Abstract
Visceral pain is one of the principal complaints of patients with irritable bowel syndrome, and this pain is reliably evoked by mechanical distension and stretch of distal colon and rectum (colorectum). This study focuses on the biomechanics of the colorectum that could play critical roles in mechanical neural encoding. We harvested the distal 30 mm of the colorectum from mice, divided evenly into three 10-mm-long segments (colonic, intermediate and rectal), and conducted biaxial mechanical stretch tests and opening-angle measurements for each tissue segment. In addition, we determined the collagen fiber orientations and contents across the thickness of the colorectal wall by nonlinear imaging via second harmonic generation (SHG). Our results reveal a progressive increase in tissue compliance and prestress from colonic to rectal segments, which supports prior electrophysiological findings of distinct mechanical neural encodings by afferents in the lumbar splanchnic nerves (LSN) and pelvic nerves (PN) that dominate colonic and rectal innervations, respectively. The colorectum is significantly more viscoelastic in the circumferential direction than in the axial direction. In addition, our SHG results reveal a rich collagen network in the submucosa and orients approximately ±30° to the axial direction, consistent with the biaxial test results presenting almost twice the stiffness in axial direction versus the circumferential direction. Results from current biomechanical study strongly indicate the prominent roles of local tissue biomechanics in determining the differential mechanical neural encoding functions in different regions of the colorectum.
NEW & NOTEWORTHY Mechanical distension and stretch—not heat, cutting, or pinching—reliably evoke pain from distal colon and rectum. We report different local mechanics along the longitudinal length of the colorectum, which is consistent with the existing literature on distinct mechanotransduction of afferents innervating proximal and distal regions of the colorectum. This study draws attention to local mechanics as a potential determinant factor for mechanical neural encoding of the colorectum, which is crucial in visceral nociception.
Keywords: biaxial test, irritable bowel syndromes, mechanotransduction, second harmonic generation, visceral pain
INTRODUCTION
Irritable bowel syndrome (IBS) is a frequent cause of patient visits to gastroenterologists with the principal complaint of prolonged visceral pain (12). Unlike other types of pain, visceral pain has a unique biomechanical component: it is mechanical distension and stretch of hollow visceral organs—not heat, cutting, or pinching—that reliably evoke pain from these organs (32). Accordingly, the distension of the distal colon and rectum (colorectum), i.e., colorectal distension, is an effective means to cause visceral pain in both IBS patients and healthy volunteers (33, 50). In addition, heightened pain perception to rectal or colonic distension (visceral hypersensitivity) is considered a biomarker for IBS (5).
Colorectal mechanotransduction, i.e., encoding and transmission of mechanical stimuli to the colorectum to inform the central nervous system is undertaken by sensory innervations of the colorectum, which consist of sensory afferents in both lumbar splanchnic nerves (LSN) and pelvic nerves (PN). Both direct mechanical stretch of sensory nerve endings (7) and indirect effect from smooth muscle contraction (spasm) (40) can effectively activate mechanosensitive colorectal afferents. In the colorectum, the proximal portion (mostly colonic) is predominantly innervated by afferent endings in the LSN pathway, whereas the distal portion (mostly rectal) is innervated by the PN pathway (2, 8). The mechanical neural encoding by colorectal afferents has been systematically characterized by us and others via single-unit recordings of action potentials from afferent nerve axons in the LSN and PN attached to the colorectum (7–11, 13, 14). In particular, we implemented an electrical stimulation protocol to unbiasedly identify all afferent endings in the colorectum, characterized their neural encoding functions, and classified them based on response, or lack thereof, to three distinct mechanical stimuli applied to the colorectum: punctate probing, mucosal stroking, and circumferential stretching (8). Together, mechanosensitive afferent classes contribute to more than 67 and 77% of the sensory innervation in the LSN and PN pathways, respectively; mechanically insensitive afferents (MIA) contribute the rest (8). In the diseased condition of prolonged visceral hypersensitivity, ~70% MIAs in the PN pathway and ~20% in the LSN pathway became sensitized and acquired mechanosensitivity to generate de novo input to the central nervous system (8, 13, 14, 23). In addition to the different innervation regions in the colorectum, the LSN and PN afferents also have different neural encoding profiles to mechanical stimuli: the neural encoding of circumferential colorectal stretch and mucosal stroking is mainly undertaken by the PN afferents while most LSN afferents encode punctate probing (8, 12). Also, a nerve lesion study indicated that a functioning PN, not LSN, pathway is necessary for evoking behavioral nociceptive responses in mice via noxious colorectal distension (22).
