Abstract
Aims and introduction
We aimed to explore concomitant orally administered antidiabetic agent (OAD) regimens used in basal supported oral therapy (BOT) with insulin glargine in a real-life setting, and to assess the efficacy and safety of each regimen using data from the Add-on Lantus® to Oral Hypoglycemic Agents 2 study, a 24-week observational study in Japanese type 2 diabetes patients.
Materials and methods
Among 1629 insulin-naïve patients who had a glycosylated hemoglobin (HbA1c) value of ≥6.5 % during the previous 4 weeks and were treated with BOT during the observational period, 1227 patients who retained the same concomitant OAD regimens throughout the period were included in the analysis.
Results
Sulfonylurea (71.5 %), dipeptidyl peptidase-4 inhibitor (DPP-4i; 60.7 %), and biguanide (BG; 48.6 %) were commonly administered OADs in BOT. The HbA1c level decreased in patients taking BG alone (−2.76 %) and DPP-4i alone (−2.46 %). Of the three OADs, mean doses of the most frequently administered OAD changed from baseline to the final evaluation point: 2.5–2.3 mg for glimepiride, 59.1–58.7 mg for sitagliptin, and 1145.6–1168.2 mg for metformin. No significant difference in the hypoglycemia incidence rate was found between regimens (p = 0.3765), with incidence rates of 1.9 % (DPP-4i alone) to 7.0 % (other regimens) observed.
Conclusions
DPP-4i plays a major role in BOT with insulin glargine in a real-life setting. The incidence of hypoglycemia did not differ significantly between BOT regimens, including DPP-4i. Insulin glargine added to DPP-4i is a potential therapeutic approach.
Keywords: Basal supported oral therapy, Dipeptidyl peptidase-4 inhibitors, Insulin glargine, Real-life observational study, Type 2 diabetes
Introduction
Basal supported oral therapy (BOT) is a widely used treatment for diabetes [1]. BOT regimens include a long-acting insulin injection, such as insulin glargine, which is merely added to ongoing treatment with orally administered antidiabetic agents (OADs) in patients with type 2 diabetes. The mere addition of basal insulin to OADs is a convenient strategy when monotherapy is unlikely to achieve and/or maintain glycosylated hemoglobin (HbA1c) at a target level [2].
Earlier research on BOT with insulin glargine demonstrated its effectiveness at reducing glycemic levels with a low risk of hypoglycemia [3–6]. Similarly, our earlier Add-on Lantus® to Oral Hypoglycemic Agents (ALOHA) study, a 24-week, prospective, open-label, multicenter observational study in Japan, showed that the BOT regimen with insulin glargine significantly improved blood-glucose levels from baseline to 24 weeks in patients with type 2 diabetes [7].
Broader treatment options are now available for patients with type 2 diabetes (e.g., glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitor [DPP-4i]). As an OAD, DPP-4i is expected to provide benefits such as a low risk of hypoglycemia and weight gain [8]. Recent studies have also revealed that DPP-4i is effective with insulin therapy [8, 9]. Considering the new treatment options available, it is important to re-evaluate the efficacy and safety of concomitant OAD regimens utilized in BOT with insulin glargine in a real-life setting. Therefore, we conducted an observational study, the Add-on Lantus® to Oral Hypoglycemic Agents 2 (ALOHA2) study, to assess the safety and effectiveness of BOT with insulin glargine [7]. Using data from the ALOHA2 study, we previously found that the baseline factors associated with achieving HbA1c < 7.0 % were a shorter duration of diabetes, a pre-study regimen of one OAD, absence of neuropathy, and a lower HbA1c level, suggesting that early initiation of BOT leads to good HbA1c control in insulin-naïve patients [10]. We conducted the present analysis to reveal the current use of OADs in combination with insulin glargine in Japanese real-life practice and to evaluate the safety and efficacy of each regimen.
