Abstract
The human cell line HK-2 is most commonly used as a model of renal proximal tubular epithelial cells (PTECs) for various studies despite the absence or low expression of transporters characteristic of parental PTECs. In an effort to develop reliable PTEC models, several human cell lines have been newly established over the last decade. In contrast, reliable mouse PTEC models are still unavailable. In this study, we established immortalized renal cortex tubule cell lines derived from p53 knockout mice and evaluated various PTEC characteristics toward the development of reliable mouse PTEC models. Here, we focus on MuRTE61, one of 13 newly established clonal cell lines. Albumin uptake in MuRTE61 cells was verified by incubation with fluorescent dye-labeled albumin. RT-PCR confirmed the expression of efflux transporter genes characteristic of PTECs in the MuRTE61 cells. MuRTE61 cells exhibited high sensitivity to treatment with cisplatin, a nephrotoxic agent, accompanied by upregulated expression of the uptake transporter Slc22a2 gene. Furthermore, MuRTE61 cells consistently formed spheroids with a lumen and apicobasal polarity in three-dimensional Matrigel cultures. Apical brush border microvilli were also observed in the spheroids by transmission electron microscopy. These data validate that MuRTE61 can be characterized as a reliable mouse PTEC line. In future, detailed analysis of reliable mouse and human PTEC lines will provide an accurate extrapolation of results of experiments using mice and humans, and may help resolve apparent inconsistencies between mouse and human nephrotoxicity.
Keywords: Renal proximal tubular epithelial cells, Cell line, 3D cell culture, Cell spheroids, Matrigel
Introduction
Renal proximal tubular epithelial cells (PTECs) are responsible for the reabsorption of numerous solutes from glomerular filtrate and excretion of metabolic waste products or xenobiotics from peritubular capillaries, contributing to renal clearance and, on occasions, nephrotoxicity. Therefore, PTEC transport mechanisms are the subject of much physiological and pharmacological research (Li et al. 2003; Brown et al. 2008). PTECs are also an important tool for studying kidney diseases such as renal fibrosis, ischemic kidney injury and diabetic nephropathy (Havasi and Borkan 2011; Phillips 2003; Gewin 2018). However, the number of population doubling PTECs is quite limited in vitro, so that a number of renal tubular cell lines have been established (Bens and Vandewalle 2008). The human cell line HK-2 is most commonly used as a PTEC model for the study of basic cell biology, pharmacology, and toxicology as well as various kidney diseases, despite the absence or low expression of transporters characteristic of the parental PTECs (Jenkinson et al. 2012). In an effort to develop reliable PTEC models, several human cell lines, such as ciPTEC, RPTEC/TERT1 and SA7K, have been newly established over the last decade (Wieser et al. 2008; Wilmer et al. 2010; Li et al. 2017). In contrast, reliable mouse PTEC models are still unavailable.
In this study, we established immortalized renal cortex tubule cell lines derived from p53-deficient mice and evaluated the various PTEC characteristics toward the development of reliable mouse PTEC models.
Materials and methods
Mice
The p53-deficient mice (Tsukada et al. 1993) were generated by crossing heterozygous mice (Trp53+/−) on the C57BL/6N background (#CDB0001K). The heterozygous mice were provided by the RIKEN BioResource Center (Tsukuba, Japan).
Primary culture of mouse PTECs
Isolation of PTECs was performed as previously described (Gai et al. 2010), with minor modifications. Kidneys from 8-week-old male mice were rinsed twice with Ca2+- and Mg2+-free phosphate-buffered saline (PBS). The renal cortex slices were minced, and digested with TrypLE Express dissociation reagent (Thermo Fisher Scientific, Waltham, MA, USA) while rotating at 37 °C for 15 min. The resultant mixture was diluted with renal epithelial cell growth medium (REGM, renal epithelial cell basal medium supplemented with 0.5% fetal bovine serum, recombinant human epidermal growth factor, insulin, hydrocortisone, epinephrine, Triiodothyronine, transferrin, 30 µg/mL gentamicin and 15 ng/mL Amphotericin) from Lonza (Basel, Switzerland), and then transferred into a 50-mL tube through a 100-µm cell strainer. The tissue debris remaining on the cell strainer was gently pressed with the plunger from a 2.5-mL syringe, and rinsed with REGM. The collected cell suspension was passed through a new 100-µm cell strainer to eliminate viscous substances, consisting mostly of genomic deoxyribonucleic acid, and then centrifuged at 75g for 5 min. The cell pellet was suspended in REGM, centrifuged again, and resuspended in REGM. The washed cells were plated on culture dishes with the Nunclon Delta surface treatment (Thermo Fisher Scientific), and incubated at 37 °C in 5% carbon dioxide. The growth medium was exchanged every 2 days until the first passage. Almost all renal glomeruli were removed by the first medium change. Cells were passaged using TrypLE Express dissociation reagent when they became 80% confluent. After the first passage, the growth medium was exchanged every 3 days.
Cloning of immortalized mouse PTECs
Immortalization in p53-deficient PTECs occurs spontaneously, or senescence occurs in the early passage cultures as in p53-intact PTECs (Hanazono et al. 1997). After seeding 1000 cells at passage 10 onto a 100-mm dish, colonies of homogenous cell populations were scraped and aspirated using a micropipette, and separately transferred to 96-well plates. Cell lines with poor proliferative capacity were withdrawn within four sequential passages. To obtain clonal cell lines, 100 cells at passage 15 were seeded onto a 6-well plate. The colonies were again isolated and cultured as described above. Clonal cells were routinely passaged to a ratio of 1:4 once every 3 days. Experiments were carried out at passage 25–35.
HK-2 cells
HK-2 cells were provided by the American Type Culture Collection (Manassas, VA, USA) and cultured in REGM.
RT-PCR analysis
The total RNA from cells was extracted and purified using NucleoSpin RNA (Macherey–Nagel, Düren, Germany). Reverse transcription of RNA to cDNA was performed with oligo dT primer and ReverTra Ace transcriptase enzyme (Toyobo, Osaka, Japan). Reverse transcription-PCR was performed with TaKaRa ExTaq HS polymerase enzyme (Takara Bio, Kusatsu, Japan). Quantitative real-time PCR was performed in triplicate, according to the manufacturer’s protocol in KAPA SYBR FAST qPCR Master Mix Kit (Kapa Biosystems, Wilmington, MA, USA), using an Eco Real-Time PCR System (Illumina, San Diego, CA, USA). Relative quantitative levels of samples were determined by the 2−ΔΔCq method. PCR primers are shown in Table 1.
