Abstract
Background
Although nodding syndrome is a catastrophic epileptic encephalopathy, it is reported only from Africa so far. We describe the first case from the Indian sub-continent.
Methods
A ten-year-old child who had an episode of Guillain Barre syndrome with incomplete recovery developed emaciation secondary to bulbar palsy and depression. Subsequently, nine months later she developed head nodding, spastic quadriparesis, choreo-athetoid movement disorder, global aphasia and depression. She improved with sodium valproate, nutritional rehabilitation and anti-spasticity and anti-depressant medications.
Results
First case of nodding syndrome is described from India where possible etiology is malnutrition. She had anemia, her electroencephalography revealed parieto-occipital inter-ictal epileptiform discharges and Magnetic Resonance Imaging showed diffuse cerebral atrophy.
Conclusion
Nodding syndrome is an epileptic encephalopathy of nutritional origin beyond geographical barriers but amenable to anti-convulsants and nutritional rehabilitation.
Keywords: Nodding syndrome, India
Introduction
Nodding syndrome (NS)is a devastating epidemic epilepsy of unknown etiology reported since 1960's in sub-Saharan African countries of South Sudan1–3, Uganda4–6, and Tanzania7–9. Studies done in Tanzania reveal that nodding syndrome is a symptomatic generalized epilepsy4,6,7. It is characterized by head bobs4 or dorsoventral head movements occurring repeatedly around 5– 20 times in a minute and patient is usually unresponsive during the episode. It usually affects children of the age group 5–15 years and nodding usually starts at sight of food leading the child to become stunted but this is not the only trigger, others may include cold food or cold breeze. The child becomes malnourished, dehydrated, cognitively retarded and has multiple types of seizures6–7. Multiple etiologies have been considered including infestation by Onchocerca volvulus1,5,7,13 or a neurotropic virus such as Measles virus or Rubella virus.10 The relationship between nodding and malnutrition and stunting has also not been established but nourishment improves the health of children with Nodding syndrome. Though epidemics of nodding syndrome (NS) have been reported from Africa, to the best of our knowledge, this is the first probable case to be reported from India.
Case description
A ten-year-old female child was admitted to our hospital with complaints of excessive weight loss and loss of speech and interest in others for 3 months. This was followed by increased stiffness and choreo-athetoid movements of all four limbs and vertical head nodding movements, 18–20 episodes per minute that persisted even during sleep for one month. She had become withdrawn and mostly kept her eyes closed. There was no other abnormal movement or history suggestive of other cranial nerve palsies. She did not indicate any physical needs and was fed once or twice a day by parents with rice gruel on most days and chicken gruel occasionally for six months prior to onset of head nods.
The past history showed that there were no significant episodes of chronic illness/ prior hospitalization till about nine months before the current illness when she had an episode of an upper respiratory tract infection. This was followed fifteen days later, with ascending quadriparesis with non- ambulation that was diagnosed as acute inflammatory demyelinating polyneuropathy (AIDP) based on clinical examination and nerve conduction studies. She received intravenous immunoglobulin with partial improvement. Prior to onset of the current symptomatology, she had regained the ability to turn over and sit with support but still could not stand. She had nasal twang and occasional nasal regurgitation but no choking episodes. She needed to be fed by parents. She had received immunizations as per Indian National immunization schedule for tuberculosis, diphtheria, pertussis, tetanus, polio, measles, mumps, rubella and typhoid. There was no past history of measles or blood transfusion.
Her birth and developmental history revealed that she was born by full term by vaginal delivery at a primary health center to a fourth gravida mother with normal antenatal and perinatal period. Her birth weight was 2.6 Kg. She had acquired her previous motor and cognitive milestones as per her chronological age and was a student of class five with good academic performance till nine months prior to admission.
The family belonged to a lower socio-economic status residing in a village and were further driven to poverty by expenditure on her previous illness. The father is a farmer by profession and mother a housewife but assisted in farmland. She was fourth in birth order amongst 5 siblings. The elder three siblings were eighteen and sixteen-year-old married sisters, fifteen-year-old brother studying in class eight and a seven-year-old younger brother studying in second class. All the other family members had average nutritional build and there were no similarly affected persons in the family or in neighborhood.
