Abstract
Background
This study was a response to the dearth of information on the timing of menarche in low-income countries, and the need to update knowledge on the condition. It thereby enables the provision of adequate support to young girls during menarche. The study determined the timing and range of onset of menarche and identified the factors influencing the timing.
Methods
We used data on girls' sexual and reproductive processes from a nationally representative population survey of girls aged 15–24 years in Nigeria. Descriptive statistics, and survival analysis techniques were used for data analysis at p = 0·05.
Finding
A quarter of the respondents (26%) had commenced menstruation by age 12. Almost all, (90%) had experienced menstruation by age 17. Girls aged 20–24 years reported later menarche (time ratio 1·066, 95% CI: 1·045-1·087) compared to those aged 15–19 years. An increase of respondents age by one year resulted in 0·8% delay in onset timing. Significant differences were also found in the zone of residence among the sampled population. Compared with girls from the South East, the timing of menstruation was generally delayed among the girls from South-South by 5%, North Central by 9%, South West by 10%, North East by 16% and 17% among girls from the North West.
Interpretation
There was a wide range in menarcheal age in Nigerian girls with a peak at 13–14 years and the possibility of a secular trend in the timing of onset. Early family life education is recommended.
Keywords: Reproductive medicine, Public health, Pediatrics
1. Introduction
Puberty is a critical time in the life of a woman. It is when many dynamic and sometimes complicated physical and biological developments occur [1], which are propelled by hormonal changes. There are obvious challenges in measuring most of these physiological changes; however, the commencement of menstruation, otherwise known as menarche, is accepted as the beginning of maturity among girls [2, 3]. Although the onset of menstruation is the last of all the secondary indicators of puberty as it usually starts about 27 months after breast budding and 18 months after pubic hair growth [4, 5], it nevertheless indicates sexual maturity in girls. Menarche is highly correlated with breast budding and is therefore adjudged to be an indicator of the early onset of puberty [4]. Literature is replete with the significance of onset of menstruation in a girl's life [5, 6] and its timely commencement is an indicator that all other puberty and reproductive changes are simultaneously taking place.
The scarcity of empirical information on the timing of menarche in low-income countries, provides a gap in knowledge that affects caregivers', caretakers' and clinicians' ability to manage this landmark period in the lives of young girls. Therefore, there is a need to update knowledge, address the dearth of information, and provide an agreeable report on the timing of menarche in Nigerian girls.
While reliable and empirical information on age at menarche is readily available in most high-income countries, this is not the case in many low-income countries where such information is often belated, not empirical and usually regional in outlook [7]. In many high-income countries, most girls experience menarche between age 10–14 years [7]. This estimate of 10–14 years of age was based on the findings of a study by Deligeoroglou et al. which reported that age at menarche ranges between 12 and 13 years in several nations of the world [8]despite global variations. For instance, Pinyerd et al put the onset of menstruation at an average age of 12·8 years (11–13 years) in the USA [6], while a slightly lower age at 12·4 years (SD = 1·3 years) was reported among Italian secondary school girls [2, 9]. Also, menarche occurred at age 12.1 years (SD = 1·2) among African-American girls and at age 12·9 years (SD = 1.20) among white girls in the USA [10]. A study among Chinese girls showed that menstruation started at 12·27 years (95% CI: 12·16-12·39) [11]. A median and mean age of 12 and 11·98 ± 0·96 years respectively were found among school girls in Alexandria, Egypt in 2007 [3]. The menarcheal age seemed to be higher in other Africa countries, considering the reported 13 years in South Africa [1], 13·85 years in Sudan [12], 13·66 years in Morocco [13], 13·9 years in Mozambique [14] and 13·7 years in Nigeria [15]. In particular, Ikaraoha et al. found a rural-urban variance in reported menarcheal mean age in Nigeria with rural as13.2 years and urban–14.2 years [15].
In the last century, a decreasing trend in age at menarche was reported in most parts of the world [3, 5, 9, 16] with signs of puberty appearing at younger ages. The underlying causes have not been completely unravelled [3]. For instance, a cross-sectional study across 13 countries by Cherry et al stated that while menarcheal age has decreased steadily by 0·34 year per decade among rural girls, a decrease of 0·73 year per decade has been established among urban girls and 0·46 year per decade for the combined groups since the 1940s [4]. A systematic review of studies on adolescent pregnancy and its contributing factors showed a global variation in mean estimates of age at menarche. The mean ages at menarche were: Argentina (12.6), Philippines (13.6), Australia (13.0), Portugal (12.5), Canada (12.7), Russia (13.0), Chile (13.0), South Africa (12.5), Columbia (12.8), South Korea (13.9), Germany (12.8), Spain (12.3), India (14.3), Sweden (13.1), Indonesia (13.0), Switzerland (13.0), Ireland (13.5), Turkey (13.3), Japan (12.5), USA (12.5), Netherlands (13.2), Uganda (13.4), Nicaragua (14.0), United Kingdom (12.9), Nigeria (13.7) and Vietnam (12.7) [4]. Accessibility to more or better nutritional intakes to urban girls was found to be responsible for this difference. Specifically, an American study reported an earlier rise of 0·34 year age at menarche in 2009, compared with the 1973 estimates among US girls [17]. This position is contrary to the report of Pinyerd et al. that menarche has been fairly constant at 12·8 years within the last 60 years [6].
Literature has posited that place of residence, education and socio-economic status influence age at menarche [1, 18]. Also, mother's menarcheal age, body mass index (BMI), family size, ethnicity and age at data collection were found to be significantly associated with age at menarche [1, 4, 16, 18]. For instance, Wu et al in a study among US girls, found that the mean age at onset of menarche was 12·1 years for black girls; 12·2 years for Mexican-American girls; and 12·7 years for white girls [19]. Similar results have been reported again in the USA by Herman-Giddens et al. [10].