Table 1.
Sequences of the PCR primers
| Gene symbol | Ref seq | Forward primer | Reverse primer | Product size | Application |
|---|---|---|---|---|---|
| Gapdh |
NM_001289726 NM_008084 |
CGACTTCAACAGCAACTC | GCCGTATTCATTGTCATACCAG | 106 | RT-PCR, real-time PCR |
| Ggt1 |
NM_001305992 NM_008116 |
TGCCTTGTGCGAGGTGTTCTGCCGC | TGGCAGCCACAGCACTGCCATCCT | 623 | RT-PCR |
| Slc22a2 |
NM_013667 NM_001355767 |
TTGGTCGCCGCTATCCCTGGGCTGT | TCCCCCAGCAACAAGGCCAACCAC | 326 | RT-PCR |
| Slc47a1 | NM_026183 | TTGGTCCTGGCGGGTCCTGCGTTCT | TGGGCTCCCAACTCCACCATGCCA | 770 | RT-PCR |
| TTGGTCCTGGCGGGTCCTGCGTTCT | TGGCTCCCGTATGTCTGGGAGATGAGC | 206 | Real-time PCR | ||
| Abcc2 | NM_013806 | TCCAACCGGTGGCTTGCCATTCGCC | TCGAAGCACGGCCCTACCCAGGCAT | 753 | RT-PCR |
| TTGGCTCTTGGCGCCTTGGCAACT | GCATTGCCTGCAGTGTTGGATCACC | 250 | Real-time PCR | ||
| Abcc4 |
NM_001033336 NM_001163675 |
ATGAGGCTGCGGGTTGCCATGTGCC | ACCCAGCACTGCGCTCAACAGCGA | 891 | RT-PCR |
| CATCGCGGTAACCGTCCTC | CCGCAGTTTTACTCCGCAG | 134 | Real-time PCR | ||
| Abcb1a | NM_011076 | ATCCCACCCGACCCAGCATCCCAGT | TGCCGTGCTCCTTGACCTTGCCGT | 636 | RT-PCR |
| CAGCAGTCAGTGTGCTTACAA | ATGGCTCTTTTATCGGCCTCA | 205 | Real-time PCR |
Stimulation with BSA or TGF-β
Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20 µg/mL bovine serum albumin (BSA, Merck, Darmstadt, Germany) or 5 ng/mL recombinant human transforming growth factor beta 1 (TGF-β1, R&D Systems, Minneapolis, MN, USA) for 24 h, and imaged using an EVOS FLoid Cell Imaging Station (Thermo Fisher Scientific).
Western blot analysis
Cells were lysed in RIPA buffer supplemented with protease and phosphatase inhibitors (#04080 and #07574-61, Nacalai Tesque Inc., Kyoto, Japan) and boiled with 2% sodium dodecyl sulfate (SDS) and 5% 2-mercaptoethanol. Lysates were electrophoresed in a SDS–polyacrylamide gel and blotted on polyvinylidene difluoride membranes (GE Healthcare, Chicago, IL, USA). For blocking non-specific binding, the membranes were incubated in Blocking One reagent (Nacalai Tesque) for 1 h at room temperature. The membranes were incubated with primary antibodies against alpha smooth muscle actin (α-SMA) or Gapdh (for use as an internal control) (Table 2) for 1 h at room temperature, followed by incubation with the secondary horseradish peroxidase (HRP)-conjugated antibodies shown in Table 2 for 1 h at room temperature. After incubation with ECL Prime detection reagent (GE Healthcare), the blots were imaged using an Omega Lum C imaging system (Gel Company, San Francisco, CA, USA).
Table 2.
Primary and secondary antibodies
| Antibody | Cat no. | Dilution | Company |
|---|---|---|---|
| Primary antibodies | |||
| Rabbit anti-Pax2 | 71-6000 | 1:100 | Thermo Fisher Scientific (Waltham, MA, USA) |
| Rabbit anti-uromodulin | sc-20631 | 1:100 for monolayer 1:50 for kidney |
Santa Cruz Biotechnology (Dallas, TX, USA) |
| Mouse anti-Atp1a1 | ab7671 | 1:50 | Abcam (Cambridge, UK) |
| Mouse anti-megalin | sc-515750 | 1:50 | Santa Cruz Biotechnology |
| Mouse anti-β-catenin | sc-7963 | 1:100 for monolayer 1:50 for spheroid |
Santa Cruz Biotechnology |
| Rat anti-E-cadherin | sc-59778 | 1:100 for monolayer 1:50 for spheroid |
Santa Cruz Biotechnology |
| Rat anti-ZO-1 | sc-33725 | 1:100 for monolayer 1:50 for spheroid |
Santa Cruz Biotechnology |
| Rabbit anti-Gapdh | ab9485 | 1:25,000 | Abcam |
| Mouse anti-α-SMA | sc-32251 | 1:10,000 | Santa Cruz Biotechnology |
| Secondary antibodies | |||
| Anti-rabbit Alexa Fluor 488-conjugated | 4412 | 1:1000 | Cell Signaling Technology (Danvers, MA, USA) |
| Anti-mouse Alexa Fluor 568-conjugated | A-11031 | 1:1000 for monolayer 1:500 for spheroid |
Thermo Fisher Scientific |
| Anti-rat Alexa Fluor 488-conjugated | 4416 | 1:1000 for monolayer 1:500 for spheroid |
Cell Signaling Technology |
| Anti-rabbit HRP-conjugated | ab6721 | 1:10,000 | Abcam |
| Anti-mouse HRP-conjugated | NA931 | 1:5000 | GE Healthcare (Chicago, IL, USA) |
Albumin uptake assay
BSA was labeled with HiLyte Fluor 555 (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s protocol. Cells on chamber slides (#154534, Thermo Fisher Scientific) were incubated with labeled albumin (equivalent to 8 µg/mL BSA) for 0, 15 and 60 min under renal epithelial growth conditions. After incubation, culture slides were washed five times with ice-cold PBS. Cells were fixed with 4% paraformaldehyde (PFA) and mounted in ProLong Diamond Antifade Mountant with DAPI (Thermo Fisher Scientific). The samples were imaged using an LSM 710 confocal microscope (Carl Zeiss, Oberkochen, Germany).
