Abstract
Resilin is an elastomeric protein abundant in insect cuticle. Its exceptional properties, which include high resilience and efficient energy storage, motivate its potential use in tissue engineering and drug delivery applications. Our lab has previously developed recombinant proteins based on the resilin-like sequence derived from Anopheles gambiae and demonstrated their promise as a scaffold for cartilage and vascular engineering. In this work, we describe a more thorough investigation of the physical properties of crosslinked resilin-like hydrogels. The resilin-like proteins rapidly form crosslinked hydrogels in physiological conditions. We also show that the mechanical properties of these resilin-like hydrogels can be modulated simply by varying the protein concentration or the stoichiometric ratio of crosslinker to crosslinking sites. Crosslinked resilin-like hydrogels were hydrophilic and had a high water content when swollen. In addition, these hydrogels exhibited moderate resilience values, which were comparable to those of common synthetic rubbers. Cryo-scanning electron microscopy showed that the crosslinked resilin-like hydrogels at 16 wt% featured a honeycomb-like structure. These studies thus demonstrate the potential to use recombinant resilin-like proteins in a wide variety of applications such as tissue engineering and drug delivery due to their tunable physical properties.
Keywords: protein engineering, biomaterials, tissue engineering, resilience, hydrogels
Graphical Abstract
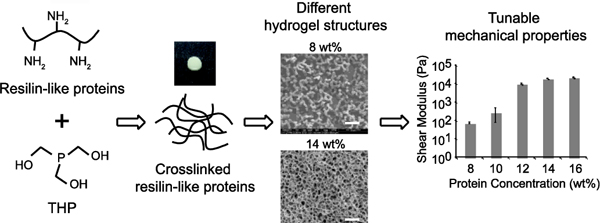
1. INTRODUCTION
Cells respond to different cues in their microenvironment, such as interactions with other cells or matrix stiffness.1 Scaffolds, therefore, not only provide cells with a 3-dimensional (3D) structural support but also play an important role in regulating cell behavior. Researchers have developed various systems using both natural and synthetic materials in an attempt to understand how matrix properties such as stiffness regulate cell behaviors including cell migration and stem cell differentiation.2 Natural materials such as chitosan, hyaluronic acid, and collagen usually have better interactions with cell surface receptors and can thus regulate cell function. On the other hand, the use of synthetic materials including poly(ethylene glycol) (PEG), poly(N-isopropylacrylamide) (poly(NiPAAm)), and poly(2-hydroxythyl methacrylate) (HEMA) allows more control over mechanical properties, degradation rate, microstructure, and permeability. New materials that exhibit the beneficial properties of both natural and synthetic materials are needed, and recombinant protein-based biomaterials have emerged to fulfill that need.
Recombinant proteins result in biomaterials that can be produced at a large scale and have a number of advantages. Recombinant protein design allows the facile combination of different functional domains without disrupting the functionality of the individual domains.3 Furthermore, protein-based biomaterials have several additional advantages, including precise control over molecular weight and amino acid sequence, modular swapping of functional domains, and tunable physical properties.
Structural domains determine the mechanical properties of recombinant proteins. A typical mechanical domain is composed of repetitive sequences derived from elastomeric proteins such as elastin, abductin, or resilin.4 Our lab has designed and developed a number of resilin-like biomaterials.5–9 Natural resilin is an elastomeric protein found in the insect cuticle.10 The extraordinary rubber-like properties of resilin, such as low stiffness and high resilience, were first described by Weis-Fogh in the early 1960s.10–12 In 2001, Ardell and Anderson reported the CG15290 gene as a precursor for dragonfly resilin,13 and in 2005, Elvin et al. manufactured recombinant rec1-resilin based on the first exon of CG15290 gene.14 They showed that crosslinked rec1-resilin also exhibited high resilience. Thereafter, resilin consensus sequences from other species such as the mosquito were reported, and recombinant resilin-like proteins based on these sequences were developed.15
Resilin-like proteins have shown potential in various tissue engineering applications.16 For example, the Kiick group developed recombinant RLP12 polypeptides based on resilin sequences from D. melanogaster.17 RLP12 exhibited high resilience and mechanical properties similar to human vocal fold tissues under high frequency.18 It could also be incorporated with distinct bioactive domains and crosslinked with PEG for cardiovascular engineering applications.19–20 Lv et al. explored a chimeric protein consisting of globular protein GB1 and resilin-like protein to mimic the mechanical properties of muscle tissues.21 Sbrana et al. also demonstrated that chimeric proteins with resilin-like, collagen-like, and elastin-like domains self-assembled into fibers and could potentially direct stem cell morphology.22 Our lab previously reported development of recombinant RZ10-RGD proteins based on resilin-like sequences from A. gambiae.5–6 We demonstrated that crosslinked RZ10-RGD hydrogels are promising biomaterials for cartilage engineering by showing that they have mechanical properties similar to those of natural cartilage.5 We also demonstrated that by incorporating different bioactive domains, RZ10-based proteins could promote cell adhesion as well as osteogenic or endothelial differentiation of human mesenchymal stem cells (hMSCs).7, 9
Materials with tunable mechanical properties serve as a powerful platform to study cell behavior in tissue engineering because scaffold stiffness significantly influences cell behavior. For example, Engler et al. showed that by changing matrix stiffness from soft (0.1 – 1 kPa) to moderate (10 kPa) to hard (34 kPa), mesenchymal stem cells (MSCs) cultured on the matrices differentiated toward neuron-like, muscle-like, and bone-like cells, respectively.23 Therefore, to further expand applications of RZ10-RGD in tissue engineering, this study expands the range of mechanical properties of crosslinked RZ10-RGD hydrogels by varying factors such as protein concentration and stoichiometric crosslinking ratio. We also characterized the resilience, internal network structure, and other physical properties of these hydrogels. By understanding the effects of protein concentration and stoichiometric crosslinking ratio on various properties of crosslinked RZ10-RGD hydrogels, we will be able to modulate the properties of crosslinked RZ10-RGD hydrogels for versatile biological applications such as tissue engineering.