Girls' experiences of embarrassment, shame, fear, and confusion in most countries, coupled with the challenges of managing menstruation, especially at the onset, are widely documented [4, 20]. Some girls have indicated that they have poor knowledge and insufficient orientation on what to expect and how to plan for their first menstrual experience [21, 22]. Knowledge gaps in normal time for commencement of menstruation were found to influence adolescents', caretakers' and clinicians' ability to monitor and manage menstruation-related issues [9]. In fact, the knowledge of the differentials in the age of onset of menarche is important in understanding and responding to adolescent fertility and pregnancy.
Deligeoroglou et al reiterated the need for healthcare providers to timely detect conditions that may suggest substantial pathologies, which may have consequences on women's reproductive health in the future. Also, there is a need to give adequate support to girls so that they can be well informed about their sexual health [8]. A few studies have identified early menarche as one of the risk factors for the development of breast cancer later in life [23, 24]. Hence, having the knowledge and determining menarche on time is crucial because it could help gynaecologists provide treatment options and screen for breast cancer among women.
This study was premised on the need to update the body of knowledge, address the dearth of information and provide a way forward on the conflicting reports on the timing of menarche. In addition, it aims to share the Nigerian experience on age at menarche. The study sought to provide healthcare practitioners with current information on the range of menarcheal age in girls so that they can provide adequate and effective support for adolescent girls. The aim of this study was, therefore, to provide up-to-date information to guide the provision of support to young girls and their caregivers on the physical development of young girls, particularly on menarche. Secondly, since the study sought to determine the ‘normal’ range of menarche and identify socio-cultural factors influencing the onset of menarche, the findings would equip girls, parents as well as healthcare professionals with information on the range, dynamics and determinants of onset of menstruation to enable early detection of diseases such as breast cancer and improve reproductive health care to young girls.
The researchers assessed the range and timing of onset of menarche among young girls and determined the factors influencing the timing using data obtained from a nationally representative population survey on girls' sexual and reproductive processes. The study provided parents and healthcare providers with information on the range and determinants of age at onset of menstruation to aid early detection of disease/abnormality and improve reproductive health care to young girls. Findings of this study would allay the anxiety of some adolescent girls who may seek medical help for “menstrual delay”. Also, the study findings may help to correct myths that the “delay” is abnormal or that it may affect fertility. However, it is unknown to what extent the psychological status of the girls affects the onset of menstruation. This is a subject for future research.
2. Methods
2.1. Study area
The study was conducted in Nigeria, a country of about 180 million inhabitants with an annual growth rate of 2·4% [25]. About 19·6% of the population are youths with a male-female ratio of 1.04 [25]. Nigeria is divided into six geopolitical zones: North West, North East, North Central, South East, South West, and South-South with an overall urban population of 49·4% [25].
2.2. Design
The study used data from a national survey conducted in 2017. The survey was conducted to assess the sexual and reproductive health behaviour of adolescents and young adults in Nigeria. The respondents were male and female Nigerian youths aged 15–24 years who were in school and out of school. The youths were selected from households in rural and urban areas using the updated master sample frame of localities and Enumeration Areas (EA) developed by the National Population Commission (NPC) [26].
The study was conducted in the 6 geopolitical zones of the country. The probability sampling, using a multi-stage cluster sampling method, was adopted to select eligible respondents. In Stage 1, using simple random sampling technique a state each was selected from all the states that constituted the zones. In Stage 2, the one rural and one urban Local Government Areas (LGA) was selected in each state using a simple random sampling technique. Stage 3 involved the selection of 15 EAs within the selected rural and urban LGAs. In stage 4, 16 households were selected in each of the selected EAs, while stage 5 involved the selection of one youth per household for data collection. Of the 3000 respondents invited for the study, 2952 youths were interviewed of which 1366 were girls. The analysis was limited to the female respondents.
A semi-structured, interviewer-administered questionnaire was used to collect information. The questionnaire addressed issues on youths' sexuality, knowledge on HIV/AIDS transmission and prevention including condom use, childbirth and marriage was administered on the respondents in June 2017. The section on menstruation had a series of questions and obtained information on whether the girls have experienced menstruation or not. Those who answered in the affirmative were then asked to supply the date when they first experienced it. Each respondent was assumed to represent each selected household and they filled the questionnaire in a private area in their homes. Of the 1366 girls who participated in the study, 1097 provided valid responses to questions on the onset of menstruation. The 269 missing dates had inconsistencies with other key responses (such as age, location, zone) and their menarcheal dates. The missing responses were mostly by girls with no education and who resided in rural communities of Northern Nigeria. The analysis was therefore based on the responses of the 1097 respondents.
2.3. Data analysis
Descriptive statistics were used to describe the study population and key variables. The dependent variable was the age at onset of menstruation, while the independent variables included the location of residence, zone of the country, marital status, education, wealth status, religion, and ethnicity. Among those who already attained menarche, Shapiro–Wilk test was used to ascertain normality of age at menarche. One-way analysis of variance was used to check equality of mean values of age at menarche across the independent variables; the survival analysis technique was used to explore the timing of menarche, the incidence rate and factors influencing the timing of menarche among the respondents. Statistical significance was determined at p = 0.05 and all statistical analysis was performed using STATA 14 and IBM SPSS (Statistical Program for Social Sciences) software version 25·0.
The study design allowed the collection of data from girls who had attained menarche as well as those who were yet to attain. Bias will be introduced if persons who have not experienced menarche (the event of interest) were excluded from further analysis. It is recognised that girls who have not experienced menarche might exhibit unique characteristics that could affect their timing. In such a case, the usual method of data analysis, such as comparison of means and test of proportions are invalid. Survival analysis techniques are the only possible method for analyzing data where time duration until one or more events of interest is the independent variable. The survival analysis is also known as “the history of event”, wherein individuals that are likely to have the event of interest (menarche in this case) are studied till they experience menarche or until they withdraw or the study came to an end (See supplementary notes for more details).