Cytotoxicity assay
Cells were incubated with cisplatin (1, 5 or 10 µM) for 24 h under renal epithelial growth conditions. Cells were harvested by collecting the supernatants and by treatment with TrypLE Express dissociation reagent, and stained with Muse Count and Viability reagent (Merck) according to manufacturer’s instructions. Cell viability was measured using a Muse Cell Analyzer (Merck).
Three-dimensional cell culture
Three-dimensional (3D) renal tubular epithelial cell culture was performed as previously described (Kaminski et al. 2016), with minor modifications. Cells were suspended in growth factor reduced Matrigel (#356230, Corning, Corning, NY, USA) after enzymatic and mechanical dissociation using TrypLE Express dissociation reagent and a 25-µm cell strainer. Aliquots of single-cell suspensions (400 cells/20 µL) were dispensed onto chamber slides with a glass coverslip bottom (#80827, Ibidi, Martinsried, Germany) and 24-well plates for immunofluorescent staining and transmission electron microscopy, respectively. The droplets were spread thinly using the tip of a micropipette, and incubated at 37 °C for 15 min to induce Matrigel polymerization. After gelation, cells were cultured under renal epithelial growth condition for 7–14 days. For immunofluorescent staining, cells were fixed in 4% PFA for 30 min at room temperature followed by 30 min at 4 °C, and incubated with 0.5% Triton X-100 in PBS (0.5% PBST) for 20 min at room temperature.
Immunofluorescent staining
Cells were washed twice in PBS at 37 °C, fixed in 4% PFA for 20 min at room temperature, and incubated with 0.3% PBST for 20 min at room temperature. For blocking non-specific binding, cells were incubated with 5% goat serum in 0.3% PBST for 1–2 h at room temperature. Kidneys from adult mice were fixed with 4% PFA at 4 °C overnight. The PFA-fixed paraffin sections (5 µm thick) were subjected to normal histological processes and antigen retrieval with sodium citrate buffer (pH 6.0). Sections were incubated with 10% goat serum in 0.3% PBST for 1 h at room temperature. After blocking, cells and sections were incubated with the primary antibodies listed in Table 2 at 4 °C overnight, followed by incubation with the secondary fluorescence-conjugated antibodies listed in Table 2 for 30–60 min at room temperature. For actin filament staining, cells were incubated with Alexa Fluor 594-conjugated phalloidin (Thermo Fisher Scientific, diluted in PBS 1:20) for 1 h at room temperature. Monolayer cells and sections were mounted in ProLong Diamond Antifade Mountant with DAPI, while spheroid cells were incubated with 1 µg/mL Hoechst 33342 (Thermo Fisher Scientific) for 30 min at room temperature and mounted in ProLong Gold Antifade Mountant (Thermo Fisher Scientific). The sample was imaged using an LSM 710 confocal microscope or an EVOS FLoid Cell Imaging Station.
Transmission electron microscopy
The spheroids were fixed by immersion in Karnovsky solution (2% glutaraldehyde, 2% PFA in 50 mM cacodylate buffer, pH 7.4) and isolated from Matrigel by cutting the gel into small pieces and pipetting strongly. After rinsing in 0.1 M phosphate buffer, the spheroids were post-fixed at room temperature in buffered 1% osmium tetroxide for 2 h. The spheroids were then dehydrated and embedded in epoxy resin. Embedded specimens were sectioned at a thickness of 60 nm using an ultramicrotome (Ultracut N, Reichert-Nissei, Vienna, Austria), and stained using uranyl acetate followed by lead citrate. Stained sections were examined using an H-7650 transmission electron microscope (Hitachi High-Technologies, Tokyo, Japan).
Statistical analysis
Dunnett’s multiple comparison test was carried out using GraphPad Prism 5 software (MDF, Tokyo, Japan), and used for comparisons among three independent groups. A p value < 0.05 was considered statistically significant.
Results
Characterization of MuRTE61 in monolayer culture
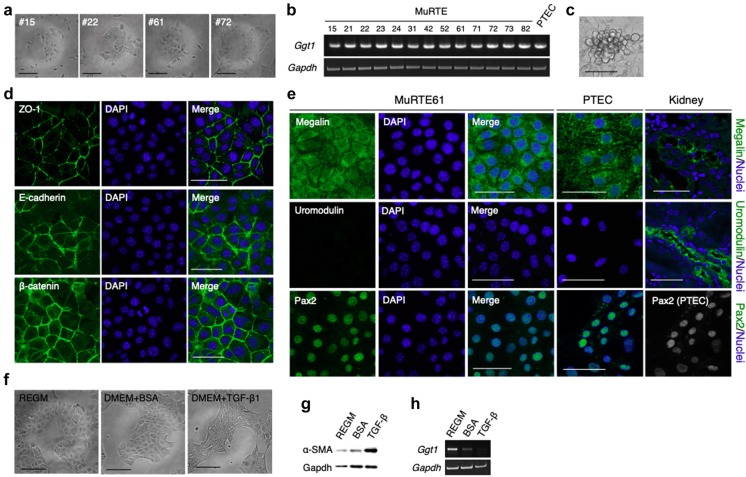
Thirteen clones, designated MuRTE cell lines, were established from the renal cortices of p53-deficient mice. MuRTE cells exhibited a rounded and slightly elongated morphology when cultured on plastic (Fig. 1a). MuRTE cells also exhibited expression of the Ggt1 gene encoding a gamma-glutamyltransferase, which is a brush border enzyme of PTEC (Fig. 1b) (Spater et al. 1982). Furthermore, when densely seeded, MuRTE61 alone exhibited a dome-like formation, which is a hallmark of cultured PTECs and is considered indicative of the vectorial active transport of ions and water (Fig. 1c) (Lever 1979; Sens et al. 1999; Kim et al. 2002). We then performed further experiments, focusing on MuRTE61 by virtue of its unique ability to form dome-like structures.
Fig. 1.
