Abstract
Purpose
Tumor grade is one of the more controversial factors with limited prognostic information in squamous cell carcinomas (SCC) of the uterine cervix.
Methods
Histologic slides of 233 surgically treated cervical SCC (FIGO IB1) were re-examined regarding the prognostic impact of the WHO-based grading system, using the different degree of keratinization, categorizing the tumors in G1, G2 and G3 (conventional tumor grade).
Results
45.1% presented with well-differentiated tumors (G1), 29.2% with moderate (G2) and 25.8% with poor differentiation (G3). Tumor grade significantly correlated with decreased recurrence-free and overall survival. However, detailed analyses between G1- and G2-tumors failed to show any correlation with either recurrence-free or overall survival. G1- and G2-tumors were therefore merged into low-grade tumors and were compared to the high-grade group (G3-tumors). This binary conventional grading system showed an improved 5-years recurrence-free (low-grade: 90.2% vs. high-grade: 71.6%; p = 0.001) and overall survival rates (low-grade: 89.9% vs. high-grade: 71.1%; p = 0.001) for low-grade tumors. On multivariate analysis adjusted for lymph node metastasis, high-grade tumors represented a hazard ratio of 2.4 (95% CI 1.3–4.7) for reduced recurrence-free and 2.4 (95% CI 1.2–4.6) for overall survival. High-grade tumors showed a significantly higher risk for pelvic lymph node involvement [OR 2.7 (95% CI 1.4–5.5); p = 0.003]. The traditional three-tiered grading system failed to predict pelvic lymph node metastases.
Conclusion
A binary grading model for the conventional tumor grade (based on the degree of keratinization) in SCC of the uterine cervix may allow a better prognostic discrimination than the traditionally used three-tiered system.
Keywords: Cervix, Cancer, Prognosis, Grading, Histopathology, Treatment, Squamous cell, Survival
Introduction
Regardless of the treatment modalities, parametrial invasion and lymph node involvement are well-accepted prognostic factors in carcinomas of the uterine cervix (Takeda et al. 2002; Vinh-Hung et al. 2007; Wagner et al. 2013). One of the most controversial factors of all histopathological prognosticators is tumor grade (Singh and Arif 2004), especially in squamous cell carcinomas (SCC). Previous studies showed a prognostic impact on survival (Delgado et al. 1990; Vinh-Hung et al. 2007). Several other studies failed to show a prognostic impact (Takeda et al. 2002; Wagner et al. 2013; Zaino et al. 1992). Especially large studies evaluating the SEER data base include a variety of different tumor stages and treatment approaches (Vinh-Hung et al. 2007; Wagner et al. 2013) which may impact the results. Additionally, squamous cell and adenocarcinomas were not usually analyzed separately.
Traditionally, cervical SCCs are graded based on the degree of keratinization, cytological atypia and mitotic activity (Broders 1926; Wentz and Reagan 1959). This grading system has been adopted by the WHO classification and is still in use (Stoler et al. 2014; Wells et al. 2003). The present study was designed to evaluate the prognostic impact of tumor grade within a cohort of surgically treated and histopathologically staged SCCs.
Materials and methods
Data form consecutive patients with upfront surgery were obtained from our medical files. Patients who received any neoadjuvant treatment, those with incomplete tumor resection and tumors of other histologic type than pure squamous cell carcinomas were excluded from the study. Patients who showed microinvasive disease and those with parametrial involvement after histopathologic examination were also excluded. Prior to the introduction of the TMMR-technique (Höckel et al. 2003) all women were treated by radical hysterectomy Piver type III (Piver et al. 1974) at our institution. All patients with histologically proven lymph node involvement were treated with combined adjuvant radiation therapy without concurrent chemotherapy.
The pathological examination was performed in a standardized manner (Kurman and Amin 1999). All tumors were classified and staged according to the WHO- and TNM-classification (Brierley et al. 2017; Stoler et al. 2014).