2. MATERIALS AND METHODS
2.1. Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Avantor Performance Materials (Center Valley, PA) unless stated otherwise.
2.2. Protein Expression and Purification
We previously published detailed information regarding the amino acid sequence of RZ10-RGD and methods for large-scale protein expression and purification, which we utilized with slight modifications.5 Briefly, protein expression was performed with a 10 L culture in a 14 L capacity fermentor (BioFlo 110 New Brunswick, Enfield, CT) and induced with 2.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) (EMD Chemicals) when the optical density at 600 nm reached 5 – 6. Protein purification was conducted via a combination method of salting-out and heating. Salted-out pellets were resuspended in Milli-Q water at a concentration up to 250 mg/mL (based on the wet weight of the pellet) before heating. The resuspended solution was heated at 75 – 80 °C for 10 – 15 min. Dialysis was used to remove residual salts from the salting-out method. Protein expression and purification were confirmed via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot. Protein purity was assessed through densitometry analysis using Image J (NIH, Bethesda, MD).24
2.3. Formation of Covalently Crosslinked Protein-based Hydrogels
Customized molds were assembled by placing 500 μm-thick silicone sheets with a 4.5-mm-diameter circular hole on top of a glass slide. All silicone sheets and glass slides were treated with Rain-X® to aid in the removal of hydrogels. Lyophilized RZ10-RGD was dissolved in ice-cold phosphate buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4). To slow down the crosslinking reaction during sample handling, the RZ10-RGD solution mixed with the tris(hydroxymethyl)phosphine (THP, Strem Chemicals Inc., Newburyport, MA) crosslinker solution (in PBS) was kept on ice as well. The solution was loaded into the silicone mold, and another silicone sheet was placed on top. The loaded molds were incubated at 37 °C in a humidified incubator for at least 2 h. Crosslinked hydrogels were cooled to room temperature before being removed from the molds.
2.4. Crosslinking Efficiency
Crosslinking efficiency was assessed via amino acid analysis performed by the Molecular Structure Facility at the University of California, Davis. The total number of reactive primary amine groups in RZ10-RGD is 8 (7 lysines and an N-terminal amine). The stoichiometric crosslinking ratio (×) was defined as the ratio of the number of hydroxyl groups in the THP crosslinker to the number of primary amines in RZ10-RGD. For 100% crosslinking efficiency at a stoichiometric crosslinking ratio of 0.8×, 5.6 lysines would be reacted. On the other hand, for 100% crosslinking efficiency at both crosslinking ratios of 1× and 5×, all 7 lysines would be reacted. Amino acid analysis was used to detect the number of unreacted lysines remaining in crosslinked hydrogels. Data are reported as an average of three replicates ± standard deviation.
2.5. Swelling Ratio and Water Content Measurements
RZ10-RGD hydrogels were prepared as described above. After formation, hydrogels were immersed in PBS at 37 °C and weighed every 24 h until the swollen weight of the hydrogels equilibrated. Swollen hydrogels were rinsed with Milli-Q water (EMD Millipore, Billerica, MA) before being lyophilized to remove salts from the PBS. Dry weights of hydrogels were measured after lyophilization. The swelling ratio (q) and water content (WC) of hydrogels were evaluated as q = Ws/Wd and WC = 100 × (Ws − Wd)/Ws, where Ws and Wd are the swollen and dry weights of the hydrogels, respectively. Data are reported as an average of five replicates ± standard deviation.
2.6. Oscillatory Rheology
Rheological properties of crosslinked resilin-like hydrogels were assessed on an AR2000 rheometer (TA Instruments, New Castle, DE) using a 15-mm-diameter plate-on-plate geometry. To prevent crosslinking during sample loading, the bottom plate was set to 4 °C before testing. The ice-cold protein and THP solutions were mixed together and immediately loaded onto the bottom plate. The top plate was then lowered to a gap distance of 825 μm. To prevent evaporation during measurements, low-viscosity mineral oil was applied to the edge of the protein solution. The temperature of the bottom plate was then raised to 37 °C. After the protein solution had equilibrated for 1 min at 37 °C, an oscillatory time sweep was performed for at least 90 min at an angular frequency of 1 rad/s and 1% strain. Sweeps of oscillatory frequency and strain were performed to verify that all measurements were conducted within the linear viscoelastic region (LVR). Frequency sweeps were conducted from 0.1 – 100 rad/s at a 1% strain. Strain sweeps were conducted from 0.1 – 100% strain at an angular frequency of 1 rad/s. Data are reported as an average of four replicates ± standard deviation.
2.7. Compression and Resilience Testing
Protein and THP solutions were prepared as described in the section on hydrogel formation but with molds constructed of 1000 μm-thick silicone sheets. Hydrogels were incubated in PBS at 37 °C until fully swollen. Calipers were used to measure the dimensions of the swollen hydrogels. Unconfined compression testing was conducted at room temperature in air on an Electroforce 3220 series III instrument (Bose Corporation, Eden Prairie, MN) with two parallel plates and a 100 g load cell. Fully swollen hydrogels were placed on the bottom plate, and the top plate was lowered until a preload force of 10 g was applied to the hydrogels. The hydrogels were compressed at a speed of 10 μm/s, and the normal force data were recorded. The compression modulus was calculated from the slope between 0 – 2% strain on the stress-strain curve, and data are reported as an average of three replicates ± standard deviation. For resilience measurements, hydrogels were compressed to 10% strain at a strain rate of 10 μm/s and then released back to 0% strain one time to ensure full contact between the gels and the plates. Afterwards, hydrogels were successively subjected to 10%, 20%, 40%, and 60% strain with 5 cycles for each strain percentage. Stress-strain data were recorded, and the resilience was calculated as the ratio of the area under the unloading curve to the area under the loading curve. Data are reported as an average of three replicates ± standard deviation.