Materials and methods
Study design and patients
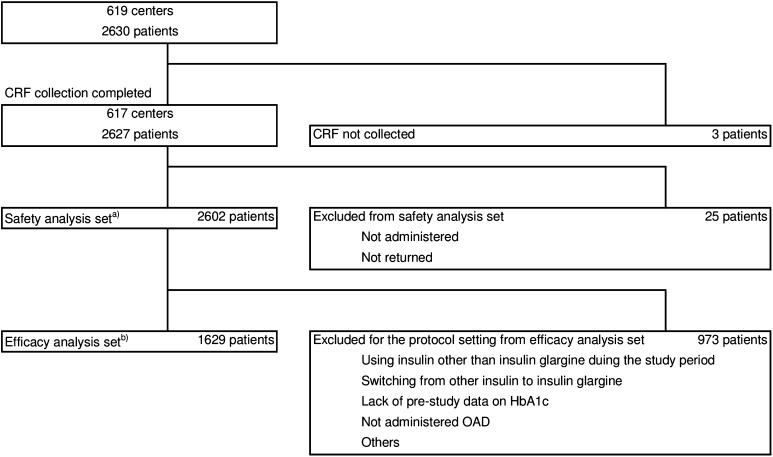
The ALOHA2 study was conducted as a special drug-use surveillance study between 2012 and 2013 in 619 hospitals and clinics across Japan. Participating patients were observed in a clinical setting and a total of 2630 patients were enrolled. Among those patients, 2602 and 1629 patients comprised the safety and efficacy analysis sets, respectively (Fig. 1).
Fig. 1.
Patient disposition. aPatients who had received insulin glargine at least once, bpatients who had never been treated with insulin previously and who were treated with BOT throughout the observational period. CRF case report form, HbA1c glycosylated hemoglobin, OAD orally administered antidiabetic agent, BOT basal supported oral therapy
The ALOHA2 study is a sequel to the ALOHA study, which was conducted between 2007 and 2009. The results of the ALOHA study have been reported previously [11–14]. Patients were eligible for the ALOHA2 study upon written consent if they (1) were type 2 diabetes patients aged 20 years or older, (2) were being treated with OAD therapy, (3) had based on the International Federation of Clinical Chemistry (IFCC) or National Glycohemoglobin Standardization Program (NGSP) HbA1c values of ≥6.5 % or a Japan Diabetes Society (JDS) HbA1c value of ≥6.1 % in the 4 weeks prior to the study, and (4) were due to start BOT with insulin glargine. Data on HbA1c levels were originally collected and recorded as the JDS, IFCC, or NGSP values. For data analysis, the JDS and IFCC values were converted into the NGSP values using the following formulae: NGSP (%) = 1.02 × JDS (%) + 0.25 %; or NGSP (%) = 1.02 × (IFCC [%] − 0.4 %) + 0.25 % [15]. Patients with a JDS HbA1c value of 6.1 % or an IFCC value of 6.5 %, converted into an NGSP value of 6.47 %, were also enrolled.
The study was conducted in accordance with the ethical principles for Good Post-marketing Study Practice in Japan.
Treatment, follow-up, and assessment
All treatment decisions were made by the attending physicians. Data on patient characteristics, safety, and effectiveness were collected over 24 weeks. Effectiveness parameters included the HbA1c level, fasting plasma glucose (FPG) level, two-hour postprandial plasma glucose (2h-PPG) level without information on the meal test performance, and weight. All adverse events reported during the observational period were documented by the physician based on patient-reported hypoglycemic episodes, including any hypoglycemic episodes and symptoms derived from hypoglycemia.
Statistical analysis
Data on insulin-naïve patients who were not administered insulin for at least 12 weeks prior to the study and who started BOT with insulin glargine during the observational period were taken from the ALOHA2 study (n = 1629; Fig. 1). Patients, including those with HbA1c < 7.0 % at baseline, were retrospectively stratified into groups based on the OADs that were commonly administered and the incorporation of concomitant OAD regimens into BOT with insulin glargine at baseline (initiation of insulin glargine). Descriptive statistics and the efficacy and safety of the concomitant OAD regimens incorporated into BOT with insulin glargine were evaluated based on the data for the patients who remained on the same concomitant OAD regimen continuously during the observational period (n = 1227).
Descriptive statistics on demographic and clinical characteristics, such as diabetic complications, body mass index (weight in kilograms divided by the square of the height in meters at baseline), and HbA1c, FPG, and 2h-PPG levels were obtained. Differences in these characteristics were assessed using the Kruskal–Wallis test for continuous variables and Fisher’s exact for binominal variables.
Glargine doses at baseline and at the final evaluation point (at 24 weeks or the last visit) were calculated. Dose differences among the concomitant OAD regimens were assessed using the Kruskal–Wallis test. Similarly, doses of the OAD agent at baseline and at the final evaluation point were also calculated.
The change in HbA1c between baseline and the final evaluation point was assessed using a paired t-test. Changes in FPG and 2h-PPG levels were not, however, included in the assessments because of the small number of patients assigned to some regimens. To assess safety, the likelihood of hypoglycemic episodes was assessed using Fisher’s exact test.
