Abstract
Purpose
There is no targeted drug therapy for NF2 patients, and surgery or radiosurgery is not always effective. Therefore, the exploration of new therapeutic pathways is urgently needed.
Methods
We analyzed the expression of cytokines in the serum of NF2 patients and determined the percentage of HLA-DR−CD33+CD11b+ cells in blood and NF2-associated schwannomas. Furthermore, we analyzed the role of HLA-DR−CD33+CD11b+ cells in inhibiting T-cell proliferation, cytokine production, and transforming growth factor expression.
Results
NF2 patients are in an immunosuppressed state with elevated IL-10 and TGF-β expression in plasma and the lymphocytes from NF2 patients secrete less IFN-γ and CD3+ T cells proliferate slower than normal healthy donors. HLA-DR−CD33+CD11b+ cells frequency significantly increased in the PBMCs and infiltrated in the tumor, these cells express higher iNOS, NOX2 and TGF-β, and induce TGF-β secretion to inhibit CD8+ T-cell proliferation, and induce T-cell transformation to a CD4+CD25+Foxp3+ regulatory T cells phenotype. NF2-associated schwannoma cells induced monocytes transformation into an HLA-DR−CD33+CD11b+ phenotype, and surgical removal of the tumor reduced the percentage of these cells.
Conclusions
HLA-DR−CD33+CD11b+ cells may represent a population of MDSCs in NF2 patients. Dissecting the mechanisms behind these suppressive mechanisms will be helpful for the design of effective immunotherapeutic protocols and likely provide a new effective treatment for NF2 patients.
Electronic supplementary material
The online version of this article (10.1007/s00432-018-02825-8) contains supplementary material, which is available to authorized users.
Keywords: NF2, MDSCs, Immunosuppression, TGF-β
Introduction
Neurofibromatosis type 2 (NF2) is an autosomal dominant disorder (Asthagiri et al. 2009) with characteristic bilateral vestibular schwannomas (Dewan et al. 2015), sometimes accompanied by meningiomas, ependymomas, gliomas or ocular abnormalities (Tanaka et al. 2013). The NF2- associated tumors are benign in histology; however, NF2 patients experience significant morbidity and mortality related to the location of the tumors related to their disease and the effects of treatments (Dirks et al. 2012). And, for nearly 30 years, there have been very few treatments that convey optimism to NF2 patients. Therefore, it is imperative that new effective treatments for these tumors be discovered or explored.
Cancer immunotherapy is designed to boost or engineer immune cells (in particular T cells) to fight against cancer cells while leaving normal cells untouched (Wang and Wang 2017). Recently, T cell-based immunotherapy has been successfully used to treat many human cancers, such as melanoma, lymphoma, breast cancer and renal cell carcinoma, with varying degrees of tumor regression (Assadipour et al. 2017; Chandran et al. 2017; Wang and Wang 2012). And now there is general agreement that there is grand opportunity for immune-driven therapies in brain cancer (Rossi et al. 1990). Thus, by re-focusing and increasing awareness about the role of the immune system in NF2, the future may hold very promising success. Immunotherapy could provide a new avenue for NF2 treatment.
To enhance antitumor immunity, it is necessary to remove the roadblocks so that T cells can be fully activated and functional for the eradication of cancer cells (Wang and Wang 2017). Over the past 10 years, there has been a growing awareness of the role of tumor-induced immune suppression in preventing the efficacy of cancer immunotherapies. Recent studies have also highlighted the contextual immunosuppressive nature of the immune system in brain tumors, and data from clinical trials also suggest that future immunotherapeutic strategies should target the removal or depletion of immunosuppressive cells and molecules in brain tumors, which will promote normal immune cell-mediated tumor rejection.
Multiple cell types are involved in tumor-mediated immune suppression, including regulatory T cells (Tregs), tumor-associated macrophages (TAMs), type 2 NKT cells and myeloid-derived suppressor cells (MDSCs) (Katoh and Watanabe 2015). MDSCs, a heterogeneous population of myeloid cells, are potent inhibitors of the antitumor immune response through negative regulation of T-cell function; they arise from myeloid progenitor cells that fail to differentiate into mature dendritic cells, granulocytes or macrophages, and they are a major obstacle for both immunotherapy and antitumor immunity (Haverkamp et al. 2011).
In mice, MDSCs express the granulocytic cell surface marker Gr1 and the monocyte/macrophage cell surface marker CD11b. Due to the lack of a human analog for mouse Gr1, there is extensive heterogeneity with respect to the expression and level of cell surface markers on human MDSCs (Ugel et al. 2009). Therefore, the phenotype of human MDSCs is not as clearly defined as the phenotype of mouse MDSCs. Generally, MDSCs in humans are broadly characterized as being CD33+, CD11b+, and HLA-DRlow/−, and additional markers may also be present depending on the type of cancer. Multiple MDSC populations have been described in patients with solid tumors. For example, Lin−HLA−DR−CD33+ cells in melanoma (Sade-Feldman et al. 2016), Lin−HLA−DR− cells in breast cancer, CD11b+CD33+HLA−DR− cells in neuroblastoma (Gowda et al. 2013), CD14+CD33+HLA-DR−/low in glioblastoma (Raychaudhuri et al. 2011).