Because there is no detailed description of different grades for SCC of the uterine cervix in the current WHO classification (Stoler et al. 2014), tumors were graded in accordance to previous studies (Delgado et al. 1990; Stock et al. 1994; Travis et al. 2004; Zaino et al. 1992). In well-differentiated tumors (G1) the tumor cell nests were composed of keratinocyte-like cells with easily visible keratinization features (layered or cytoplasmic keratin). In the poorly differentiated tumors (G3) squamous morphology was only noticeable in a small area of the tumor. The moderately differentiated tumors (G2) showed an intermediate degree of squamous differentiation that was between the well- and poorly differentiated ones.
The original H&E-stained slides were re-examined on low-power magnification (× 25). If necessary, the infiltrating tumor cell nests were screened at intermediate-power fields (× 100) for single cell keratinization and intercellular bridges.
Follow-up information was obtained from the medical charts. Written informed consent was obtained from all patients. The study was approved by the Institutional Review Board.
Recurrence-free survival (RFS) was calculated from the day of diagnosis until tumor recurrence or end of follow-up. Overall survival (OS) was calculated from the day of diagnosis until death or end of follow-up. Kaplan–Meier survival curves and log rank test were used to analyze survival data. Cox regression analyses were fitted to estimate the impact of grading. Odds ratios (OR) with 95% confidence intervals (CI) were determined to describe the chance (risk) for pelvic lymph node involvement depending on tumor grade. All statistical analyses were performed with IBM SPSS Statistics version 24.0.
Results
A total of 233 cases with a median follow-up of 71 (range 2–182) months were available for review. The patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics (N = 233)
| Follow-up (months) | |
| Median (range) | 71 (2–182) |
| Age (years) | |
| Median (range) | 39 (23–67) |
| Mean ± SD | 40.7 ± 10.4 |
| Post-surgical stage distribution | |
| pT1b1 | 233 (100%) |
| Pelvic lymph node involvement | |
| No (pN0) | 189 (81.1%) |
| Yes (pN1) | 44 (18.9%) |
| Histological tumor type | |
| Squamous cell carcinoma | 233 (100%) |
| Tumor grade | |
| G1 | 105 (45.1%) |
| G2 | 68 (29.2%) |
| G3 | 60 (25.8%) |
| Lymphovascular space involvement | |
| None | 109 (46.8%) |
| Yes | 124 (53.2%) |
| Recurrent disease | |
| No | 195 (83.7%) |
| Yes | 38 (16.3%) |
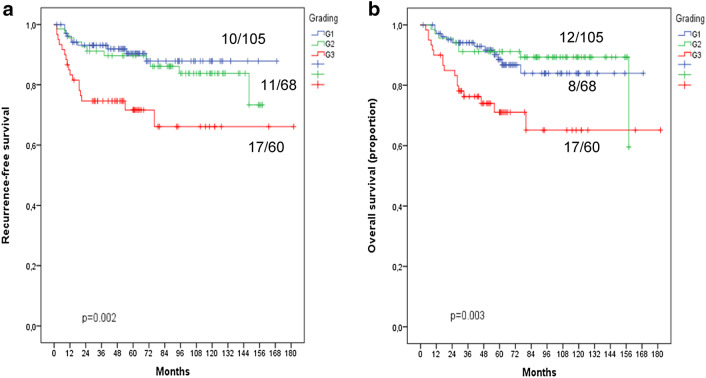
45.1% of the tumors were well-differentiated (G1), 29.2% showed a moderate differentiation (G2) and 25.8% were poorly differentiated (G3). There was a significant decrease in both recurrence-free and overall survival with increasing tumor grade (Fig. 1; Table 2). The detailed statistical analyses between the different grading groups failed to show a difference between well (G1) and moderately (G2) differentiated tumors both in recurrence-free as well as in overall survival (Table 2). As illustrated in Fig. 1, the Kaplan–Meier curves for recurrence-free as well as for overall survival run closely together.
Fig. 1.