The researchers considered the Cox-proportional Hazard model and the well-known parametric models, also referred to as Accelerated Failure Time (AFT) models. The AFT models include the exponential, Weibull, Gompertz, lognormal, log-logistic, and generalized gamma models to analyse the data. The “streg” package in STATA was used to estimate the maximum likelihood estimate for the parametric regression survival-time models. Basically, the accelerated failure time implies a deceleration of time or, in other terms, an increment in the expected waiting time for an event of interest to occur. The Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC) and -2loglikelihood were used to select the best model for the data. The Generalised Gamma Model (GGM) consistently had the best performance for all the 3 criteria used (not shown in the Tables).
The gamma model is implemented by parameterizing and treating the parameters k and σ as ancillary parameters to be estimated from the data. The hazard function of the GGM has many shapes. A special case is Weibull distribution when k = 1, the exponential when k = 1 and σ = 1, and the lognormal distribution when k = 0. The flexibility of the GGM has enhanced its usability. For each covariate in the model, a Time Ratio (TR) > 1 implies that an individual experiences the event at a later timing, TR = 1 suggest no difference in the timings, while TR < 1 suggest an earlier timing.
The details of the statistical theory used in modelling the menarche age are shown below.
The survivor function S(t) estimates the probability that a girl “survives” beyond time t without starting menstruation, while the hazard function h(t) estimates the instantaneous failure rate per unit time to start menstruation after time t, given that the girl has not started menstruation before that time. The survival and hazard functions are estimated respectively as
| (1) |
and
| (2) |
The time and the censoring index are two essential variables in survival analysis. The “survival time” or “follow-up time”, is a discrete random variable that takes on only positive integer. It is the time period (age) to the attainment of menarche among those that had started menstruation in the current study. Since all female participants in this study are at the “risk” of menstruation, the equivalent “survival time”, otherwise called the “censoring time” is the current age of girls who have not started menstruation as of the survey date. We set the censoring index for girls who had started menstruation as “1” and “0” for those who have not started.
Kaplan-Meier estimates as shown in Eq. (3) was used to compute the survival functions at time t where nj is the number of participants who are at the “risk” of menarche at time tj and dj is the number of subjects that had started menstruation at time tj.
| (3) |
Unlike other parametric models, the GGM is implemented only in the Accelerated Failure Time (AFT) form. The three-parameter generalized gamma survivor and density functions are shown in Eqs. (4) and (5).
| (4) |
And
| (5) |
respectively, where
is the standard normal cumulative distribution function, and
is the incomplete gamma function
The followings are the operational definitions used in this study:-
-
•
Early menarche is the appearance of menstruation at or before age 10 years, while late menarche is having first menstruation after age 14 years [2].
-
•
The incidence rate (IR), is the likelihood that an individual would have started menstruation at age tk+1 given that she has not had it by age tk.
Ethical approval was obtained from the Ethical Board of the Obafemi Awolowo University (IPHOAU/12/582). Also, written informed consent for participation in the study was obtained from all the participants. The anonymity and confidentiality of the data collected were ensured. Permission was obtained from the community leaders, village or area chiefs in all the study communities and feedback were given to participating communities on completion of the study.
3. Results
Of the 1097 respondents studied, 703 (64%) were aged 15–19 years, while 394 (36%) were aged 20–24 years. Most (940 = 86%) of the respondents were never married and 54% resided in the urban areas (Table 1). Among the girls who had started menstruation, the overall mean and median ages at onset of menstruation were 13·536 ± 1.789 and 14 years respectively. The age at onset of menstruation was 13·250 ± 1.591 years among those aged 15–19 years, compared to 14·042 ± 2.025 years among those aged 20–24 years. There were significant differences in age at first menstruation among the girls viz-a-viz their age, marital status, the zone of residence, ethnicity, religion and wealth status. The overall median survival time to menarche was 14 (IQR = 13–15) years, while 274 (25%) of the respondents would have had menarche by age 13 years and 823 (75%) by age 15 years. The incidence rate of menstruation was 0·065 per year and this was higher among Ibo girls (0·070) than Yoruba girls (0·063) and Hausa/Fulani girls (0·057).