To date, most studies pertain only to peripheral MDSCs (Gros et al. 2012). Studies have revealed that peripheral MDSC levels are significantly increased and mediated immunosuppressive function in many types of cancers (Jiang et al. 2014; Danilin et al. 2012; Koinis et al. 2016; Zhang et al. 2016; Xu et al. 2016; Karakasheva et al. 2015). MDSCs use several mechanisms to suppress antitumor immunity that have been demonstrated both in vivo and in vitro. The mechanisms include cysteine sequestration to decrease proliferation (Srivastava et al. 2010), activation and differentiation of T cells, depletion of L-arginine to arrest T cells in mitosis, induction of FoxP3+ Tregs, downregulation of CD4+ and CD8+ T cell homing to lymph nodes (Hanson et al. 2009), and conversion of antitumor M1 cells into tumor-promoting M2 cells (Ostrand-Rosenberg 2010; Sinha et al. 2007). MDSCs also promote tumor vasculature via their production of different angiogenic proteins. Therefore, it is necessary to study the molecular and cellular mechanisms used by MDSCs to develop successful immunotherapy.
Until now, the study of NF2 has focused primarily on the genetic changes in the NF2 gene, and thus far, immunological changes have not been studied in NF2. Here, we detected the immune status in NF2 patients and identified a subpopulation of MDSCs with an immunosuppressive function. Further elucidation of such NF2-associated tumor-initiated suppressive mechanisms may suggest possible therapeutic interventions to overcome inhibitory effects and improve the efficacy of immunotherapy protocols. It is imperative to identify all the suppressive mechanisms employed by MDSCs so potential therapies can be fully evaluated for their efficacy.
Materials and methods
Patients and healthy donors
All procedures performed involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Our study protocols were approved by the institutional review boards of the Beijing Tiantan Hospital, and written informed consent was obtained from all of the patients prior to their participation. Patient samples were donated from NF2-associated patients who had received a histologically confirmed diagnosis of NF2 by neuropathologists at Beijing Tiantan Hospital. The following were used in this project.
NF2 (n = 23): patients met the diagnostic criteria for NF2, with bilateral acoustic neuroma and other central nervous system tumors, despite treatments with drugs or radiation therapy.
Healthy control (n = 13): healthy volunteers were age and gender-matched to NF2 patients.
Details of healthy and NF2 patients are described in Table 1.
Table 1.
Details of healthy and NF2 patients
| Details of healthy and NF2 patients | Numbers (%) |
|---|---|
| All patients (n = 23) | |
| Median age | 28 ± 9.6 |
| Gender | |
| Male | 9 (39.13%) |
| Female | 14 (60.87%) |
| Healthy donors (n = 13) | |
| Median age | 32.8 ± 7.7 |
| Gender | |
| Male | 6 (46.15%) |
| Female | 7 (53.85%) |
Enzyme-linked immunosorbent assay (ELISA)
Venous blood samples were taken from all subjects and immediately centrifuged at a speed of 4000 rpm for 10 min to obtain serum. All serum samples of the participants were stored at − 80 °C instantly after separation from peripheral blood prior to the analysis. Serum IL-1β, IL-6, IL-10, TNF-α, TGF-β and IFN-γ concentrations were determined by specific ELISA kits (eBioscience, San Diego, CA, USA) according to the manufacturer’s recommendations. The absorbance of samples was measured at 450 nm using a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The IL-1β, IL-6, IL-10, TNF-α, TGF-β and IFN-γ concentrations were determined by interpolation on the standard curve created by the absorbance of the standards. Serum IL-1β, IL-6, IL-10, TNF-α, TGF-β and IFN-γ concentrations are expressed in pg/ml.
To measure the intracellular cytokine production potential, the cells were stimulated with PMA (12.5 ng/ml) plus ionomycin (500 ng/ml) in the presence of Brefeldin-A (5 µg/ml) for 4 h before analysis.
T-cell BrdU proliferation assay
Lymphocytes were incubated with 1 µg of PHA/1 × 106 lymphocytes for 48 h, and then pulsed with 10 µM of BrdU/1 × 106 lymphocytes. After 24 h, the lymphocytes were harvested, stained with CD3-percp-cy5.5, then fixed and permeabilized. Next, the cells were treated with DNase for 1 h and then stained with anti-BrdU-FITC.
Lymphocytes proliferation was measured by the percentage of BrdU+ cells in gated CD3+ cells.
Analysis of MDSC percentage in tumors
Primary schwannoma samples were collected from 11 NF2 patients. Schwannoma tissues were dissected into pieces, digested with collagenase type I (160 U/ml, Sigma) and dispase grade I (1.25 U/ml, Roche, West Sussex, UK), and then filtered using 70 µm cell strainers (BD Falcon). Thereafter, the single cell suspension was subjected to density gradient centrifugation using Ficoll (Biochrom, Berlin, Germany) to enrich for mononuclear cells and to remove debris, followed by re-suspension with FACS staining buffer and staining with anti-CD33 APC, anti-HLA-DR Percp-cy5.5, and anti-CD11b FITC antibodies, along with appropriate isotype controls (all from eBioscience) for flow cytometry acquisition (BD Aria II) and analysis using FlowJo software (TreeStar, Inc., Ashland, OR, USA).
Analysis of MDSC percentages in peripheral blood
Peripheral blood was obtained prior (n = 23) to or 7 days after surgery (n = 5) from NF2 patients from whom we had tumor samples. Controls (n = 13) were available from healthy donors. Blood was subjected to density gradient centrifugation with Ficoll to separate the mononuclear cells and then stained with the same antibodies used for the tumor in the flow cytometry analysis.
MDSCs were defined as HLA-DR−CD33+CD11b+ cells. The percentage of MDSCs was defined as the number of CD33+CD11b+ cells divided by the total number of HLA-DR− cells × 100%.
