Abstract
Regarding challenges in isolation and maintenance of cultured heart cells, introduction of new in vitro heart model that is stable and exhibits long-term spontaneously contracting cell aggregates (SCCs), whose electrophysiological properties are comparable to the human heart, is required. This research aimed to establish cardiac primary cells and to evaluate the effects of culture conditions. Also the effect of fish age on beating SCC and cardiac cell morphology were studied. Twelve healthy grass carps (Ctenopharyngodon idella) were divided into four groups based on their age. Non-enzymatic explant culture was used and heart explants were incubated at 21–31 °C for 60 days. After proliferation of the cardiac primary cells, changes in their morphology and their beatings were recorded. The results showed that the explants derived from different age of grass carp fish are fully viable and proliferative with formation of SCC for a long period of time. By increasing the number of adhered cells, the cardiac primary cells became more flat and elongated. Increasing the medium temperature and fetal bovine serum concentration in culture medium led to decline and enhancement in beat frequencies of heart cell aggregates, respectively. Also, grass carp heart explant had high potential in regeneration (especially in young fish) and thus high feasibility to generate stable long-term cultures. In general, it seems that explant culture of heart from grass carp can be considered as a promising tool in heart research area.
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0281-x) contains supplementary material, which is available to authorized users.
Keywords: Cardiac primary cells, Heart, Explant culture, Grass carp, Temperature
Introduction
Nowadays, the number of patients with cardiovascular disease (CVD) is quickly increasing throughout the world and CVD is considered as one of the most important causes of morbidity and mortality (Parameswaran et al. 2013). Recent scientific advances clarified some causes of cardiac disease, and focused on the pathophysiology of the disease and the therapeutic applications using various animal models. Since the highly specific conditions for human cardiocyte culture and the correlated ethical issues prevent the use of human tissue for research, our understanding and knowledge about the heart at cellular and molecular level is poor (Parameswaran et al. 2013). Hence, finding the animal models that provide easiest and most reliable source of cardiac primary cells is important in development of heart research. So far, the main model used to investigate human diseases is the murine model. However, some deviations from human cardiac physiology have hindered the use of murine model in large-scale genetic and therapeutic screening (Brette et al. 2008; Hodgson et al. 2018). Because of difference in ion channel isoforms and consequent electrical activities, the basal heart rate in mouse is 7–10 times higher than human (Carmeliet 2004), leading to very short action potential without a distinct plateau (Carmeliet 2004); while the action potentials in human ventricular myocytes has a long prominent plateau phase due to the presence of IKr (rapid delayed rectifier). Although mouse cardiac model provides useful viewpoint about pathophysiological mechanisms of cardiac arrhythmia but better animal models should be looked for in genetic and cellular cardiac research (Brette et al. 2008; Hodgson et al. 2018). In spite of obvious anatomical differences, fish is particularly useful model for cellular cardiac research (Grunow et al. 2011; Matrone et al. 2017; Sander et al. 2013), because of similarity in the cardiac physiology of fish and human (Pieperhoff et al. 2014). Fish heart has a long potential duration, long plateau phase and cardiac primary cells can regenerate via proliferation in a way that is different from rodent heart model. In fish electrocardiogram, same to human, QT-interval depends upon the beating frequency (Kikuchi et al. 2010; Milan et al. 2006; Shiels et al. 2006).
Compared to immortalized cell lines, cardiac primary cells represent physiologically more similarity to the in vivo condition (Sander et al. 2013), especially to the explant culture condition, reflecting in vivo state of the myocardium and can be regarded as a valuable method for predicting processes in the living animal. Moreover, cardiac primary cells are easy to work and do not require complex facilities, exhibiting regeneration potential (Kikuchi et al. 2010) and long-term contraction in spontaneously contracting cell aggregates without the need of medium replacement or electrical stimulation (Grunow et al. 2011), as well as do not require O2/CO2 buffering for maintaining physiological properties of the heart culture (Warren et al. 2001). Regarding the importance of the culture condition on heart cell morphology and behavior, their optimization is essential.