Kaplan–Meier curves for prognostic impact of conventional tumor grading in squamous cell carcinoma of the uterine cervix using a 3-tiered grading system (please see text). a Recurrence-free survival. b Overall survival
Table 2.
5-year rate for recurrence-free (RFS) and overall survival (OS) for the different grading groups using the conventional three-tiered grading system for squamous cell carcinomas of the uterine cervix

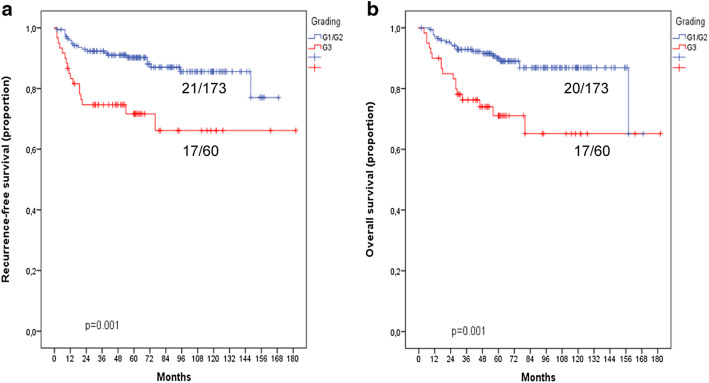
For further analyses, a binary grading model was established. The G1- and G2-tumors were merged into a low-grade group, whereas the G3-tumors formed the high-grade group. The majority of the tumors were part of the low-grade group (173/233; 74.2%) and 25.8% showed high-grade morphology. Patients within the low-grade group were found to have a significantly increased 5-year recurrence-free survival when compared to the high-grade group [90.2% (95% CI 85.7–94.7%) versus 71.6% (59.5–83.7%); p = 0.001; Table 3 and Fig. 2]. The 5-year overall survival rate was significantly higher in the low-grade compared to the high-grade group [89.9% (95% CI 85.2–94.6%) versus 71.1% (95% CI 58.7–83.4%); p = 0.001; Table 3 and Fig. 2b]. In the multivariate Cox regression analysis that included the pelvic lymph node status and binary tumor grade, there was a 2.4-fold increase in the risk for recurrent disease and death from the tumor for high-grade tumors (Table 4).
Table 3.
5-year rate for recurrence-free (RFS) and overall survival (OS) for the different grading groups using a binary approach for the conventional grading system for squamous cell carcinomas of the uterine cervix
| 5-year recurrence-free survival | p values | |
|---|---|---|
| Low-grade (G1/G2) | 90.2% (95% CI 85.7–94.7%) | 0.001 |
| High-grade (G3) | 71.6% (95% CI 59.5–83.7%) |
| 5-year overall survival | p values | |
|---|---|---|
| Low-grade (G1/G2) | 89.9% (95% CI 85.2–94.6%) | 0.001 |
| High-grade (G3) | 71.1% (95% CI 58.7–83.4%) |
Fig. 2.
Kaplan–Meier curves for the prognostic impact of conventional tumor grading in squamous cell carcinoma of the uterine cervix using a 2-tiered grading system (please see text). a Recurrence-free survival. b Overall survival
Table 4.
Cox regression analyses for recurrence-free and overall survival
| HR | p values | |
|---|---|---|
| Recurrence-free survival | ||
| Pelvic lymph node involvement | ||
| No (pN0) | Ref. | |
| Yes (pN1) | 3.3 (95% CI 1.7–6.4) | < 0.001 |
| Histological tumor grade | ||
| Low-grade (G1 + G2) | Ref. | |
| High-grade (G3) | 2.4 (95% CI 1.3–4.7) | 0.009 |
| Overall survival | ||
| Pelvic lymph node involvement | ||
| No (pN0) | Ref. | |
| Yes (pN1) | 4.0 (95% CI 2.1–7.7) | < 0.001 |
| Histological tumor grade | ||
| Low-grade (G1 + G2) | Ref. | |
| High-grade (G3) | 2.4 (95% CI 1.2–4.6) | 0.011 |
The frequency of pelvic lymph node involvement within the different grading is summarized in Table 5.