In the present subanalysis, missing data were filled using the last observation carried forward approach. All statistical tests were two-tailed, and p < 0.05 was considered to indicate statistical significance. All the statistical analyses were conducted using SAS for Windows, version 9.2 or higher (SAS Institute Inc., Cary, NC, USA).
Results
Commonly used OAD and concomitant OAD regimens in BOT with insulin glargine in a real-life setting
Almost half of the 1629 insulin-naïve patients were treated with sulfonylurea (SU), DPP-4i, and biguanides (BG). The most commonly administered OAD was SU (71.5 %), followed by DPP-4i (60.7 %), BG (48.6 %), α-glucosidase inhibitor (α-GI; 30.1 %), thiazolidinedione (16.5 %), and glinide (6.7 %; Table 1). The concomitant OAD regimens commonly incorporated into BOT were mainly single agents or a combination of SU, DDP-4i, and BG as follows: SU/DPP-4i (12.1 %), SU alone (11.9 %), BG/SU/DPP-4i (11.7 %), BG/SU (8.4 %), DPP-4i alone (7.7 %), BG alone (5.2 %), and BG/DPP-4i (3.8 %; Table 2). Of the 1629 patients, 1227 (75.3 %) remained on their concomitant OAD regimens continuously throughout the observational period, and subsequent analyses were performed using data from those 1227 patients.
Table 1.
Most commonly administered OADs in BOT with insulin glargine (n = 1629)
| n | (%) | |
|---|---|---|
| SU | 1164 | (71.5) |
| DPP-4i | 988 | (60.7) |
| BG | 792 | (48.6) |
| α-GI | 490 | (30.1) |
| TZD | 269 | (16.5) |
| Glinide | 109 | (6.7) |
OAD orally administered antidiabetic agent, BOT basal supported oral therapy, SU sulfonylurea, DPP-4i dipeptidyl peptidase-4 inhibitor, BG biguanide, α-GI α-glucosidase inhibitor, TZD thiazolidinedione
Table 2.
Concomitant OAD regimens used in the glargine BOT
| Baseline | Continuous administration of the same OAD regimen throughout the observational period | ||
|---|---|---|---|
| n | (%) | n | |
| Overall | 1629 | (100.0) | 1227 |
| SU alone | 193 | (11.9) | 122 |
| DPP-4i alone | 125 | (7.7) | 104 |
| BG alone | 84 | (5.2) | 58 |
| SU + DPP-4i | 197 | (12.1) | 143 |
| BG + DPP-4i | 62 | (3.8) | 49 |
| BG + SU | 137 | (8.4) | 112 |
| BG + SU + DPP-4i | 191 | (11.7) | 156 |
| Others | 640 | (39.3) | 483 |
Totals may not sum to 100 % due to rounding
OAD orally administered antidiabetic agent, BOT basal supported oral therapy, SU sulfonylurea, DPP-4i dipeptidyl peptidase-4 inhibitor, BG biguanide
Baseline characteristics based on concomitant OAD regimens
Descriptive statistics of the patients who remained on a concomitant OAD single agent or a combination regimen of SU, DPP-4i, and BG continuously during the observational period are shown in Table 3. Most patients were male: 57.4 % (SU alone), 68.3 % (DPP-4i alone), 70.7 % (BG alone), 69.2 % (SU/DPP-4i), 67.4 % (BG/DPP-4i), 59.8 % (BG/SU), 59.0 % (BG/SU/DPP-4i), and 63.6 % (other regimens). The mean (standard deviation) age of the patients on each concomitant OAD regimen was 62.8 ± 13.0 years (SU alone), 61.9 ± 13.8 years (DPP-4i alone), 56.8 ± 12.4 years (BG alone), 65.0 ± 12.6 years (SU/DPP-4i), 55.0 ± 12.3 years (BG/DPP-4i), 58.4 ± 12.1 years (BG/SU/DPP-4i), 59.3 ± 10.9 years (BG/SU), and 63.3 ± 12.2 years (other regimens).
Table 3.