On examination at admission, she was severely emaciated with BMI less than 3rd centile, contractures in all limbs, head nodding episodes 18–20/ minute, Glasgow coma score of 7/15 (E2V2M3), global aphasia, pupils (bilateral) were 4 mm in size, normally reacting to light, normal fundus, there was no facial asymmetry or drooling, clasp knife spasticity in lower limbs and cogwheel rigidity in upper limbs. Deep tendon reflexes were not elicitable but plantars were bilateral extensor. There were intermittent choreo-athetoid movements of all four limbs.
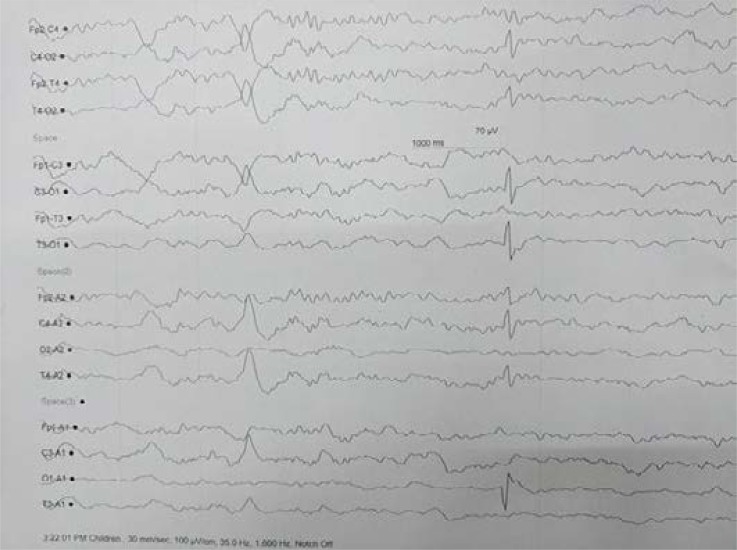
The child was started on supportive management and investigated as a Gray Matter Degenerative Brain disease. She had a hemoglobin level of 8 g/d% with microcytic hypochromic picture on peripheral smear. Liver, kidney function tests, serum electrolytes including sodium, potassium, calcium, phosphate were normal. Serum magnesium could not be done because of financial constraints. MRI brain revealed diffuse cerebral atrophy without predilection for any specific region. Nerve conduction studies (NCS) revealed normal CMAP amplitudes, distal latency and conduction velocities but with absent F waves. Cerebrospinal fluid analysis showed no cells and normal protein and sugar. HIV serology was negative. Electroencephalography (EEG) revealed occipito-temporal focal inter-ictal epileptiform discharges. (Figure 1)
Figure 1.
Electroencephalography showing occipitotemporal sharp wave discharges
Nutritional rehabilitation was done with slow escalation of nasogastric tube feeding to achieve 1800 Kcal/day and 40-gram proteins per day ultimately and multivitamin and mineral supplementation were started on day one. For contractures, physiotherapy was also started. Spasticity and choreo-athetoid movements were treated with trihexyphenidyl and baclofen. Significant improvement was seen with the start of valproate and head nodding movements subsided. Gradually tone reduced, child started indicating the need for micturition and defecation and hunger with gestures and starting speaking few words. She was evaluated for depression by psychiatrist as she had poor eye contact and preferred to keep her eyes closed and was started on anti-depressants. She was discharged on valproate, anti-depressants, multivitamins and nutritional advice. On her last follow up visit after 4 months of starting treatment, she was seizure and head nods free, had achieved weight between 0 to 1 SD, was not depressed and was able to walk with support.
Discussion
Nodding syndrome may be a possibly partially reversible epileptic encephalopathy of unknown etiology with multiple seizure types in form of nodding, atonic seizures or tonic clonic seizures.