2.8. Cryo-scanning Electron Microscopy Imaging
Cryo-scanning electron microscopy (cryo-SEM) images were acquired by the Life Science Microscopy Facility (Purdue University, West Lafayette, IN). As described above, hydrogel samples were prepared with a thickness of 1000 μm, and the hydrogels were immersed in Milli-Q water until fully swollen. Samples were plunged quickly into liquid nitrogen to preserve network structure for imaging. SEM images were taken on an FEI NOVA nanoSEM field emission scanning electron microscope (FEI Company, Hillsboro, OR) using an Everhart-Thornley (ET) detector or the high-resolution through-the-lens (TLD) detector operating at an acceleration voltage of 5 kV. Three replicates of each gel were examined.
2.9. Statistical Analysis
All data are presented as the mean ± standard deviation and were analyzed using a one-way analysis of variance (ANOVA). Tukey’s post hoc analysis was performed to organize treatments into statistically different (p < 0.05) subgroups using Minitab (State College, PA). When groups exhibited unequal variances, Welch’s ANOVA coupled with Games-Howell post hoc analysis was performed instead. For all statistical tests, a significance threshold of α = 0.05 was chosen.
3. RESULTS AND DISCUSSION
3.1. Protein Expression and Purification
A schematic of the protein design of RZ10-RGD is shown in Figure 1, and the detailed amino acid sequence is shown in Figure S1. RZ10-RGD consists of 10 repeats of the repeating resilin motif derived from A. gambiae and an Arg-Gly-Asp (RGD) cell-binding domain. One lysine (K) residue is introduced into every two resilin repeats and serves as a crosslinking site. Large-scale protein fermentation and purification were conducted as previously reported.5 Moderate yields (up to 70 mg/L) were achieved. Purified RZ10-RGD showed high purity (>95%) from SDS-PAGE, and these results are similar to those reported in a previous publication.5
Figure 1.

RZ10-RGD protein design and successful production. (A) The design scheme and protein sequence of recombinant RZ10-RGD. There are a total of 10 resilin repeats (gray rectangles) with one lysine residue (black line) introduced into every two repeats. Lysines serve as crosslinking sites to create a crosslinked RZ10-RGD network. Near the C-terminus there is a cell-binding domain (RGD) to promote cell interactions. (B) SDS-PAGE gel showed successful purification of RZ10-RGD (1 mg/mL in Milli-Q water). The expected molecular weight of RZ10-RGD is 18.3 kDa, and the purity of RZ10-RGD was calculated to be >95%.
3.2. Formation of Crosslinked RZ10 Hydrogels
RZ10-RGD hydrogels were crosslinked by forming aminomethylphosphine bonds between primary amines of RZ10 and the crosslinker THP (Scheme 1). Gelation was confirmed by visual observation of the clear protein solution becoming opaque and through characterization of rheological properties on a rheometer.
Scheme 1.
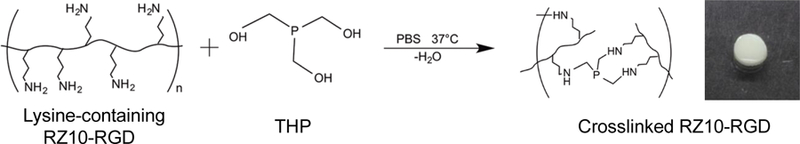
Crosslinking scheme in which the primary amine groups of RZ10-RGD react with the hydroxyl groups of THP. The reaction happens under physiological conditions and is biocompatible. Crosslinked RZ10-RGD is opaque and forms a free-standing 3D hydrogel.
3.3. Crosslinking Efficiency
The crosslinking efficiencies of crosslinked RZ10-RGD hydrogels can be determined by detecting the difference in the number of lysine residues between uncrosslinked and crosslinked resilin-like proteins. This characterization can be performed via amino acid analysis because the alkyamine linkage formed during the crosslinking reaction can be acid-hydrolyzed. Amino acid analysis was performed on 12 wt% resilin-like hydrogels with varying crosslinking ratios to determine the crosslinking efficiencies. Amino acid analysis results showed that crosslinking ratios of 5×, 1×, and 0.8× resulted in crosslinking efficiencies of 85.5 ± 1.4%, 36.5 ± 5.2%, and 47.2 ± 14.9%, respectively. Low crosslinking efficiencies were also observed when crosslinking RLP12 with [tris(hydroxymethyl)phosphino] propionic acid (THPP).18
3.4. Water Content and Swelling Ratio
RZ10-RGD hydrogels were immersed in PBS at 37 °C after formation, and their wet weights were monitored every 24 h. Most hydrogels equilibrated after 72 h and showed no significant differences in weight after this time. Before lyophilization, hydrogels were rinsed with Milli-Q water to remove residual salts from the PBS. The water content and swelling ratios of crosslinked RZ10-RGD hydrogels were calculated as described in the Materials and Methods section and are shown in Figure 2, Table 1, and Table 2.
Figure 2.

(A, B) Swelling ratio and (C, D) water content of RZ10-RGD hydrogels. (A,C) Welch’s ANOVA coupled with a Games-Howell post hoc analysis were performed for hydrogels with a stoichiometric crosslinking ratio of 5× and different protein concentrations. (B,D) ANOVA and Tukey’s honestly significant difference post hoc tests were performed for 12 wt% hydrogels with various crosslinking ratios. Letters indicate groups that are not significantly different from each other (p > 0.05).
Table 1.
Water content and swelling ratios for RZ10-RGD hydrogels with a stoichiometric crosslinking ratio of 5× and varying protein concentration.
| Protein concentration (wt%) | Water content (%) | Swelling ratio |
|---|---|---|
| 8 | 89.9 ± 0.8 | 10.0 ± 0.9 |
| 10 | 88.1 ± 2.1 | 8.6 ± 1.5 |
| 12 | 88.3 ± 2.6 | 8.9 ± 1.9 |
| 14 | 85.5 ± 1.5 | 6.9 ± 0.7 |
| 16 | 87.2 ± 0.3 | 7.8 ± 0.2 |
Table 2.