Colorectal mechanotransduction occurs at afferent nerve endings embedded in the colorectal wall and relies on a biomechanical process, namely transmission of bulk mechanical deformation (e.g., colorectal distension) to local stress and strain distributions in micrometer-thick afferent nerve endings that trigger generation of action potentials at the spike initiation zone (15). Thus, knowledge of the biomechanics of distal colon and rectum is crucial in understanding colorectal mechanotransduction; however, such knowledge is scarce in contrast to the available neurophysiological data. So far, no studies have attempted to contrast the biomechanical properties of distal colon (predominantly innervated by the LSN) and rectum (predominantly innervated by the PN). In the present study, we systematically conducted biaxial tissue testing on harvested mouse intestinal tissues of 7×7 mm2 and discovered significant differences in tissue biomechanical properties along the axial length of the mouse colorectum. In addition, colorectal tissue showed significant anisotropy between the axial and circumferential directions. We also quantified residual stresses in the colorectum by measuring opening angles and recorded gradually increased residual stress from colonic to rectal regions.
In addition, we imaged with second harmonic generation (SHG) to visualize through-thickness fiber orientations and discovered a rich network of collagen fibers in the submucosal regions of the colorectum, new anatomic evidence to support submucosa as a load-carrying structure for the large intestine.
MATERIALS AND METHODS
All experiments were reviewed and approved by the University of Connecticut Institutional Animal Care and Use Committee.
Colorectal tissue harvesting.
Twenty one mice of 11 males and 10 females were used in this study (C57BL/6, Taconic, Germantown, NY), aged 8–12 wk, and weighing 20–30 g. All of the mice used in this study were raised in individually ventilated cages (up to five mice per cage), bedded with aspen sani-chip bedding, fed an irradiated Teklad global diet, and provided light from 7 am to 9 pm. On an experimental day, one mouse was transferred to a fume hood in the morning for anesthesia by isoflurane inhalation, euthanized by exsanguination after perforating the right atrium, and transcardially perfused with oxygenated Krebs solution (in mM: 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4·7H2O, 2.5 CaCl2, 11.1 d-glucose, 2 butyrate, and 20 acetate) bubbled with carbogen (95% O2-5% CO2). A midline laparotomy was performed, and the pubic symphysis was transected to expose the pelvic floor organs. The distal 30 mm of the large bowel, including the distal colon and rectum, was dissected free of connective tissues and transferred to modified Krebs solution, to which was added nifedipine (4 μM; L-type calcium channel antagonist to block muscle activities), penicillin-streptomycin (100 U/ml), and protease inhibitors (P2714; Sigma-Aldrich, St. Louis, MO). To be consistent with prior electrophysiological and behavioral studies that implemented colorectal distension (e.g., 43), the tissue was cannulated and distended by PBS at ascending levels of graded intraluminal pressure: 15, 30, 45, and 60 mmHg, 10 s for each distension. Graded distension was performed at least four times on each colorectum in modified Krebs solution at room temperature.
Opening angle measurements.
Assuming the anus as zero coordinate, the 30-mm colorectum was divided into three even segments: 0–10 mm (rectal), 10–20 mm (intermediate), and 20–30 mm (colonic), as shown in Fig. 1 (see Ref. 7). After performing the graded distension, one tissue ring of 2-mm length was collected from the proximal end of each segment. A photograph was taken of the cross section of the rings (the no-load state). Then, each ring was cut radially under a stereomicroscope (M165; Leica Microsystems, Wetzlar, Germany), which may open into a sector to release residual stresses. Following a prior report (18), we allowed a 30-min period for complete release of the residual stress before taking another photograph (the zero-stress state). The opening angle is defined as the angle subtended by two radii drawn from the midpoint of the inner wall to the inner tips of two ends of the sector.
