Abstract
Background:
Bronchoscopic lung cryobiopsy (BLC) is a novel technique for obtaining lung tissue for the diagnosis of diffuse parenchymal lung diseases. The procedure is performed using several different variations of technique, resulting in an inconsistent diagnostic yield and a variable risk of complications. There is an unmet need for standardization of the technical aspects of BLC.
Methodology:
This is a position statement framed by a group comprising experts from the fields of pulmonary medicine, thoracic surgery, pathology, and radiology under the aegis of the Indian Association for Bronchology. Sixteen questions on various technical aspects of BLC were framed. A literature search was conducted using PubMed and EMBASE databases. The expert group discussed the available evidence relevant to each question through e-mail and a face-to-face meeting, and arrived at a consensus.
Results:
The experts agreed that patients should be carefully selected for BLC after weighing the risks and benefits of the procedure. Where appropriate, consideration should be given to perform alternate procedures such as conventional transbronchial biopsy or subject the patient directly to a surgical lung biopsy. The procedure is best performed after placement of an artificial airway under sedation/general anesthesia. Fluoroscopic guidance and occlusion balloon should be utilized for positioning the cryoprobe to reduce the risk of pneumothorax and bleeding, respectively. At least four tissue specimens (with at least two of adequate size, i.e., ≥5 mm) should be obtained during the procedure from different lobes or different segments of a lobe. The histopathological findings of BLC should be interpreted by an experienced pulmonary pathologist. The final diagnosis should be made after a multidisciplinary discussion. Finally, there is a need for structured training for performing BLC.
Conclusion:
This position statement is an attempt to provide practical recommendations for the performance of BLC in DPLDs.
KEY WORDS: Bronchoscopy, hypersensitivity pneumonitis, idiopathic pulmonary fibrosis, interstitial lung disease, interventional pulmonology, lung biopsy, sarcoidosis, video-assisted thoracoscopic surgery
INTRODUCTION
Diffuse parenchymal lung diseases (DPLDs) or interstitial lung diseases (ILDs) are a heterogeneous group of disorders characterized by varying degrees of inflammation and fibrosis of the lung parenchyma.[1] A combination of clinical, radiological, and histological features is required to make a diagnosis of DPLD during a multidisciplinary discussion (MDD).[2,3,4] Bronchoalveolar lavage and conventional transbronchial lung biopsy (TBLB) are minimally invasive techniques for obtaining cytological/histopathological samples in DPLDs.[5,6,7,8] However, both are associated with a low accuracy in the diagnosis of most DPLDs.[5,6,7,8,9,10,11] Surgical lung biopsy (SLB) is considered the reference standard for providing lung tissue for the histologic examination in DPLD.[1] However, SLB is fraught with several limitations. It is an invasive procedure with significant mortality (pooled mortality of 3.6%) and a generally high (pooled yield, 95%) yet variable diagnostic yield (42%–100%).[12] Importantly, expertise in SLB, especially video-assisted thoracoscopic surgery (VATS), may not be available, particularly in resource-constrained settings.[13]
Bronchoscopic lung cryobiopsy (BLC) is a novel bronchoscopic technique of obtaining lung biopsy [Figure 1].[14,15] It involves the bronchoscopic placement of a flexible cryoprobe inside the lung parenchyma, freezing the probe and shearing out the lung tissue frozen around the tip. BLC yields tissue specimen larger than the conventional TBLB, resulting in a significantly higher diagnostic yield.[16] However, there is a caveat. There is no prospective, randomized trial comparing BLC with conventional TBLB performed with a large (7.3 mm cusp), fenestrated, alligator forceps. Most studies have used small (5 mm) nonfenestrated, cup forceps to obtain TBLB which may have spuriously led to a lower yield compared to BLC.[16] If BLC is not available, in selected cases, a conventional TBLB using a large alligator, fenestrated forceps may be performed.
Figure 1.

Classic features of usual interstitial pneumonia in a bronchoscopic lung cryobiopsy specimen. Single specimen highlighting the spatial and temporal heterogeneity of usual interstitial pneumonia (normal alveolar paces [arrow] and an area of collagenous fibrosis [open arrow]) (H and E, ×40). Inset shows fibroblastic foci (H and E, ×200)
Several centers across the world have reported the yield and safety of BLC in patients with DPLD.[17,18,19,20,21] However, there is no uniformity in the procedural aspects of BLC, which partially explains the varying yield and complications of the procedure. Several centers in India have also started performing this procedure with different methods.[22] Standardizing the method for performing BLC will ensure that the procedure is performed safely and effectively.[23] To address this unmet need, the Indian Association for Bronchology formed an expert group from the specialties of pulmonary medicine, thoracic surgery, radiology, and pathology from different parts of the country. This article is a position statement describing the technical aspects of BLC in the diagnosis of DPLDs.
METHODOLOGY
A systematic search of PubMed and EMBASE databases was performed for articles describing the use of BLC in DPLD. The relevant questions concerning different aspects of BLC were identified and circulated by e-mail among the members of the expert group. The questions were refined according to the suggestions of the experts. Next, the evidence relevant to each question was assimilated. The key points of evidence available with regard to each question were circulated. The suggestions of the members were incorporated after discussion in the e-mail group, and draft recommendations were framed. Finally, a face-to-face meeting was held (February 9, 2018; Coimbatore) where the evidence related to various questions was discussed; the draft recommendations were modified accordingly. A level of evidence and grade of recommendation was assigned to each recommendation according to a modified GRADE system [Table 1].[24] The final draft of the document was circulated among all expert members for suggestions and comments.
Table 1.
Categorization of level of evidence and grading of recommendation

What are the indications of bronchoscopic lung cryobiopsy in diffuse parenchymal lung diseases? How is bronchoscopic lung cryobiopsy positioned vis-a-vis other procedures for the diagnosis of common diffuse parenchymal lung disease?
The indications of BLC are similar to those for SLB, namely the failure to arrive at a confident diagnosis of a specific subtype of the DPLD after a detailed history, physical examination, and laboratory and radiological investigations.[1] In a meta-analysis, the pooled diagnostic yields of BLC and VATS lung biopsy in suspected DPLDs were 83.7% and 92.7%, respectively.[25] The 30–60-day mortality after BLC and VATS was 0.7% and 1.8%, respectively. In another study comparing BLC and VATS lung biopsy, the median hospitalization times were 2.6 days versus 6.1 days, respectively. Thus, BLC may have a lower yield compared to SLB but possibly has a better safety profile with shorter hospitalization. However, the yield and safety of both SLB and BLC may vary between centers.[12,26,27] Importantly, the complications related to BLC are immediate while those related to SLB can be delayed and may be attributed to the underlying DPLD.[28]
The major DPLDs include sarcoidosis, idiopathic pulmonary fibrosis (IPF), connective tissue disease (CTD)-associated ILDs, hypersensitivity pneumonitis (HP), and non-IPF idiopathic interstitial pneumonias.[29,30] The indication of lung biopsy and the choice between procedures for the commonly suspected DPLD subtypes are summarized in Table 2.[31,32,33,34,35,36,37,38] For all patients, in whom BLC is nondiagnostic, SLB is indicated for a histopathological diagnosis after weighing the risks and benefits of that approach.