Water content and swelling ratios for 12 wt% RZ10-RGD hydrogels with various stoichiometric crosslinking ratios.
| Stoichiometric crosslinking ratio | Water content (%) | Swelling ratio |
|---|---|---|
| 5× | 88.3 ± 2.6 | 8.9 ± 1.9 |
| 1× | 93.3 ± 1.0 | 15.3 ± 2.6 |
| 0.8× | 98.1 ± 1.2 | 12.9 ± 1.9 |
At a crosslinking ratio of 5×, 8 wt% RZ10-RGD hydrogels had a higher water content and swelling ratio than 14 or 16 wt% hydrogels. Similarly, water content and swelling ratio for 12 wt% hydrogels were higher for gels with crosslinking ratios of 0.8× and 1× compared to gels crosslinked at 5×. This trend is comparable to what has been observed for other resilin-like hydrogels.18 RLP12 hydrogels at 20 wt% had decreasing swelling ratios with increasing crosslinking ratios.
All RZ10-RGD hydrogels investigated in this study possessed a high swelling ratio (>6) and high water content (>85%) (Figure 2). These water content values are comparable to those of natural resilin (60%) and other recombinant resilin hydrogels (82 – 96%).10, 18, 20 The high water content and swelling ratios suggest that RZ10-RGD is hydrophilic. Hydrophilic materials are promising for use in the fields of tissue engineering and drug delivery as biocompatibility is promoted by high water content.25
3.5. Rheological Properties
Rheological properties of crosslinked RZ10-RGD were characterized on a rheometer. Protein and crosslinker solutions were mixed and loaded on a rheometer, and time sweeps were performed to monitor the gelation of the crosslinking reaction. Gelation time was determined as the time when the storage modulus (G’) surpassed the loss modulus (G’’) on a time sweep (Figures 3A and 3B) and when the phase angle (δ) decreased below 45°. Gelation times of crosslinked RZ10-RGD are shown in Table 3 for different protein concentrations and Table 4 for different stoichiometric crosslinking ratios. The gelation time was ~5 – 6 min for crosslinked RZ10-RGD with a stoichiometric crosslinking ratio of 5× (Table 3). Decreasing crosslinking ratios resulted in increased gelation times. For 12 wt% hydrogels, when the crossinking ratio decreased from 5× to 1×, the gelation time increased from 3 min to 9 min (Table 4). When the crosslinking ratio was further reduced to 0.8×, the gelation time increased to 11 min.
Figure 3.

Rheological properties of 8 wt% and 14 wt% crosslinked RZ10-RGD. (A) Dynamic oscillatory time sweeps were used to monitor gelation of crosslinked RZ10-RGD. Both moduli reached equilibrium after 30 min with G’ >> G’’, indicating formation of steady-state gels. (B) Graphing the first 10 min of gelation revealed that fast gelation occurred for both 8 wt% and 14 wt% hydrogels. Gelation occurs when the storage modulus (G’) crosses over the loss modulus (G’’). (C) Oscillatory frequency sweeps and (D) oscillatory strain sweeps showed that for both 8 wt% and 14 wt% RZ10-RGD hydrogels, G’ was not a function of frequency or strain within the frequency range of 0.1 – 10 rad/s and the strain range of 0.1 – 10%.
Table 3.
Gelation times of crosslinked RZ10-RGD with various protein concentrations and a stoichiometric crosslinking ratio of 5×.
| Protein concentration (wt%) | Gelation time (s) |
|---|---|
| 8 | 200.6 ± 69.5 |
| 10 | 142.6 ± 60.7 |
| 12 | 160.0 ± 75.5 |
| 14 | 177.4 ± 31.8 |
| 16 | 321.4 ± 55.4 |
Table 4.
Gelation times of 12 wt% crosslinked RZ10-RGD with different stoichiometric crosslinking ratios.
| Stoichiometric crosslinking ratio | Gelation time (s) |
|---|---|
| 0.8× | 652.5 ± 116.5 |
| 1× | 536.4 ± 90.0 |
| 5× | 160.0 ± 75.5 |
Dynamic oscillatory storage moduli of crosslinked RZ10-RGD were measured when G’ and G’’ reached plateau regions. Oscillatory frequency (Figure 3C) and strain (Figure 3D) sweeps were performed to verify that the angular frequency and strain used during testing were within the linear region. We previously reported that crosslinked 16 wt% RZ10-RGD possessed a storage modulus of 22 kPa.5 To investigate the range of mechanical properties that crosslinked RZ10-RGD can span, mechanical properties of four different protein concentrations (8, 10, 12, and 14 wt%) with a stoichiometric crosslinking ratio of 5× were investigated. As expected, results showed that with decreasing protein concentration, the moduli of crosslinked RZ10-RGD decreased. The shear modulus decreased from 22 kPa to 75 Pa when the protein concentration decreased from 16 wt% to 8 wt% (Figure 4). Interestingly, there seemed to be a sharp change in the shear modulus when the protein concentration decreased from 12 wt% to 10 wt%. To further investigate the change of shear modulus within this range, we investigated two more stoichiometric crosslinking ratios (1× and 0.8×) at the protein concentration of 12 wt%. Decreasing the stoichiometric crosslinking ratio from 5× to 0.8× lowered the shear modulus from 10 kPa to 2.8 kPa. Tables 5 and 6 contain a summary of rheological properties of crosslinked RZ10-RGD under different protein concentrations and stoichiometric crosslinking ratios.
Figure 4.