Table 1.
Distribution of time to menarche and incidence rate by sex and selected characteristics.
| Characteristics | n | a% |
bTime to Menarche |
cSurvival Time |
Incidence Rate | |||
|---|---|---|---|---|---|---|---|---|
| Mean | Std. Dev. | 25% | d50% | 75% | ||||
| Current Age | ||||||||
| 15–19 | 703 | 64.1 | *13.250 | 1.591 | 12 | 13 | 15 | 0.067 |
| 20–24 | 394 | 35.9 | 14.042 | 2.025 | 13 | 14 | 16 | 0.062 |
| Current Marital Status | ||||||||
| Never Married | 940 | 85.7 | *13.492 | 1.748 | 13 | 14 | 15 | 0.066 |
| Currently Married | 103 | 9.4 | 13.978 | 1.999 | 13 | 14 | 16 | 0.061 |
| Separated/Widowed | 17 | 1.5 | 14.235 | 2.305 | 13 | 14 | 15 | 0.070 |
| Not married but LWSP | 34 | 3.1 | 13.031 | 1.802 | 12 | 13 | 14 | 0.070 |
| Zone | ||||||||
| North Central | 191 | 17.4 | *13.407 | 1.617 | 12 | 14 | 15 | 0.065 |
| North East | 128 | 11.7 | 14.191 | 1.448 | 13 | 15 | 15 | 0.059 |
| North West | 126 | 11.5 | 13.918 | 2.065 | 13 | 15 | 15 | 0.044 |
| South East | 208 | 19.0 | 12.849 | 1.601 | 12 | 13 | 14 | 0.073 |
| South South | 222 | 20.2 | 13.306 | 1.773 | 12 | 13 | 14 | 0.074 |
| South West | 222 | 20.2 | 14.023 | 1.862 | 13 | 14 | 15 | 0.068 |
| Location | ||||||||
| Urban | 596 | 54.3 | 13.591 | 1.782 | 12 | 14 | 15 | 0.067 |
| Rural | 501 | 45.7 | 13.465 | 1.813 | 13 | 14 | 15 | 0.063 |
| Education | ||||||||
| None | 30 | 2.7 | 13.409 | 1.556 | 12 | 14 | . | 0.051 |
| Pry/Quran | 145 | 13.2 | 13.288 | 1.842 | 12 | 14 | 15 | 0.062 |
| Secondary | 793 | 72.3 | 13.561 | 1.746 | 13 | 14 | 15 | 0.067 |
| Higher | 129 | 11.8 | 13.667 | 2.010 | 12 | 14 | 16 | 0.064 |
| Ethnicity | ||||||||
| Hausa/Fulani | 274 | 25.0 | *14.075 | 1.887 | 13 | 15 | 16 | 0.057 |
| Yoruba | 223 | 20.3 | 13.706 | 1.956 | 13 | 14 | 15 | 0.063 |
| Ibo | 233 | 21.2 | 12.986 | 1.557 | 12 | 13 | 14 | 0.070 |
| Othersˆ | 357 | 32.5 | 13.433 | 1.682 | 12 | 13 | 15 | 0.070 |
| Religion | ||||||||
| Christianity | 674 | 61.4 | *13.404 | 1.791 | 12 | 13 | 15 | 0.069 |
| Islam | 395 | 36.0 | 13.829 | 1.749 | 13 | 14 | 16 | 0.059 |
| Others# | 25 | 2.3 | 12.739 | 1.480 | 12 | 13 | 14 | 0.070 |
| Household Wealth Status | ||||||||
| Lowest | 363 | 33.1 | *13.372 | 1.810 | 12 | 13 | 15 | 0.069 |
| Average | 374 | 34.1 | 13.564 | 1.721 | 13 | 14 | 15 | 0.064 |
| Highest |
360 |
32.8 |
13.678 |
1.859 |
13 |
14 |
15 |
0.064 |
| Total | 1097 | 100 | 13.536 | 1.789 | 13 | 14 | 15 | 0.065 |
aComputed column wise bComputed among those that have started menstruation *significantly different at 5% F-test cComputed among all girls.
#Include Isan, Tiv, Kanuri ˆInclude traditional, no religion, Judaism etc LWSP Living with Sexual Partner dMedian.
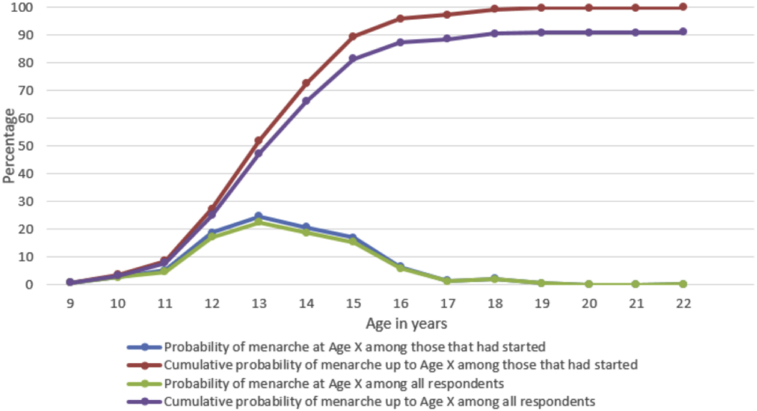
As shown in Fig. 1 and Table 2, the onset of menstruation peaked at ages 13 and 14 with 25% and 21% of respondents respectively. However, a quarter of the 1097 respondents had already started menstruating by age 12, while nearly 90% had experienced menstruation by age 17 years. About 9% of the respondents were yet to experience menstruation.
Fig. 1.
Distribution of respondents by their age at commencement of menstruation among those that had started and among all respondents.
Table 2.
Probabilities of commencement of menstruation at age x among those that had started and among all respondents.
| Age at Menarche (x) | Among those that already had menarche |
Among all respondents |
||
|---|---|---|---|---|
| At Age x | Cumulative to Age x | At Age x | Cumulative to Age x | |
| 9 | 0.70 | 0.70 | 0.64 | 0.64 |
| 10 | 2.80 | 3.50 | 2.55 | 3.19 |
| 11 | 5.11 | 8.61 | 4.65 | 7.84 |
| 12 | 18.72 | 27.33 | 17.05 | 24.89 |
| 13 | 24.62 | 51.95 | 22.42 | 47.31 |
| 14 | 20.62 | 72.57 | 18.78 | 66.09 |
| 15 | 16.92 | 89.49 | 15.41 | 81.49 |
| 16 | 6.41 | 95.90 | 5.83 | 87.33 |
| 17 | 1.40 | 97.30 | 1.28 | 88.61 |
| 18 | 2.10 | 99.40 | 1.91 | 90.52 |
| 19 | 0.50 | 99.90 | 0.46 | 90.98 |
| 20 | 0.00 | 99.90 | 0.00 | 90.98 |
| 21 | 0.00 | 99.90 | 0.00 | 90.98 |
| 22 | 0.10 | 100.00 | 0.09 | 91.07 |
| Not yet | 8.93 | 100.00 | ||
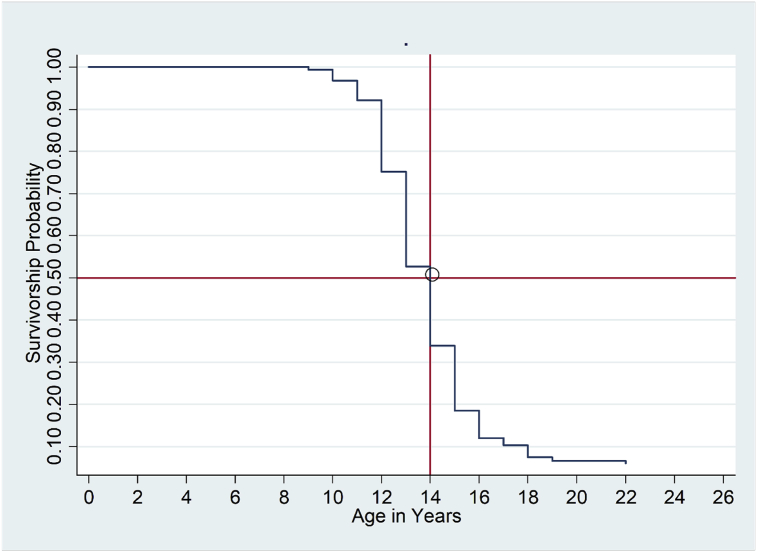
The overall survival curve of age at onset of menstruation is presented in Fig. 2. A total of 1097 girls were at risk and 98 were censored. The curve showed that the median survival time was 14 years. The curve became steep at age 12 years through age 15 years.