Renal proximal tubular epithelial cell type identification of MuRTE61 cells. a Relief phase contrast images of MuRTE cell lines at low density on culture plastic. Cells exhibited a rounded and slightly elongated morphology. b RT-PCR for the renal proximal tubular epithelial marker gene, Ggt1, in MuRTE cell lines. c Relief phase contrast image of the dome-like structure formed in the confluent MuRTE61 culture. d Immunofluorescent staining for epithelial cell adhesion molecules and renal tubular epithelial markers in MuRTE61 cells. e Immunofluorescent staining for megalin, uromodulin and Pax2 in MuRTE15 cells, primary cultured PTECs and kidney. Immunofluorescent staining for megalin showed specific staining of renal proximal tubules in kidney and punctate cytoplasmic staining in MuRTE61 cells and primary cultured PTECs. The thick ascending limb of loop of Henle marker, uromodulin, was not detected in MuRTE61 cells. A transcription factor Pax2 was not detected in renal cortex (data not shown) but nuclear staining of Pax2 was seen in MuRTE61 cells and primary cultured PTECs. f Relief phase contrast images of MuRTE61 before and after stimulation with 20 µg/mL BSA or 5 ng/mL TGF-β1. TGF-β1-stimulated MuRTE61s exhibited a spindle-shaped morphology. g Western blot analysis of α-SMA in cell lysates from MuRTE61 cells before and after stimulation with 20 µg/mL BSA or 5 ng/mL TGF-β1. h RT-PCR for Ggt1 in MuRTE61 cells before and after stimulation with 20 µg/mL BSA or 5 ng/mL TGF-β1. Ggt1 expression was clearly decreased in the BSA-stimulated MuRTE61 cells, or lost in TGF-β1-stimulated MuRTE61 cells. Scale bars, 100 µm (a, e) or 50 µm (c–e)
Lateral membrane localization of E-cadherin and β-catenin, which are components of epithelial adherens junction (Aberle et al. 1996), and tight junction protein ZO-1 (Umeda et al. 2006) in confluent MuRTE61 cells was confirmed by immunofluorescence staining (Fig. 1d). Immunofluorescence staining also demonstrated that MuRTE61 cells and primary cultured PTECs were positive for megalin, a multiligand endocytic receptor responsible for albumin reabsorption in PTECs (Cui et al. 1996), and Pax2, a transcription factor for kidney development or renal proximal tubular dedifferentiation (Dressler and Douglass 1992; Kusaba et al. 2014), but were negative for uromodulin, a marker for the thick ascending limb of loop of Henle (Hoyer et al. 1979) (Fig. 1e). Incubation of MuRTE61 with TGF-β1, a known inducer of epithelial–mesenchymal transition (EMT) in epithelial cells including PTECs (Xu et al. 2009; Yang and Liu 2001), induced fibroblast-like morphologic transformation (Fig. 1f), increased EMT marker α-SMA protein expression (Fig. 1g), and abolished Ggt1 mRNA expression (Fig. 1h). The transcription of Ggt1 in MuRTE61 was clearly decreased (Fig. 1h), without accompanying changes in morphology or α-SMA production (Fig. 1f, g), when exposed to an in vitro model of proteinuria (20 µg/mL albumin) (Lee et al. 2011; Hu et al. 2015). Thus, MuRTE61 showed an epithelial cell phenotype and, at least partially, a PTEC phenotype.
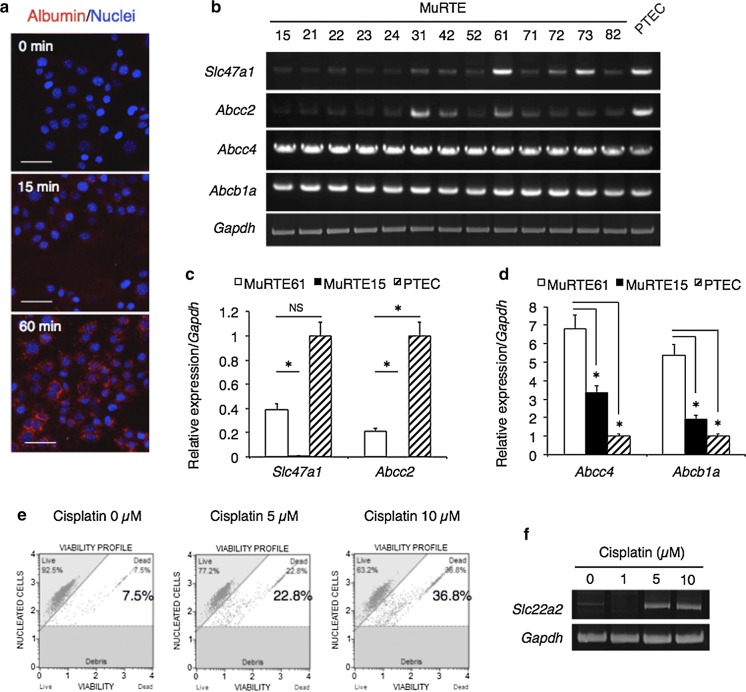
Next, we performed evaluation of the functional profile of PTECs. Consistent with the megalin expression, endocytotic activity in MuRTE61 was functionally confirmed by albumin uptake assay with fluorescent dye-labeled albumin (Fig. 2a). RT-PCR and quantitative real-time PCR analyses revealed higher or comparable expression levels of efflux transporter genes, Slc47a1 (encoding multidrug and toxin extrusion protein 1, Mate1), Abcc2 (encoding multidrug resistance-associated protein 2, Mrp2), Abcc4 (encoding Mrp4) and Abcb1a (encoding multidrug resistance protein 1A, Mdr1a), compared with those in the primary cultured PTECs (Fig. 2b–d). These transporters are located in the apical membrane of PTECs and play a role in the urinary excretion of organic cations and anions, as well as drugs (König et al. 2013). PTECs are vulnerable to toxicity due to their physiological function in mediating the excretion of metabolic waste products and xenobiotics, including toxicants (George et al. 2017). MuRTE61 cell death was increased in a dose-depending manner on treatment with the nephrotoxic agent cisplatin (Fig. 2e), which was accompanied by upregulated expression of the Slc22a2 gene encoding organic cation transporter 2 (Oct2), an uptake transporter that is located in the basolateral membrane of PTECs and is involved in cisplatin transport (Fig. 2f) (Karbach et al. 2000; Yokoo et al. 2007; Kim et al. 2015).
Fig. 2.
