroenterol. 2014;38(4):467–474. doi: 10.1016/j.clinre.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Jeon J, Watkins A, Wagener G, et al. Complex hepatectomy under total vascular exclusion of the liver: impact of ischemic preconditioning on clinical outcomes. World J Surg. 2013;37(4):838–846. doi: 10.1007/s00268-012-1865-9. [DOI] [PubMed] [Google Scholar]
- 14.van Buijtenen JM, Lamme B, Hesselink EJ. Spontaneous splenic rupture during Pringle maneuver in liver surgery. World J Hepatol. 2010;2(6):243–245. doi: 10.4254/wjh.v2.i6.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teoh NC. Hepatic ischemia reperfusion injury: contemporary perspectives on pathogenic mechanisms and basis for hepatoprotection—the good, bad and deadly. J Gastroenterol Hepatol. 2011;26(Suppl 1):180–187. doi: 10.1111/j.1440-1746.2010.06584.x. [DOI] [PubMed] [Google Scholar]
- 16.Nijkamp MW, van der Bilt JD, Snoeren N, et al. Prolonged portal triad clamping during liver surgery for colorectal liver metastases is associated with decreased time to hepatic tumour recurrence. Eur J Surg Oncol. 2010;36(2):182–188. doi: 10.1016/j.ejso.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Ni JS, Lau WY, Yang Y, et al. A prospective randomized controlled trial to compare Pringle manoeuvre with hemi-hepatic vascular inflow occlusion in liver resection for hepatocellular carcinoma with cirrhosis. J Gastrointest Surg. 2013;17(8):1414–1421. doi: 10.1007/s11605-013-2236-z. [DOI] [PubMed] [Google Scholar]
- 18.Wang HQ, Yang JY, Yan LN. Hemihepatic versus total hepatic inflow occlusion during hepatectomy: a systematic review and meta-analysis. World J Gastroenterol. 2011;17(26):3158–3164. doi: 10.3748/wjg.v17.i26.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu SY, Lau WY, Li GG, et al. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. Am J Surg. 2011;201(1):62–69. doi: 10.1016/j.amjsurg.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 20.Feng L, Wang L, Rong W, et al. Initial comparison of regional ischemic preconditioning and hemi-hepatic vascular inflow occlusion in resection of hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 2015;37(3):186–189. [PubMed] [Google Scholar]
- 21.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 22.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 23.Gerits N, Mikalsen T, Kostenko S, et al. Modulation of F-actin rearrangement by the cyclic AMP/cAMP-dependent protein kinase (PKA) pathway is mediated by MAPK-activated protein kinase 5 and requires PKA-induced nuclear export of MK5. J Biol Chem. 2007;282(51):37232–37243. doi: 10.1074/jbc.M704873200. [DOI] [PubMed] [Google Scholar]
- 24.Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22(6):631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Ravingerova T, Barancik M, Strniskova M. Mitogen-activated protein kinases: a new therapeutic target in cardiac pathology. Mol Cell Biochem. 2003;247(1–2):127–138. doi: 10.1023/A:1024119224033. [DOI] [PubMed] [Google Scholar]
- 26.Li SQ, Liang LJ, Huang JF, Li Z. Hepatocyte apoptosis induced by hepatic ischemia-reperfusion injury in cirrhotic rats. Hepatobiliary Pancreat Dis Int. 2003;2(1):102–105. [PubMed] [Google Scholar]
- 27.Yang Y, Fu SY, Lau WY, et al. Selective main portal vein clamping to minimize the risk of recurrence after curative liver resection for hepatocellular carcinoma. Hepatogastroenterology. 2012;59(117):1560–1565. doi: 10.5754/hge10174. [DOI] [PubMed] [Google Scholar]
- 28.Jin S, Dai CL. Attenuation of reperfusion-induced hepatocyte apoptosis is associated with reversed bcl-2/bax ratio in hemi-hepatic artery-preserved portal occlusion. J Surg Res. 2012;174(2):298–304. doi: 10.1016/j.jss.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Lau WY, Lai EC, Lau SH. Methods of vascular control technique during liver resection: a comprehensive review. Hepatobiliary Pancreat Dis Int. 2010;9(5):473–481. [PubMed] [Google Scholar]
- 30.Wang S, Ma K, Chen L, et al., TAZ promotes cell growth and inhibits Celastrol-induced cell apoptosis, Biosci Rep. 2016; 36(5). [DOI] [PMC free article] [PubMed]
- 31.Zhou C, Liu S, Zhou X, et al. Overexpression of human pituitary tumor transforming gene (hPTTG), is regulated by beta-catenin /TCF pathway in human esophageal squamous cell carcinoma. Int J Cancer. 2005;113(6):891–898. doi: 10.1002/ijc.20642. [DOI] [PubMed] [Google Scholar]
- 32.Si T, Chen Y, Ma D, et al. Preoperative transarterial chemoembolization for resectable hepatocellular carcinoma in Asia area: a meta-analysis of random controlled trials. Scand J Gastroenterol. 2016;51(12):1512–1519. doi: 10.1080/00365521.2016.1216588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clavien PA, Selzner M, Rudiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238(6):843–850. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xing J, Kornhauser JM, Xia Z, et al. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol. 1998;18(4):1946–1955. doi: 10.1128/MCB.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crenesse D, Laurens M, Gugenheim J, et al. Intermittent ischemia reduces warm hypoxia-reoxygenation-induced JNK(1)/SAPK(1) activation and apoptosis in rat hepatocytes. Hepatology. 2001;34(5):972–978. doi: 10.1053/jhep.2001.28709. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2(9):717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 37.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. doi: 10.1042/bj3510289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88(2):581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Bian HJ, Li XX, et al. ERK-MAPK signaling opposes rho-kinase to reduce cardiomyocyte apoptosis in heart ischemic preconditioning. Mol Med. 2010;16(7–8):307–315. doi: 10.2119/molmed.2009.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 41.Kovalska M, Kovalska L, Pavlikova M, et al. Intracellular signaling MAPK pathway after cerebral ischemia-reperfusion injury. Neurochem Res. 2012;37(7):1568–1577. doi: 10.1007/s11064-012-0752-y. [DOI] [PubMed] [Google Scholar]
- 42.Kovalska M, Kovalska L, Mikuskova K, et al. p-ERK involvement in the neuroprotection exerted by ischemic preconditioning in rat hippocampus subjected to four vessel occlusion. J Physiol Pharmacol. 2014;65(6):767–776. [PubMed] [Google Scholar]
- 43.Kobayashi M, Takeyoshi I, Yoshinari D, et al. The role of mitogen-activated protein kinases and the participation of intestinal congestion in total hepatic ischemia-reperfusion injury. Hepatogastroenterology. 2006;53(68):243–248. [PubMed] [Google Scholar]
- 44.Kobayashi M, Takeyoshi I, Yoshinari D, et al. P38 mitogen-activated protein kinase inhibition attenuates ischemia-reperfusion injury of the rat liver. Surgery. 2002;131(3):344–349. doi: 10.1067/msy.2002.121097. [DOI] [PubMed] [Google Scholar]
- 45.Massip-Salcedo M, Casillas-Ramirez A, Franco-Gou R, et al. Heat shock proteins and mitogen-activated protein kinases in steatotic livers undergoing ischemia-reperfusion: some answers. Am J Pathol. 2006;168(5):1474–1485. doi: 10.2353/ajpath.2006.050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative cases of immunohistochemistry staining for pJNK, pp38, and pERK in RIP group and HHV group are shown. Magnification: ×100. (PNG 819 kb)