Rheological properties of crosslinked RZ10-RGD with varying protein concentrations and stoichiometric crosslinking ratios. The shear moduli of crosslinked RZ10-RGD can be modulated from 75 Pa to 22 kPa by increasing the protein concentration and the stoichiometric crosslinking ratio. (A) Welch’s ANOVA coupled with a Games-Howell post hoc analysis were performed for hydrogels with a stoichiometric crosslinking ratio of 5× and different protein concentrations. (B) ANOVA and Tukey’s honestly significant difference post hoc tests were performed for 12 wt% hydrogels with various stoichiometric crosslinking ratios. Letters indicate groups that are not significantly different from each other (p > 0.05).
Table 5.
Storage moduli (G’), loss moduli (G’’), and shear moduli for crosslinked RZ10-RGD with a stoichiometric crosslinking ratio of 5× and varying protein concentration.
| Protein concentration (wt%) |
G’ (Pa) | G’’ (Pa) | Shear modulus (Pa) |
|---|---|---|---|
| 8 | 74.5 ± 7.6 | 1.7 ± 0.3 | 74.5 ± 7.6 |
| 10 | 285.7 ± 207.7 | 7.0 ± 4.1 | 285.8 ± 207.8 |
| 12 | 10008 ± 646.4 | 222.3 ± 41.5 | 10010.5 ± 646.6 |
| 14 | 19690 ± 884.6 | 432.6 ± 25.7 | 19694.8 ± 884.9 |
| 16 | 22350 ± 1411.0 | 100.6 ± 6.1 | 22350.2 ± 1411.0 |
Table 6.
Storage moduli (G’), loss moduli (G’’), and shear moduli for crosslinked 12 wt% RZ10-RGD with various stoichiometric crosslinking ratios.
| Stoichiometric crosslinking ratio |
G’ (Pa) | G’’ (Pa) | Shear modulus (Pa) |
|---|---|---|---|
| 5× | 10008 ± 646.4 | 222.3 ± 41.5 | 10010.5 ± 646.6 |
| 1× | 4745.3 ± 826.0 | 39.1 ± 5.4 | 4745.4 ± 825.9 |
| 0.8× | 2769.6 ± 720.5 | 23.6 ± 10.1 | 2769.7 ± 720.5 |
We demonstrated that rheological properties of crosslinked RZ10-RGD could be tuned by varying the protein concentration and the stoichiometric crosslinking ratio. The shear moduli of crosslinked RZ10-RGD ranged from 75 Pa – 22 kPa. To our knowledge, this range of mechanical properties is the largest reported for recombinant resilin-like proteins. Li et al. demonstrated that crosslinking RLP12 with similar chemistry resulted in shear moduli ranging from 0.6 – 20 kPa.18, 26–27 McGann et al. showed that RLP hydrogels crosslinked with PEG had storage moduli of 0.4 – 13 kPa.19–20, 28
Researchers have investigated using materials that possess similar mechanical properties to native tissues for stem cell differentiation.29 The wide range of stiffness that crosslinked RZ10-RGD spans matches the stiffness of soft tissues such as brain tissue (0.1 – 1 kPa)30 to stiffer tissues such as muscle (8 – 17 kPa).31 This feature demonstrates the potential to use RZ10-RGD to investigate the ways in which the mechanical properties of the microenvironment influence stem cell fate in tissue engineering.
3.6. Compressive Modulus
The unconfined compressive moduli of RZ10-RGD hydrogels were characterized by applying a constant uniaxial strain to hydrated RZ10-RGD on the Bose Electroforce instrument. By changing the protein concentration from 12 wt% to 16 wt%, the unconfined compressive moduli of RZ10-RGD hydrogels were modulated from 1.0 – 1.7 kPa (Table 7). Materials with similar mechanical properties have been used to differentiate stem cells to various tissues including neurons (0.1 – 1 kPa),23, 32–33 pancreatic beta cells (2.1 kPa),34 adipocytes (~1 – 2 kPa),35–36 and chondrocytes (~1 – 3.5 kPa).35, 37–38 Given that the values of compressive moduli of our crosslinked RZ10-RGD are comparable to the stiffness of a variety of tissues, RZ10-RGD offers a new platform to investigate the role of mechanical properties on cell functions such as differentiation of stem cells.
Table 7.
Compressive moduli of crosslinked, hydrated RZ10-RGD with various protein concentrations and a stoichiometric crosslinking ratio of 5×.
| Protein concentration (wt%) | Compressive Modulus (Pa) |
|---|---|
| 12 | 1006± 475.3 |
| 14 | 1243 ± 224.8 |
| 16 | 1676 ± 96.7 |
We previously demonstrated high compressive modulus of RZ10-RGD (2.5 MPa) for potential applications in engineering cartilage,5 a tissue subjected to compressive forces. However, hydrogels with the same protein concentration (16 wt%) and crosslinking ratio (5×) showed lower compressive modulus in this study. One major difference between the two studies is that the hydrogels tested in the current study were immersed in PBS until fully hydrated whereas the hydrogels in the previous study were not. When poly(vinyl alchol)-chitosan based hydrogels were fully hydrated, they demonstrated a reduced compressive modulus compared to non-hydrated hydrogels.39 Therefore, we believe that the discrepancy in the stiffness resulted from different hydration levels of hydrogels.
3.7. Resilience
The resilience of crosslinked RZ10-RGD hydrogels was characterized by repeated loading and unloading strain cycles in a compression format. All hydrogels were subjected to 10%, 20%, 40%, and 60% strain consecutively to determine the resilience at each strain. Typical stress-strain curves under different strains are shown in Figures 5 and S2. Resilience was calculated as the ratio of the area under the unloading curve to the area under the loading curve in one cycle. Soft (G’ < 10 kPa) RZ10-RGD hydrogels showed minimal resilience (data not shown). Therefore, only RZ10-RGD hydrogels with high mechanical properties (12 wt%, 14 wt%, and 16 wt% at a crosslinking ratio of 5×) were further investigated in resilience tests. Results are shown in Table 8 for each hydrogel at different strains.
Figure 5.