Table 2.
The indication of lung biopsy and the choice of procedures for the major subtypes of diffuse parenchymal lung diseases suspected on clinical and radiological findings
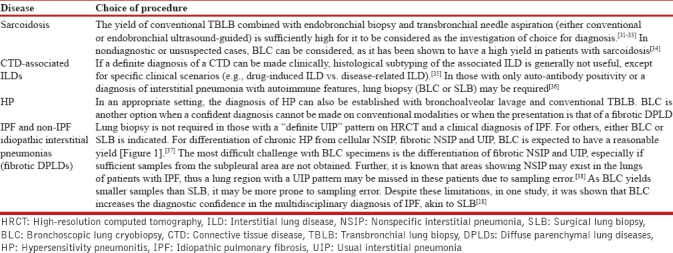
Recommendations
We recommend that BLC should be considered in all patients with DPLDs, where information obtained from clinical and radiological characteristics is not enough to arrive at a confident diagnosis of a specific subtype of the DPLD (2A)
We suggest that for the differentiation between fibrotic nonspecific interstitial pneumonia (NSIP) and IPF, either BLC or SLB may be performed after carefully weighing the risks and benefits of the two procedures (UPP).
What are the investigations required before performing bronchoscopic lung cryobiopsy?
The investigations required before performing BLC are as follows:[15,39,40]
-
Essential
- Computed tomography (CT) of the chest
- Complete blood count and coagulation profile
- Serum creatinine
- Spirometry and pulse oximetry
- Electrocardiogram and echocardiography
-
Desirable
- Diffusion capacity of carbon monoxide (DLCO)
- Arterial blood gases
- Serology against human immunodeficiency virus and hepatitis B and C viruses.
Recommendations
We recommend that the following investigations should be obtained before performing BLC in all patients: high-resolution CT chest, complete blood counts, clotting profile, serum creatinine, spirometry, pulse oximetry, electrocardiogram, and echocardiography (UPP)
We suggest that the following investigations may preferably be obtained in most patients planned for BLC: DLCO, arterial blood gases, and serology against human immunodeficiency virus and hepatitis B and C viruses (UPP).
What are the contraindications of bronchoscopic lung cryobiopsy?
The contraindications for BLC can be broadly divided into absolute and relative [Table 3].[15,39,40] Subjects who are on antiplatelet or anticoagulation therapy should stop the drugs as per the standard guidelines [Table 4].[41,42]
Table 3.
Contraindications for performing bronchoscopic lung cryobiopsy

Table 4.
Standard recommendations for peri-procedure use of anticoagulant and antiplatelet agents

Recommendations
We recommend that the following conditions be considered as absolute contraindications for BLC: American Society of Anesthesiologists category 4–6; hemodynamic instability; pulmonary hypertension; uncorrected bleeding diathesis; significant hypoxemia; pregnancy; cystic DPLDs (UPP)
We suggest that the following be considered as relative contraindications for BLC: hemoglobin <8 g/dL; reduced lung function; DLCO <30% of predicted obesity (UPP)
We recommend that any antiplatelet and anticoagulant medications be stopped as per the standard guidelines (UPP).
Should bronchoscopic lung cryobiopsy be performed with an artificial airway in place or without a protected airway? Which is the preferred type of artificial airway during the performance of bronchoscopic lung cryobiopsy: Endotracheal tube, laryngeal mask airway, or rigid bronchoscope?
No head-to-head study has compared the outcomes of BLC with or without the placement of an artificial airway (AA). Most studies describe the use of an endotracheal tube (ET), laryngeal mask airway (LMA), or the rigid bronchoscope [Table 5]. There are, however, a few studies that have described BLC without an AA.[17,45,46,47,54] Although the yield of BLC in these studies (66%–98%)[17,45] is similar to that with the use of AA (51%–98%),[26,49] certain aspects need consideration.First, the use of an AA allows rapid re-entry into the airway after performing the biopsy without having to renavigate through the nasal or oral cavity and the vocal cords.[44] Second, it also obviates the risk of the frozen distal end of the cryoprobe getting adhered to the upper tracheal wall or the vocal cords at the time of withdrawal. Third, the use of an ET or rigid bronchoscope allows isolation and ventilation of the contralateral lung, in the event of significant bleeding.[19] Fourth, if an AA is not used, the patient has to be maintained in a lighter plane of anesthesia that may be associated with vigorous coughing, thus increasing the chance of slippage of the occlusion balloon (OB).[58]
Table 5.
Studies describing the use of bronchoscopic lung cryobiopsy and their technical aspects in the diagnosis of diffuse parenchymal lung diseases
Only a few studies conducted by expert operators at high-volume centers have described BLC without the use of an AA.[17,45,54] As this technique gets adopted at less experienced centers, the risks of performing the procedure without an AA may become more evident. A case series has described the occurrence of difficult-to-control bleeding when AA was not used, which prompted the operators to switch to rigid bronchoscopy for performing BLC.[59] Thus, the importance of the use of an AA during BLC cannot be overemphasized.
There is no direct comparison between the different types of AA. In contrast to LMA, both ET and rigid bronchoscope offer the opportunity of lung isolation in the event of a massive bleed. Also, when compared to an ET, rigid bronchoscope offers a larger lumen, provides a better control and maneuverability inside the airway, and facilitates the placement of the OB inside the lobar/segmental bronchus.
Recommendations
We recommend that an AA be used while performing BLC (2A)
We suggest that an ET or rigid bronchoscope may be preferred over LMA (UPP).
What type of sedation/anesthesia should be used for performing bronchoscopic lung cryobiopsy?
No study has investigated the effect of different levels of sedation on the yield and safety of BLC. There appears no difference in the diagnostic yield between studies using conscious sedation (66%–98%)[17,45] or deep sedation/general anesthesia (GA) (51%–98%).[26,49] The rate of complications was also similar in studies, independent of the level of sedation, or the use of GA. The choice of the depth of sedation (conscious/moderate sedation, deep sedation, or GA) mainly depends on the type of AA used. Conscious/moderate sedation has been used in the absence of an AA.[45] Any level of sedation (moderate/deep/GA) can be used with ET/LMA, while rigid bronchoscopy is uniformly performed under deep sedation/GA.[18,20,26,44,56]
Recommendations
We recommend that BLC should be performed under either moderate (or conscious) sedation, deep sedation, or GA (2A)
We recommend that if rigid bronchoscopy is used, deep sedation or GA should be employed (2A).
Should an occlusion balloon be routinely used during bronchoscopic lung cryobiopsy?
Several studies have described the prophylactic placement of an OB in the segmental or lobar bronchus.[15,39,48] The OB is inflated immediately after performing each biopsy to avoid spillage of blood to other parts of the airway, in case massive bleeding occurs. Further, the tamponade effect produced by the OB helps in controlling the bleeding. Different types of OBs have been used in various studies: Balloon occlusion catheter (Fogarty; Edwards Lifesciences, Irvine, CA, USA), endobronchial blocker (Arndt; Cook Medical, Bloomington, IN, USA), and percutaneous transluminal angioplasty balloon catheter (ATB Advance, Cook Medical).[15,19,49] The usual size of the OB used in bronchial blockade is 5–7 Fr.