Fig. 2.
Survivorship curves showing probabilities of starting menstruation at different ages (The red lines show age at which 50% of the girls had Menarche).
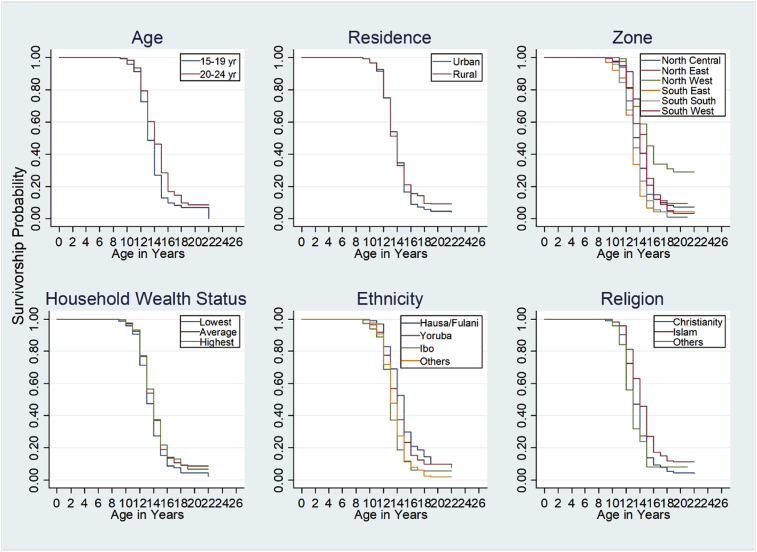
The survivorship probabilities of the “failure time” of menstruation among the girls are presented in Fig. 3. The survivorship lines viz-a-viz the current age of the girls showed that the timing of onset of menstruation was significantly different among the two cohorts of girls studied. The log-rank tests performed on the timing of menstruation among the girls by their zones of residence, household wealth category, ethnicity, and religion showed that the survival curves were significantly different among the categories of each characteristic.
Fig. 3.
Survivorship curves showing probabilities of starting menstruation at different ages by selected characteristics.
The outcome of the bivariate generalised gamma model fitted to each of the girls' characteristics is presented in Table 3. For every one-year increase in the girls' age, there was a 0·8% chance of delayed menstruation. Also, girls aged 20–24 years had later timing of menstruation by 5% than the girls aged 15–19 years. The time ratio of the onset of menarche among the currently married girls, compared to the never married girls who were living with a sexual partner(s) was 1·084 (1·021-1·150). This suggests a delay in the onset of menarche among girls who were never married but living with a sexual partner(s). There were significant differences in timing of onset of menarche by the girls' marital status, the zone of residence, ethnicity, religion and household wealth category.
Table 3.
Unadjusted and adjusted determinants of timing of the onset of menarche.
| Characteristics | Unadjusted Determinants |
Adjusted Determinants |
||||
|---|---|---|---|---|---|---|
| Time Ratio | 95% CI | p-value | Time Ratio | 95% CI | p-value | |
| Age (continuous) | *1.008 | 1.005–1.011 | 0.000 | |||
| 15–19 years | ||||||
| 20–24 |
*1.051 |
1.031–1.071 |
0.000 |
*1.066 |
1.045–1.087 |
0.000 |
| Marital Status (NMLWSP) | ||||||
| Never Married | 1.043 | 0.989–1.099 | 0.118 | 1.044 | 0.993–1.098 | 0.094 |
| Currently Married | *1.084 | 1.021–1.150 | 0.008 | 1.050 | 0.991–1.112 | 0.096 |
| Separated/Widowed/Divorced |
1.048 |
0.959–1.147 |
0.301 |
1.015 |
0.932–1.107 |
0.730 |
| Zone (South East | ||||||
| North Central | *1.063 | 1.033–1.094 | 0.000 | *1.090 | 1.041–1.142 | 0.000 |
| North East | *1.140 | 1.104–1.178 | 0.000 | *1.164 | 1.106–1.224 | 0.000 |
| North West | *1.159 | 1.122–1.197 | 0.000 | *1.172 | 1.118–1.229 | 0.000 |
| South South | 1.027 | 0.999–1.056 | 0.062 | *1.053 | 1.009–1.099 | 0.017 |
| South West |
*1.086 |
1.057–1.117 |
0.000 |
*1.104 |
1.054–1.156 |
0.000 |
| Location (Urban) | ||||||
| Rural |
1.005 |
0.986–1.023 |
0.620 |
|||
| Education (None) | ||||||
| Pry/Quranic | *0.941 | 0.886–1.000 | 0.050 | |||
| Secondary | 0.953 | 0.901–1.009 | 0.097 | |||
| Higher |
0.958 |
0.901–1.019 |
0.175 |
|||
| Ethnicity (Ibo) | ||||||
| Hausa/Fulani | *1.107 | 1.079–1.137 | 0.000 | 0.975 | 0.933–1.019 | 0.256 |
| Yoruba | *1.058 | 1.029–1.088 | 0.000 | 0.974 | 0.932–1.018 | 0.249 |
| Others |
*1.032 |
1.007–1.059 |
0.012 |
0.967 |
0.929–1.007 |
0.107 |
| Religion (Christianity) | ||||||
| Islam | *1.060 | 1.040–1.080 | 0.000 | 1.022 | 0.999–1.045 | 0.061 |
| Others |
0.968 |
0.911–1.029 |
0.295 |
0.990 |
0.933–1.050 |
0.727 |
| Wealth Category (Lowest) | ||||||
| Average | *1.030 | 1.007–1.053 | 0.010 | 1.011 | 0.989–1.033 | 0.326 |
| Highest | *1.034 | 1.012–1.058 | 0.003 | 1.005 | 0.983–1.027 | 0.688 |
| _cons | 11.646 | 11.03–12.295 | 0.000 | |||
| /ln_sig | −1.944 | −1.990–1.898 | 0.000 | |||
| /kappa | −0.551 | −0.696–0.405 | 0.000 | |||
| Sigma | 0.143 | 0.137–0.150 | 0.000 | |||
NMLWSP Not Married but Living with Sexual Partner *Significant at 5% The adjusted models are when the effects of a variable is determined while controlling for other variables by accounting for the effect due to all the additional variables included in the analysis. That is, other susceptible variables are included in the model so as to account for cofounders.