Cyclic compression tests on hydrated 16 wt% RZ10-RGD hydrogels at a stoichiometric crosslinking ratio of 5×. Five strain loading and unloading cycles were performed up to 10% (black) and 20% (gray) strain. Resilience was calculated as the ratio of the area under the unloading curve to the area under the loading curve at each cycle.
Table 8.
Resilience data for RZ10-RGD hydrogels with a stoichiometric crosslinking ratio of 5× and varying protein concentration.
| Protein concentration (wt%) |
Strain | |||
|---|---|---|---|---|
| 10% | 20% | 40% | 60% | |
| 12 | 58.4 ± 15.6 | 29.0 ± 16.6 | − | − |
| 14 | 71.2 ± 10.4 | 59.7 ± 10.9 | 56.4 ± 9.4 | 61.5 ± 5.7 |
| 16 | 70.0 ± 7.4 | 49.8 ± 5.5 | 46.3 ± 5.0 | 54.0 ± 6.6 |
RZ10-RGD hydrogels at 14 wt% and 16 wt% showed moderate resilience (~70%) at 10% strain, but the resilience decreased to 50 – 60% at higher strains (20%, 40%, and 60%). For 12 wt% RZ10-RGD hydrogels, moderate resilience (~60%) was observed at 10% strain; however, the resilience decreased rapidly to ~30% when the strain was increased to 20%. No resilience data was collected for 12 wt% hydrogels strains higher than 20% because of hydrogel breakdown.
Resilience of RZ10-RGD hydrogels was comparable to common synthetic rubbers (56 – 80%) but relatively lower when compared to native resilin (92%) and other recombinant resilin-based proteins (82 – 100%).11, 14, 17–20, 22, 40–42 Notably, the resilience of other resilin-based proteins was determined primarily using scanning probe microscopy (SPM). SPM has recently become a routine method to evaluate the resilience of materials.43 However, the measured resilience of viscoelastic materials highly depends on experimental parameters such as probe size, strain rate, and maximum strain. These conditions can be significantly different between SPM and bulk compression tests and thus result in different absolute values of material resilience. In particular, SPM probes penetrate a few hundred nanometers into the material surface whereas the Bose Electroforce instrument used in our experiments deforms hydrogels on the micrometer scale. At the molecular level, more cooperative segmental motion is required for polymer chains to accommodate greater deformation; thus, it is harder for materials to recover rapidly without energy loss.43 This difference could potentially offer an explanation of the lower resilience of RZ10-RGD compared to the resilience of other resilin-based proteins measured using SPM.14, 22, 40–42 On the other hand, RLP12-based hydrogels showed high resilience (>87%) up to 100% strain in bulk tensile tests.18, 20 It should be noted that there are major differences in the sequences of these resilin-like proteins. Resilin-like sequences of RZ10-RGD and RLP12 are derived from different species, and our previous results showed that RZ10-RGD is stiffer than RLP12.5
3.8. Cryo-scanning Electron Microscopy
Cryo-SEM was performed to examine the network structures of RZ10-RGD hydrogels. Hydrogels with low protein concentration (8 wt% and 10 wt%) showed rounded protein particles with little to no connectivity. Images of 8 wt% protein particles are shown in Figure 6A. Hydrogels at 10 wt% showed similar connectivity but had larger particles than the 8 wt% hydrogels (data not shown). Connectivity of the protein networks increased with increasing protein concentration. Hydrogels at 12 wt% and a stoichiometric crosslinking ratio of 5× showed connected networks but many incomplete pore walls (data not shown). Decreasing the stoichiometric crosslinking ratios of 12 wt% RZ10-RGD hydrogels resulted in inhomogeneous network structures with various pore sizes and morphologies (data not shown), and there was no obvious relationship between network connectivity and crosslinking ratio. RZ10-RGD hydrogels at 14 wt% exhibited a well-connected network with an average pore size of ~3 – 4 μm (Figure 6B).
Figure 6.

Cryo-SEM images of (A) 8 wt% and (B) 14 wt% RZ10-RGD hydrogels. Hydrogels at 8 wt% showed rounded protein particles with little connectivity. On the other hand, 14 wt% hydrogels showed well-connected networks. Scale bar represents 10 μm in images on the left and 5 μm in images on the right.
The structures of RZ10-RGD hydrogels may describe the trends observed in the mechanical properties. Hydrogels at low protein concentrations formed poorly-connected networks and resulted in soft hydrogels. Higher protein concentrations resulted in well-connected structures and stiffer hydrogels. It is possible that the inhomogeneity of 12 wt% hydrogels could be an intermediate state between particles with little connectivity and a fully crosslinked network. Thus, the presence of partially connected structures could potentially explain the sharp increase in shear moduli at 12 wt% (Figure 4).
4. CONCLUSIONS
Our lab has previously designed and manufactured the modular recombinant resilin-like protein, RZ10-RGD. This study presents a more thorough examination of these hydrogels and demonstrates ways in which to tune the mechanical properties. By varying the protein concentration or stoichiometric crosslinking ratio, the gelation times (3 to 10 min), shear moduli (75 Pa to 22 kPa), compressive moduli (1.0 to 1.7 kPa), and network structures were modulated. Resilience measurements showed that crosslinked RZ10-RGD exhibited resilience values comparable to those of common synthetic rubbers. The wide range of tunable properties that RZ10-RGD hydrogels can achieve demonstrates their potential application as a platform to study the mechanical niche on cell behavior or for use as a drug delivery carrier.
Supplementary Material
Figure S1: full amino acid sequence of RZ10-RGD. Figure S2: additional stress-strain curves during cyclic compression tests.
Highlights.
Resilin is an elastomeric protein found in insect cuticle.
Resilin-like hydrogels are hydrophilic and crosslinked rapidly (3–10 min).
Properties were modulated by varying protein concentration or crosslinking ratio.
Shear moduli (75 Pa to 22 kPa) and compressive moduli (1.0 to 1.7 kPa) were tuned.