Baseline characteristics of the patients who remained on concomitant OAD regimens during the observational period
| Single-OAD regimens | Multiple-OAD regimens | Others (n = 483) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SU alone (n = 122) |
DPP-4i alone (n = 104) |
BG alone (n = 58) |
SU + DPP-4i (n = 143) |
BG + DPP-4i (n = 49) |
BG + SU (n = 112) |
BG + SU + DPP-4i (n = 156) |
||||||||||
| Sex | ||||||||||||||||
| Men | 70 | 57.4 % | 71 | 68.3 % | 41 | 70.7 % | 99 | 69.2 % | 33 | 67.4 % | 67 | 59.8 % | 92 | 59.0 % | 307 | 63.6 % |
| Women | 52 | 42.6 % | 33 | 31.7 % | 17 | 29.3 % | 44 | 30.8 % | 16 | 32.7 % | 45 | 40.2 % | 64 | 41.0 % | 176 | 36.4 % |
| Age (years) | 119 | 62.8 ± 13.0 | 103 | 61.9 ± 13.8 | 56 | 56.8 ± 12.4 | 140 | 65.0 ± 12.6 | 47 | 55.0 ± 12.3 | 111 | 59.3 ± 10.9 | 152 | 58.4 ± 12.1 | 471 | 63.3 ± 12.2 |
| Duration of diabetes (years) | 84 | 11.3 ± 8.7 | 70 | 9.3 ± 7.8 | 42 | 9.0 ± 9.5 | 117 | 11.4 ± 8.3 | 34 | 7.7 ± 7.2 | 93 | 12.3 ± 7.1 | 120 | 11.1 ± 6.7 | 374 | 12.3 ± 7.7 |
| BMI (kg/m2) | 94 | 24.5 ± 4.5 | 85 | 24.1 ± 4.3 | 45 | 26.0 ± 4.7 | 114 | 24.9 ± 4.6 | 41 | 24.9 ± 5.4 | 99 | 25.4 ± 4.8 | 132 | 26.5 ± 4.2 | 405 | 24.8 ± 5.0 |
| HbA1c (%) | 122 | 9.41 ± 1.74 | 104 | 9.73 ± 2.20 | 58 | 10.43 ± 2.63 | 143 | 9.34 ± 1.41 | 49 | 9.79 ± 1.71 | 112 | 9.67 ± 1.77 | 156 | 9.48 ± 1.44 | 483 | 9.35 ± 1.68 |
| FPG (mg/dL) | 41 | 226.3 ± 84.3 | 44 | 211.0 ± 87.4 | 26 | 222.4 ± 79.8 | 50 | 198.2 ± 75.0 | 17 | 212.8 ± 122.6 | 28 | 188.3 ± 62.9 | 57 | 193.9 ± 61.1 | 199 | 192.5 ± 69.2 |
| 2h-PPG (mg/dL) | 37 | 256.5 ± 69.7 | 15 | 330.3 ± 115.6 | 10 | 339.7 ± 162.0 | 35 | 275.4 ± 88.3 | 11 | 246.9 ± 82.2 | 35 | 275.3 ± 99.6 | 34 | 243.6 ± 77.8 | 153 | 266.2 ± 90.8 |
| Diabetic retinopathy | 10 | 8.2 % | 9 | 8.7 % | 9 | 15.5 % | 22 | 15.4 % | 8 | 16.3 % | 21 | 18.8 % | 26 | 16.7 % | 65 | 13.5 % |
| Diabetic nephropathy | 25 | 20.5 % | 20 | 19.2 % | 5 | 8.6 % | 35 | 24.5 % | 16 | 32.7 % | 21 | 18.8 % | 45 | 28.9 % | 98 | 20.3 % |
| Diabetic neuropathy | 15 | 12.3 % | 7 | 6.7 % | 7 | 12.1 % | 26 | 18.2 % | 7 | 14.3 % | 16 | 14.3 % | 24 | 15.4 % | 78 | 16.2 % |
Each datum is a frequency, mean ± standard deviation, or percentage. OAD orally administered antidiabetic agent, SU sulfonylurea, DPP-4i dipeptidyl peptidase-4 inhibitor, BG biguanide, BMI body mass index, HbA1c glycosylated hemoglobin, FPG fasting plasma glucose, 2h-PPG two-hour postprandial plasma glucose, SD standard deviation
The duration of diabetes (± SD) was relatively short for patients taking DPP-4i alone (9.3 ± 7.8 years), BG alone (9.0 ± 9.5 years), and BG/DPP-4i (7.7 ± 7.2 years), but longer for patients taking SU alone (11.3 ± 8.7 years), SU/DPP-4i (11.4 ± 8.3 years), BG/SU (12.3 ± 7.1 years), and BG/SU/DPP-4i (11.1 ± 6.7 years). More patients on the BG/DPP-4i regimen had diabetic nephropathy (32.7 %) while fewer patients on the BG-alone regimen experienced diabetic nephropathy (8.6 %). Patients on the SU-alone and the DPP-4i-alone regimens reported fewer cases of retinopathy (8.2 and 8.7 %, respectively) than patients on the other regimens (range, 13.5–18.8 %). Diabetic neuropathy was observed in a relatively small proportion of the patients on the DPP-4i-alone regimen (6.7 %), whereas the corresponding proportions for the other regimens were 12.1–18.2 %.