However, after controlling for other variables, age significantly influenced the onset of menarche with the older girls starting menstruation later than the younger girls. The time ratio of the timing between the two cohorts was 1·066 (95% CI: 1·045-1·087) against those aged 15–19 years. Compared with girls from the South East, the timing of menstruation was generally delayed among the girls from South-South by 5%, North Central by 9%, South West by 10%, North East by 16% and 17% among girls from the North West. Marital status was not significantly related to the onset of menarche in the adjusted model (Table 3).
4. Discussion
This study was designed to determine the timing of menarche and thereby provide updated information on the timing of onset of menstruation among Nigerian girls. Lack of knowledge on normal time for commencement of menstruation has been identified to influence adolescents, caretakers and clinicians ability to monitor and manage menstruation-related issues in adolescent girls [9]. While some girls started menstruation as early as age 9 years, the majority had menarche between ages 13 and 15 years; the age at onset of menstruation peaked at ages 13 and 14 years.
We observed that the onset of menstruation occurred in about 1 of every 10 Nigerian girls before their eleventh birthday. This result is in consonance with the findings of Chumela et al., who found that less than one-tenth of US girls had menarche before age 11 years [17]. Similarly, the average age at menarche ranged between 13 to 14, which is similar to the findings of Sommer et al. [7].
The finding of this study is also in agreement with the submission that age at menarche is on a gradual decline in many countries and perhaps stabilized at ages 12 and 13 years [8]. The fact that the average time to onset of menstruation among girls aged 15–19 years was shorter than among those aged 20–24 years suggests that a secular trend exists in these timings in Nigeria. Nevertheless, our estimates appear to be longer than those found in previous studies. For instance, Pinyerd et al. found a lower mean of 12·8 years at menarche for girls aged 11–13 years [6] and similarly, a lower mean age of 12·3 years was observed among Chinese girls [11]. Ma et al reported that 95% of Chinese girls had their menarche between age 12·16 and 12·39 years [11]. Also, a lower mean age of 12·4 years was found among Italian secondary school girls, 12·2 years among African-American girls, 12·9 years among white girls and a much lower mean age among girls in Egypt [2, 3, 9, 10], compared with 13·5 years found in this study. However, the mean age found in this study is similar to the 13·51 ± 1.04 years and 13·67 ± 0.8 years reported for urban and rural areas in India respectively [27].
Another departure from the existing age at onset of menarche was observed in the estimates of this study when compared with a mean age at menstruation reported earlier in Nigeria and in some other African countries. The mean menarcheal ages of 13·7 years, 13·7 years and 13·9 years reported in Morocco, Nigeria and Mozambique [13, 14, 15] respectively between 1997 and 2005 were slightly higher than the mean age of 13·5 found in this study. This probably suggests a menarcheal experience that was not previously observed in Nigeria and perhaps in Africa. The departure also gave credence to the finding of this study that the age at menarche is declining.
The observed decline in mean age of onset of menarche we found compared to available figures suggests a secular trend, particularly among those aged 15–19 years. This may be due to changes in the prevailing socio-economic and cultural factors affecting the girls. Cherry et al had already noted that improved socio-economic situations of families quicken onset of menstruation. We found that the geographical location or residence of the girls was a major risk factor in the timing of first menstruation among Nigerian girls. There is a major divide in the wealth distribution between urban and rural areas of Nigeria [28]. This may also account for the difference in rural-urban age at menarche, as rural girls were found to have an older age at menarche compared with their urban counterparts with widest differences occurring in the low-income countries [4]. One plausible explanation for higher/older age at menarche among rural girls is the poor nutritional status of these girls. Also, a girl-child receives less nurturing” compared with the boy-child. Also, school dropouts arising from early marriage are only peculiar to girls and more prevalent among rural girls. Poorer health and nutritional indices among rural girls will result in slower physical maturation [4].
The geographical and ethnic variation in the onset of menarche is in consonance with the report of a US study on ethnic differentials at menarche [29]. It was clear that the Black and Mexican-American girls, unlike the White girls, had younger ages at onset of pubic hair, breast development and menarche, which possibly explained the disparities in the earlier and later commencement of menarche. It is unsurprising that girls' ethnicity was no longer a significant variable after the adjustment for other variables. The effect of ethnicity was masked by the geographical zones in the model because each zone is primarily dominated by the one ethnic group. The North West and North East are predominantly the Hausa/Fulanis, the South West by the Yorubas, while the South East by the Ibos [28,30,31]. The Ibo girls who predominantly live in the South East of Nigeria had the earliest timing of first menstruation, followed by girls from the North Central, the Yoruba girls in the South West, while the later timings were common among the Hausa Fulani girls in the North. Similar ethnic and geographic divides had been reported earlier [2, 7, 9].