Hydrogels exhibited resilience values comparable to those of synthetic rubbers.
6. ACKNOWLEDGMENTS
This work was supported by the Department of Defense Lung Cancer Research Program (W81XWH-14-1-0012 to J.C.L.), the National Institutes of Health (NIDCR R03DE021755 to J.C.L.), the American Heart Association Scientist Development Grant (12SDG8980014 to J.C.L.), a 3M Nontenured Faculty Award (J.C.L.), a Bilsland Dissertation Fellowship (R.S.-C.S.), and the Purdue Summer Undergraduate Research Fellowship Program (E.E.G.). We thank Dr. Sherry Voytik-Harbin (Purdue University) for access to her rheometer, Dr. Chia-Ping Huang (Life Science Microscopy Facility, Purdue University) for help obtaining cryo-SEM images, and Dr. John Schulze (Molecular Structure Facility, University of California, Davis) for performing amino acid analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Army, or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATIONS OF INTEREST
None.
8. REFERENCES
- 1.Langer RS; Vacanti JP, Tissue Engineering: The Challenges Ahead. Scientific American 1999, 280 (4), 86–89. [DOI] [PubMed] [Google Scholar]
- 2.Burdick JA; Vunjak-Novakovic G, Engineered Microenvironments for Controlled Stem Cell Differentiation. Tissue Engineering: Part A 2009, 15 (2), 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sengupta D; Heilshorn SC, Protein-engineered Biomaterials: Highly Tunable Tissue Engineering Scaffolds. Tissue Engineering, Part B: Reviews 2010, 16, 285–293. [DOI] [PubMed] [Google Scholar]
- 4.Li L; Charati MB; Kiick KL, Elastomeric Polypeptide-Based Biomaterials. Polymer Chemistry 2010, 1 (8), 1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renner JN; Cherry KM; Su RS-C; Liu JC, Characterization of Resilin-based Materials for Tissue Engineering Applications. Biomacromolecules 2012, 13 (11), 3678–3685. [DOI] [PubMed] [Google Scholar]
- 6.Renner JN; Kim Y; Cherry KM; Liu JC, Modular Cloning and Protein Expression of Long, Repetitive Resilin-based Proteins. Protein Expression and Purification 2012, 82 (1), 90–96. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y; Renner JN; Liu JC, Incorporating the BMP-2 Peptide in Genetically-engineered Biomaterials Accelerates Osteogeneic Differentiation. Biomaterials Science 2014, 2 (8), 1110–1119. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y; Gill EE; Liu JC, Enzymatic Cross-Linking of Resilin-Based Proteins for Vascular Tissue Engineering Applications. Biomacromolecules 2016, 17 (8), 2530–2539. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y; Liu JC, Protein-engineered microenvironments can promote endothelial differentiation of human mesenchymal stem cells in the absence of exogenous growth factors. Biomaterials Science 2016, 4 (12), 1761–1772. [DOI] [PubMed] [Google Scholar]
- 10.Weis-Fogh T, A Rubber-like Protein in Insect Cuticle. Journal of Experimental Biology 1960, 37, 889–907. [Google Scholar]
- 11.Weis-Fogh T, Molecular Interpretation of the Elasticity of Resilin, A Rubber-like Protein. Journal of Molecular Biology 1961, 3 (5), 648–667. [Google Scholar]
- 12.Weis-Fogh T, Thermodynamic Properties of Resilin, A Rubber-like Protein. Journal of Molecular Biology 1961, 3 (5), 520–531. [Google Scholar]
- 13.Ardell DH; Andersen SO, Tentative Identification of A Resilin Gene in Drosphila Melanogaster. Insect Biochemistry and Molecular Biology 2001, 31 (10), 965–970. [DOI] [PubMed] [Google Scholar]
- 14.Elvin CM; Carr AG; Huson MG; Maxwell JM; Pearson RD; Vuocolo T; Liyou NE; Wong DCC; Merritt DJ; Dixon NE, Synthesis and Properties of Crosslinked Recombinant Pro-resilin. Nature 2005, 437, 999–1002. [DOI] [PubMed] [Google Scholar]
- 15.Lyons RE; Lesieur E; Kim M; Wong DCC; Huson MG; Nairn KM; Brownlee AG; Pearson RD; Elvin CM, Design and Facile Production of Recombinant Resilin-like Polypeptides: Gene Construction and A Rapid Protein Purification Method. Protein Engineering, Design and Selection 2007, 20 (1), 25–32. [DOI] [PubMed] [Google Scholar]
- 16.Su RS-C; Kim Y; Liu JC, Resilin: Protein-based elastomeric biomaterials. Acta Biomaterialia 2014, 10, 1601–1611. [DOI] [PubMed] [Google Scholar]
- 17.Charati MB; Ifkovits JL; Burdick JA; Linhardt JG; Kiick KL, Hydrophilic Elastomeric Biomaterials Based on Reilin-like Polypeptides. Soft Matter 2009, 5, 3412–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L; Teller S; Clifton RJ; Jia X; Kiick KL, Tunable Mechanical Stability and Deformation Response of A Resilin-based Elastomer. Biomacromolecules 2011, 12 (6), 2302–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGann CL; Levenson EA; Kiick KL, Resilin-Based Hybrid Hydrogels for Cardiovascular Tissue Engineering. Macromolecular Chemistry and Physics 2013, 214 (2), 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGann CL; Akins RE; Kiick KL, Resilin-PEG Hybrid Hydrogels Yield Degradable Elastomeric Scaffolds with Heterogeneous Microstructure. Biomacromolecules 2016, 17 (1), 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv S; Dudek DM; Cao Y; Balamurali MM; Gosline J; Li H, Designed Biomaterials to Mimic the Mechanical Properties of Muscles. Nature 2010, 465, 69–73. [DOI] [PubMed] [Google Scholar]