Table 5.
Pelvic lymph node involvement within different grading groups
| Conventional grading | |||||
|---|---|---|---|---|---|
| Three-tiered grading system | Binary grading system | ||||
| G1 (n = 105) | G2 (n = 68) | G3 (n = 60) | Low-grade (n = 173) | High-grade (n = 60) | |
| pN0 | 87.6% | 82.4% | 68.3% | 85.5% | 68.3% |
| pN1 | 12.4% | 17.6% | 31.7% | 14.5% | 31.7% |
| p value | 0.009 | 0.003 | |||
Overall, between the conventional three-tiered tumor grading and the pelvic lymph node involvement was a significant association (p = 0.009).
The risk for lymph node involvement for G2 tumors compared to G1 tumors was increased slightly [OR = 1.52 (95% CI 0.65–3.56)] and not significantly. More pronounced by a factor of about two was the risk for lymph node involvement for G3 tumors related to G2 tumors [OR = 2.16 (95% CI 0.94–4.95); p < 0.05].
However, using the binary grading system, there was a relevant and significant increased risk for lymph node involvement for high-grade tumors compared to low-grade tumors [OR 2.74 (95% CI 1.38–5.47), p = 0.003].
Discussion
Lymph node status, tumor stage and tumor size are the most powerful and well-accepted prognostic factors in cervical carcinoma (Piura et al. 2006; Singh and Arif 2004; Takeda et al. 2002; Wagner et al. 2013). Data about the impact of histopathological tumor grade are more controversial, particularly in squamous cell cancers of the uterine cervix (SCC; Singh and Arif 2004).
Historically, cervical SCCs were graded using Broders system (Broders 1926) or modifications thereof based on the degree of keratinization (Wentz and Reagan 1959). This particular grading system of cervical SCCs is still mentioned in the last two WHO classifications (Stoler et al. 2014; Wells et al. 2003) and is referred to as conventional grading in order to differentiate it from other grading systems. In an earlier GOG study, the 3-year disease-free interval correlated significantly with the conventional tumor grade (G1: 90.6%, G2: 86.0% and for G3: 76.1%; p = 0.001; 4). Metindir and Bilir (2007) reported that tumor grade was a prognostic factor regarding 5-year disease-free survival. In a larger study of 331 node-negative patients with FIGO stages IB–IIA, grading had no prognostic relevance regarding 5-year disease-free survival (Sartori et al. 2007). Tumor grade was no predictor for vaginal and/or parametrial invasion or pelvic lymph node involvement in FIGO stage IB patients (Koleli et al. 2014; Kong et al. 2016; Silva-Filho et al. 2005; Xie et al. 2016).
In the present study, conventional tumor grade was of prognostic impact both on recurrence-free and overall survival (Fig. 1; Table 2). However, separate analyses revealed no difference between well-differentiated (G1) and moderately differentiated (G2) tumors with regard to recurrence-free and overall survival (Fig. 1; Table 2). After merging G1 and G2 tumors into low-grade tumors, this two-tiered conventional grading showed a statistically more powerful impact both on recurrence-free and overall survival (Fig. 2; Table 3). The prognostic power of the two-tiered conventional grading persisted in multivariate analyses including lymph node status (Table 4). The high-grade tumors showed a hazard ratio of 2.4 (95% CI 1.3–4.7) and of 2.4 (95% CI 1.2–4.6) for recurrent disease and death from the disease, respectively.
With an odds ratio of 1.52 (95% CI 0.65–3.56) there was no difference regarding the risk of pelvic lymph node involvement using the three-tiered conventional grading system. Using the binary grading system, high-grade tumor represented a near threefold increase in the frequency of pelvic lymph node metastases [OR 2.74 (95% CI 1.38–5.47); p = 0.003].