This study confirmed findings of earlier studies that found significant regional differences between nutritional outcomes of children in Nigeria [32,33]. Kandala et al found a striking variation in overweight/obesity prevalence across ethnic groups and by place of residence. Coincidentally, the highest and lowest prevalence rates were found in Cross River State [2.32 (1.62, 3.40)] and Osun State [0.48 (0.36, 0.61)] [32], both in Southern Nigeria. However, the prevalence of obesity was higher among the states of the Southern region compared with the North generally. This in agreement with the findings of Okafor et al, and it indicates significant regional differences in the prevalence of obesity and clinical characteristics. The authors reported that obesity and overweight were common in the urban areas with accompanying regional differences and that the “burden and mean values of obesity were particularly higher among participants from the Southern region” [33]. Nigeria has a diverse ecosystem which is similar within regions but different across the regions. It is, therefore, possible that the influence of region on age at menarche is as a result of social differences such as access to fast food, the proximity of schools etc.
Although not significant in the adjusted model, the study found an earlier timing of menarche among girls from households in the wealthiest category, and that concluded that socioeconomic status may influence the menarcheal age. Most of the differences in age at menarche are not among countries but by wealth distribution within countries [4]. A previous study by Fagbamigbe et al had reported regional divide in wealth distribution among the households in Nigeria. The researcher found that more households in the South East and South West zones belonged to the wealthiest quintiles than those in the Northern zones [28]. This is in agreement with other studies that have reported that wealth distribution can considerably affect girls nutritional well-being [1, 3, 18]. Poor nutritional intake could, therefore, recede the pace of menstruation [4] and thereby interact with the physiology/maturation of individuals studied [4, 5].
There appears to be a distinct connection between the regional variations in menarcheal age found in this study, with the differences in obesity prevalence across Nigeria [32,33] as well as the differences in social economic status in the country [28]. Having established that children from wealthier households (more concentrated in the Southern region) are more likely to be obese and have earlier menarche, the study hypothesized that age at menarche is related to obesity, as an offshoot of nutritional intake and social economic status.
On one hand, these findings would allay anxiety wherein some adolescent-girls seek medical help for purported “menstrual delay,” which may indeed be within the normal range. Conversely, it would correct the wrong notion among girls who may be unaware that the “delay” is abnormal and thereby result in long-term health consequences.
5. Conclusion
The menarcheal age in Nigeria ranged widely between age 9 and 22 years and peaked at age 13–14 years with a mean age at 13·536 years. Most of the girls would have experienced menarche before attaining age 18 years and there seemed to be a secular trend in the timing of onset of menstruation among the girls. Every one year added to the age of the girls resulted in 0.8% delay in the timing of the onset. Diversities in the onset of menarche among the girls were clearly observed in their ages (as a proxy for the cohort of births) and their zone of residence, which may be ascribed to the social, ecosystem, gene, hormonal and nutritional effects.
The public health and social implications of the study are multifarious. Based on the decline in age at onset of menarche we observed, health education on fertility should be commenced at an early age. Family life education focusing on reproductive and sexual health, HIV transmission and prevention is also necessary. The findings of this study will guide healthcare providers to care for adolescent girls. Parents, guardians and community members should provide early care and support for adolescent girls. Thus, information provided in this study will assist caregivers and clinicians in offering timely care and management that could aid prevention, diagnosis, and treatment of breast cancer and other sexual and reproductive health issues among women and girls in particular.
While this study is easily reproducible, its findings could be generalized across Nigeria because a representative sample of the population of youths was obtained. The ages at onset of menarche were self-reported by respondents and could not be validated. Thus, responses were subject to recall bias. However, the good representativeness of the sample and large number of respondents involved in the study would have reduced the effect of information bias in our estimates. Although, the researchers could not assess the diet and nutritional intake of the girls before and at the onset of menarche, despite this, we have succeeded in providing information on menarche onset in Nigeria and the factors influencing it.
Declarations
Author contribution statement
Adeniyi Fagbamigbe: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mary Obiyan, Oluwafunmilayo Fawole: Analyzed and interpreted the data; Contributed materials, analysis tools or data; Wrote the paper.
Funding statement
This research was supported by the Consortium for Advanced Research Training in Africa (CARTA). CARTA is jointly led by the African Population and Health Research Center and the University of the Witwatersrand and funded by the Carnegie Corporation of New York (Grant No--B 8606.R02), Sida (Grant No: 54100113), the DELTAS Africa Initiative (Grant No: 107768/Z/15/Z) and Deutscher Akademischer Austauschdienst (DAAD). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (UK) and the UK government. The statements made and views expressed are solely the responsibility of the Fellow.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ramathuba D.U. Menstrual knowledge and practices of female adolescents in Vhembe district, Limpopo Province, South Africa. Curationis. 2015;38(1):1–6. doi: 10.4102/curationis.v38i1.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Sanctis V., Bernasconi S., Bianchin L. Onset of menstrual cycle and menses features among secondary school girls in Italy: a questionnaire study on 3,783 students. Indian J. Endocrinol. Metab. 2014;18(Suppl 1):S84–S92. doi: 10.4103/2230-8210.140251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mounir G.M., El-sayed N.A., Mahdy N.H., Khamis S.E. Nutritional factors affecting the menarcheal state of adolescent school girls in Alexandria. J. Egypt. Publ. Health Assoc. 2007;82(3-4):1–22. [PubMed] [Google Scholar]