- 22.Sbrana F; Fotia C; Bracalello A; Baldini N; Marletta G; Ciapetti G; Bochicchio B; Vassalli M, Multiscale Characterization of A Chimeric Biomimetic Polypeptide for Stem Cell Culture. Bioinspiration and Biomimetics 2012, 7. [DOI] [PubMed] [Google Scholar]
- 23.Engler AJ; Sen S; Sweeney HL; Discher DE, Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126 (4), 677–689. [DOI] [PubMed] [Google Scholar]
- 24.Schneider CA; Rasband WS; Eliceiri KW, NIH Image to ImageJ: 25 Years of Image Analysis. Nature Methods 2012, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J, Chitosan-based Gels for the Drug Delivery System. In Chitosan-based Hydrogels: Functions and Applications, Yao K; Li J; Yao F; Yin Y, Eds. CRC Press: 2011. [Google Scholar]
- 26.Li L; Kiick KL, Transient Dynamic Mechanical Properties of Resilin-based Elastomeric Hydrogels. Frontiers in Chemistry 2014, 2 (21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L; Mahara A; Tong Z; Levenson EA; McGann CL; Jia X; Yamaoka T; Kiick KL, Recombinant Resilin-Based Bioelastomers for Regenerative Medicine Applications. Advanced Healthcare Materials 2016, 5, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGann CL; Dumm RE; Jurusik AK; Sidhu I; Kiick KL, Thiol-ene Photocrosslinking of Cytocompatible Resilin-Like Polypeptide-PEG Hydrogels. Macromolecular Bioscience 2016, 16, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao S; Chan CK; Ramakrishna S, Stem Cells and Biomimetic Materials Strategies for Tissue Engineering. Material Science and Engineering C 2008, 28 (8), 1189–1202. [Google Scholar]
- 30.Flanagan LA; Ju YE; Marg B; Osterfield M; Janmey PA, Neurite Branching on Deformable Substrates. Neuroreport 2002, 13 (18), 2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engler AJ; Griffin MA; Sen S; Bönnemann CG; Sweeney HL; Discher DE, Myotubes Differentiate Optimally on Substrates with Tissue-Like Stiffness: Pathological Implications for Soft Or Stiff Microenvironments. The Journal of Cell Biology 2004, 166 (6), 877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kothapalli CR; Kamm RD, 3D Matrix Microenvironment for Targeted Differentiation of Embryonic Stem Cells into Neural and Glial Lineages. Biomaterials 2013, 34 (25), 5995–6007. [DOI] [PubMed] [Google Scholar]
- 33.Her GJ; Wu H-C; Chen M-H; Chen M-Y; Chang S-C; Wang T-W, Control of Three-Dimensional Substrate Stiffness to Manipulate Mesenchymal Stem Cell Fate toward Neuronal or Glial Lineages. Acta Biomaterialia 2013, 9 (2), 5170–5180. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan K; Lim VY; Shen J; Tan ZW; Rajendran D; Luo S-C; Gao S; Wan ACA; Ying JY, Extracellular Matrix-Mediated Differentiation of Human Embryonic Stem Cells: Differentiation to Insulin-Secreting Beta Cells. Tissue Engineering: Part A 2014, 20 (1&2), 424–433. [DOI] [PubMed] [Google Scholar]
- 35.Park JS; Chu JS; Tsou AD; Diop R; Tang Z; Wang A; Li S, The Effect of Matrix Stiffness on the Differentiation of Mesenchymal Stem Cells in Response to TGF-β. Biomaterials 2011, 32 (16), 3921–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young DA; Choi YS; Engler AJ; Christman KL, Stimulation of Adipogenesis of Adult Adipose-derived Stem Cells Using Substrates that Mimic the Stiffness of Adipose Tissue. Biomaterials 2013, 34 (34), 8581–8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon HJ; Yasuda K, Chondrogenesis on Sulfonate-coated Hydrogels is Regulated by Their Mechanical Properties. Journal of the Mechanical Behavior of Biomedical Materials 2013, 17, 337–346. [DOI] [PubMed] [Google Scholar]
- 38.Bian L; Hou C; Tous E; Rai R; Mauck RL; Burdick JA, The Influence of Hyaluronic Acid Hydrogel Crosslinking Density and Macromolecular Diffusivity on Human MSC Chondrogenesis and Hypertrophy. Biomaterials 2013, 34 (2), 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S-Y; Pereira BP; Yusol N; Selvaratnam L; Yu Z; Abbas AA; Kamarul T, Unconfined Compression Properties of a Porous Poly(vinyl alcohol)-chitosan-based Hydrogel After Hydration. Acta Biomaterialia 2009, 5 (6), 1919–1925. [DOI] [PubMed] [Google Scholar]
- 40.Qin G; Rivkin A; Lapidot S; Hu X; Preis I; Arinus SB; Dgany O; Shoseyov O; Kaplan DL, Recombianant exon-encoded resilins for elastomeric bioamterials. Biomaterials 2011, 32, 9231–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyons RE; Nairn KM; Huson MG; Kim M; Dumsday G; Elvin CM, Comparisions of Recombinant Resilin-like Proteins: Repetitive Domains are Sufficient to Confer Resilin-like Properties. Biomacromolecules 2009, 10 (11), 3009–3014. [DOI] [PubMed] [Google Scholar]
- 42.Bracalello A; Santopietro V; Vassalli M; Marletta G; Gaudio RD; Bochicchio B; Pepe A, Design and Production of a Chimeric Resilin-, Elastin-, and Collagen-like Engineered polypeptide. Biomacromolecules 2011, 12, 2957–2965. [DOI] [PubMed] [Google Scholar]
- 43.Huson MG; Maxwell JM, The Measurement of Resilience with A Scanning Probe Microscope. Polymer Testing 2006, 25 (1), 2–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: full amino acid sequence of RZ10-RGD. Figure S2: additional stress-strain curves during cyclic compression tests.