The present case fulfills the case definition of probable nodding syndrome as evidenced by the previously proposed case definitions5. Most common age group of nodding syndrome has been cited as 5–15 years as in studies done in Sudan15 and patient is developmentally normal until onset of nodding5,12,8 as in the current case report. Although the present case fulfills the case definitions, there are several atypical features that have not been described so far like prior Guillain Barre Syndrome and loss of speech; increased stiffness and choreo-athetoid movements at onset of head nodding; head nodding persisting in sleep and disappearance of head nodding with nutritional and medication support. This is in contrast to a Ugandan case where marked improvement with such treatment is described but seizure episodes are usually reduced (up to 57%) in frequency not eliminated.11
Moreover, in a study by de Polo et al, none of the patients achieved good seizure control even if all of them received antiepileptic treatment (carbamazepine alone [43%] or in association with phenobarbitone or phenytoin), our patient responded well to administration of valproate. The partial symptomatic improvement in nodding syndrome and nodding that may be a form of atonic seizures with valproate has been documented in a cohort in Uganda. The same drug has been used in the present case.
Previous studies have postulated that there is no cure for NS, and treatment is symptomatic with common anti-convulsants to improve the quality of life.13 However, the remission of seizures in the present case could also be because of short latency between the onset of symptoms of 3 months and presentation to the hospital in the present case as against a long duration in the Ugandan series where the median age at onset of symptoms was 6 (range 4–10) years and the median duration of symptoms was 8.5 (range 2–11) years.11
This child had associated depression. The previous study from Uganda also describes associated psychiatric manifestations including wandering, aggression, depression and disordered perception.11
The etiology in this child seems to be macro and micronutrient deficiency secondary to her neurological condition and depression. Although other family members were not malnourished as the family is a rural family with access to fresh food, however the index case became malnourished. This was consequent to her non-ambulation and depression and dependence on the parents for food intake. She was predominantly fed rice gruel once or twice a day by parents who both had to work in farms to sustain family. This could have led to macro and micronutrient deficiency. It may be akin to re-feeding syndrome as the child had prolonged periods of starvation and only occasional protein and vitamin rich chicken meal diet prior to onset of head nodding.
Amongst other proposed etiologies like slow virus infections, both progressive rubella panencephalitis and subacute sclerosing panencephalitis (SSPE) are progressive disorders and would not improve with rehabilitation as in the present case. Moreover, the child had a younger age of onset, did not have cortical blindness, was immunized for measles and rubella and had no past history of measles infection. EEG also did not show pseudoperiodic R complexes as are seen in SSPE. So, these seem unlikely. There was no similar family history to suggest a genetic etiology. There was also no history suggestive of any exposure to toxins. The association with Onchocerciasis is puzzling as in a study in Tanzania and south Sudan1,7 which reveals negative PCR samples of CSF for onchocerca nematode. India has only occasional case reports of ocular onchocerciasis from NorthEastern region with high flowing rivers. The patient neither had ocular features nor belonged to this geographical belt.
MRI of the brain revealed cerebral atrophy in a study done in Sudan6 which is similar to finding in this case report. In contrast, Brain imaging of Ugandan children have suggested more atrophy in the occipital lobes or the parieto-occipital regions than anteriorly. Cerebellar atrophy has also been noted in NS. Hippocampal sclerosis, probably from inflammation, was reported in some patients in a Tanzanian cohort.8
In a previous study, de Polo et al demonstrated epileptiform abnormalities in EEG in 18 / 21 (85%) subjects that pointed NS to be an epileptic encephalopathy.14 The EEG in their study showed 2–3.5 Hz spike-and-wave discharges often intermingled with sharp waves. Ictal EEG in three patients showed head nodding episodes came in clusters during hyperventilation. In the present study too, inter-ictal Electroencephalography (EEG) revealed occipitotemporal focal epileptiform discharges. Ictal EEG was not captured.
Most of the previously described cases have come from marginalized people who are facing food scarcity. In the present case too, the cognitive decline and nodding episodes started after the child was chronically deprived of food due to her preceding neurological ailment, poverty of family and her dependence on caregivers for food.
The reporting of this case highlights that NS is not confined to Africa and the etiology may be more than that ascribable to geographical location or river blindness. Thus, nodding syndrome may be an epileptic encephalopathy of nutritional origin beyond geographical barriers but amenable to anti-convulsants, appropriate slow nutritional rehabilitation and treatment of comorbid psychiatric condition like depression as in the present study.
Conflict of interest
None.