The advantage of the present study may be the analysis of a well-defined study population including only histopathologically staged T1b1-tumors with pure squamous cell histology treated by a primary surgical approach.
Many attempts regarding the definition of grading systems for SCCs of the cervix uteri have been made (Kristensen et al. 1999; Stock et al. 1994). An invasive front grading was suggested by Kristensen et al. (1999). This type of grading was first introduced in head and neck SCCs (Bryne et al. 1992) and evaluates the degree of keratinization, nuclear pleomorphism, pattern of invasion and peritumoral host response at the infiltrative edge of SCCs. In cervical cancer, however, data on this grading system are very limited (Kristensen et al. 1999). Not uncommonly, studies assessing tumor grade as a potential prognostic variable provide no details on the grading system applied. The definition of grading criteria is also an issue in large multicenter investigations such as the SEER analyses (Macdonald et al. 2009; Sartori et al. 2007; Vinh-Hung et al. 2007).
The dilemma of the definition of a grading system in carcinomas with squamous cell histology is not limited to the uterine cervix but is also evident in lung cancer (Kadota et al. 2017; Travis et al. 2004). A recent study evaluated the grade of tumor budding in pulmonary SCC (Kadota et al. 2017). Tumor budding was initially described in colorectal cancer and is defined as the presence of isolated small tumor nests composed of < 5 tumor cells within the stroma of the invasive front of the tumor (Karamitopoulou et al. 2013; Prall 2007). A high number of tumor buds is associated with poor outcome both in colorectal adenocarcinomas and squamous cell cancers of the lung (Kadota et al. 2017; Karamitopoulou et al. 2013). Studies have shown that tumor cell budding may be associated with epithelial–mesenchymal transition and increased cell migration (Kalluri and Weinberg 2009; Taira et al. 2012). Similar to the different patterns of invasion including a finger-like and spray-like growth pattern in SCC of the uterine cervix (Horn et al. 2006, 2012), tumor budding signifies the histopathologic feature of tumor cell dissociation. The invasive front grading (Kristensen et al. 1999) and growth pattern analyses at the invasive tumor front (Horn et al. 2006) may be included in future approaches to define a grading system in SCC of the uterine cervix.
At the present time, no particular grading system(s) has achieved universal acceptance nor has one been recommended by the most recent WHO classification (Stoler et al. 2014). According to the most recent International Collaboration on Cancer Reporting (ICCR 2017) recommendations for cervical cancer, grading of SCCs of the uterine cervix has uncertain clinical value. Finally, additional studies are required to define standardized and reproducible criteria for the grading of SCC of the uterine cervix.
Funding
The authors declare that there was no funding of the study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- Brierley JD, Gospodarowicz MK, Wittekind C (2017) Cervix uteri. TNM classification of malignant tumors. Wiley-Blackwell, Chichester, pp 166–170 [Google Scholar]
- Broders AC (1926) Carcinoma grading and practical application. Arch Pathol 2:376–381 [Google Scholar]
- Bryne M, Koppang HS, Lilleng R, Kjaerheim A (1992) Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol 166(4):375–381 [DOI] [PubMed] [Google Scholar]
- Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F (1990) Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol 38(3):352–357 [DOI] [PubMed] [Google Scholar]