Magnetic-activated cell sorting (MACS)
CD8+ T cells were isolated from peripheral blood from NF2 patients with MACS using a CD8 T Kit (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Briefly, 107 cells in 100 µl of buffer were incubated with 20 µl of CD8+ T-cell MicroBead Cocktail. The sample was mixed well and incubated for 10 min at 2–8 °C. Cells were washed with 1 ml of buffer and centrifuged at 300g for 10 min, and underwent magnetic sorting, and then, the CD8+ cells were collected. Purity was assessed with a flow cytometry analysis, and all preparations were more than 98% pure.
CD14+ cells were purified as stated above using a CD14+ monocyte isolation Kit.
Cell isolation and sorting
PBMCs were isolated from fresh blood by density gradient centrifugation with Ficoll as described previously. For isolation of HLA-DR− CD33+CD11b+ cells, PBMCs were sorted into HLA-DR− CD33+CD11b+ and CD33− cells using a BECKMAN cell sorting system (Becton Dickinson, Heidelberg, Germany). The purity of the cells after sorting was more than 98%.
Suppression assay
HLA-DR− CD33+CD11b+ cells were purified and sorted as described. The responder CD8+ T cells acquired from NF2 patients were stained with 1.5 µM CFSE (Molecular Probe/Invitrogen) and then stimulated with a T-cell activation and expansion kit (Miltenyi Biotech) and incubated with HLA-DR− CD33+CD11b+ cells at 4:1 for 5 days. Flow cytometry was used to measure the proliferation of T cells based on the CFSE dilution. Briefly, CFSE-labeled responder cells were harvested and then washed with PBS containing 0.1% NaN3 and 0.1% FBS. For blocking experiments, anti-TGF-β neutralizing monoclonal antibody (10 µg/ml; R&D Systems) was used. The recovery rate of proliferative T cells was determined as follows:
For determination of IFN-γ responses, after 48 h of culture, ELISA (eBioscience) was used to examine the supernatants from the suppression assay according to the manufacturer’s instructions.
For functional analysis of in vitro generated HLA-DR−CD33+CD11b+ cells, HLA-DR− CD33+CD11b+ cells were sorted as described. The PBMC were co-cultured with NF2-associated schwannoma cells (≤ 4 passages) for 3 days. Cells were analyzed after 3 days by gating the HLA-DR− CD33+CD11b+ population.
Quantitative polymerase chain reaction (RT-PCR)
The HLA-DR−CD33+CD11b+ cells and CD33− cells were sorted as described previously. Total RNA was extracted using TRIzol reagent (Transgene), and RNA purity and concentrations were measured using a Nanodrop 1000 spectrophotometer (Nanodrop Technologies). cDNA was prepared using a PrimeScript™ RT reagent Kit with gDNA Eraser (TAKARA, Kyoto, Japan), and qRT-PCR were performed with SYBR® Premix Ex Taq™ (TAKARA) using GAPDH as a control for normalization of the data. Oligonucleotide primers for human ARG1, INOS, NOX2, TGFβ and GAPDH were from Origene Co. (Rockville, MD, USA). Gene expression was normalized to GAPDH expression using the method.
Statistical analysis
Differences in quantitative normally distributed variables between two groups were tested with Student’s t test and p value less than 0.05 were considered to indicate statistical significance. Data are expressed as the mean ± SEM, and analyses were performed using Prism 6 software, GraphPad Software (La Jolla, CA, USA).
Results
Patient characteristics
An overview of the clinical details of NF2 patients is given in Supplemental Table 1. All the patients underwent enhanced cranial magnetic resonance imaging examinations and fulfilled the Manchester criteria for diagnosis of NF2. Among the 23 patients with NF2, 9 (39.13%) were male, and 14 (60.87%) were female. The age at diagnosis ranged from 14 to 46 years, with a mean age of 28 years (standard deviation (SD) ± 9.6 years). And we also identified the tumor infiltrating lymphocytes (TILs) and their immunophenotype in the resected schwannoma tissue through immunohistochemical staining. The results show that there are CD4+, CD8+ and CD20+ TILs in the resected schwannoma tissue (Supplemental Fig. 1).
Immunosuppression of NF2 patients versus healthy donors
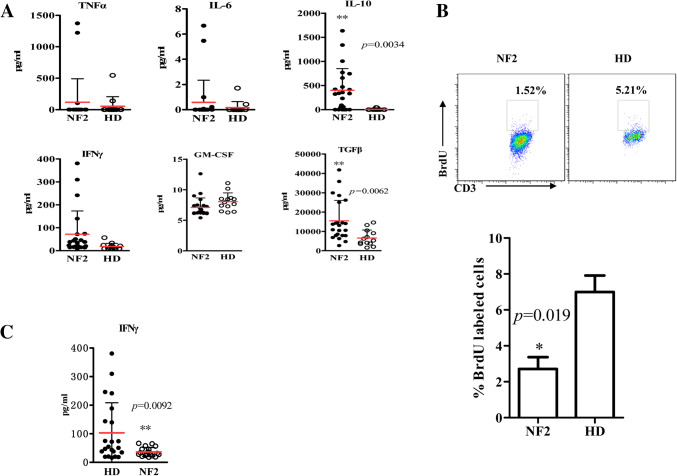
We first assessed the immune status of NF2 patients compared to healthy subjects. Analysis of serum samples showed that TGF-β (p = 0.0062) and IL-10 (p = 0.0034) levels were significantly higher in NF2 patients (n = 23) compared to healthy donors (n = 13) (Fig. 1a). We also observed lymphocytes from NF2 patients proliferated slower (p = 0.019) (Fig. 1b) and secreted less IFN-γ after stimulation than healthy donors (p = 0.0092) (Fig. 1c).
Fig. 1.