MuRTE61 cells show gene expression and functional properties related to renal proximal tubular epithelial cells. a Fluorescence images of MuRTE61 cells after incubation with fluorescent dye-labeled albumin for 0, 15, or 60 min. b RT-PCR for renal proximal tubular transporter genes in the MuRTE cell lines. Expression data for Gapdh used as an internal control is identical to that in Fig. 2b. c, d Relative expression level of Slc47a1, Abcc2, Abcc4 and Abcb1a in the MuRTE cell lines (MuRTE61 and MuRTE15) and the primary cultured PTECs. Asterisk, p < 0.001. NS, not significant. e Graphical representation of the viability of MuRTE61 cells after incubation with cisplatin (0, 5, or 10 µM) for 24 h. In the plots, the upper left quadrant represents live cells and the upper right quadrant represents dead cells. f RT-PCR for Slc22a2 in MuRTE61 cells after incubation with cisplatin (0, 1, 5, or 10 µM) for 24 h. Scale bars, 50 µm (a)
Characterization of MuRTE61 in the three-dimensional matrix culture
A single MuRTE61 cell showed the ability to form a spheroid with a lumen in the 3D Matrigel culture (Fig. 3a, b). In contrast, MuRTE15 and HK-2 cells did not form spheroids or proliferate in the 3D Matrigel culture (Fig. 3a, b). MuRTE61-derived spheroids exhibited apicobasal polarity and junction formation with apical/lateral border localization of ZO-1 and basolateral membrane localization of E-cadherin and β-catenin (Fig. 3c, d). The Na+/K+ ATPase alpha-1 subunit (Atp1a1), which plays a key role in active transport in renal tubule cells (Katz 1982), was also detected at the basolateral membrane in MuRTE61-derived spheroids, while the accumulation of F-actin at the apical surface was confirmed by phalloidin staining (Fig. 3c, d). Consistent with the immunostaining for ZO-1 and F-actin in the spheroids, electron-dense tight junctions and tightly packed microvilli were observed by transmission electron microscopy (Fig. 3e). Endocytic structures were observed on the apical membrane and cytoplasm of the spheroids (Fig. 3e), and open lateral space was also seen between the cells (Fig. 3e).
Fig. 3.
Three-dimensional cultured MuRTE61 cells. a Relief phase contrast and immunofluorescent images of MuRTE61 and HK-2 cells grown in Matrigel. b Percentage of spheroids with a lumen per cell grown in Matrigel; n = 5 wells. MuRTE15 and HK-2 cells did not form spheroids. Error bar represents standard deviation. ND, not detectable. c Confocal immunofluorescent images of MuRTE61-derived spheroids grown in Matrigel. d Confocal immunofluorescent images of reconstructed z-stack 3D structures of MuRTE61-derived spheroids grown in Matrigel. e Transmission electron microscopy images of MuRTE61-derived spheroids grown in Matrigel. Nu, nucleus; Mv, microvilli; TJ, tight junction; M, mitochondria; P, peroxisome; arrows, endocytic structures. Scale bars, 20 µm (a, c) or 2 µm (e)
Discussion
We established 13 MuRTE cell lines from the renal cortices of p53-deficient mice and characterized MuRTE61, the only MuRTE cell line possessing the ability to form dome-like structures, which is a hallmark of cultured PTECs and is considered indicative of vectorial active transport of ions and water (Lever 1979; Sens et al. 1999; Kim et al. 2002). MuRTE61 cells exhibited epithelial morphologies and cell–cell junctions, and underwent EMT upon TGF-β1 stimulation. MuRTE61 cells expressed high levels of efflux transporter genes related to PTEC function. Although the expression level of an uptake transporter Oct2 was low in MuRTE61 cells, its expression was highly induced in response to treatment with the nephrotoxic agent cisplatin, and was accompanied by increased cell death. It has been reported that p53 is partially responsible for cisplatin-induced nephrotoxicity (Wei et al. 2007; Jiang et al. 2009). Thus, p53 deficiency might influence the sensitivity to cisplatin by inhibiting apoptosis in MuRTE61 cell lines. However, by virtue of their ability to uptake cisplatin, the apoptotic rate of MuRTE61 cells to cisplatin was within a similar range to those of several primary cultured PTECs and reliable PTEC lines (Camano et al. 2010; Secker et al. 2017; Nieskens et al. 2018). Furthermore, MuRTE61 cells expressed a PTEC-specific endocytic receptor megalin and showed endocytic uptake activity for albumin. MuRTE61 cells also expressed a transcription factor Pax2. Pax2 is not expressed normally in renal proximal tubules but it is expressed there during regeneration after ischemia reperfusion injury (Kusaba et al. 2014) and in developing kidney (Dressler and Douglass 1992). In addition, we found that Pax2 is expressed in primary cultured PTECs. While these observations indicate that MuRTE61 cells (and even primary cultured PTECs) are not identical to PTECs, MuRTE61 cells demonstrate several key characteristics and functionally resemble PTECs.
In general, a 3D culture model improves the differentiation status of the cultured cells and more accurately imitates the in vivo cells in comparison with a monolayer culture (Antoni et al. 2015; Secker et al. 2017). As observed in the induced renal tubular epithelial cells (iRECs) as well as in several other mammalian epithelial cells (Kaminski et al. 2016), MuRTE61 cells formed spheroids with a central lumen (also called cysts) in the 3D Matrigel culture. The localization of ZO-1 (Gonzalez-Mariscal et al. 2000), E-cadherin (Marciano et al. 2011) and Atp1a1 (Kumar et al. 2015) in the MuRTE61-derived spheroids is consistent with their subcellular localization in PTECs in vivo. The apical tightly packed microvilli seen in MuRTE61-derived spheroids are found on the surface of PTECs (brush borders), but not on that of the other nephron segments (Christensen et al. 2012). The observed endocytic structures on the apical membrane and cytoplasm of the spheroids indicate endocytosis of luminal solutes, similar to proximal tubule apical endocytosis. Furthermore, the open lateral space, the apical side of which is sealed with a tight junction, seen in the spheroids is found in the S3 segment of renal proximal tubules, but not in the S1/S2 segments (Faarup et al. 2011). The scattered mitochondria seen in the spheroids are also characteristic of the S3 segment, whereas in the S1/S2 segments of renal proximal tubules they are elongated along the apicobasal axis of the cell (Zhuo and Li 2013; Christensen et al. 2012). In addition, the basolateral localization of β-catenin seen in the spheroids is unique to the S3 segment among renal proximal tubules (Prozialeck et al. 2004). Taken together, these results indicate that MuRTE61-derived spheroids could be an in vitro model for the S3 segment of proximal tubules.