Cardiac primary cell is powerful tool toward research on function and regulation of heart electrophysiology at cellular level, intracellular calcium fluxes, contractile mechanics, and protein expression (Gut et al. 2017; Parameswaran et al. 2013). Heart cell culture allows microscopic visualization of the cells, especially those adhering to the plate in a primary cell culture (Parameswaran et al. 2013), and investigations on the cell morphology. Moreover, isolated heart cells can be used as a replacement for whole-heart or whole-animal to perform mechanistic and comparative studies on cardiotoxic compounds (Hodgson et al. 2018; Remião et al. 2001). The primary monolayer cultured heart cells are not completely similar to heart cells in “in vivo” condition, and change in cell–cell interactions affect results of experiments (Horrobin 2003). This problem could be solved by using spontaneously contracting cell aggregates (SCC) containing developed heart muscle cells, as the best available 3D in vitro heart model, with cell–cell connections and a pacemaker center (Grunow et al. 2010, 2011).
Regarding challenges in isolation and maintenance of cultured heart cells, introduction of new in vitro fish heart model that is stable and exhibits long-term spontaneously contracting cell aggregates (SCC) whose electrophysiological properties are comparable to the human heart is required (Nemtsas et al. 2010; Vornanen and Hassinen 2016). Compared to mammals, less evolved vertebrate animal models (e.g. fish) are more ethically acceptable and cheaper to be set up. Therefore, knowing how cardiac primary cells can be cultured and what culture condition is better for obtaining stable and long-term spontaneously contracting cell aggregates (SCC) is necessary.
For the first time, here we describe a method for grass carp cardiac primary cells by non-enzymatic method. Also the effects of changes in some culture conditions (FBS concentration, temperature) and age of fish on spontaneously contracting cell aggregates (beating) and the cell morphology are studied.
Materials and methods
Fish and anesthetizing
Twelve healthy grass carp (Ctenopharyngodon idella) were obtained from Kamangar fish farm (Babol City, Mazandaran Province, Iran), divided into four groups based on their age including under 4 months (weighing 15–20 g), 1 year (0.850–1 kg), 2 years (2–3 kg) and 3 years (3.5–5 kg) old and fed on commercial food prior to the isolation of heart. Fish were physically anesthetized through Blunt trauma method. Briefly, after restraining the fish in the net or by the tail, anesthetizing was carried out through forceful blow to the head just behind the eyes by a fish bonker. All experiments were performed according to Babol University Animal Scientific Procedures Act.
Isolation and culture of heart cells
The tissue were cut into small pieces using sterile small scissor, transferred into the empty flasks (which is uncoated) for tissues attachment. Then flasks incubated for 25 min at 23 °C were cautiously washed with sterile PBS to remove unattached tissues. Subsequently complete L-15 medium with 5–20% fetal bovine serum (FBS) was added and incubated at 21–31 °C for 60 days. Half of the medium was replaced with new one every week. After proliferation of the cardiac primary cells, their morphology and beating were assayed and recorded under an inverted microscope (Nikon Eclipse TS100, Japan).
Heart rate recording
Observation and recording of SCC were performed under an inverted microscope (Nikon Eclipse TS100, Japan) equipped with video recording system. The contraction frequency in each experiment was calculated for 1 min. After all experiments, recording the beating was performed at 26 °C, which is the range of physiological temperatures for grass carp populations in the wild (Cudmore and Mandrak 2004).
Statistical analysis
Quantitative data were presented as mean ± standard deviation. The results were tested for normality via Kolmogorov–Smirnov Test. Two-way ANOVA test was used to analyses differences between the different experimental conditions followed by Tukey’s test. Significance level was defined at 5% level. All analyses were performed using OriginPro 2016, Version b9.3.226.
Results
Cell aggregate morphology and beating frequency
Cardiac primary cells obtained from four age groups of fish were successfully established and maintained under laboratory conditions up to 60 days after the isolation. After 3–4 days of culture in groups 1 and 2 and after 4–7 days of culture in groups 3 and 4, some cells from the heart tissue began to divide and became attached to the flask. Most of these cells were fibroblast like cells (Fig. 1). The proliferated cells expanded around the tissue heart and formed mass of epithelial and fibroblast like cells. At this stage, formation of spontaneously contracting areas that had detectable beating was observed and average rate of beats per minute (BPM) for groups of 1–4 was 73 ± 2.51 BPM, 65 ± 6.02 BPM, 48 ± 3.6 BPM and 37 ± 1.9 BPM, respectively. Heart explants with one or two contraction centers were detectable (supplementary video 1 and 2). With increasing the number of adhered cells, the heart explant became more flat and elongated (after 40–43 days of the culture in groups 1 and 2 and after 35–40 days of the culture in groups 3 and 4). The intrinsic phenotype of heart explant was slowly disappeared and frequency of beating were gradually weakened (supplementary video 3) (group 1 stops beating after 68 days of the culture but in other groups the beatings were stopped in the 60th, 64th and 58th day).