- Höckel M, Horn LC, Hentschel B, Höckel S, Naumann G (2003 Nov-Dec) Total mesometrial resection: high resolution nerve-sparing radical hysterectomy based on developmentally defined surgical anatomy. Int J Gynecol Cancer 13(6):791–803 [DOI] [PubMed] [Google Scholar]
- Horn LC, Fischer U, Raptis G, Bilek K, Hentschel B, Richter CE, Braumann UD, Einenkel J (2006) Pattern of invasion is of prognostic value in surgically treated cervical cancer patients. Gynecol Oncol 103(3):906–911 [DOI] [PubMed] [Google Scholar]
- Horn LC, Hommel N, Roschlau U, Bilek K, Hentschel B, Einenkel J (2012) Peritumoral stromal remodeling, pattern of invasion and expression of c-met/HGF in advanced squamous cell carcinoma of the cervix uteri, FIGO stages III and IV. Eur J Obstet Gynecol Reprod Biol 163(1):76–80 [DOI] [PubMed] [Google Scholar]
- ICCR (2017) http://www.iccr-cancer.org/datasets/datasets-under-consultation/datasets-for-comment/iccr-cervix-bookmarked-guide-1st-edition-v0-5-with. Accessed 10 Dec 2018
- Kadota K, Miyai Y, Katsuki N, Kushida Y, Matsunaga T, Okuda M, Yokomise H, Kanaji N, Bandoh S, Haba RA (2017) Grading system combining tumor budding and nuclear diameter predicts prognosis in resected lung squamous cell carcinoma. Am J Surg Pathol 41(6):750–760 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119(6):1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamitopoulou E, Zlobec I, Kölzer V, Kondi-Pafiti A, Patsouris ES, Gennatas K, Lugli A (2013) Proposal for a 10-high-power-fields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol 26(2):295–301 [DOI] [PubMed] [Google Scholar]
- Koleli IK, Ozdogan E, Sariibrahim B, Ozturk LB, Karateke A (2014) Prognostic factors affecting lymph node involvement in cervical cancer. Eur J Gynaecol Oncol 35(4):425–428 [PubMed] [Google Scholar]
- Kong TW, Kim J, Son JH, Kang SW, Paek J, Chun M, Chang SJ, Ryu HS (2016) Preoperative nomogram for prediction of microscopic parametrial infiltration in patients with FIGO stage IB cervical cancer treated with radical hysterectomy. Gynecol Oncol 142(1):109–114 [DOI] [PubMed] [Google Scholar]
- Kristensen GB, Abeler VM, Risberg B, Trop C, Bryne M (1999) Tumor size, depth of invasion, and grading of the invasive tumor front are the main prognostic factors in early squamous cell cervical carcinoma. Gynecol Oncol 74(2):245–251 [DOI] [PubMed] [Google Scholar]
- Kurman RJ, Amin MB (1999) Protocol for the examination of specimens from patients with carcinomas of the cervix: a basis for checklists. Cancer Committee, College of American Pathologists. Arch Pathol Lab Med 123(1):55–61 [DOI] [PubMed] [Google Scholar]
- Macdonald OK, Chen J, Dodson M, Lee CM, Gaffney DK (2009) Prognostic significance of histology and positive lymph node involvement following radical hysterectomy in carcinoma of the cervix. Am J Clin Oncol 32(4):411–416 [DOI] [PubMed] [Google Scholar]
- Metindir J, Bilir G (2007) Prognostic factors affecting disease-free survival in early-stage cervical cancer patients undergoing radical hysterectomy and pelvic-paraaortic lymphadenectomy. Eur J Gynaecol Oncol 28(1):28–32 [PubMed] [Google Scholar]
- Piura B, Rabinovich A, Friger M (2006) Surgical pathologic factors in patients with early-stage cervical carcinoma treated with radical hysterectomy and pelvic lymph node dissection: association with administration of adjuvant radiotherapy and effect on survival. Eur J Gynaecol Oncol 27(6):573–578 [PubMed] [Google Scholar]
- Piver MS, Rutledge F, Smith JP (1974) Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol 44(2):265–272 [PubMed] [Google Scholar]