The characteristics of MuRTE61 and preexisting mouse proximal tubular cell lines are summarized in Table 3 for comparison. Few preexisting cell lines have been analyzed for pleiotropic PTEC functions, while the MuRTE61 cell line was analyzed for brush border enzymes and structure, transcription factor, endocytic activity, efflux and uptake transporters and sensitivity to nephrotoxic agents. For example, TKPTS, which is the most commonly used mouse proximal tubular cell line with 22 citations, was analyzed for only Mdr1-mediated efflux using an Mdr1 inhibitor in the original article (Ernest and Bello-Reuss 1995). It is noteworthy that our results showed Abcb1a (encoding Mdr1a) gene expression was maintained in all the MuRTE cell lines compared with the primary cultured PTECs. Moreover, several studies used their own established cell lines (not listed in the table) as proximal tubular cell lines even without characterization (Kröning et al. 1999; Lee et al. 2002; L’Hoste et al. 2007; Woost et al. 2007; Takahashi et al. 2012; Adedoyin et al. 2017). This is in part because it is generally accepted that isolated nephron segments per se have their own properties; however, there is no guarantee that immortalized cultured cells retain the properties of their parent cells. This concern is particularly significant for PTECs, which rapidly dedifferentiate and lose their characteristic properties when cultured (Bens and Vandewalle 2008). In addition, we should pay attention to the genetic backgrounds of the cell lines as, for instance, the enzymatic activity of renal tubules differs between mouse strains (Tanaka-Kagawa et al. 1998).
Table 3.
Characteristics of renal proximal tubule cell lines derived from mouse
| Cell line | Origin | Genetic background | Characterisitics related to PTEC-specific function analyzed | References | |
|---|---|---|---|---|---|
| Expression/activity | Miscellaneous | ||||
| PKSV-PCT | Proximal tubule (S2) | Mixed | Alanyl aminopeptidase (Apn), Ggt1, villin | Brush border | Cartier et al. (1993) |
| PKSV-PR | Proximal tubule (S3) | Mixed | Alanyl aminopeptidase (Apn), Ggt1, villin | Brush border | Cartier et al. (1993) |
| TKPTS | Proximal tubule | C57BL/6J | Mdr1 | Mdr1-mediated efflux | Ernest and Bello-Reuss (1995) |
| PCT-VimTts | Proximal tubule (S2) | Mixed | Response of cAMP to parathyroid hormone (PTH) | (Cluzeaud et al. 1996) | |
| tsMPT | Renal cortex | Mixed | Angiotensinogen (Agt), Angiotensin converting enzyme (Ace), Ggt1 | Response of cAMP to PTH, Na+-dependent uptake of phosphate, d-glucose and amino acid | Loghman-Adham et al. (1997) |
| MuRTE61 | Renal cortex | C57BL/6N, Trp53 (−/−) | Ggt1, megalin, Mate1, Mrp2, Mrp4, Mdr1a, Oct2a | Albumin uptake, Sensitive to cisplatin-induced cytotoxicity, brush border | This study |
aUpregulated by cisplatin treatment
In conclusion, we established a comparatively reliable proximal tubular cell line from the mouse kidney. In general, reliable mouse cell lines help develop (3D) cell culture models recapitulating the in vivo system, which contributes to the accurate prediction of experimental results in mice. Furthermore, the detailed analysis of reliable mouse and human cell lines will provide an accurate extrapolation of these results to humans, and may help resolve the apparent inconsistencies between mouse and human toxicity.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Glant-in-Aid for Scientific Research, KAKENHI (16K16606) to HS and KAKENHI (25430083) to NS. The funders had no role in the design of the study and collection, analysis, decision to publish, interpretation of data or preparation of the manuscript.
Conflict of interest
The authors declare no conflict interest.
Human and animal rights
All research was conducted according to the Regulation for the Care and Use of Laboratory Animals of Kitasato University. Animal experimentation protocol was approved by President of Kitasato University through the judgment by Institutional Animal Care and Use Committee of Kitasato University (Approval ID: No. 15-168).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hayato Sasaki, Phone: +81-176-24-9496, Email: hsasaki@vmas.kitasato-u.ac.jp.
Makoto Sugiyama, Email: masugi@vmas.kitasato-u.ac.jp.
Nobuya Sasaki, Email: nobsasa@vmas.kitasato-u.ac.jp.