Fig. 1.
Explant culture of heart from grass carp after 3–7 days. a Heart explant culture from group 1 after 3 days. Epithelium and fibroblast like cells are observed. b Heart explant culture from group 2 after 3 days. Most fibroblast like cells regularly and densely grew. c Heart explant culture from group 3 after 5 days. Fibroblast like cells are observed around the heart tissue. The rounded and detached cells (arrows) are dead. d Heart explant culture from group 4 after 7 days. Fibroblast cells grew in low density with increased number of dead cells (arrows)
Temperature and beating frequency
Results showed that increasing temperature led to a significant decrease in SCC beating frequency in all age groups (except at 21 and 23 °C in the groups 1 and 2) (P < 0.05) (Fig. 2). Generally, alterations in beating rate in all groups were regular, and maximum beating rate was observed at 21–23 °C. The beating rate severely decreased (P < 0.05) at 31 °C, and most of attached cells, especially in groups 3 and 4, were dead and made mass of debris.
Fig. 2.
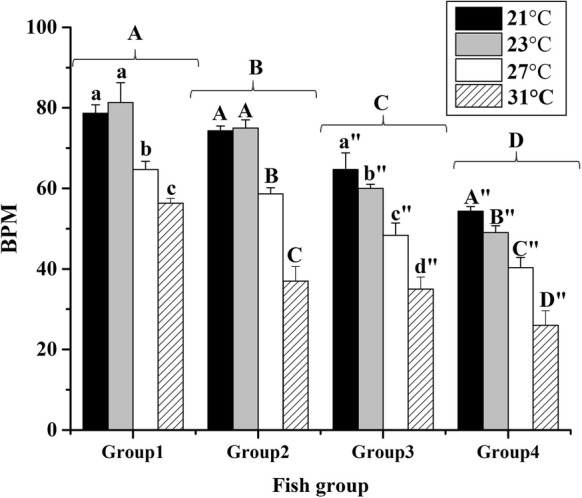
Effect of temperature on beat frequency of spontaneously contracting heart cell aggregates from explant culture of heart from grass carp in different age groups. Different letters show significant difference (P < 0.05) in each group
FBS concentration and beating frequency
In our study, increasing FBS concentration led to significant and constant increase in the beating rate in groups 3 and 4 (P < 0.05). In these groups, maximum and minimum beating rates observed were 20% and 5%, respectively. However, no regular alteration of beating rate in different concentrations of FBS was detected (Fig. 3).
Fig. 3.
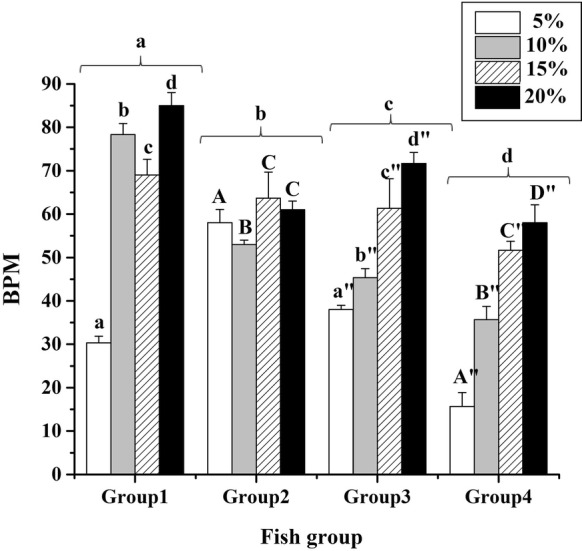
Effect of FBS concentrations on beat frequency of spontaneously contracting cell aggregates derived from grass carp heart in different age groups. Different letters show significant difference (P < 0.05) in each group
Discussion
Nowadays, remained as a great challenge, introduction of cardiac model systems providing high quality data, high throughput and low costs for genetic, biochemistry and toxicological studies is required. In spite of anatomical differences between human and fish hearts, there is a high degree of conservation in the molecular and cellular basis of relevant processes and mechanisms in both hearts (Noguera et al. 2017). In this scenario, recently fish heart has been considered as one of the best in vitro cardiac models in human and veterinary medicine (Matrone et al. 2017; Noguera et al. 2017; Phillips and Westerfield 2014; Pieperhoff et al. 2014).