References
- 1.Tumwine JK, Vandemaele K, Chungong S, Richer M, Anker M, Ayana Y, et al. Clinical and epidemiologic characteristics of nodding syndrome in MundriCounty, southern Sudan. Afr Health Sci. 2012;12:242–248. doi: 10.4314/ahs.v12i3.1. Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer PS, Vandemaele K, Richer M, Palmer VS, Chungong S, Anker M, et al. Nodding syndrome in Mundri county, South Sudan: environmental, nutritional and infectious factors. Afr Health Sci. 2013;13:183–204. doi: 10.4314/ahs.v13i2.2. Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyungura JL, Akim T, Lako A, Gordon A, Lejeng L, William G. Investigation into the Nodding syndrome in Witto Payam, Western Equatoria State, 2010. Southern Sudan Med J. 2011;4:3–6. [Google Scholar]
- 4.Sejvar JJ, Kakooza AM, Foltz JL, Makumbi I, Atai-Omoruto AD, Malimbo M, et al. Clinical, neurological, and electrophysiological features of nodding syndrome in Kitgum, Uganda: an observational case series. Lancet Neurol. 2013;12:166–174. doi: 10.1016/S1474-4422(12)70321-6. Pubmed. [DOI] [PubMed] [Google Scholar]
- 5.Foltz JL, Makumbi I, Sejvar JJ, Malimbo M, Ndyomugyenyi R, Atai-Omoruto AD, et al. An epidemiologic investigation of potential risk factors for nodding syndrome in Kitgum District, Uganda. PloS ONE. 2013;8:e66419. doi: 10.1371/journal.pone.0066419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Idro R, Opoka RO, Aanyu HT, Kakooza-Mwesige A, Piloya-Were T, Namusoke H, et al. Nodding syndrome in Ugandan children—clinical features, brain imaging and complications: a case series. BMJ Open. 2013;3:e002540. doi: 10.1136/bmjopen-2012-002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler AS, Friedrich K, Konig R, Meindl M, Helbok R, Unterberger I, et al. The head nodding syndrome— clinical classification and possible causes. Epilepsia. 2008;49:2008–2015. doi: 10.1111/j.1528-1167.2008.01671.x. Pubmed. [DOI] [PubMed] [Google Scholar]
- 8.Winkler AS, Friedrich K, Meindl M, Kidunda A, Nassri A, Jilek-Aall L, et al. Clinical characteristics of people with head nodding in southern Tanzania. Trop Doct. 2010;40:173–175. doi: 10.1258/td.2010.090373. Pubmed. [DOI] [PubMed] [Google Scholar]
- 9.Winkler AS, Wallner B, Friedrich K, Unterberger I, Matuja W, Jilek-Aall L, et al. A longitudinal study on nodding syndrome—a new African epilepsy disorder. Epilepsia. 2014;55:86–93. doi: 10.1111/epi.12483. Pubmed. [DOI] [PubMed] [Google Scholar]
- 10.Spencer PS, Mazumder R, Palmer VS, Lasarev MR, Stadnik RC, King P, et al. Environmental, dietary and case-control study of Nodding Syndrome in Uganda: A post-measles brain disorder triggered by malnutrition? J Neurol Sci. 2016;369:191–203. doi: 10.1016/j.jns.2016.08.023. Pubmed. [DOI] [PubMed] [Google Scholar]
- 11.Idro R, Namusoke H, Abbo C, Mutamba BB, Kakooza-Mwesige A, Opoka RO, et al. Patients with nodding syndrome in Uganda improve with symptomatic treatment: a cross-sectional study. BMJ Open. 2011;4(11):e006476. doi: 10.1136/bmjopen-2014-006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention, author. Nodding syndrome—South Sudan, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:52–54. [PubMed] [Google Scholar]
- 13.Daniël K A, Benjamin J V. Reviewing the evidence on nodding syndrome, a mysterious tropical disorder. IJID. 2013;17(3):e149–e152. doi: 10.1016/j.ijid.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 14.de Polo G, Romaniello R, Otim A, Benjamin K, Bonanni P, Borgatti R. Neurophysiological and clinical findings on Nodding Syndrome in 21 South Sudanese children and a review of the literature. Seizure. 2015;31:64–71. doi: 10.1016/j.seizure.2015.07.006. [DOI] [PubMed] [Google Scholar]