Insulin and OAD doses
Doses of insulin glargine at baseline and at the final evaluation point were calculated based on the seven most commonly used concomitant OAD regimens (Table 4). Insulin glargine dose at baseline ranged from 5.6 to 6.8 units, and the dose at the final evaluation point ranged from 7.9 to 11.1 units. A statistically significant difference was observed in the concomitant OAD regimen doses at baseline and at the final evaluation point (p = 0.0358 and p < 0.0001, respectively).
Table 4.
Dose of insulin glargine at baseline and final evaluation point based on concomitant OAD regimens
| Single-OAD regimens | Multiple-OAD regimens | Others (n = 483) |
p valuesa,b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SU alone (n = 122) |
DPP-4i alone (n = 104) |
BG alone (n = 58) |
SU + DPP-4i (n = 143) |
BG + DPP-4i (n = 49) |
BG + SU (n = 112) |
BG + SU + DPP-4i (n = 156) |
|||
| Insulin glargine dose at baseline | |||||||||
| n | 121 | 104 | 58 | 143 | 48 | 112 | 156 | 483 | |
| Units | 6.2 ± 3.4 | 6.2 ± 3.2 | 6.3 ± 3.0 | 5.6 ± 2.5 | 6.8 ± 3.1 | 6.7 ± 2.7 | 6.3 ± 3.4 | 6.5 ± 3.5 | * |
| Insulin glargine dose at final evaluation point | |||||||||
| n | 121 | 104 | 58 | 143 | 48 | 112 | 156 | 483 | |
| Units | 10.2 ± 5.3 | 8.3 ± 4.7 | 7.9 ± 4.1 | 8.7 ± 5.3 | 10.0 ± 6.5 | 11.1 ± 6.1 | 10.2 ± 5.3 | 8.8 ± 4.9 | *** |
OAD orally administered antidiabetic agent, SU sulfonylurea, DPP-4i dipeptidyl peptidase-4 inhibitor, BG biguanide
aKruskal–Wallis test, b * <0.05, *** <0.001
The mean doses of the OAD agents at baseline and at the final evaluation point were summarized. Glimepiride was the most commonly used SU agent, with a mean (SD) dose of 2.5 ± 1.9 mg at baseline and 2.3 ± 1.8 mg at the final evaluation point in patients whose OAD regimens contained an SU agent. For patients with DPP-4i, the main DPP-4i agent administered was sitagliptin, with a mean dose of 59.1 ± 22.6 mg at baseline and 58.7 ± 21.7 mg at the final evaluation point. Metformin was the primary BG agent. The mean (SD) dose in patients with BG was 1145.6 ± 921.2 mg at baseline and 1168.2 ± 922.0 mg at the final evaluation point.
Changes in glycemic level and incidence of hypoglycemia
The change in HbA1c from baseline to the final evaluation point was calculated to assess efficacy (Table 5). Overall, the HbA1c level significantly improved regardless of the regimen. The HbA1c level decreased in the BG-alone (−2.76 ± 2.71 %) and DPP-4i-alone (−2.46 ± 2.52 %) regimens. In the SU/DPP-4i, BG/DPP-4i, BG/SU, BG/SU/DPP-4i, and other regimens, the HbA1c level decreased by −1.31 to −1.55 %. The smallest reduction in HbA1c occurred for SU alone (−0.96 %).
Table 5.
HbA1c level at baseline and at the final evaluation point and changes in HbA1c
| Single-OAD regimens | Multiple-OAD regimens | Others | p valueb, c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SU alone | DPP-4i alone | BG alone | SU + DPP-4i | BG + DPP-4i | BG + SU | BG + SU + DPP-4i | |||
| HbA1c level | |||||||||
| n | 110 | 86 | 55 | 141 | 46 | 105 | 145 | 444 | |
| Baseline (%) | 9.39 ± 1.71 | 9.80 ± 2.25 | 10.13 ± 2.25 | 9.33 ± 1.42 | 9.71 ± 1.69 | 9.63 ± 1.75 | 9.44 ± 1.40 | 9.33 ± 1.63 | |
| Final evaluation point (%) | 8.44 ± 1.55 | 7.34 ± 1.31 | 7.38 ± 1.48 | 7.93 ± 1.20 | 8.37 ± 1.86 | 8.32 ± 1.50 | 8.10 ± 1.30 | 7.79 ± 1.36 | |
| Change from baseline to final evaluation pointa,b | −0.96 ± 1.90*** | −2.46 ± 2.52*** | −2.76 ± 2.71*** | −1.40 ± 1.55*** | −1.34 ± 2.14*** | −1.31 ± 1.44*** | −1.34 ± 1.41*** | −1.55 ± 1.75*** | *** |
Each datum is a frequency or a mean ± SD
HbA1c glycosylated hemoglobin, OAD orally administered antidiabetic agent, SU sulfonylurea, DPP-4i dipeptidyl peptidase-4 inhibitor, BG biguanide, SD standard deviation
aPaired t-test, b *** <0.001, c Kruskal–Wallis test
There was no significant difference in the hypoglycemia incidence rate between regimens (p = 0.3765). The hypoglycemia incidence rates were 3.3 % (SU alone), 1.9 % (DPP-4i alone), 5.2 % (BG alone), 6.3 % (SU/DPP-4i), 4.1 % (BG/DPP-4i), 3.6 % (BG/SU), 3.9 % (BG/SU/DPP-4i), and 7.0 % (other regimens; Table 6).