NF2 patients are in an immunosuppressed state. a An ELISA was performed with serum from NF2 patients or healthy subjects for IL-6, IL-10, GM-CSF, TNFα, IFN-γ and TGF-β. b Cytometric (up) and statistical (down) analysis of the proliferation of the lymphocytes from NF2 patients or healthy subjects. c PBMCs from healthy donors and NF2 patients were stimulated with PMA and ionomycin, and IFN-γ release from culture supernatants was measured. *p < 0.05, **p < 0.01, ***p < 0.005 compared to the healthy donor (HD) group
HLA-DR− CD33+CD11b+ cell frequency in PBMCs and tumors of NF2 patients
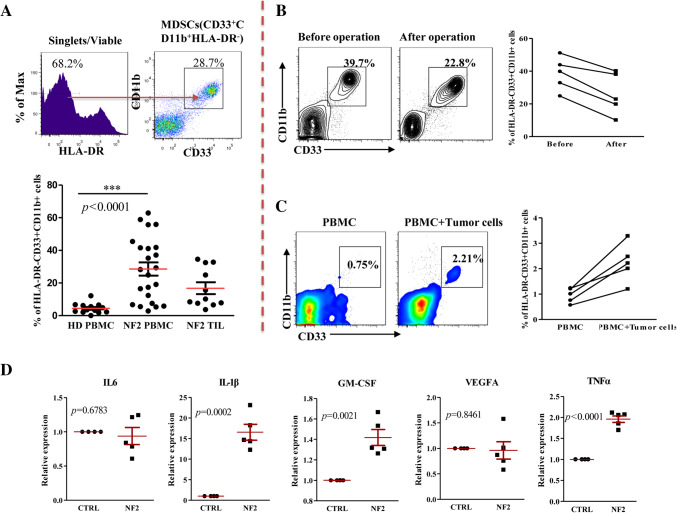
Before evaluating the suppressive function of HLA-DR−CD33+CD11b+ cells, we assessed whether this population is enriched in NF2 patients compared to healthy subjects. The percentage of MDSCs in the current study is shown as the percentage of CD33+CD11b+ in gated HLA-DR− cells (Sade-Feldman et al. 2016) (Fig. 2a). Analysis of blood samples showed that the frequency of HLA-DR− CD33+CD11b+ cells was significantly increased in the PBMCs of NF2 patients (n = 23, p = 0.0001) compared with healthy donors (n = 13) (Fig. 2a), and we also found that HLA-DR− CD33+CD11b+ cells accumulated in NF2-associated schwannomas (n = 11, 16.79 ± 3.621%) (Fig. 2a). As expected, surgical removal of the NF2-associated schwannoma reduced the percentage of HLA-DR− CD33+CD11b+ cells in PBMCs (n = 5) (Fig. 2b).
Fig. 2.
NF2 patients have an increased number of HLA-DR− CD33+CD11b+ cells in peripheral blood. a Cytometric (up) and statistical (down) analysis of the percentage of HLA-DR− CD33+CD11b+ cells in peripheral blood or tumors of NF2 patients or healthy donors. b Surgical removal of NF2-associated schwannomas reduced the percentage of HLA-DR− CD33+CD11b+ cells in PBMCs (n = 5). c NF2-associated schwannoma cells induced monocytes transformation into an HLA-DR− CD33+CD11b+ cell phenotype (n = 5). ***p < 0.005 compared to the healthy donor (HD) group. d NF2-associated schwannoma cells express higher IL-1β, GM-CSF and TNFα
NF2-associated schwannoma cells could induce monocytes transformation into an HLA-DR− CD33+CD11b+ phenotype
Previous studies have reported that melanoma can induce monocytes into a similar myeloid-derived suppressor cell population in a proximity-dependent fashion. To examine whether NF2-associated schwannoma-educated monocytes were phenotypically similar to MDSCs, we co-cultured CD14+ monocytes enriched from healthy individuals with separated primary NF2-associated schwannoma cells (≤ 4 passages) in round-bottomed 96-well plates. In line with our expectation, when cells were co-cultured with NF2-associated schwannoma cells, the percentage of HLA-DR− CD33+CD11b+ cells was increased (n = 5) (Fig. 2c). And we further detected IL-1β, IL-6, TNFα, VEGF, and GM-CSF expression in 5 cases of primary NF2-associated schwannoma cells (≤ 4 passages) using quantitative RT-PCR techniques, results show that IL-1β (p = 0.0002), TNFα (p < 0.0001), and GM-CSF (p = 0.0021) was significantly higher expression in NF2-associated schwannoma cells in comparison with primary normal nerve cells (Fig. 2d).
HLA-DR− CD33+CD11b+ cells express higher levels of iNOS, TGF-β and NOX2 mRNA and can inhibit autologous T cell proliferation
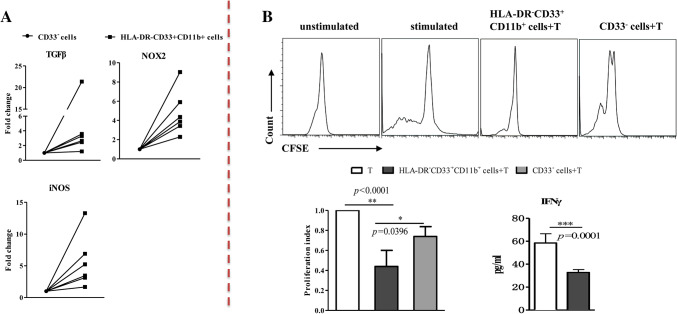
The increased frequencies of HLA-DR− CD33+CD11b+ cells detected in NF2 patients suggested immunosuppressive activity that leads to an impaired immunological status. We thus further characterized the HLA-DR−CD33+CD11b+ cells in NF2 patients. We sorted the HLA-DR− CD33+CD11b+ cells and CD33− cells from PBMCs of NF2 patients (n = 6) using flow cytometry and compared ARG-1, iNOS, NOX2 and TGF-β mRNA expression levels through RT-PCR. Representative histograms of the expression of different transcription factors are shown in Fig. 3a. The results shows that iNOS, NOX2 and TGF-β mRNA expression levels upregulated in HLA-DR− CD33+CD11b+ cells, however, we did not detect the increase in ARG-1 mRNA expression (data not shown).