In studies describing the use of OB, the incidence of bleeding has ranged from 1.4% to 30%.[15,26,48] Higher bleeding rates have been reported in studies that have not described the use of OB (up to 78% moderate-to-severe bleeding).[20,50,51] Massive bleeding after biopsy entails the risk of immediate hypoxemia and asphyxia, especially if it floods the contralateral bronchial tree. It may also trigger an acute exacerbation of the underlying ILD, resulting in the need for prolonged ventilation.[22]
The use of an OB reduced the risk of moderate-to-severe bleeding (bleeding incidence, 1.8% vs. 35.7%; adjusted odds ratio [OR], [95% confidence interval (CI)]; 0.02 [0.001–0.18]) in one study.[22] Further, two reports have described the introduction of the prophylactic use of OB into their procedural protocol after encountering serious bleeding.[19,59] It is important to ensure the correct placement of the balloon and prevent its displacement and rupture while performing BLC. There is a theoretically higher chance of the balloon getting damaged when placed adjacent to the probe in the segmental bronchus compared to its placement in the lobar bronchus.
Recommendation
We recommend that an OB should be used while performing BLC (2A).
Should fluoroscopy be used for guiding bronchoscopic lung cryobiopsy?
All but three published studies on BLC have reported the use of fluoroscopy during the procedure [Table 4].[22,27,54] Fluoroscopy is used to facilitate the appropriate placement of the cryoprobe tip before performing BLC. This is important because of several reasons. If the probe is blindly advanced up to the pleura, it may increase the risk of pneumothorax. A retrospective study found a reduced risk of pneumothorax with the use of fluoroscopy (pneumothorax incidence, 5.9% vs. 20.9%) (adjusted OR [95% CI], 0.26 [0.07–0.94]).[22] There are several other advantages of using fluoroscopy during BLC. The appropriate placement of cryoprobe “closer to” the periphery is essential for sampling the subpleural parenchyma, which is especially useful in the differentiation of IPF and fibrotic NSIP. In such a situation, when an attempt is made to sample the subpleural parenchyma, the cryobiopsy specimen would contain fragments of pleura. However, the presence of pleura does not necessarily mean that the patient will develop a pneumothorax, although the risk is higher.[15,28,57] Thirdly, inadvertent proximal sampling in the absence of fluoroscopy may lead to disruption of major bronchial vessels, resulting in significant bleeding. Finally, fluoroscopic guidance may help in screening for pneumothorax immediately after each biopsy.
The positioning of cryoprobe is a trade-off between the risk of pneumothorax, on the one hand (the probe placed too close to the pleura), and the increased risk of bleeding along with the chances of potentially missing the subpleural lung parenchyma, on the other (when the probe is placed too proximally). A large study (n = 297) with a large proportion (39%) of cases diagnosed with usual interstitial pneumonia (UIP)/NSIP (where peripheral lung sampling is most important) has described the placement of cryoprobe about 10 mm away.[53] The study reported a diagnostic yield of 83% with 20% of subjects having a pneumothorax. Probe placement 15–20 mm from the pleura, described in another study, may be associated with a lesser incidence of pneumothorax (3%) but may potentially miss out on subpleural sampling (diagnostic yield, 68%) and may be associated with an increased bleeding risk (4%).[19]
Recommendations
We recommend that fluoroscopic guidance should be used during the performance of BLC, wherever available (2A)
We suggest that the cryoprobe may be placed about 10 mm from the pleura for performing BLC (3B).
What is the size of the cryoprobe that should be used for bronchoscopic lung cryobiopsy?
Either a 1.9-mm or 2.4-mm cryoprobe has been used in different studies of BLC. There is no human study that has compared the yield and safety of these two probes. In an in vitro porcine model, the 2.4-mm cryoprobe yielded a larger sample compared to the 1.9-mm probe if the same activation time was used.[60] The diameter of the biopsy was similar with an activation time of 3 s for the 2.4-mm probe and 5 s for the 1.9-mm probe. However, appropriate placement of the 2.4-mm probe may be more difficult as it may get obstructed by a bronchial bifurcation while being advanced into the pulmonary parenchyma.[23] There is also a higher risk of bleeding due to the rupture of larger bronchial vessels due to an inadvertent proximal placement (in the absence of fluoroscopy).[28] It may be difficult to advance the larger probe into the lung periphery, especially in obese individuals,[28] and in those with extensive lung fibrosis. In view of these practical concerns, pending comparative studies of safety and yield between these probes, the 1.9-mm probe appears preferable.
Recommendations
We suggest that a 1.9-mm probe may be preferred over the 2.4-mm probe for performing BLC (UPP).
Which cryogen and what activation/freezing time should be used for bronchoscopic lung cryobiopsy?
Two different cryogens, i.e., nitrous oxide and carbon dioxide, have been used for BLC. Both the gases reach temperatures below −50°C that is sufficient for BLC.[61] Carbon dioxide may be less expensive and more readily available.[62] Almost all studies on BLC have described the freezing time from 3 to 6 s. There is no comparative human study that compares different freezing/probe activation times. In an in vivo porcine model study (using carbon dioxide as cryogen), no difference in the histological accessibility of specimen or risk of bleeding was observed between shorter (2–4 s) and longer (4–6 s) freezing times with the 1.9-mm probe.[63] In another in vivo animal study in sheep, increasing freezing time led to an increase in the BLC cross-sectional area (6.5, 7.1, 9.0, and 15.7 mm2 with 3, 4, 5, and 6 s, respectively).[64] However, the risk of bleeding and pneumothorax was higher with a 5–6 s freezing time (the cryogen used was carbon dioxide).[64] Further, the use of a 5-s activation time with a carbon dioxide probe resulted in excessive resistance encountered during withdrawal of the probe.[40] As nitrous oxide achieves a lower minimum temperature (−89°C) compared to carbon dioxide (−79°C), cooling occurs faster, with a shorter freezing time.[65,66]
Recommendations
We suggest that a freezing time of 3 s with nitrous oxide cryogen (or 4 s with carbon dioxide cryogen) may be used initially (UPP)
We suggest that if a specimen of adequate size (described later) is not obtained with this activation time, the freezing time may be increased up to 6 s (UPP).
How many specimens should be obtained?
Studies on BLC have described obtaining 1–6 specimens.[15,52] A recent study evaluated the cumulative yield of increasing number of biopsies and reported a yield of 96% when two biopsies were obtained from different segments.[40] However, this study was performed at a highly experienced center using rigid bronchoscopy, deep sedation, a 2.4-mm probe (freezing time, 5 s), Fogarty balloon, and fluoroscopy. The size of the first and second biopsies was 37.8 and 28.1 mm2, respectively. Such a result may not be achievable at all centers. A retrospective study found higher diagnostic yields with increasing number of biopsies (adjusted OR [95% CI], 2.17 [1.29–3.67]).[22] In this study, the diagnostic yield was 90.7% with four or more specimen compared to 75.3% with 1–3 specimens (unpublished data, P = 0.055). There is theoretically an increased risk of complications with increasing number of biopsies.