Table 6.
Incidence of hypoglycemia according to concomitant OAD regimen
| Single-OAD regimens | Multiple-OAD regimens | Others | p valuesa,b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SU alone | DPP-4i alone | BG alone | SU + DPP-4i | BG + DPP-4i | BG + SU | BG + SU + DPP-4i | |||
| n | 122 | 104 | 58 | 143 | 49 | 112 | 156 | 483 | |
| Patients with hypoglycemia, n (%) | 4 (3.3) | 2 (1.9) | 3 (5.2) | 9 (6.3) | 2 (4.1) | 4 (3.6) | 6 (3.9) | 34 (7.0) | n.s. |
| Incidence rate, episodes/patient-year | 0.1420 | 0.0474 | 0.1200 | 0.4626 | 0.3996 | 0.1037 | 0.1192 | 0.2639 | |
OAD orally administered antidiabetic agent, SU sulfonylurea, DPP-4i dipeptidyl peptidase-4 inhibitor, BG biguanide
aFisher’s exact test, b n.s. not significant
Discussion
The present subanalysis of data collected for the ALOHA2 study was conducted to explore concomitant OAD regimen use in BOT in a real-life setting and to assess their efficacy and safety. Our results showed that SU, DPP-4i, and BG were the commonly administered OADs in BOT. The concomitant OAD regimens commonly used in the present subanalysis were mainly single agents or a combination of these three OADs. All of these regimens reduced glycemic levels, and the incidence of hypoglycemia did not differ between these regimens.
The results showed that OAD use in recent years had substantially changed compared with our previous ALOHA study. In the ALOHA study, SU was the most commonly used OAD in BOT with insulin glargine (88.1 %), followed by BG (48.1 %) and α-GI (47.8 %) [12], while SU (71.5 %), DPP-4i (60.7 %), and BG (48.6 %) were the common OADs administered at baseline in the ALOHA2 study. These changes suggest that the use of DPP-4i in BOT currently plays a major role in treating type 2 diabetes.
Despite the status quo, early initiation of insulin therapy is still recommended to attain glycemic control [13]. The duration of diabetes was relatively short in patients on DPP-4i or BG single-agent therapy at baseline, and their glycemic levels were better controlled after the initiation of BOT with insulin glargine than when other OAD regimens were used (HbA1c level at the final evaluation point: 7.34 % for DPP-4i alone and 7.38 % for BG alone, respectively). These findings suggest that the glycemic level can be sufficiently controlled by initiating BOT at an early stage of diabetes, even with a single concomitant OAD. In addition to the relatively short duration of diabetes in patients on DPP-4i or BG single-agent therapy, a relatively high HbA1c level at baseline was also commonly observed in these patient groups. Considering the substantial reduction in the HbA1c level at the final evaluation point, early initiation of insulin therapy may benefit these groups. A substantial reduction in the HbA1c level in the BG-alone regimen may have resulted from approval of the additional indication for high-dose metformin, based on the mean dose of metformin in any OAD regimen exceeding 1000 mg. These results should be interpreted with care, however, because of the small number of patients in this group.