For the first time, this study optimized explant cardiac cell culture, using mechanical homogenization without any enzymatic tissue digestion, from grass carp and it has been maintained for more than 60 days. According to the results, spontaneously contracting cell aggregates in different age groups were appeared on different days; so that formation of contractive areas in lower age groups is earlier and cell proliferation is faster and more regular than that in higher age groups. In addition, the number of SCCs formation in lower age groups (35–40% for group 1 and 2) was more than that in higher age group (15–20% for groups 3 and 4). It seems that by increasing the age, fish heart tissue matrix become harder which hinder the cell migration and negatively affects spontaneously contracting cardiomyocyte populations. In addition, with increasing the age, the ratio of dead cells also raised in proliferative heart cells. These cells are dissociated from the network of living cells (Fig. 1). Probably, viability, proliferation, and differentiation of heart cells in the culture condition are significantly related to the age of the fish and handling of the cells during the isolation (Sander et al. 2013).
According to the results, alteration in FBS concentrations significantly affects contraction frequencies (BPM). This alteration was more constant in the fish with higher age (2 and 3 years old). An increase in FBS concentration may introduce trophic factors that are essential for fish cardiac cell proliferation (Zeng et al. 2017) and the fish with high age are more sensitive to this alteration compared to low age fish. Previous study on zebra fish showed that the increase in FBS concentrations led to cardiac cell proliferation and development (Zeng et al. 2017). Also, the present study showed that contraction frequencies over time were different (data not showed), that may be due to the change in the supply of growth factors as it occurs when the medium is changed (Grunow et al. 2011).
In the present study, the increase of culture temperature from 21 to 31 °C caused significant decrease in the contraction frequencies (Fig. 2). It has been shown that cardiac contractility is influenced by temperature in all vertebrates (Shiels et al. 2002). In atrial myocytes of rainbow trout, increasing temperature led to decrease in intracellular calcium concentration (Grunow et al. 2011). However, some other studies suggested that sarcoplasmic reticulum Ca2+, as a source for activation of contraction, may play minor role in most fish hearts (Gesser 1996). Probably, other unknown molecular factors contribute in heart contraction. Therefore, the influence of the temperature on all factors contributing in fish heart cell contraction and why alteration of temperature causes different beating rate in cell culture, should be considered in future studies.
In conclusion, cardiac primary cells derived from different grass carp fish are able to have viability, cell division feature and formation of spontaneously contracting cell aggregates for a long time period. The increase of temperature and FBS led to decline and enhancement in beating frequencies of heart cell aggregates, respectively. Furthermore, grass carp heart explant has high potential in regeneration (especially in young fish) and thus high feasibility to generate stable long-term cultures. In general, it seems that explant culture of heart from grass carp can be considered as an in vitro heart model and promising tool in the toxicological, environmental–ecological, physiological, and medical researches.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank ‘Babol University of Medical Sciences’-Deputy of Research and Technology for providing laboratory instruments and financially supporting this research.
Compliance with ethical standards
Conflict of interest
The authors declare no competing or financial interests.
References
- Brette F, Luxan G, Cros C, Dixey H, Wilson C, Shiels HA. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio) Biochem Biophys Res Commun. 2008;374:143–146. doi: 10.1016/j.bbrc.2008.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. Intracellular Ca2+ concentration and rate adaptation of the cardiac action potential. Cell Calcium. 2004;35:557–573. doi: 10.1016/j.ceca.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Cudmore B, Mandrak NE (2004) Biological synopsis of grass carp (Ctenopharyngodon idella). Canadian manuscript report of Fisheries and Aquatic Sciences, 2705
- Gesser H. Cardiac force-interval relationship, adrenaline and sarcoplasmic reticulum in rainbow trout. J Comp Physiol B. 1996;166:278–285. doi: 10.1007/BF00262872. [DOI] [Google Scholar]