Fig. 1.
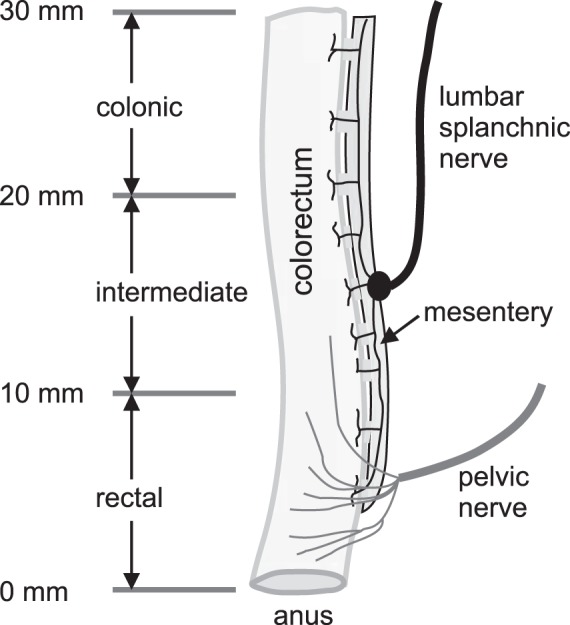
The schematic of the sensory innervations of mouse distal colon and rectum (colorectum) by lumbar splanchnic and pelvic nerves. Considering the anus as zero coordinate, the distal 30 mm of the colorectum is divided into rectal (0–10 mm), intermediate (10–20 mm), and colonic (20–30 mm) segments.
Biaxial stretch test and local strain measurement.
All three segments of colorectum were cut open one by one along the mesentery, pinned flat, and kept in the Krebs solution for at least 15 min before performing the experiments. Three square-shaped specimens (~7×7 mm2) were harvested from the colonic, intermediate, and rectal segments, as illustrated in Fig. 1, respectively. Each specimen was then mounted onto a biaxial tensile testing device via a custom-built adaptor consisting of long and narrow cantilevers to freely permit lateral deformations during the biaxial tests, as shown in Fig. 2A. The 3-D-printed cantilever was made from acrylic resin (Fig. 2B, 0.1-mm resolution; Shapeways, New York, NY) to enforce minimal lateral force during deformation (0.5 mN/mm). We aligned specimens in the circumferential and axial directions and tested them using a computer-controlled, force-displacement actuator (model 300D; Aurora Scientific, Aurora, ON, Canada). Throughout the experiments, the tissue remained submerged in the ~120 ml of aforementioned modified Krebs solution at room temperature containing nifedipine, penicillin-streptomycin, and protease inhibitor. Consistent with the electrophysiological studies, we focused on quantifying the quasi-static material properties of the specimens by applying slow ramped force (0–80 mN) and continuously recorded displacements in both circumferential and longitudinal directions. In a preliminary experiment, we determined a loading rate of 1.2 mN/s as sufficient for providing repeatable and consistent force-displacement measurements (Fig. 3). Thus, the test protocol for each specimen consisted of 30 cycles of quasi-static ramped loading (0–80 mN) and ramped unloading (80–0 mN) at 1.2 mN/s. From our preliminary study, the force-displacement response started to become repeatable after more than 25 loading cycles. Thus, the first 27 cycles are for tissue preconditioning, and the data from the last three cycles were averaged as the steady-state force-displacement response of the specimen. After completing the test, the specimen thickness was measured using a caliper under stereomicroscopy.
Fig. 2.