- Prall F (2007) Tumour budding in colorectal carcinoma. Histopathology 50(1):151–162 [DOI] [PubMed] [Google Scholar]
- Sartori E, Tisi G, Chiudinelli F, La Face B, Franzini R, Pecorelli S (2007) Early stage cervical cancer: adjuvant treatment in negative lymph node cases. Gynecol Oncol 107(1 Suppl 1):S170–S174 [DOI] [PubMed] [Google Scholar]
- Silva-Filho AL, Reis FM, Traiman P, Pedrosa MS, Miranda D, Triginelli SA (2005) Clinicopathological features influencing pelvic lymph node metastasis and vaginal and parametrial involvement in patients with carcinoma of the cervix. Gynecol Obstet Invest 59(2):92–96 [DOI] [PubMed] [Google Scholar]
- Singh N, Arif S (2004) Histopathologic parameters of prognosis in cervical cancer–a review. Int J Gynecol Cancer 14(5):741–750 [DOI] [PubMed] [Google Scholar]
- Stock RJ, Zaino R, Bundy BN, Askin FB, Woodward J, Fetter B, Paulson JA, DiSaia PJ, Stehman FB (1994) Evaluation and comparison of histopathologic grading systems of epithelial carcinoma of the uterine cervix: Gynecologic Oncology Group studies. Int J Gynecol Pathol 13(2):99–108 [DOI] [PubMed] [Google Scholar]
- Stoler M, Bergeron C, Colgan TJ, Ferency AS, Herrington CS, Kim KR, Loening T, Schneider A, Sherman ME, Wilbur DC, Wright T (2014) Squamous cell tumors of the uterine cervix and its precursors. In: Kurman RJ, Carcangiou ML, Herrington S, Young RH (eds) WHO classification of tumours of female reproductive organs. IARC Press, Lyon, pp 172–182 [Google Scholar]
- Taira T, Ishii G, Nagai K, Yoh K, Takahashi Y, Matsumura Y, Kojima M, Ohmatsu H, Goto K, Niho S, Takashima H, Inoue H, Ohe Y, Ochiai A (2012) Characterization of the immunophenotype of the tumor budding and its prognostic implications in squamous cell carcinoma of the lung. Lung Cancer 76(3):423–430 [DOI] [PubMed] [Google Scholar]
- Takeda N, Sakuragi N, Takeda M, Okamoto K, Kuwabara M, Negishi H, Oikawa M, Yamamoto R, Yamada H, Fujimoto S (2002) Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstet Gynecol Scand 81(12):1144–1151 [DOI] [PubMed] [Google Scholar]
- Travis W, Brambilla E, Muller-Hermelink H et al (2004) World Health Organization classification of tumours pathology and genetics tumours of the lung, pleura, thymus, and heart. IARC Press, Lyon [Google Scholar]
- Vinh-Hung V, Bourgain C, Vlastos G, Cserni G, De Ridder M, Storme G, Vlastos AT (2007) Prognostic value of histopathology and trends in cervical cancer: a SEER population study. BMC Cancer 7:164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AE, Pappas L, Ghia AJ, Gaffney DK (2013) Impact of tumor size on survival in cancer of the cervix and validation of stage IIA1 and IIA2 subdivisions. Gynecol Oncol 129(3):517–521 [DOI] [PubMed] [Google Scholar]
- Wells M, Östör AG, Crum CP, Franceschi S, Tommasino M, Nesland JM, Goodman AK, Sankaranarayanan R, Hanselaar AG, Albores-Saavedra J (2003) Epithelial Tumors of the uterine cervix. In: Tavassoli FA, Devilee P (eds) Tumors of the breast and female genital organs. IARC Press, Lyon, pp 261–272 [Google Scholar]
- Wentz WB, Reagan JW (1959) Survival in cervical cancer with respect to cell type. Cancer 12:384–388 [DOI] [PubMed] [Google Scholar]
- Xie X, Song K, Cui B, Jiang J, Zhang Y, Wang B, Yang X, Kong B (2016) Significance of the factors associated with parametrial involvement in stage IB to IIA cervical cancer. Int J Gynecol Cancer 26(5):939–943 [DOI] [PubMed] [Google Scholar]
- Zaino RJ, Ward S, Delgado G, Bundy B, Gore H, Fetter G, Ganjei P (1992) Frauenhoffer E Histopathologic predictors of the behavior of surgically treated stage IB squamous cell carcinoma of the cervix. A Gynecologic Oncology Group study. Cancer 69(7):1750–1758 [DOI] [PubMed] [Google Scholar]