- Grunow B, Ciba P, Rakers S, Klinger M, Anders E, Kruse C. In vitro expansion of autonomously contracting, cardiomyogenic structures from rainbow trout Oncorhynchus mykiss. J Fish Biol. 2010;76:427–434. doi: 10.1111/j.1095-8649.2009.02535.x. [DOI] [PubMed] [Google Scholar]
- Grunow B, Wenzel J, Terlau H, Langner S, Gebert M, Kruse C. In vitro developed spontaneously contracting cardiomyocytes from rainbow trout as a model system for human heart research. Cell Physiol Biochem. 2011;27:1–12. doi: 10.1159/000325212. [DOI] [PubMed] [Google Scholar]
- Gut P, Reischauer S, Stainier DY, Arnaout R. Little fish, big data: zebrafish as a model for cardiovascular and metabolic disease. Physiol Rev. 2017;97:889–938. doi: 10.1152/physrev.00038.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson P, Ireland J, Grunow B. Fish, the better model in human heart research? Zebrafish heart aggregates as a 3D spontaneously cardiomyogenic in vitro model system. Prog Biophys Mol Biol. 2018 doi: 10.1016/j.pbiomolbio.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Horrobin DF. Modern biomedical research: an internally self-consistent universe with little contact with medical reality? Nat Rev Drug Discov. 2003;2:151. doi: 10.1038/nrd1012. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, et al. Primary contribution to zebrafish heart regeneration by gata4 + cardiomyocytes. Nature. 2010;464:601. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrone G, Tucker CS, Denvir MA. Cardiomyocyte proliferation in zebrafish and mammals: lessons for human disease. Cell Mol Life Sci. 2017;74:1367–1378. doi: 10.1007/s00018-016-2404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan DJ, Jones IL, Ellinor PT, MacRae CA. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am J Physiol Heart Circ Physiol. 2006;291:H269–H273. doi: 10.1152/ajpheart.00960.2005. [DOI] [PubMed] [Google Scholar]
- Nemtsas P, Wettwer E, Christ T, Weidinger G, Ravens U. Adult zebrafish heart as a model for human heart? An electrophysiological study. J Mol Cell Cardiol. 2010;48:161–171. doi: 10.1016/j.yjmcc.2009.08.034. [DOI] [PubMed] [Google Scholar]
- Noguera PA, Grunow B, Klinger M, Lester K, Collet B, del-Pozo J. Atlantic salmon cardiac primary cultures: an in vitro model to study viral host pathogen interactions and pathogenesis. PLoS ONE. 2017;12:e0181058. doi: 10.1371/journal.pone.0181058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran S, Kumar S, Verma RS, Sharma RK. Cardiomyocyte culture—an update on the in vitro cardiovascular model and future challenges. Can J Physiol Pharmacol. 2013;91:985–998. doi: 10.1139/cjpp-2013-0161. [DOI] [PubMed] [Google Scholar]
- Phillips JB, Westerfield M. Zebrafish models in translational research: tipping the scales toward advancements in human health. Dis Models Mech. 2014;7:739–743. doi: 10.1242/dmm.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieperhoff S, et al. Heart on a plate: histological and functional assessment of isolated adult zebrafish hearts maintained in culture. PLoS ONE. 2014;9:e96771. doi: 10.1371/journal.pone.0096771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remião F, Carmo H, Carvalho F, Bastos M. The study of oxidative stress in freshly isolated Ca2+-tolerant cardiomyocytes from the adult rat. Toxicol In Vitro. 2001;15:283–287. doi: 10.1016/S0887-2333(01)00022-4. [DOI] [PubMed] [Google Scholar]
- Sander V, Suñe G, Jopling C, Morera C, Belmonte JCI. Isolation and in vitro culture of primary cardiomyocytes from adult zebrafish hearts. Nat Protoc. 2013;8:800. doi: 10.1038/nprot.2013.041. [DOI] [PubMed] [Google Scholar]
- Shiels HA, Vornanen M, Farrell AP. Effects of temperature on intracellular [Ca2+] in trout atrial myocytes. J Exp Biol. 2002;205:3641–3650. doi: 10.1242/jeb.205.23.3641. [DOI] [PubMed] [Google Scholar]
- Shiels HA, Calaghan SC, White E. The cellular basis for enhanced volume-modulated cardiac output in fish hearts. J Gen Physiol. 2006;128:37–44. doi: 10.1085/jgp.200609543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vornanen M, Hassinen M. Zebrafish heart as a model for human cardiac electrophysiology. Channels. 2016;10:101–110. doi: 10.1080/19336950.2015.1121335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KS, Baker K, Fishman MC. The slow mo mutation reduces pacemaker current and heart rate in adult zebrafish. Am J Physiol Heart Circ Physiol. 2001;281:H1711–H1719. doi: 10.1152/ajpheart.2001.281.4.H1711. [DOI] [PubMed] [Google Scholar]
- Zeng WR, Beh SJ, Bryson-Richardson RJ, Doran PM. Production of zebrafish cardiospheres and cardiac progenitor cells in vitro and three-dimensional culture of adult zebrafish cardiac tissue in scaffolds. Biotechnol Bioeng. 2017;114:2142–2148. doi: 10.1002/bit.26331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