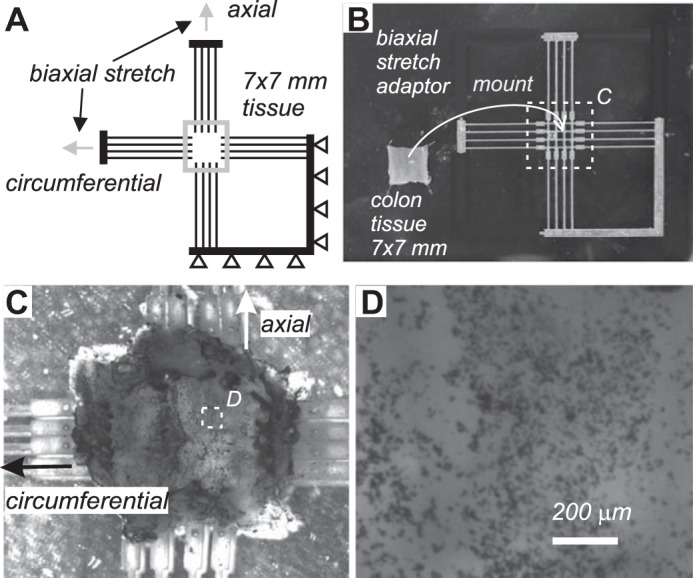
Biaxial tissue testing of specimen from mouse colorectum and local strain measurement. A: specimen (7×7 mm2 squares) were harvested from three segments of the colorectum (rectal, intermediate, and colonic) and mounted onto a custom-built adaptor for biaxial stretch tests. B: adaptor fabricated by 3-D printing was further trimmed and configured as in A to allow lateral displacement of the specimen during biaxial stretch with minimal resistance force (0.5 mN/mm). C: photo images of a specimen sprayed with carbon dots for local strain measurement by optical tracking. D: magnified view of the carbon dots in the white square region in C, showing speckle patterns of micrometers in size.
Fig. 3.
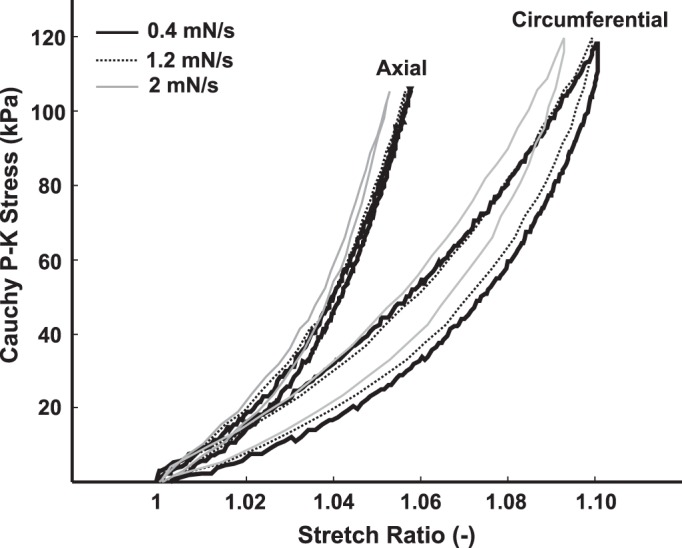
Cauchy stress-stretch ratio results from quasi-static biaxial stretch test at multiple stretch rates. Linear ramped loading (0–80 mN) and unloading (80–0 mN) forces were applied to the specimen at multiple rates: 0.4, 1.2, and 2 mN/s. Stretch rates below 1.2 mN/s provide repeatable and robust results, and ramped forces of 1.2 mN/s were used as the testing protocol throughout.
We recorded the biaxial extension of a subset of specimens with a stereo camera system (Q-400 μDIC; Dantec Dynamics, Skovlunde, Denmark) and used commercially available software Istra4D (V4.4.3.414; Dantec Dynamics) for digital-image correlation (DIC) to obtain the two-dimensional deformation field. To track the deformation, we airbrushed (CM-C Plus; ANEST IWATA, Yokohama, Japan) carbon dots (<2 µm) on the specimen, with the serosal side facing the camera. We then calculated the local circumferential, axial, and shear strain distribution throughout the specimen to verify homogenous deformations.
Imaging collagen fibers by SHG.
After mechanical testing, specimens were stretched biaxially to 80 mN, fixed with 4% paraformaldehyde for 60 min, and mounted onto a glass slide (Permount, Fisher Scientific, Hampton, NH), with the serosal side facing the coverslip (no. 1.5), for nonlinear two-photon imaging of SHG. For imaging, we used a LSM 780 (Carl Zeiss, Oberkochen, Germany) with a ×40 objective (C-Apochromat ×40/1.2 W Corr) with a working distance of 280 µm. We used a tunable two-photon light source (Chameleon, Coherent, Santa Clara, CA) to excite the SHG at 900 nm and collected the signal at 450 nm. This setup allowed label-free imaging, highly specific to collagen fibers, through the entire thickness of our specimens (see Ref. 35).