Recommendations
We recommend that at least four tissue specimens (with at least two of adequate size [see later]) be obtained during BLC (3A).
Which lobes should be selected for taking the tissue sample during bronchoscopic lung cryobiopsy? How many lobes and segments should be sampled?
The conventional practice with SLB is to sample at least two different lobes.[11] This is because discordant histopathologic features may be found in different lung areas (e.g., patients with UIP can show areas of NSIP in a significant proportion of cases).[67] Sampling of two lobes was also found to increase the diagnostic yield of BLC in one study (91% with two lobes vs. 73% with one lobe), while it did not affect the yield in another.[22,56] However, in both the studies, the number of subjects in whom two lobes were sampled was small.
Although it would be ideal to obtain BLC from two different lobes, it may not be always feasible. Certain points merit consideration. The lower lobe is generally preferred for BLC because of the technical ease and the lower lobar predominance of abnormalities in most DPLDs. Further, the dependent position of the lower lobe ensures that even if bleeding occurs, it remains confined to the same lobe. Sampling the middle lobe may be associated with a higher chance of pneumothorax as the bronchi in this lobe may be directed toward the oblique fissure.[68] Although debatable, biopsies from the middle lobe and lingula may yield nonspecific interstitial changes.[69,70,71,72] Finally, sampling the upper lobe may be technically difficult, with an increased risk of slippage of the OB and consequent increased risk of bleeding. If a single lobe is sampled, two BLC samples taken from the same segment result in a lower yield (78%) than two samples taken from different segments of the same lobe (96%).[40]
Recommendations
We suggest that it is preferable to obtain samples from two lobes (UPP)
We recommend that BLC samples should be obtained from different segments of the lower lobe, if a single lobe is being sampled (2A)
We suggest that upper lobe sampling may be attempted after gaining sufficient experience in the technique (UPP).
What are the complications of bronchoscopic lung cryobiopsy?
The complications of BLC include pneumothorax (pooled estimate [95% CI], 9.5% [5.9%–14.9%], moderate-severe endobronchial bleeding (pooled estimate [95% CI], 4.9% [2.2%–10.7%]), and acute exacerbation of the underlying ILD, need for prolonged mechanical ventilation, prolonged hypoxemia, postprocedure infection/fever, and death (0.7% [0.4%–1.2%]).[25,53,73,74] Other uncommon complications include pneumomediastinum and subcutaneous emphysema.[75] The management of pneumothorax includes observation, oxygen therapy, single time aspiration, or intercostal drainage, according to the size of the pneumothorax and the patient's symptoms.[76] The management of endobronchial bleeding includes suction, instillation of ice-cold saline, and/or epinephrine, and prolonged (>3 min) inflation of the OB to block the biopsied segment/lobe.[77] If these measures fail, intubation of the contralateral side with the rigid bronchoscope or endotracheal tube to isolate the bleeding lung may be required.[78] Patients may also develop acute exacerbation of the underlying ILD either due to BLC per se or due to the occurrence of airway bleeding.[22]
What investigations and monitoring are required in the postprocedure period? Can bronchoscopic lung cryobiopsy be performed as a daycare procedure or hospitalization is required?
Chest radiograph is required to detect a pneumothorax in the postoperative period (generally 2–3 h after the procedure).[17,57,79] Chest ultrasound performed by trained pulmonologists for the detection of pneumothorax following BLC has a sensitivity and specificity of 90% and 94%, respectively,[79] and is superior to a chest radiograph.[80] In a meta-analysis, chest ultrasonography had a pooled sensitivity and specificity of 78.6% (95% CI, 68.1–98.1) and 98.4% (95% CI, 97.3–99.5), respectively, for the detection of pneumothorax; while the sensitivity and specificity of chest radiography was 39.8% (95% CI, 29.4–50.3) and 99.3% (95% CI, 98.4–100), respectively.[81] The working group acknowledged that chest ultrasound performed by trained operators, although more sensitive, may be difficult to obtain at all centers. Hence, a chest radiograph is a good alternative especially when performed on two separate occasions.[82] For screening immediately after the procedure, fluoroscopy itself may be utilized.
If the BLC procedure is uncomplicated (no or mild bleeding, no postprocedure hypoxemia, and the absence of pneumothorax), the patient can be discharged after 4–6 h.[20,54,56,57] A follow-up by a phone call at 24 h has been described in one study.[54] In case of a complication (moderate-severe bleeding, intra- or post-procedure hypoxemia, pneumothorax), the patient requires a longer observation with monitoring of vital parameters and appropriate management. As complications of BLC can be delayed, easy access to health services is advisable even after discharge.
Recommendations
We recommend that a chest radiograph (or fluoroscopic screening) or a thoracic ultrasound should be performed immediately after the procedure (2A). Either of them may be repeated 2 h after the procedure for the detection of pneumothorax
We suggest that in case of an uncomplicated procedure, the patient can be discharged after 6 h of observation (3B)
We recommend that in case of a complicated procedure, the patient should be managed in the hospital for as long as is required (UPP).
What is the minimum size of bronchoscopic lung cryobiopsy required for histological analysis? How should the bronchoscopic lung cryobiopsy histopathology be interpreted and reported?
The size of the biopsy obtained may influence the diagnostic yield. In a study on fibrosing DPLDs, diagnostic cryobiopsies had a larger total size than nondiagnostic biopsies (mean area ± standard deviation, 42.0 ± 14.7 mm2 vs. 28.4 ± 11.7 mm2).[15] Thus, in general, a total surface area of ≥40 mm2 should be targeted. Visually adequate BLC specimens are generally 5 mm or more in diameter, much bigger than the 1–3 mm diameter specimens obtained with conventional TBLB.[16] A 5-mm diameter corresponds to a surface area of about 20 mm2. Thus, two such specimens would represent a total surface area of 40 mm2. A large biopsy sample helps in the identification of different histological features (e.g., patchy fibrosis, honeycombing, and fibroblastic foci typical of a usual interstitial pneumonia pattern) in the same specimen [Figure 1]. In other cases, the different patterns may be identified in different biopsy samples [Figure 2]. It is essential that BLC specimens are reported by a pathologist with experience in DPLDs. Reporting is best done in a systematic way so that interpretation can be made with greater confidence [Table 6].
Figure 2.

Usual interstitial pneumonia was diagnosed after considering the findings in multiple bronchoscopic lung cryobiopsy specimens (a). The individual specimens showed normal lung parenchyma and fibrous scarring (b), honeycombing (c), and fibroblastic foci (d) (H and E, ×10, ×40, ×100, and × 200, respectively)
Table 6.