Fig. 3.
HLA-DR− CD33+CD11b+ cells show immunosuppressive activity. a HLA-DR− CD33+CD11b+ cells express higher iNOS, NOX2 and TGF-β. b Sorted HLA-DR− CD33+CD11b+ cells were added to autologous anti-CD3/CD28-stimulated CD8+ T cells, and proliferation was analyzed. HLA-DR− CD33+CD11b+ cells suppressed CD8+ T-cell proliferation of autologous CD8+ T cells and IFN-γ secretion
For functional analysis, sorted HLA-DR− CD33+CD11b+ cells were added to autologous anti-CD3/CD28-stimulated CD8+ T cells, and proliferation and IFN-γ production were analyzed. HLA-DR− CD33+CD11b+ cells suppressed CD8+ T cell proliferation and IFN-γ secretion of autologous CD8+ T cells (Fig. 3b). When CD33− cells were used as controls, they failed to suppress CD8+ T cell proliferation or IFN-γ release of the responding CD8+ T cells as expected (Fig. 3b).
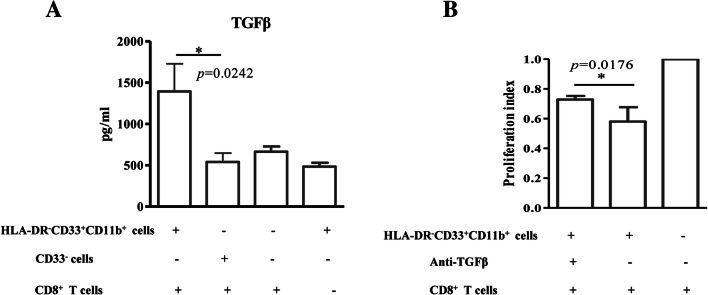
HLA-DR− CD33+CD11b+ cells induce TGF-β secretion in cultured T cells
The cytokine secretion profile of HLA-DR−CD33+CD11b+ cells was analyzed. HLA-DR−CD33+CD11b+ cells did not secrete IL-4, IL-12, IL-10 or TGF-β as measured by ELISA (data not shown). However, TGF-β was detected in cell supernatants of HLA-DR− CD33+CD11b+ cells co-cultured with autologous CD8+ T cells (Fig. 4a). Next, we tested whether TGF-β secretion impaired the proliferation of CD3/CD28-stimulated CD8+ T cells using a specific neutralizing antibody, which resulted in a 53% increase in proliferation (Fig. 4b).
Fig. 4.
HLA-DR− CD33+CD11b+ cell inhibition of T-cell proliferation partially depends on TGF-β secretion. a HLA-DR− CD33+CD11b+ cells induce TGF-β secretion in cultured T cells. b Specific neutralizing antibody reduced CD8+ T-cell proliferation
HLA-DR− CD33+CD11b+ cells induce CD4+CD25+Foxp3+ regulatory T cells
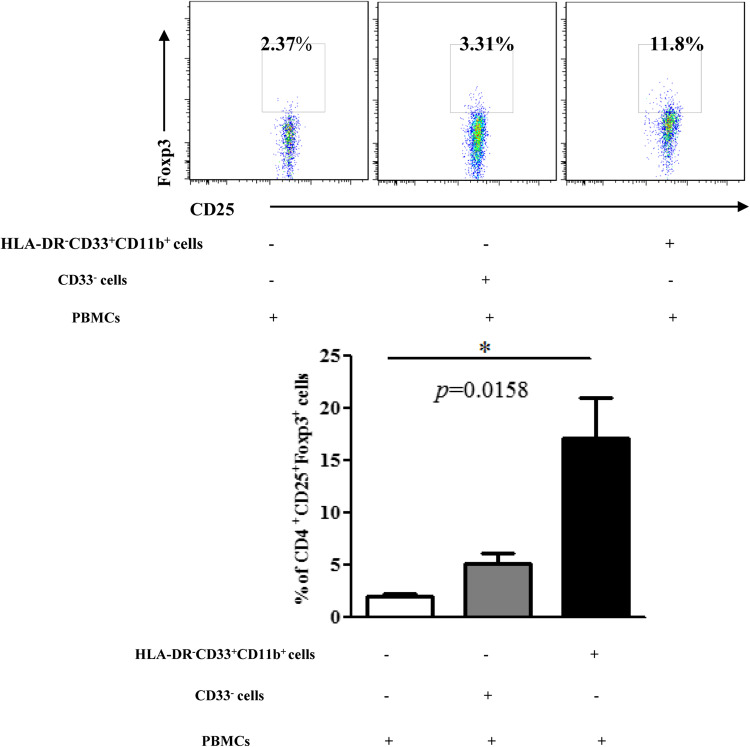
We further examined the T cells from the co-cultures for regulatory phenotype. Sorted HLA-DR−CD33+CD11b+ cells and CD33− cells were incubated with autologous CD3/CD28-stimulated CD3+ T cells for 3 days and CD4+CD25+Foxp3+ regulatory T cells percentage was evaluated by intracellular staining. A higher frequency CD4+CD25+Foxp3+ cells was detected when CD3+ T co-cultured with HLA-DR− CD33+CD11b+ cells in comparison with CD33− cells (p = 0.0361) (Fig. 5).