However, changes in the HbA1c level between baseline and the final evaluation point were small in patients on SU (−0.96 %) single-agent therapy. This may have resulted from the low HbA1c level at baseline. The highest insulin glargine dose (10.2 units) was required at the final evaluation point in the SU-alone regimen among the SU-alone, DPP-4i-alone, and BG-alone regimens, and the HbA1c level at baseline was the lowest in the SU-alone regimen. However, the proportion of patients who achieved a HbA1c level of <7.0 %, as recommended by the American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD), Canadian Diabetes Association (CDA), and the JDS [16–18], at the final evaluation point (the achievers) was low (15.6 %, data not shown) compared with the DPP-4i-alone and BG-alone regimens (39.4 and 43.1 %, respectively, data not shown). This suggests that dose titration was not sufficient to control the glycemic level, and that higher doses of insulin glargine may be required in this population. Patients receiving the SU-alone regimen may have experienced progression of diabetes because of its longer duration, suggesting that there may be less beta cell function remaining in these patients.
There was a substantial decrease in the HbA1c level in the BG-alone and DPP-4i-alone groups, and no significant difference between hypoglycemia frequency in these regimens, suggesting that early initiation of insulin therapy—rather than adding another OAD to the regimen—may help these patients to control HbA1c. The ADA and EASD position statements recommend the introduction of insulin at any stage of diabetes after metformin administration [2]. SU is commonly administered in Japanese patients before insulin therapy is introduced, as indicated by the fact that >70 % of the patients in the present analysis were taking SU. The results of the present analysis suggest that administration of SU prior to insulin therapy may not be necessary.
The present subanalysis has some limitations. The results of the present study do not prove the superiority of concomitant OAD regimens, including DPP-4i, BG, and SU, in BOT with insulin glargine. This subanalysis is a retrospective observational assessment of patients who began BOT with insulin glargine, some of whom were on other OADs, using data from the ALOHA 2 study conducted as post-marketing surveillance in a routine clinical setting after the drug was marketed. Hypoglycemia was not strictly defined and compared with clinical trial results. For this reason, hypoglycemia frequency may be underestimated. The results may have been affected by bias, because concomitant OAD regimens in BOT were not randomly allocated and/or controlled as in an interventional study, and because the results were summarized according regimen-based stratification. Therefore, the results should be interpreted with care. However, the present study showed the current use of OADs in BOT with insulin glargine in a real-life clinical setting, characteristics of concomitant OAD regimens, and the effectiveness and/or safety of each regimen using data from a large-scale study. These findings are expected to be beneficial for developing a treatment strategy for patients with type 2 diabetes.
In conclusion, our results showed that DPP-4i plays a major role in BOT treatment with insulin glargine for type 2 diabetes patients in a real-life setting. DPP-4i in combination with insulin glargine can be a reasonable therapeutic approach because it promotes effective glycemic control and because the incidence of hypoglycemia observed when implementing this combination was not significantly different from that observed for BOT regimens, meaning that this combination can help patients to achieve and maintain the ideal HbA1c level. The results of this subanalysis provide physicians with insights into DPP-4i and insulin glargine combination therapy in daily practice.
Acknowledgments
The authors are grateful to all physicians at the hospitals and clinics that participated in the ALOHA 2 study. Statistical analysis was performed by EPS Corp. and editorial support was provided by Clinical Study Support, Inc., both under contract with Sanofi K.K. This study was conducted and sponsored by Sanofi K.K.
Author contributions
Sanofi K.K. was responsible for the study design, planning the statistical analysis, and for drafting and approving the manuscript. Shoko Tsukube drafted the manuscript. Takashi Kadowaki and Masato Odawara made significant suggestions regarding the analysis and interpretation of data, and reviewed and revised the draft manuscript. All authors have reviewed and approved the final version of this manuscript.
Conflict of interest
Insulin glargine is marketed by Sanofi K.K. under the name Lantus®. Shoko Tsukube is an employee of Sanofi K.K., Tokyo, Japan. Takashi Kadowaki has served on advisory panels for Boehringer Ingelheim, Daiichi Sankyo, Novo Nordisk, Taisho, Takeda, and Mitsubishi Tanabe; has served as a consultant for Boehringer Ingelheim and MSD; has received research support from Astra Zeneca, Chugai, Daiichi Sankyo, MSD, Ono, Sanofi, Takeda, and Mitsubishi Tanabe; and has served on speakers’ bureaus for Astellas, Astra Zeneca, Boehringer Ingelheim, Daiichi Sankyo, Sumitomo Dainippon, Eli Lilly, Kowa, Kyowa Hakko Kirin, MSD, Novartis, Ono, Sanofi, Sanwa, Taisho, Takeda, and Mitsubishi Tanabe. Masato Odawara has served on advisory panels for Astra Zeneca, Boehringer Ingelheim, Novo Nordisk, Sanofi, Taisho; has received research support from Astellas, Astra Zeneca, Boehringer Ingelheim, Daiichi Sankyo, Sumitomo Dainippon, Eli Lilly, Kowa, Kyowa Hakko Kirin, MSD, Novartis, Ono, Sanofi, Taisho, Takeda, and Mitsubishi Tanabe; and has served on speakers’ bureaus for Astellas, Astra Zeneca, Boehringer Ingelheim, Daiichi Sankyo, Sumitomo Dainippon, Eli Lilly, Kowa, Kyowa Hakko Kirin, MSD, Novartis, Ono, Sanofi, Taisho, Takeda, and Mitsubishi Tanabe.