Preferred format for reporting the histopathological characteristics of bronchoscopic lung cryobiopsy specimens

Certain characteristics of the biopsy specimen should also be routinely reported [Table 6]. The maximum dimension (diameter/length) and a rough estimate of the number of alveoli in each specimen (>100 is generally considered adequate) are important parameters for judging the adequacy of specimen.[83] The presence of a sufficient number of terminal bronchioles is important if airway-centric pathology (such as bronchiolitis or HP) is a consideration [Figure 3]. The presence of larger airways, cartilage, or large vessels represents proximal sampling and can provide important feedback to the bronchoscopist. The presence of pleura represents subpleural sampling, which may be especially useful in diagnosing IPF. Freezing artifacts are rare in contrast to their frequent occurrence with surgical frozen sections.[84] Artifacts in the form of implantation of bronchiolar epithelium in the alveolar tissue and intra-alveolar hemorrhage and/or proteinaceous fluid may appear due to the thrusting of the probe through the bronchi and the trauma of the biopsy process, respectively.[84] Crush artifacts may be present if improper handling of the specimen is done, while collapsed air spaces may be encountered due to poor permeation of fixative if the BLC specimen is not shaken properly in the preservative formalin solution [Figure 4]. It is also important that the BLC sample is thawed in air. If thawing is performed in normal saline, it should be done only for a few seconds, and the sample should then immediately be transferred to formalin. Prolonged thawing in saline may lead to degeneration of antigens and DNA, which may hamper future immunohistochemical and molecular studies.
Figure 3.

Features of hypersensitivity pneumonitis in different bronchoscopic lung cryobiopsy specimens. Peribronchiolar lymphomononuclear cell infiltrate with isolated giant cells (a and b), ill-formed interstitial granulomas (c), and distortion of the architecture, bridging fibrosis with peribronchiolar inflammation and giant cells indicative of usual interstitial pneumonia-like pattern in chronic hypersensitivity pneumonitis (d) (H and E, ×100, ×200, ×100, and ×100, respectively)
Figure 4.

Artifacts encountered in bronchoscopic lung cryobiopsy specimens: Procedural hemorrhage (a), crush artifact (b), proximal sampling (c), and collapsed alveolar spaces (d)
Recommendations
We recommend that the target is to obtain a BLC specimen of at least 5 mm diameter (2A)
We recommend that BLC specimens should be examined and interpreted by a pulmonary pathologist with experience in DPLDs (UPP)
We recommend that the technical quality, histopathological characteristics, and level of diagnostic confidence should be reported (UPP).
How is the information from bronchoscopic lung cryobiopsy incorporated into multidisciplinary discussion for the diagnosis of diffuse parenchymal lung diseases?
The information from BLC is incorporated into the MDD similar to that of SLB.[18] BLC may be prone to sampling error due to its smaller size and sampling from one lobe.[15] However, it should not be forgotten that even SLB with its higher diagnostic yield may still be associated with sampling errors.[67,85] The BLC procedure is essentially a trade-off between the higher diagnostic yield of SLB and the lower complication rate (including mortality) of BLC. The information from BLC should not be viewed in isolation, but rather combined with clinical and radiologic information to arrive at a reasonable consensus during MDD.[4,86] At this stage, it is also important to assign some level of confidence to the final diagnostic impression.[87]
Recommendations
We recommend that all clinical, radiologic, and pathological findings should be discussed during a multidisciplinary team meeting to arrive at an MDD diagnosis (1A)
We recommend that a diagnostic confidence level should be assigned to the MDD diagnosis (UPP).
What is the training and competency required for performing bronchoscopic lung cryobiopsy?
There is limited information on how much training is needed to acquire proficiency in performing BLC. In one study, the diagnostic yield improved with successive procedures and plateaued after about 70 procedures.[57] The risk of complications also decreased with experience, with a lesser frequency in the last 25 of the first 100 procedures.[57] This indicates a steep curve for both diagnostic yield and safety. The working group felt that it is difficult to perform 70 supervised BLC procedures before getting certified for competency. However, it is important that a pulmonologist attempting to initiate BLC observes and performs at least a few BLC procedures under expert guidance, to learn the nuances of the procedure. The training requirements will vary according to the background skills of the bronchoscopist, especially the expertise in airway management and handling procedural complications. While a novice would certainly need to perform a greater number of procedures under supervision, an experienced bronchoscopist routinely performing diagnostic and interventional bronchoscopy is likely to require much less training. Although it is difficult to define objective criteria, a bronchoscopist having good expertise in conventional TBLB (about 50 procedures), with experience in managing endobronchial bleeding, should be able to quickly learn the technique.
Recommendations
We recommend that an experience of 50 conventional TBLB procedures, an experience in management of endobronchial bleeding, and the availability of expertise in intubation are the essential requirements for the performance of BLC (UPP).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flaherty KR, Thwaite EL, Kazerooni EA, Gross BH, Toews GB, Colby TV, et al. Radiological versus histological diagnosis in UIP and NSIP: Survival implications. Thorax. 2003;58:143–8. doi: 10.1136/thorax.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunninghake GW, Zimmerman MB, Schwartz DA, King TE, Jr, Lynch J, Hegele R. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164:193–6. doi: 10.1164/ajrccm.164.2.2101090. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty KR, King TE, Jr, Raghu G, Lynch JP, 3rd, Colby TV, Travis WD. Idiopathic interstitial pneumonia: What is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med. 2004;170:904–10. doi: 10.1164/rccm.200402-147OC. [DOI] [PubMed] [Google Scholar]
- 5.Veeraraghavan S, Latsi PI, Wells AU, Pantelidis P, Nicholson AG, Colby TV, et al. BAL findings in idiopathic nonspecific interstitial pneumonia and usual interstitial pneumonia. Eur Respir J. 2003;22:239–44. doi: 10.1183/09031936.03.00105202. [DOI] [PubMed] [Google Scholar]
- 6.Ryu YJ, Chung MP, Han J, Kim TS, Lee KS, Chun EM, et al. Bronchoalveolar lavage in fibrotic idiopathic interstitial pneumonias. Respir Med. 2007;101:655–60. doi: 10.1016/j.rmed.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Berbescu EA, Katzenstein AL, Snow JL, Zisman DA. Transbronchial biopsy in usual interstitial pneumonia. Chest. 2006;129:1126–31. doi: 10.1378/chest.129.5.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim HS, Park MS, Park IK. Histopathologic findings of transbronchial biopsy in usual interstitial pneumonia. Pathol Int. 2010;60:373–7. doi: 10.1111/j.1440-1827.2010.02528.x. [DOI] [PubMed] [Google Scholar]