Histological staining of colorectum.
After the completion of the mechanical stretch protocols, the 7×7mm colorectal patches were fixed with 4% paraformaldehyde for 60 min, embedded in paraffin, and sectioned on a microtome at 8 µm. Following staining with hematoxylin and eosin, the tissue was examined on an Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan) provided with appropriate filters and a Hamamatsu ORCA-ER, C4742-80 digital camera, using Hamamatsu Photonics Wasabi 150 software (Hamamatsu Photonics K.K., System Division, Hamamatsu City, Japan).
Data analysis.
Data processing was conducted blind to the experimental conditions. For biaxial stretch tests, abrupt change of stretch force or displacement more than 10% of the peak value within 0.5 s was considered as a sign of tissue tearing, and the data were excluded from the analysis. We assumed a cylindrical coordinate (r, θ, z) for the tubular colorectum in radial, circumferential, and axial directions, respectively. We denoted Τ as the mean thickness in the unloaded reference configuration measured by the caliper. The measured lengths of the unloaded specimens were denoted as Lθ and Lz in the circumferential and axial directions, respectively. The biaxial stretch forces were denoted as ƒθ and ƒz. The stretch ratios (λθ, λZ) were calculated using the specimen geometry after the 27 cycles of the precondition as the reference coordinates (Xθ, Xz) normalized to the deformed coordinates (χθ, χz): λθ = χθ/Χθ and λz = χz/Χz. We then computed Cauchy stresses (σ) assuming incompressibility and negligible shear using σθθ = λθƒθ/TLz, σzz = λzƒz/TLθ. Data were presented throughout as means ± SE, unless specifically noted. One-way and two-way ANOVA or repeated-measures ANOVA was performed as appropriate using SigmaPlot v9.0 (Systat Software, San Jose, CA). Bonferroni post hoc multiple comparisons were performed when F values for main effects were significant. Differences were considered significant when P < 0.05. Before data collection, the sample sizes for the experiments were determined by estimated power analysis using standard deviations from prior tissue biomechanical studies by us and others. The statistical power of the study was further validated after the data collection to be greater than 0.8.
RESULTS
Differential stress-strain behaviors from colonic to rectal region.
From the biaxial tissue testing results of force-displacement data, the Cauchy stress-stretch ratio relationships from one example specimen were calculated and displayed in Fig. 4A. The ramped loading and unloading cycles were repeated 30 times for each specimen, and the last three cycles were averaged as the test results. The 27 cycles of preconditioning stretch were plotted as gray curves, indicating viscous creep during the tissue preconditioning. The loading portion of the Cauchy stress-stretch ratio relationships from 21 colorectums were averaged and plotted in Fig. 4B for males and Fig. 4C for females. Specimens showing apparent tissue tearing and/or abrupt change in the force-displacement curve were excluded, including specimens from five colonic (three males and two females), one intermediate (male), and three rectal (two males and one female) segments. There were no sex differences between the stress-strain plots in Fig. 4, B and C (two-way, repeated-measures ANOVA, F1,14 = 0.02, P = 0.89 for colonic circumferential; F1,14 = 0.002, P = 0.91 for colonic axial; F1,18 = 0.11, P = 0.75 for intermediate circumferential; F1,18 = 0.41, P = 0.53 for intermediate axial; F1,16 = 0.29, P = 0.6 for rectal circumferential; F1,16 = 0.51, P = 0.49 for rectal axial). Thus, the data from both sexes were pooled together for subsequent analysis. The axial stress-strain properties showed significant differences between all three segments (two-way ANOVA, F2,2195 = 282.5, P < 0.001, post hoc comparison, P < 0.001 for rectal vs. intermediate, rectal vs. colonic, and intermediate vs. colonic). The same significant difference was observed also in the circumferential stress-strain properties (two-way ANOVA, F2,2117 = 225.3, P < 0.001, post hoc comparison, P < 0.001 for rectal vs. intermediate, rectal vs. colonic, and intermediate vs. colonic). Collectively, the distal colorectum showed a progressive increase in compliance from proximal to distal segments in both axial and circumferential directions. In addition, specimens from all three segments showed significant anisotropy between the circumferential and axial directions (two-way ANOVA, F1,30 = 43.9, P < 0.001 for colonic; F1,38 = 68.9, P < 0.001 for intermediate; F1,34 = 105.3, P < 0.001 for rectal segments).