Fig. 5.
HLA-DR− CD33+CD11b+ cells induce CD4+CD25+Foxp3+ regulatory T cells. Cytometric (up) and statistical (down) analysis of the percentage of CD4+CD25+Foxp3+ regulatory T cells
Discussion
In this study, we show that NF2 patients are in an immunosuppressed state with elevated IL-10 and TGF-β expression in plasma, and the lymphocytes from NF2 patients proliferated slower and secreted less IFN-γ than those from normal healthy subjects. In addition, our study also shows that HLA-DR−CD33+CD11b+ cells are significantly increased in peripheral blood and infiltrated into the tumor of NF2 patients. These cells expressed higher levels of iNOS, NOX2 and TGF-β. When co-cultured with autologous CD8+ T cells, HLA-DR−CD33+CD11b+ cells from NF2 patients induced TGF-β secretion in cultured T cells to inhibit T cells proliferation. Thus, these cells may represent a population of MDSCs in NF2. Further research is required for the development of novel strategies for tumor immunotherapy, which could provide a new effective treatment for NF2 patients.
An immunosuppressive tumor microenvironment plays a key role in tumor progression, and it is also a major obstacle for efficient tumor immunotherapy (Danelli et al. 2015; Kalathil and Thanavala 2016). Accordingly, there is now unanimous agreement that host immune cells succumb to immunosuppressive forces induced by brain tumor cells and therefore become converted into active participants that promote tumor progression (Wainwright et al. 2012). Based on this central tenet, it is now understood that for brain tumor treatments to be maximally effective, immunotherapeutic strategies must both reverse the immunosuppression in leukocytes and target the central hubs within tumor cells that regulate those immunosuppression-inducing pathways (Lieberman et al. 2016).
Here, our study is the first to report that NF2 patients are in an immunosuppressed state, and interestingly, we found that IL-10 and TGF-β expression in the plasma of NF2 patients is significantly up regulated. These two cytokines are two of the major immunosuppressive cytokines secreted by MDSCs, and they are major players in tumor-mediated immunosuppression. Therefore, we next evaluated the MDSCs in NF2 patients.
MDSCs were first characterized in mice and identified as Gr1+CD11b+ cells (Gabrilovich and Nagaraj 2009), while in humans, MDSCs do not have 100% sensitive or specific markers, and markers can even differ among tumor types (Youn and Gabrilovich 2010). Generally, MDSCs are characterized by the expression of the myeloid markers CD11b and CD33 and low or absent HLA-DR. Therefore, we next examined the percentage of HLA-DR− CD33+CD11b+ cells in NF2 patients.
As expected, the percentage of HLA-DR− CD33+CD11b+ cells was significantly higher in NF2 patient PBMCs compared to those from healthy individuals. In addition, we found that HLA-DR− CD33+CD11b+ cells infiltrated into NF2-associated schwannomas; surgical removal of the tumor reduced the percentage of these cells in PBMCs, and tumor cells could transform monocytes into an HLA-DR− CD33+CD11b+ phenotype. Studies have reported that MDSC-induction capacity correlated directly with tumor cell line expression of IL-1β, IL-6, TNFα, VEGF, and GM-CSF (Lechner et al. 2011), so we further detected these five genes expression in five cases of primary NF2-associated schwannoma cells (≤ 4 passages) using quantitative RT-PCR techniques, results show that IL-1β, TNFα, and GM-CSF was significantly higher expression in NF2-associated schwannoma cells in comparison with primary normal nerve cells (Fig. 2d), thus it may partly illustrate that the ability of the mechanism of NF2-associated schwannoma cells inducing MDSC.
And further we also evaluate the NF2 mutation relative to immunosuppression, we selected 20 cases of NF2 patients which already get mutation information to detect the peripheral MDSC percentage, including 6 cases of truncating mutations (3 cases of nonsense mutations and 3 cases of frame shift mutations), 5 cases of splice site mutations, 5 cases of large deletions and 4 cases of missense mutations (the mutation type data are shown in Supplemental Table 3), and next we analyzed the possible correlation between the mutation type and the percentage of HLA-DR−CD33+CD11b+ cells. As shown in Supplemental Fig. 1, we divided the measured data into two groups: Truncating mutations group, represent severe clinical symptoms, and other mutations groups, represents less severe symptoms. We found that truncating mutations group has a higher MDSC percentage in comparison with other mutations groups (p < 0.0001), however, there was no significant difference among different mutations within the truncating mutations group or other mutations groups. The above results illustrated that it may be not the certain mutation type but the severity of the disease has an influence on the immunosuppression.
Suppression of T-cell activation is a hallmark of human MDSC activity (Youn and Gabrilovich 2010). As most human MDSCs have no or very low levels of MHC II, most suppression of CD4+ T cells is not considered being antigen-specific (Trikha and Carson 2014). In vitro studies also demonstrated that cell-to-cell contact or very close proximity is essential for MDSC-mediated suppression (De Wilde et al. 2009), and thus, we chose CD8+ T cells and co-cultured them with HLA-DR− CD33+CD11b+ cells in round-bottomed 96-well plates for suppression assays. As expected, HLA-DR− CD33+CD11b+ cells significantly inhibited autologous T-cell proliferation, indicating that HLA-DR− CD33+CD11b+ cells contribute to the immune suppressive microenvironment in NF2 patients.