- 9.Tomassetti S, Cavazza A, Colby TV, Ryu JH, Nanni O, Scarpi E, et al. Transbronchial biopsy is useful in predicting UIP pattern. Respir Res. 2012;13:96. doi: 10.1186/1465-9921-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheth JS, Belperio JA, Fishbein MC, Kazerooni EA, Lagstein A, Murray S, et al. Utility of transbronchial vs. Surgical lung biopsy in the diagnosis of suspected fibrotic interstitial lung disease. Chest. 2017;151:389–99. doi: 10.1016/j.chest.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj R, Raparia K, Lynch DA, Brown KK. Surgical lung biopsy for interstitial lung Diseases. Chest. 2017;151:1131–40. doi: 10.1016/j.chest.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Han Q, Luo Q, Xie JX, Wu LL, Liao LY, Zhang XX, et al. Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: A systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2015;149:1394–4010. doi: 10.1016/j.jtcvs.2014.12.057. [DOI] [PubMed] [Google Scholar]
- 13.Dhooria S, Agarwal R. Idiopathic pulmonary fibrosis in India. Chest India. 2015;6:1–3. doi: 10.4103/0970-2113.148396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babiak A, Hetzel J, Krishna G, Fritz P, Moeller P, Balli T, et al. Transbronchial cryobiopsy: A new tool for lung biopsies. Respiration. 2009;78:203–8. doi: 10.1159/000203987. [DOI] [PubMed] [Google Scholar]
- 15.Casoni GL, Tomassetti S, Cavazza A, Colby TV, Dubini A, Ryu JH, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 2014;9:e86716. doi: 10.1371/journal.pone.0086716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pajares V, Puzo C, Castillo D, Lerma E, Montero MA, Ramos-Barbón D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: A randomized trial. Respirology. 2014;19:900–6. doi: 10.1111/resp.12322. [DOI] [PubMed] [Google Scholar]
- 17.Ramaswamy A, Homer R, Killam J, Pisani MA, Murphy TE, Araujo K, et al. Comparison of transbronchial and cryobiopsies in evaluation of diffuse parenchymal lung disease. J Bronchology Interv Pulmonol. 2016;23:14–21. doi: 10.1097/LBR.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomassetti S, Wells AU, Costabel U, Cavazza A, Colby TV, Rossi G, et al. Bronchoscopic lung cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:745–52. doi: 10.1164/rccm.201504-0711OC. [DOI] [PubMed] [Google Scholar]
- 19.Lentz RJ, Taylor TM, Kropski JA, Sandler KL, Johnson JE, Blackwell TS, et al. Utility of flexible bronchoscopic cryobiopsy for diagnosis of diffuse parenchymal lung diseases. J Bronchology Interv Pulmonol. 2018;25:88–96. doi: 10.1097/LBR.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sriprasart T, Aragaki A, Baughman R, Wikenheiser-Brokamp K, Khanna G, Tanase D, et al. A single US center experience of transbronchial lung cryobiopsy for diagnosing interstitial lung disease with a 2-scope technique. J Bronchology Interv Pulmonol. 2017;24:131–5. doi: 10.1097/LBR.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhooria S, Sehgal IS, Aggarwal AN, Behera D, Agarwal R. Diagnostic yield and safety of cryoprobe transbronchial lung biopsy in diffuse parenchymal lung diseases: Systematic review and meta-analysis. Respir Care. 2016;61:700–12. doi: 10.4187/respcare.04488. [DOI] [PubMed] [Google Scholar]
- 22.Dhooria S, Mehta RM, Srinivasan A, Madan K, Sehgal IS, Pattabhiraman V, et al. The safety and efficacy of different methods for obtaining transbronchial lung cryobiopsy in diffuse lung diseases. Clin Respir J. 2018;12:1711–20. doi: 10.1111/crj.12734. [DOI] [PubMed] [Google Scholar]
- 23.Hetzel J, Maldonado F, Ravaglia C, Wells AU, Colby TV, Tomassetti S, et al. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: Expert statement from the cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration. 2018;95:188–200. doi: 10.1159/000484055. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R, Dhooria S, Aggarwal AN, Maturu VN, Sehgal IS, Muthu V, et al. Guidelines for diagnosis and management of bronchial asthma: Joint ICS/NCCP (I) recommendations. Lung India. 2015;32:S3–42. doi: 10.4103/0970-2113.154517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iftikhar IH, Alghothani L, Sardi A, Berkowitz D, Musani AI. Transbronchial lung cryobiopsy and video-assisted thoracoscopic lung biopsy in the diagnosis of diffuse parenchymal lung disease. A Meta-analysis of diagnostic test accuracy. Ann Am Thorac Soc. 2017;14:1197–211. doi: 10.1513/AnnalsATS.201701-086SR. [DOI] [PubMed] [Google Scholar]
- 26.Ussavarungsi K, Kern RM, Roden AC, Ryu JH, Edell ES. Transbronchial cryobiopsy in diffuse parenchymal lung disease: Retrospective analysis of 74 cases. Chest. 2017;151:400–8. doi: 10.1016/j.chest.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 27.DiBardino DM, Haas AR, Lanfranco AR, Litzky LA, Sterman D, Bessich JL, et al. High complication rate after introduction of transbronchial cryobiopsy into clinical practice at an academic medical center. Ann Am Thorac Soc. 2017;14:851–7. doi: 10.1513/AnnalsATS.201610-829OC. [DOI] [PubMed] [Google Scholar]
- 28.Linhas R, Marçôa R, Oliveira A, Almeida J, Neves S, Campainha S, et al. Transbronchial lung cryobiopsy: Associated complications. Rev Port Pneumol (2006) 2017;23:331–7. doi: 10.1016/j.rppnen.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Singh S, Collins BF, Sharma BB, Joshi JM, Talwar D, Katiyar S, et al. Interstitial lung disease in India. Results of a prospective registry. Am J Respir Crit Care Med. 2017;195:801–13. doi: 10.1164/rccm.201607-1484OC. [DOI] [PubMed] [Google Scholar]
- 30.Dhooria S, Agarwal R, Sehgal IS, Prasad KT, Garg M, Bal A, et al. Spectrum of interstitial lung diseases at a tertiary center in a developing country: A study of 803 subjects. PLoS One. 2018;13:e0191938. doi: 10.1371/journal.pone.0191938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta D, Dadhwal DS, Agarwal R, Gupta N, Bal A, Aggarwal AN, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs. Conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547–56. doi: 10.1378/chest.13-2339. [DOI] [PubMed] [Google Scholar]
- 32.Muthu V, Gupta N, Dhooria S, Sehgal IS, Bal A, Aggarwal AN, et al. Aprospective, randomized, double-blind trial comparing the diagnostic yield of 21 – And 22-gauge aspiration needles for performing endobronchial ultrasound-guided transbronchial needle aspiration in sarcoidosis. Chest. 2016;149:1111–3. doi: 10.1016/j.chest.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Madan K, Dhungana A, Mohan A, Hadda V, Jain D, Arava S, et al. Conventional transbronchial needle aspiration versus endobronchial ultrasound-guided transbronchial needle aspiration, with or without rapid on-site evaluation, for the diagnosis of sarcoidosis: A Randomized controlled trial. J Bronchology Interv Pulmonol. 2017;24:48–58. doi: 10.1097/LBR.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 34.Aragaki-Nakahodo AA, Baughman RP, Shipley RT, Benzaquen S. The complimentary role of transbronchial lung cryobiopsy and endobronchial ultrasound fine needle aspiration in the diagnosis of sarcoidosis. Respir Med. 2017;131:65–9. doi: 10.1016/j.rmed.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ. 2016;352:h6819. doi: 10.1136/bmj.h6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46:976–87. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Jones KD, Urisman A, Elicker BM, Urbania T, Johannson KA, et al. Pathologic findings and prognosis in a large Prospective cohort of chronic hypersensitivity pneumonitis. Chest. 2017;152:502–9. doi: 10.1016/j.chest.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Flaherty KR, Travis WD, Colby TV, Toews GB, Kazerooni EA, Gross BH, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164:1722–7. doi: 10.1164/ajrccm.164.9.2103074. [DOI] [PubMed] [Google Scholar]