Fig. 4.
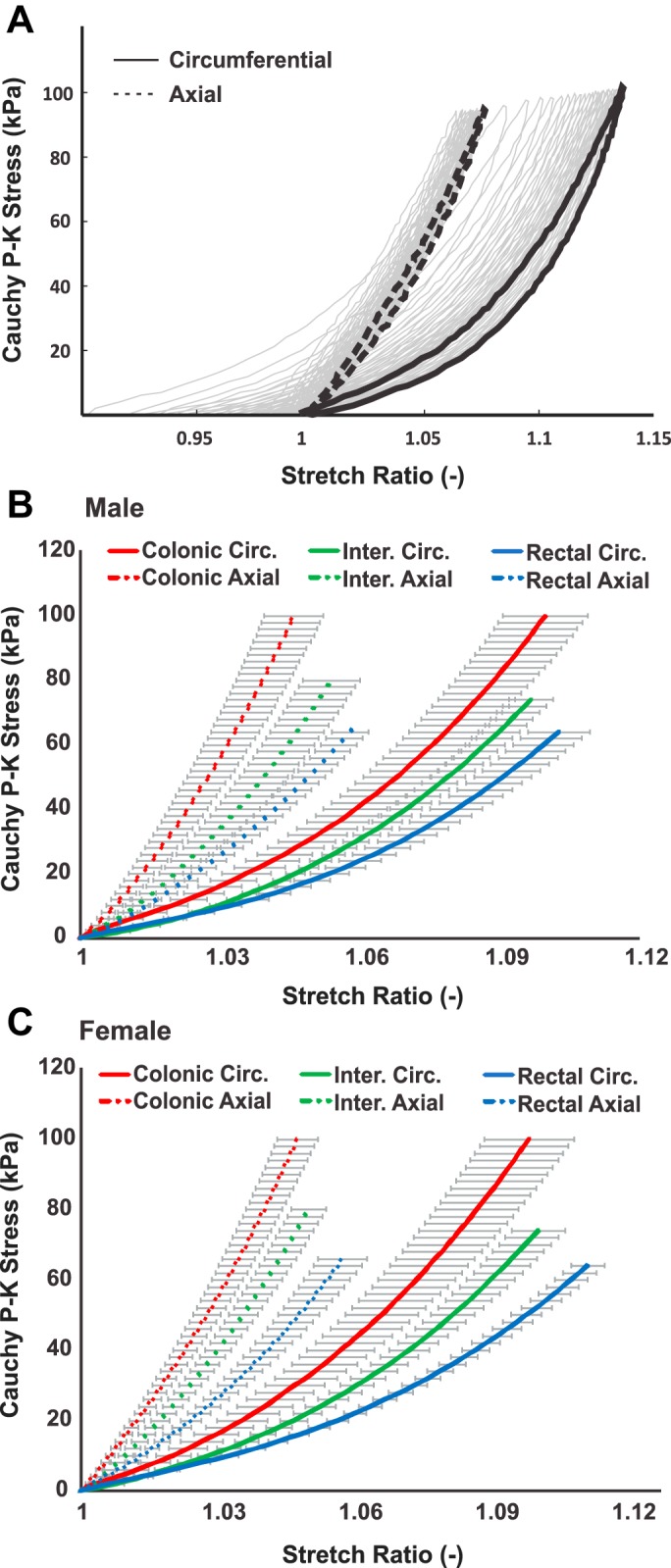
Colorectal stress-strain behavior determined by biaxial tissue stretch tests. A: representative Cauchy stress-stretch ratio curves (solid colored lines) calculated as the average of the last three loading/unloading cycles of the total 30 cycles. Displayed in gray lines were the first 27 loading/unloading cycles for tissue preconditioning. The Cauchy stress-stretch ratio curves in the loading cycle at colonic, intermediate, and rectal segments from male (B) and female (C) mice, respectively. Repeatable stretch forces were delivered to all specimens, and the average stretch ratios were plotted along with the standard error of the mean. For male colorectum, data from 8 colonic, 10 intermediate, and 9 rectal specimens were analyzed. For female colorectum, data from 8 colonic, 10 intermediate, and 9 rectal specimens were analyzed.