References
- Aberle H, Schwartz H, Kemler R. Cadherin–catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4<514::AID-JCB4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Adedoyin O, Boddu R, Traylor AM, Lever JM, Bolisetty S, George J, Agarwal A. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am J Physiol Renal Physiol. 2017;314:F702–F714. doi: 10.1152/ajprenal.00044.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015;16:5517–5527. doi: 10.3390/ijms16035517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bens M, Vandewalle A. Cell models for studying renal physiology. Pflugers Arch. 2008;457:1–15. doi: 10.1007/s00424-008-0507-4. [DOI] [PubMed] [Google Scholar]
- Brown CD, Sayer R, Windass AS, Haslam IS, De Broe ME, D’Haese PC, Verhulst A. Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol Appl Pharmacol. 2008;233:428–438. doi: 10.1016/j.taap.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Camano S, Lazaro A, Moreno-Gordaliza E, Torres AM, Lucas C, Humanes B, Milagros Gomez-Gomez LM, Bosca L, Tejedor A. Cilastatin attenuates cisplatin-induced proximal tubular cell damage. J Pharmacol Exp Ther. 2010;334:419–429. doi: 10.1124/jpet.110.165779. [DOI] [PubMed] [Google Scholar]
- Cartier N, Lacave R, Vallet V, Hagege J, Hellio R, Robine S, Pringault E, Cluzeaud F, Briand P, Kahn A. Establishment of renal proximal tubule cell lines by targeted oncogenesis in transgenic mice using the L-pyruvate kinase-SV40 (T) antigen hybrid gene. J Cell Sci. 1993;104:695–704. doi: 10.1242/jcs.104.3.695. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Wagner CA, Kaissling B. Uriniferous tubule: structural and functional organization. Compr Physiol. 2012;2:805–861. doi: 10.1002/cphy.c100073. [DOI] [PubMed] [Google Scholar]
- Cluzeaud F, Bens M, Wu MS, Li Z, Vicart P, Paulin D, Vandewalle A. Relationships between intermediate filaments and cell-specific functions in renal cell lines derived from transgenic mice harboring the temperature-sensitive T antigen. J Cell Physiol. 1996;167:22–35. doi: 10.1002/(SICI)1097-4652(199604)167:1<22::AID-JCP3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Cui S, Verroust PJ, Moestrup SK, Christensen EI. Megalin/gp330 mediates uptake of albumin in renal proximal tubule. Am J Physiol. 1996;271:F900–F907. doi: 10.1152/ajprenal.1996.271.4.F900. [DOI] [PubMed] [Google Scholar]
- Dressler GR, Douglass EC. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc Natl Acad Sci USA. 1992;89:1179–1183. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest S, Bello-Reuss E. Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol. 1995;269:C323–C333. doi: 10.1152/ajpcell.1995.269.2.C323. [DOI] [PubMed] [Google Scholar]
- Faarup P, Holstein-Rathlou NH, Nørgaard T, Harrison AP, Bastholm L, Thatt L, Johansen FF, Hegedüs V. Functionally induced changes in water transport in the proximal tubule segment of rat kidneys. Int J Nephrol Renovasc Dis. 2011;4:73–84. doi: 10.2147/IJNRD.S15459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai Z, Zhou G, Gui T, Itoh S, Oikawa K, Uetani K, Muragaki Y. Trps1 haploinsufficiency promotes renal fibrosis by increasing Arkadia expression. J Am Soc Nephrol. 2010;21:1468–1476. doi: 10.1681/ASN.2009121201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George B, You D, Joy MS, Aleksunes LM. Xenobiotic transporters and kidney injury. Adv Drug Deliv Rev. 2017;116:73–91. doi: 10.1016/j.addr.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewin LS. Renal fibrosis: primacy of the proximal tubule. Matrix Biol. 2018;68–69:248–262. doi: 10.1016/j.matbio.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S, Valencia L, Muriel P, Ponce L, Reyes JL. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int. 2000;57:2386–2402. doi: 10.1046/j.1523-1755.2000.00098.x. [DOI] [PubMed] [Google Scholar]
- Hanazono M, Tomisawa H, Tomooka Y, Hirabayashi K, Aizawa S. Establishment of uterine cell lines from p53-deficient mice. In Vitro Cell Dev Biol Anim. 1997;33:668–671. doi: 10.1007/s11626-997-0121-3. [DOI] [PubMed] [Google Scholar]
- Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer JR, Sisson SP, Vernier RL. Tamm-Horsfall glycoprotein: ultrastructural immunoperoxidase localization in rat kidney. Lab Invest. 1979;41:168–173. [PubMed] [Google Scholar]
- Hu J, Zhu Q, Li PL, Wang W, Yi F, Li N. Stem cell conditioned culture media attenuated albumin-induced epithelial–mesenchymal transition in renal tubular cells. Cell Physiol Biochem. 2015;35:1719–1728. doi: 10.1159/000373984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson SE, Chung GW, van Loon E, Bakar NS, Dalzell AM, Brown CD. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch. 2012;464:601–611. doi: 10.1007/s00424-012-1163-2. [DOI] [PubMed] [Google Scholar]
- Jiang M, Wang C-Y, Huang S, Yang T, Dong Z. Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. Am J Physiol Renal Physiol. 2009;296:F983–F993. doi: 10.1152/ajprenal.90579.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski MM, Tosic J, Kresbach C, Engel H, Klockenbusch J, Müller AL, Pichler R, Grahammer F, Kretz O, Huber TB, Walz G, Arnold SJ, Lienkamp SS. Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors. Nat Cell Biol. 2016;18:1269–1280. doi: 10.1038/ncb3437. [DOI] [PubMed] [Google Scholar]
- Karbach U, Kricke J, Meyer-Wentrup F, Gorboulev V, Volk C, Loffing-Cueni D, Kaissling B, Bachmann S, Koepsell H. Localization of organic cation transporters OCT1 and OCT2 in rat kidney. Am J Physiol Renal Physiol. 2000;279:F679–F687. doi: 10.1152/ajprenal.2000.279.4.F679. [DOI] [PubMed] [Google Scholar]
- Katz AI. Renal Na-K-ATPase: its role in tubular sodium and potassium transport. Am J Physiol. 1982;242:F207–F219. doi: 10.1152/ajpcell.1982.242.3.C207. [DOI] [PubMed] [Google Scholar]
- Kim D, Garrett SH, Sens MA, Somji S, Sens DA. Metallothionein isoform 3 and proximal tubule vectorial active transport. Kidney Int. 2002;61:464–472. doi: 10.1046/j.1523-1755.2002.00153.x. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park DJ, Kim JH, Jeong EY, Jung MH, Kim TH, Yang JI, Lee GW, Chung HJ, Chang SH. Glutamine protects against cisplatin-induced nephrotoxicity by decreasing cisplatin accumulation. J Pharmacol Sci. 2015;127:117–126. doi: 10.1016/j.jphs.2014.11.009. [DOI] [PubMed] [Google Scholar]