Informed consent
This study was conducted as special drug use surveillance, and complied with the pharmaceutical affairs law enacted by the Health Authority in Japan and the ministerial ordinance of Good Post-marketing Study Practice (GPSP) in Japan. It was conducted after a contract had been signed with each medical institution that participated in the survey. Informed consent or a substitute for it was obtained from all patients before they were included in the ALOHA2 study.
References
- 1.Riddle MC, Rosenstock J, Gerich J, et al. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 3.Yki-Järvinen H, Dressler A, Ziemen M, et al. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130–1136. doi: 10.2337/diacare.23.8.1130. [DOI] [PubMed] [Google Scholar]
- 4.Fritsche A, Schweitzer MA, Häring HU, et al. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138:952–959. doi: 10.7326/0003-4819-138-12-200306170-00006. [DOI] [PubMed] [Google Scholar]
- 5.Rosenstock J, Schwartz SL, Clark CM, Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE901) and NPH insulin. Diabetes Care. 2001;24:631–636. doi: 10.2337/diacare.24.4.631. [DOI] [PubMed] [Google Scholar]
- 6.Dunn CJ, Plosker GL, Keating GM, et al. Insulin glargine: an updated review of its use in the management of diabetes mellitus. Drugs. 2003;63:1743–1778. doi: 10.2165/00003495-200363160-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi M, Tsukube S, Ikeda Y, et al. Safety and efficacy of combination therapy with insulin glargine and oral hypoglycaemic agents including DPP-4 inhibitors in Japanese T2DM patients: ALOHA 2 study, a post-marketing surveillance for Lantus®. J Diabetes Mellit. 2014;4:273–289. doi: 10.4236/jdm.2014.44039. [DOI] [Google Scholar]
- 8.Seufert J, Pegelow K, Bramlage P. Efficacy and safety of insulin glargine added to a fixed-dose combination of metformin and a dipeptidyl peptidase-4 inhibitor: results of the GOLD observational study. Vasc Health Risk Manag. 2013;9:711–717. doi: 10.2147/VHRM.S54362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilsbøll T, Rosenstock J, Yki-Järvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:167–177. doi: 10.1111/j.1463-1326.2009.01173.x. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda Y, Tsukube S, Kadowaki T, et al. Predictors for achieving target glycemic control in Japanese patients with type 2 diabetes after initiation of basal supported oral therapy using insulin glargine: sub-analysis of the ALOHA2 study, drug use surveillance in Japan. Diabetol Int. 2015. doi:10.1007/s13340-015-0236-9. [DOI] [PMC free article] [PubMed]
- 11.Kadowaki T, Ohtani T, Odawara M. Potential formula for the calculation of starting and incremental insulin glargine doses: ALOHA subanalysis. PLoS ONE. 2012;7:e41358. doi: 10.1371/journal.pone.0041358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odawara M, Ohtani T, Kadowaki T. Dosing of insulin glargine to achieve the treatment target in Japanese type 2 diabetes on a basal supported oral therapy regimen in real life: ALOHA study subanalysis. Diabetes Technol Ther. 2012;14:635–643. doi: 10.1089/dia.2011.0220. [DOI] [PubMed] [Google Scholar]
- 13.Kadowaki T, Ohtani T, Odawara M. Baseline predictive factors for glycemic control in Japanese type 2 diabetes patients treated with insulin glargine plus oral antidiabetic drugs: ALOHA study subanalysis. Diabetol Int. 2013;4:16–22. doi: 10.1007/s13340-012-0087-6. [DOI] [Google Scholar]
- 14.Odawara M, Kadowaki T, Naito Y. Incidence and predictors of hypoglycemia in Japanese patients with type 2 diabetes treated by insulin glargine and oral antidiabetic drugs in real-life: ALOHA post-marketing surveillance study sub-analysis. Diabetol Metab Syndr. 2014;6:20. doi: 10.1186/1758-5996-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2008;32:S1–S201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Japan Diabetes Society . Treatment guide for diabetes 2014–2015. Tokyo: Bunkodo Co., Ltd.; 2014. [Google Scholar]