- 39.Cascante JA, Cebollero P, Herrero S, Yagüe A, Echegoyen A, Elizalde J, et al. Transbronchial cryobiopsy in interstitial lung disease: Are we on the right path? J Bronchology Interv Pulmonol. 2016;23:204–9. doi: 10.1097/LBR.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 40.Ravaglia C, Wells AU, Tomassetti S, Dubini A, Cavazza A, Piciucchi S, et al. Transbronchial lung cryobiopsy in diffuse parenchymal lung disease: Comparison between biopsy from 1 segment and biopsy from 2 segments – Diagnostic yield and complications. Respiration. 2017;93:285–92. doi: 10.1159/000456671. [DOI] [PubMed] [Google Scholar]
- 41.Tafur A, Douketis J. Perioperative management of anticoagulant and antiplatelet therapy. Heart. 2018;104:1461–7. doi: 10.1136/heartjnl-2016-310581. [DOI] [PubMed] [Google Scholar]
- 42.Abuqayyas S, Raju S, Bartholomew JR, Abu Hweij R, Mehta AC. Management of antithrombotic agents in patients undergoing flexible bronchoscopy. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0001-2017. pii: 170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pajares V, Torrego A, Puzo C, Lerma E, Gil De Bernabé MA, Franquet T, et al. Transbronchial lung biopsy using cryoprobes. Arch Bronconeumol. 2010;46:111–5. doi: 10.1016/j.arbres.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Kropski JA, Pritchett JM, Mason WR, Sivarajan L, Gleaves LA, Johnson JE, et al. Bronchoscopic cryobiopsy for the diagnosis of diffuse parenchymal lung disease. PLoS One. 2013;8:e78674. doi: 10.1371/journal.pone.0078674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fruchter O, Fridel L, El Raouf BA, Abdel-Rahman N, Rosengarten D, Kramer MR, et al. Histological diagnosis of interstitial lung diseases by cryo-transbronchial biopsy. Respirology. 2014;19:683–8. doi: 10.1111/resp.12296. [DOI] [PubMed] [Google Scholar]
- 46.Griff S, Schönfeld N, Ammenwerth W, Blum TG, Grah C, Bauer TT, et al. Diagnostic yield of transbronchial cryobiopsy in non-neoplastic lung disease: A retrospective case series. BMC Pulm Med. 2014;14:171. doi: 10.1186/1471-2466-14-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gershman E, Fruchter O, Benjamin F, Nader AR, Rosengarten D, Rusanov V, et al. Safety of cryo-transbronchial biopsy in diffuse lung diseases: Analysis of three hundred cases. Respiration. 2015;90:40–6. doi: 10.1159/000381921. [DOI] [PubMed] [Google Scholar]
- 48.Hernández-González F, Lucena CM, Ramírez J, Sánchez M, Jimenez MJ, Xaubet A, et al. Cryobiopsy in the diagnosis of diffuse interstitial lung disease: Yield and cost-effectiveness analysis. Arch Bronconeumol. 2015;51:261–7. doi: 10.1016/j.arbres.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Echevarria-Uraga JJ, Pérez-Izquierdo J, García-Garai N, Gómez-Jiménez E, Aramburu-Ojembarrena A, Tena-Tudanca L, et al. Usefulness of an angioplasty balloon as selective bronchial blockade device after transbronchial cryobiopsy. Respirology. 2016;21:1094–9. doi: 10.1111/resp.12827. [DOI] [PubMed] [Google Scholar]
- 50.Hagmeyer L, Theegarten D, Wohlschläger J, Treml M, Matthes S, Priegnitz C, et al. The role of transbronchial cryobiopsy and surgical lung biopsy in the diagnostic algorithm of interstitial lung disease. Clin Respir J. 2016;10:589–95. doi: 10.1111/crj.12261. [DOI] [PubMed] [Google Scholar]
- 51.Hagmeyer L, Theegarten D, Treml M, Priegnitz C, Randerath W. Validation of transbronchial cryobiopsy in interstitial lung disease - interim analysis of a prospective trial and critical review of the literature. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:2–9. [PubMed] [Google Scholar]
- 52.Pourabdollah M, Shamaei M, Karimi S, Karimi M, Kiani A, Jabbari HR, et al. Transbronchial lung biopsy: The pathologist's point of view. Clin Respir J. 2016;10:211–6. doi: 10.1111/crj.12207. [DOI] [PubMed] [Google Scholar]
- 53.Ravaglia C, Bonifazi M, Wells AU, Tomassetti S, Gurioli C, Piciucchi S, et al. Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: A Comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration. 2016;91:215–27. doi: 10.1159/000444089. [DOI] [PubMed] [Google Scholar]
- 54.Bango-Álvarez A, Ariza-Prota M, Torres-Rivas H, Fernández-Fernández L, Prieto A, Sánchez I, et al. Transbronchial cryobiopsy in interstitial lung disease: Experience in 106 cases – How to do it. ERJ Open Res. 2017;3 doi: 10.1183/23120541.00148-2016. pii: 00148-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kronborg-White S, Folkersen B, Rasmussen TR, Voldby N, Madsen LB, Rasmussen F, et al. Introduction of cryobiopsies in the diagnostics of interstitial lung diseases – Experiences in a referral center. Eur Clin Respir J. 2017;4:1274099. doi: 10.1080/20018525.2016.1274099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marçôa R, Linhas R, Apolinário D, Campainha S, Oliveira A, Nogueira C, et al. Diagnostic yield of transbronchial lung cryobiopsy in interstitial lung diseases. Rev Port Pneumol (2006) 2017;23:296–8. doi: 10.1016/j.rppnen.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Almeida LM, Lima B, Mota PC, Melo N, Magalhães A, Pereira JM, et al. Learning curve for transbronchial lung cryobiopsy in diffuse lung disease. Rev Port Pneumol (2006) 2017 doi: 10.1016/j.rppnen.2017.09.005. pii: S2173-5115(17)30148-3. [DOI] [PubMed] [Google Scholar]
- 58.Madan K, Mittal S, Gupta N, Hadda V, Mohan A, Guleria R. Cryoprobe transbronchial lung biopsy – How to do it? Lung India. 2018;35:520–2. doi: 10.4103/lungindia.lungindia_52_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhooria S, Sehgal IS, Bal A, Aggarwal AN, Behera D, Agarwal R, et al. Transbronchial lung biopsy with a flexible cryoprobe during rigid bronchoscopy: Standardizing the procedure. Lung India. 2016;33:248–9. doi: 10.4103/0970-2113.177463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franke KJ, Szyrach M, Nilius G, Hetzel J, Hetzel M, Ruehle KH, et al. Experimental study on biopsy sampling using new flexible cryoprobes: Influence of activation time, probe size, tissue consistency, and contact pressure of the probe on the size of the biopsy specimen. Lung. 2009;187:253–9. doi: 10.1007/s00408-009-9156-4. [DOI] [PubMed] [Google Scholar]
- 61.Winkler JL, Jeronimo J, Singleton J, Janmohamed A, Santos C. Performance of cryotherapy devices using nitrous oxide and carbon dioxide. Int J Gynaecol Obstet. 2010;111:73–7. doi: 10.1016/j.ijgo.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 62.Cordero I. Ensuring proper and safe use of the cryotherapy machine. Community Eye Health. 2014;27:77. [PMC free article] [PubMed] [Google Scholar]
- 63.Yarmus LB, Semaan RW, Arias SA, Feller-Kopman D, Ortiz R, Bösmüller H, et al. A randomized controlled trial of a novel sheath cryoprobe for bronchoscopic lung biopsy in a porcine model. Chest. 2016;150:329–36. doi: 10.1016/j.chest.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 64.Ing M, Oliver RA, Oliver BG, Walsh WR, Williamson JP. Evaluation of transbronchial lung cryobiopsy size and freezing time: A Prognostic animal study. Respiration. 2016;92:34–9. doi: 10.1159/000447329. [DOI] [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health Publication. 1999. Jan, [Last accessed on 2018 Feb 28]. Available from: https://www.cdc.gov/niosh/docs/99-105/pdfs/99-105.pdf .