- 4.Cherry A.L. Biological determinants and influences affecting adolescent pregnancy. In: ALC, MED, editors. International Handbook of Adolescent Pregnancy. Springer Science+Business Media; New Delhi: 2014. pp. 39–55. [Google Scholar]
- 5.Sarpolis K. 2011. First Menstruation: Average Age and Physical Signs. Young Women, Sexual Health.http://www.obgyn.net/sexual-health/first-menstruation-average-age-and-physical-signs [Google Scholar]
- 6.Pinyerd B., Zipf W.B. Puberty—timing is everything! J. Pediatr. Nurs. 2005;20(2):75–82. doi: 10.1016/j.pedn.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Sommer M. Menarche: a missing indicator in population health from low-income countries. Publ. Health Rep. 2013;128:399–401. doi: 10.1177/003335491312800511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deligeoroglou E., Creatsas G. Menstrual disorders. Endocr. Dev. 2012;22:160–170. doi: 10.1159/000331697. [DOI] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet. Gynecol. 2015;126 doi: 10.1097/AOG.0000000000001210. http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Adolescent-Health-Care/Menstruation-in-Girls-and-Adolescents-Using-the-Menstrual-Cycle-as-a-Vital-Sign (Committee Opinion No. 651.):e143–6. [DOI] [PubMed] [Google Scholar]
- 10.Herman-Giddens M., Slora E., Wasserman R. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings Network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 11.Ma H.M., Du M.L., Luo X.P. Onset of breast and pubic hair development and menses in urban Chinese girls. Pediatrics. 2009;124(2):e269–e277. doi: 10.1542/peds.2008-2638. [DOI] [PubMed] [Google Scholar]
- 12.Attallah N.L., Matta W.M., El-Mankoushi M. Age at menarche of schoolgirls in Khartoum. Ann. Hum. Biol. 1983;10(2):185–188. doi: 10.1080/03014468300006321. [DOI] [PubMed] [Google Scholar]
- 13.Montero P., Bernis C., Loukid M., Hilali K., Baali A. Characteristics of menstrual cycles in Moroccan girls: prevalence of dysfunctions and associated behaviours. Ann. Hum. Biol. 1999;26(3):243–249. doi: 10.1080/030144699282741. [DOI] [PubMed] [Google Scholar]
- 14.Padez C. Age at menarche of schoolgirls in Maputo, Mozambique. Ann. Hum. Biol. 2003;30(4):487–495. doi: 10.1080/0301446031000111401. [DOI] [PubMed] [Google Scholar]
- 15.Ikaraoha C.I., Mbadiwe I.N.C., Igwe C.U., Allagua D.O., Mezie O., Iwo G.T.O. Menarchial age of secondary school girls in urban and rural areas of rivers state, Nigeria. Online J. Health Allied Sci. 2005;2:4. [Google Scholar]
- 16.Parent A.S., Teilmann G., Juul A., Skakkebaek N.E.N., Toppari J., Bourguignon J.J.-P. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev. 2003;24:669–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 17.Chumlea W., Schubert C., Roche A. Age at menarche and racial comparisons in US girls. Pediatrics. 2003;111(1):101–103. doi: 10.1542/peds.111.1.110. https://www.ncbi.nlm.nih.gov/pubmed/12509562 [DOI] [PubMed] [Google Scholar]
- 18.WHO . 2011. The Sexual and Reproductive Health of Younger Adolescents: Research Issues in Developing Countries. Geneva, Switzerland. [Google Scholar]
- 19.Wu T., Mendola P., Buck G.M. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics. 2002;110(4):752–757. doi: 10.1542/peds.110.4.752. https://www.ncbi.nlm.nih.gov/pubmed/12359790 [DOI] [PubMed] [Google Scholar]
- 20.Sommer M., Caruso B.A., Sahin M., Calderon T., Cavill S. A Time for Global Action : addressing Girls ’ Menstrual Hygiene Management Needs in Schools. PLoS Med. 2016;13(2) doi: 10.1371/journal.pmed.1001962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason L., Nyothach E., Alexander K., Odhiambo F., Eleveld A., Vulule J. We keep it secret so no one should know’—a qualitative study to explore young schoolgirls attitudes and experiences with menstruation in rural Western Kenya. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thakur H., Aronsson A., Bansode S., Lundborg C., Dalvie S., Faxelid E. Knowledge, practices and restrictions related to menstruation among young women from low socioeconomic community in Mumbai, India. Front Public Heal. 2014;2:2–7. doi: 10.3389/fpubh.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nwajana A.N., Oboma Y.I. Relationship between menarche, oral contraceptive and breast cancer in abuja, Nigeria. Int. J. Adv. Res. 2016;4(2):9–14. http://www.journalijar.com/article/7394/relationship-between-menarche-and-oral-contraceptive-and-breast-cancer-in-abuja,-nigeria/ [Google Scholar]
- 25.CIA World Factbook . CIA; 2017. Nigeria Demographics Profile 2018.https://www.indexmundi.com/nigeria/demographics_profile.html [Google Scholar]
- 26.National Population Commission (Nigeria) and ICF International . Abuja; Nigeria: 2014. Nigeria Demographic and Health Survey 2013. [Google Scholar]
- 27.Dambhare D.G., Wagh S.V., Dudhe J.Y. Age at menarche and menstrual cycle pattern among school adolescent girls in Central India. Global J. Health Sci. 2011;4(1):105–111. doi: 10.5539/gjhs.v4n1p105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fagbamigbe A.F., Bamgboye E.A., Yusuf B.O. The Nigeria wealth distribution and health seeking behaviour : evidence from the 2012 national HIV/AIDS and reproductive health survey. Health Econ. Rev. 2015;5(5):e1–e10. doi: 10.1186/s13561-015-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu T., Mendola P., Buck G. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics. 2002;110:752–757. doi: 10.1542/peds.110.4.752. [DOI] [PubMed] [Google Scholar]
- 30.Doctor H., Findley S., Afenyadu G., Uzondu C., Ashir G. Awareness, use, and unmet need for family planning in rural northern Nigeria. Afr. J. Reprod. Health. 2013;17(4):107–117. https://www.ncbi.nlm.nih.gov/pubmed/24558787 [PubMed] [Google Scholar]
- 31.Adebowale A.S., Fagbamigbe A.F., Adebayo A.M. Regional differences in adolescent childbearing in Nigeria. J. Popul. Soc. Stud. 2016;24(2):101–116. [Google Scholar]
- 32.Kandala N.B., Stranges S. Geographic variation of overweight and obesity among women in Nigeria: a case for nutritional transition in Sub-Saharan Africa. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okafor C.I., Gezawa I.D., Sabir A.A., Raimi T.H., Enang O. Obesity, overweight, and underweight among urban Nigerians. Niger. J. Clin. Pract. 2014;17(6):743–748. doi: 10.4103/1119-3077.144389. [DOI] [PubMed] [Google Scholar]