- König J, Müller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65:944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- Kröning R, Katz D, Lichtenstein AK, Nagami GT. Differential effects of cisplatin in proximal and distal renal tubule epithelial cell lines. Br J Cancer. 1999;79:293–299. doi: 10.1038/sj.bjc.6690047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Liu J, Pang P, Krautzberger AM, Reginensi A, Akiyama H, Schedl A, Humphreys BD, McMahon AP. Sox9 activation highlights a cellular pathway of renal repair in the acutely injured mammalian kidney. Cell Rep. 2015;12:1325–1338. doi: 10.1016/j.celrep.2015.07.034. [DOI] [PubMed] [Google Scholar]
- Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA. 2014;111:1527–1532. doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WK, Jang SB, Cha SH, Lee JH, Lee KH, Kim J, Jo YH, Endou H. Different sensitivity to nephrotoxic agents and osmotic stress in proximal tubular and collecting duct cell lines derived from transgenic mice. Toxicol In Vitro. 2002;16:55–62. doi: 10.1016/S0887-2333(01)00097-2. [DOI] [PubMed] [Google Scholar]
- Lee JY, Chang JW, Yang WS, Kim SB, Park SK, Park JS, Lee SK. Albumin-induced epithelial–mesenchymal transition and ER stress are regulated through a common ROS-c-Src kinase-mTOR pathway: effect of imatinib mesylate. Am J Physiol Renal Physiol. 2011;300:F1214–F1222. doi: 10.1152/ajprenal.00710.2010. [DOI] [PubMed] [Google Scholar]
- Lever JE. Inducers of mammalian cell differentiation stimulate dome formation in a differentiated kidney epithelial cell line (MDCK) Proc Natl Acad Sci USA. 1979;76:1323–1327. doi: 10.1073/pnas.76.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hoste S, Poet M, Duranton C, Belfodil R, é Barriere H, Rubera I, Tauc M, Poujeol C, Barhanin J, Poujeol P. Role of TASK2 in the control of apoptotic volume decrease in proximal kidney cells. J Biol Chem. 2007;282:36692–36703. doi: 10.1074/jbc.M703933200. [DOI] [PubMed] [Google Scholar]
- Li W, Choy DF, Lam MS, Morgan T, Sullivan ME, Post JM. Use of cultured cells of kidney origin to assess specific cytotoxic effects of nephrotoxins. Toxicol In Vitro. 2003;17:107–113. doi: 10.1016/S0887-2333(02)00128-5. [DOI] [PubMed] [Google Scholar]
- Li S, Zhao J, Huang R, Steiner T, Bourner M, Mitchell M, Thompson DC, Zhao B, Xia M. Development and application of human renal proximal tubule epithelial cells for assessment of compound toxicity. Curr Chem Genom Transl Med. 2017;11:19–30. doi: 10.2174/2213988501711010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loghman-Adham M, Rohrwasser A, Helin C, Zhang S, Terreros D, Inoue I, Lalouel JM. A conditionally immortalized cell line from murine proximal tubule. Kidney Int. 1997;52:229–239. doi: 10.1038/ki.1997.325. [DOI] [PubMed] [Google Scholar]
- Marciano DK, Brakeman PR, Lee CZ, Spivak N, Eastburn DJ, Bryant DM, Beaudoin GM, Hofmann I, Mostov KE, Reichardt LF. p120 catenin is required for normal renal tubulogenesis and glomerulogenesis. Development. 2011;138:2099–2109. doi: 10.1242/dev.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieskens TTG, Peters JGP, Dabaghie D, Korte D, Jansen K, Van Asbeck AH, Tavraz NN, Friedrich T, Russel FGM, Masereeuw R, Wilmer MJ. Expression of organic anion transporter 1 or 3 in human kidney proximal tubule cells reduces cisplatin sensitivity. Drug Metab Dispos. 2018;46:592–599. doi: 10.1124/dmd.117.079384. [DOI] [PubMed] [Google Scholar]
- Phillips AO. The role of renal proximal tubular cells in diabetic nephropathy. Curr Diab Rep. 2003;3:491–496. doi: 10.1007/s11892-003-0013-1. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC, Appelt DM. Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol. 2004;4:10. doi: 10.1186/1472-6793-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secker PF, Luks L, Schlichenmaier N, Dietrich DR. RPTEC/TERT1 cells form highly differentiated tubules when cultured in a 3D matrix. Altex. 2017;35:223–234. doi: 10.14573/altex.1710181. [DOI] [PubMed] [Google Scholar]
- Sens DA, Detrisac CJ, Sens MA, Rossi MR, Wenger SL, Todd JH. Tissue culture of human renal epithelial cells using a defined serum-free growth formulation. Exp Nephrol. 1999;7:344–352. doi: 10.1159/000020632. [DOI] [PubMed] [Google Scholar]
- Spater HW, Poruchynsky MS, Quintana N, Inoue M, Novikoff AB. Immunocytochemical localization of gamma-glutamyltransferase in rat kidney with protein A-horseradish peroxidase. Proc Natl Acad Sci USA. 1982;79:3547–3550. doi: 10.1073/pnas.79.11.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Kimura T, Takabatake Y, Namba T, Kaimori J, Kitamura H, Matsui I, Niimura F, Matsusaka T, Fujita N, Yoshimori T, Isaka Y, Rakugi H. Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol. 2012;180:517–525. doi: 10.1016/j.ajpath.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Tanaka-Kagawa T, Suzuki M, Naganuma A, Yamanaka N, Imura N. Strain difference in sensitivity of mice to renal toxicity of inorganic mercury. J Pharmacol Exp Ther. 1998;285:335–341. [PubMed] [Google Scholar]
- Tsukada T, Tomooka Y, Takai S, Ueda Y, Nishikawa S, Yagi T, Tokunaga T, Takeda N, Suda Y, Abe S. Enhanced proliferative potential in culture of cells from p53-deficient mice. Oncogene. 1993;8:3313–3322. [PubMed] [Google Scholar]
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol. 2007;293:1282–1291. doi: 10.1152/ajprenal.00230.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser M, Stadler G, Jennings P, Streubel B, Pfaller W, Ambros P, Riedl C, Katinger H, Grillari J, Grillari-Voglauer R. hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am J Physiol Renal Physiol. 2008;295:F1365–F1375. doi: 10.1152/ajprenal.90405.2008. [DOI] [PubMed] [Google Scholar]
- Wilmer MJ, Saleem MA, Masereeuw R, Ni L, van der Velden TJ, Russel FG, Mathieson PW, Monnens LA, van den Heuvel LP, Levtchenko EN. Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res. 2010;339:449–457. doi: 10.1007/s00441-009-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woost PG, Kolb RJ, Chang CH, Finesilver M, Inagami T, Hopfer U. Development of an AT2-deficient proximal tubule cell line for transport studies. In Vitro Cell Dev Biol Anim. 2007;43:352–360. doi: 10.1007/s11626-007-9061-1. [DOI] [PubMed] [Google Scholar]
- Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol. 2007;74:477–487. doi: 10.1016/j.bcp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Zhuo JL, Li XC. Proximal nephron. Compr Physiol. 2013;3:1079–1123. doi: 10.1002/cphy.c110061. [DOI] [PMC free article] [PubMed] [Google Scholar]