- 66.McNabb JW, Pfenninger JL. Cryosurgery. In: Pfenninger JL, Fowler GC, editors. Procedures for Primary Care. 3rd ed. Philadephia, PA, USA: Mosby; 2011. pp. 93–106. [Google Scholar]
- 67.Katzenstein AL, Zisman DA, Litzky LA, Nguyen BT, Kotloff RM. Usual interstitial pneumonia: Histologic study of biopsy and explant specimens. Am J Surg Pathol. 2002;26:1567–77. doi: 10.1097/00000478-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Andersen HA. Transbronchoscopic lung biopsy for diffuse pulmonary diseases. Results in 939 patients. Chest. 1978;73:734–6. [PubMed] [Google Scholar]
- 69.Gaensler EA, Carrington CB. Open biopsy for chronic diffuse infiltrative lung disease: Clinical, roentgenographic, and physiological correlations in 502 patients. Ann Thorac Surg. 1980;30:411–26. doi: 10.1016/s0003-4975(10)61291-x. [DOI] [PubMed] [Google Scholar]
- 70.Newman SL, Michel RP, Wang NS. Lingular lung biopsy: Is it representative? Am Rev Respir Dis. 1985;132:1084–6. doi: 10.1164/arrd.1985.132.5.1084. [DOI] [PubMed] [Google Scholar]
- 71.Miller RR, Nelems B, Müller NL, Evans KG, Ostrow DN. Lingular and right middle lobe biopsy in the assessment of diffuse lung disease. Ann Thorac Surg. 1987;44:269–73. doi: 10.1016/s0003-4975(10)62071-1. [DOI] [PubMed] [Google Scholar]
- 72.Ayed AK. Video-assisted thoracoscopic lung biopsy in the diagnosis of diffuse interstitial lung disease. A prospective study. J Cardiovasc Surg (Torino) 2003;44:115–8. [PubMed] [Google Scholar]
- 73.Johannson KA, Marcoux VS, Ronksley PE, Ryerson CJ. Diagnostic yield and complications of transbronchial lung cryobiopsy for interstitial lung disease. A Systematic review and metaanalysis. Ann Am Thorac Soc. 2016;13:1828–38. doi: 10.1513/AnnalsATS.201606-461SR. [DOI] [PubMed] [Google Scholar]
- 74.Tomic R, Cortes-Puentes GA, Murugan P, Joo Kim H, Amin K, Dincer HE, et al. Acute exacerbation of interstitial lung disease after cryobiopsy. J Bronchology Interv Pulmonol. 2017;24:319–22. doi: 10.1097/LBR.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 75.Madan K, Mehta S, Gupta N, Hadda V, Mohan A, Guleria R, et al. Pneumomediastinum and extensive subcutaneous emphysema after cryoprobe transbronchial lung biopsy. Ann Am Thorac Soc. 2016;13:2101–3. doi: 10.1513/AnnalsATS.201605-395LE. [DOI] [PubMed] [Google Scholar]
- 76.MacDuff A, Arnold A, Harvey J BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British thoracic society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii18–31. doi: 10.1136/thx.2010.136986. [DOI] [PubMed] [Google Scholar]
- 77.Du Rand IA, Barber PV, Goldring J, Lewis RA, Mandal S, Munavvar M, et al. British thoracic society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax. 2011;66(Suppl 3):iii1–21. doi: 10.1136/thoraxjnl-2011-200713. [DOI] [PubMed] [Google Scholar]
- 78.Sakr L, Dutau H. Massive hemoptysi: An update on the role of bronchoscopy in diagnosis and management. Respiration. 2010;80:38–58. doi: 10.1159/000274492. [DOI] [PubMed] [Google Scholar]
- 79.Viglietta L, Inchingolo R, Pavano C, Tomassetti S, Piciucchi S, Smargiassi A, et al. Ultrasonography for the diagnosis of pneumothorax after transbronchial lung cryobiopsy in diffuse parenchymal lung diseases. Respiration. 2017;94:232–6. doi: 10.1159/000477818. [DOI] [PubMed] [Google Scholar]
- 80.Kumar S, Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Role of ultrasonography in the diagnosis and management of pneumothorax following transbronchial lung biopsy. J Bronchology Interv Pulmonol. 2015;22:14–9. doi: 10.1097/LBR.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 81.Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: Review of the literature and meta-analysis. Crit Care. 2013;17:R208. doi: 10.1186/cc13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Facciolongo N, Patelli M, Gasparini S, Lazzari Agli L, Salio M, Simonassi C, et al. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis. 2009;71:8–14. doi: 10.4081/monaldi.2009.370. [DOI] [PubMed] [Google Scholar]
- 83.Arcasoy SM, Berry G, Marboe CC, Tazelaar HD, Zamora MR, Wolters HJ, et al. Pathologic interpretation of transbronchial biopsy for acute rejection of lung allograft is highly variable. Am J Transplant. 2011;11:320–8. doi: 10.1111/j.1600-6143.2010.03382.x. [DOI] [PubMed] [Google Scholar]
- 84.Colby TV, Tomassetti S, Cavazza A, Dubini A, Poletti V. Transbronchial cryobiopsy in diffuse lung disease: Update for the pathologist. Arch Pathol Lab Med. 2017;141:891–900. doi: 10.5858/arpa.2016-0233-RA. [DOI] [PubMed] [Google Scholar]
- 85.Rabeyrin M, Thivolet F, Ferretti GR, Chalabreysse L, Jankowski A, Cottin V, et al. Usual interstitial pneumonia end-stage features from explants with radiologic and pathological correlations. Ann Diagn Pathol. 2015;19:269–76. doi: 10.1016/j.anndiagpath.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Walsh SL. Multidisciplinary evaluation of interstitial lung diseases: Current insights: Number 1 in the series “Radiology” edited by Nicola Sverzellati and Sujal Desai. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0002-2017. pii: 170002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ryerson CJ, Corte TJ, Lee JS, Richeldi L, Walsh SLF, Myers JL, et al. Astandardized diagnostic ontology for fibrotic interstitial lung disease. An international working group perspective. Am J Respir Crit Care Med. 2017;196:1249–54. doi: 10.1164/rccm.201702-0400PP. [DOI] [PMC free article] [PubMed] [Google Scholar]