MDSCs affect the metabolism of arginine and tryptophan, which leads to inhibition of the T-cell antitumor immune response. ARG-1 and NOS production is a major suppressive mechanism of MDSCs (Obermajer et al. 2012). The enzyme iNOS (inducible nitric oxide synthase) converts arginine into citrulline and NO (Lechner et al. 2005), and ARG-1 converts arginine into ornithine and urea (Yeon et al. 2016). This conversion downregulates the TCR-associated z chain, which is critical for T-cell activation because it activates cyclin D3 and cyclin-dependent kinase, which initiate cell proliferation (Zeng et al. 2014). And our results shows that iNOS, NOX2 and TGF-β mRNA expression levels upregulated in HLA-DR− CD33+CD11b+ cells compared to CD33− cells. However, we did not detect higher expression of ARG-1 mRNA in HLA-DR− CD33+CD11b+ cells. In support of our data, Roberta Valenti et al. have proved that CD14+HLA-DR−/low cells identified in melanoma did not show any arginase activity with TGF-β-mediated immunosuppressive activity (Valenti et al. 2006). And in current study, we found that HLA-DR− CD33+CD11b+ cells suppressed CD8+ T-cell proliferation and IFN-γ secretion of autologous CD8+ T cells in a dose-dependent manner, and TGF-β was detected in cell supernatants of HLA-DR− CD33+CD11b+ cells co-cultured with autologous CD8+ T cells. TGF-β has been a focus throughout tumor biology for nearly 4 decades (Costanza et al. 2017; Lawrence 1985). It became a major focus due to its effects on the maintenance of tumor progression (Sun et al. 2016). In addition, studies have demonstrated the important role of TGF-β signaling in MDSCs. Using a specific antibody, we confirmed that TGF-β also plays a key role in the immunosuppressive activity of HLA-DR− CD33+CD11b+ cells from NF2 PBMCs.
Tregs are an important composition of tumor-related immune suppression subject to regulatory influences by MDSCs (Holmgaard et al. 2015; Lee et al. 2016). Tregs are recruited by MDSC release of IL-10 and TGF-β (Coosemans et al. 2016). Some research has successfully proved that MDSC-derived TGF-β and retinoic acid could transform Th17 cells into Foxp3 positive Tregs (Hoechst et al. 2011). And our results also suggests that HLA-DR− CD33+CD11b+ cells in NF2 patients could induce CD4+CD25+Foxp3+ regulatory T cells, further illustrating the immunosuppressive function of HLA-DR− CD33+CD11b+ cells.
In summary, we describe an immunosuppressive MDSC population characterized by an HLA-DR− CD33+CD11b+ phenotype in peripheral blood and tumors of NF2 patients, and we are the first to report that NF2 patients are in an immunosuppressed state. Dissecting the mechanisms behind these suppressive mechanisms will be helpful for the design of effective immunotherapeutic protocols and likely provide a new effective treatment for NF2 patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- NF2
Neurofibromatosis type 2
- MDSC
Myeloid-derived suppressor cells
- TGF-β
Transforming growth factor-β
- iNOS
Inducible nitric oxide synthase
- NOX2
Non-phagocytic cell oxidase 2
- ARG-1
Arginase-1
- PBMC
Peripheral blood mononuclear cell
- TCR
T-cell receptor
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 81502453) and Natural Science Foundation of Beijing Municipality (No. 7162057).
Compliance with ethical standards
Conflict of interest
The authors declare no potential conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- Assadipour Y, Zacharakis N, Crystal JS, Prickett TD, Gartner JJ, Somerville RP, Xu H, Black MA, Jia L, Chinnasamy H et al (2017) Characterization of an immunogenic mutation in a patient with metastatic triple negative breast cancer. Clin Cancer Res 23:4347–4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthagiri AR, Parry DM, Butman JA, Kim HJ, Tsilou ET, Zhuang Z, Lonser RR (2009) Neurofibromatosis type 2. Lancet 373:1974–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran SS, Somerville RP, Yang JC, Sherry RM, Klebanoff CA, Goff SL, Wunderlich JR, Danforth DN, Zlott D, Paria BC et al (2017) Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol 18:792–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coosemans A, Decoene J, Baert T, Laenen A, Kasran A, Verschuere T, Seys S, Vergote I (2016) Immunosuppressive parameters in serum of ovarian cancer patients change during the disease course. Oncoimmunology 5:e1111505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza B, Umelo IA, Bellier J, Castronovo V, Turtoi A (2017) Stromal Modulators of TGF-beta in cancer. J Clin Med 6:E7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danelli L, Frossi B, Pucillo CE (2015) Mast cell/MDSC a liaison immunosuppressive for tumor microenvironment. Oncoimmunology 4:e1001232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilin S, Merkel AR, Johnson JR, Johnson RW, Edwards JR, Sterling JA (2012) Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology 1:1484–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wilde V, Van Rompaey N, Hill M, Lebrun JF, Lemaitre P, Lhomme F, Kubjak C, Vokaer B, Oldenhove G, Charbonnier LM et al (2009) Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transpl 9:2034–2047 [DOI] [PubMed] [Google Scholar]
- Dewan R, Pemov A, Kim HJ, Morgan KL, Vasquez RA, Chittiboina P, Wang X, Chandrasekharappa SC, Ray-Chaudhury A, Butman JA et al (2015) Evidence of polyclonality in neurofibromatosis type 2-associated multilobulated vestibular schwannomas. Neuro-oncology 17:566–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks MS, Butman JA, Kim HJ, Wu T, Morgan K, Tran AP, Lonser RR, Asthagiri AR (2012) Long-term natural history of neurofibromatosis Type 2-associated intracranial tumors. J Neurosurg 117:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda M, Payne KK, Godder K, Manjili MH (2013) HLA-DR expression on myeloid cells is a potential prognostic factor in patients with high-risk neuroblastoma. Oncoimmunology 2:e26616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA (2012) Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res 18:5212–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S (2009) Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol 183:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp JM, Crist SA, Elzey BD, Cimen C, Ratliff TL (2011) In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur J Immunol 41:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F (2011) Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood 117:6532–6541 [DOI] [PubMed] [Google Scholar]
- Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, Merghoub T, Wolchok JD (2015) Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell Rep 13:412–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Guo W, Liang X (2014) Phenotypes, accumulation, and functions of myeloid-derived suppressor cells and associated treatment strategies in cancer patients. Hum Immunol 75:1128–1137 [DOI] [PubMed] [Google Scholar]
- Kalathil SG, Thanavala Y (2016) High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. Cancer Immunol Immunother 65:813–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakasheva TA, Waldron TJ, Eruslanov E, Kim SB, Lee JS, O’Brien S, Hicks PD, Basu D, Singhal S, Malavasi F, Rustgi AK (2015) CD38-expressing myeloid-derived suppressor cells promote tumor growth in a murine model of esophageal cancer. Cancer Res 75:4074–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Watanabe M (2015) Myeloid-derived suppressor cells and therapeutic strategies in cancer. Mediat Inflamm 2015:159269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinis F, Vetsika EK, Aggouraki D, Skalidaki E, Koutoulaki A, Gkioulmpasani M, Georgoulias V, Kotsakis A (2016) Effect of first-line treatment on myeloid-derived suppressor cells’ subpopulations in the peripheral blood of patients with non-small cell lung cancer. J Thorac Oncol 11:1263–1272 [DOI] [PubMed] [Google Scholar]
- Lawrence DA (1985) Transforming growth factors—an overview. Biol Cell 53:93–98 [DOI] [PubMed] [Google Scholar]
- Lechner M, Lirk P, Rieder J (2005) Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin. Semin Cancer Biol 15:277–289 [DOI] [PubMed] [Google Scholar]
- Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, Epstein AL (2011) Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med 9:90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Kwak Y, Yang T, Han JH, Park SH, Ye MB, Lee W, Sim KY, Kang JA, Kim YC et al (2016) Myeloid-derived suppressor cells are controlled by regulatory T cells via TGF-beta during murine colitis. Cell Rep 17:3219–3232 [DOI] [PubMed] [Google Scholar]
- Lieberman NA, Moyes KW, Crane CA (2016) Developing immunotherapeutic strategies to target brain tumors. Expert Rev Anticancer Therapy 16:775–788 [DOI] [PubMed] [Google Scholar]
- Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P (2012) PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Investig 41:635–657 [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 59:1593–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, Garcia J, Vogelbaum MA, Finke J (2011) Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro-oncology 13:591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi ML, Jones NR, Esiri MM, Havas L, Nakamura N, Coakham HB (1990) Mononuclear cell infiltrate, HLA-Dr expression and proliferation in 37 acoustic schwannomas. Histol Histopathol 5:427–432 [PubMed] [Google Scholar]
- Sade-Feldman M, Kanterman J, Klieger Y, Ish-Shalom E, Olga M, Saragovi A, Shtainberg H, Lotem M, Baniyash M (2016) Clinical significance of circulating CD33+CD11b+HLA-DR− myeloid cells in patients with stage IV melanoma treated with ipilimumab. Clin Cancer Res 22:5661–5672 [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S (2007) Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 179:977–983 [DOI] [PubMed] [Google Scholar]
- Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res 70:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Taguchi A, Hanash S (2016) Switching roles of TGF-beta in cancer development: implications for therapeutic target and biomarker studies. J Clin Med 5:E109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Eskin A, Chareyre F, Jessen WJ, Manent J, Niwa-Kawakita M, Chen R, White CH, Vitte J, Jaffer ZM et al (2013) Therapeutic potential of HSP90 inhibition for neurofibromatosis type 2. Clin Cancer Res 19:3856–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikha P, Carson WE III (2014) Signaling pathways involved in MDSC regulation. Biochim Biophys Acta 1846:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, Zilio S, Bronte V (2009) Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol 9:470–481 [DOI] [PubMed] [Google Scholar]
- Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L (2006) Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res 66:9290–9298 [DOI] [PubMed] [Google Scholar]
- Wainwright DA, Nigam P, Thaci B, Dey M, Lesniak MS (2012) Recent developments on immunotherapy for brain cancer. Expert Opin Emerg Drugs 17:181–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Wang RF (2012) Enhancing cancer immunotherapy by intracellular delivery of cell-penetrating peptides and stimulation of pattern-recognition receptor signaling. Adv Immunol 114:151–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF, Wang HY (2017) Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res 27:11–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XD, Hu J, Wang M, Peng F, Tian R, Guo XJ, Xie Y, Qin RY (2016) Circulating myeloid-derived suppressor cells in patients with pancreatic cancer. Hepatobiliary Pancreat Dis Int 15:99–105 [DOI] [PubMed] [Google Scholar]
- Yeon JT, Choi SW, Kim SH (2016) Arginase 1 is a negative regulator of osteoclast differentiation. Amino Acids 48:559–565 [DOI] [PubMed] [Google Scholar]
- Youn JI, Gabrilovich DI (2010) The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 40:2969–2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng QL, Yang B, Sun HQ, Feng GH, Jin L, Zou ZS, Zhang Z, Zhang JY, Wang FS (2014) Myeloid-derived suppressor cells are associated with viral persistence and downregulation of TCR zeta chain expression on CD8(+) T cells in chronic hepatitis C patients. Mol Cells 37:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ye YL, Li MX, Ye SB, Huang WR, Cai TT, He J, Peng JY, Duan TH, Cui J et al (2016) CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene 36:2095–2104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.