Differential viscoelastic behaviors between axial and circumferential directions.
As illustrated in Fig. 5A, the viscoelastic behaviors of the specimen were quantified from the force-displacement relationship as the area between the loading and unloading curves normalized by the total area under the loading curve, indicating the fraction of energy dissipation during the symmetric loading-unloading cycle. Displayed in Fig. 5B are the average fractions of energy loss from colonic, intermediate, and rectal segments in axial and circumferential directions, respectively. The fraction of energy dissipation is significantly higher in the circumferential direction than the axial one (two-way ANOVA, F1,102 = 102.4, P < 0.001), indicating significant viscous properties in the circumferential direction. In contrast, the colorectum is comparatively elastic in the axial direction with minimal viscous dissipation (<5% energy dissipation). The fraction of energy dissipation is not different across different segments (F1,2 = 0.63, P = 0.53)
Fig. 5.
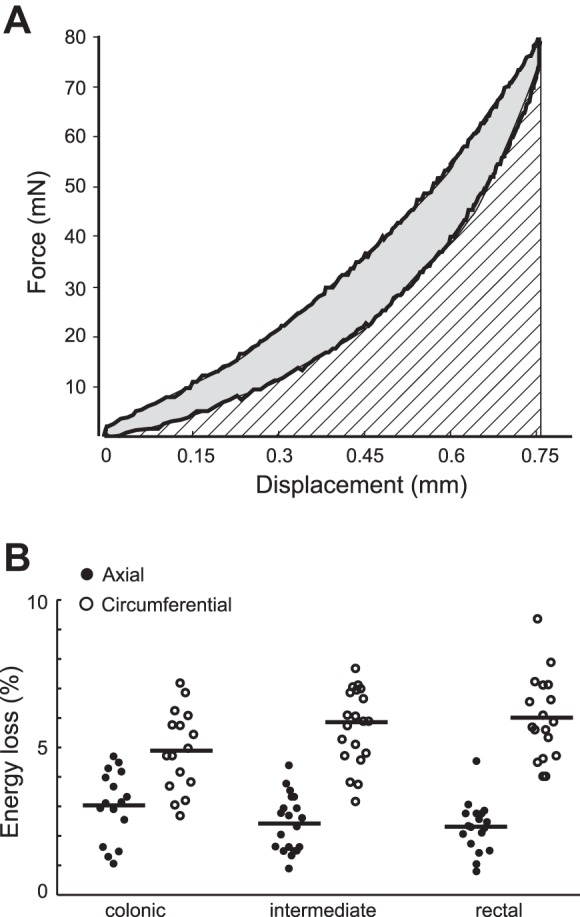
Colorectal tissue viscoelasticity quantified as a fraction of energy loss. A: force-displacement curves were used to calculate the fraction of energy loss using the gray area enclosed by the loading and unloading curves normalized by the total area under the loading curve (gray plus hatched area). B: fraction of energy loss from 16 colonic, 20 intermediate, and 18 rectal segments. The horizontal bars indicate the average fraction of energy loss.
Increased residual stress from colonic to rectal region.
The opening angles were measured from 2-mm-thick tissue rings harvested from the colorectums with representative photos shown in Fig. 6A. Because no significant sex difference was detected in the above biomechanical tests, opening angles measured from both sexes were pooled together. Opening angles from seven colorectums were averaged and displayed in Fig. 6B, showing significantly higher opening angle in the rectal segments than in the colonic and intermediate segments (one-way ANOVA, F2,18 = 134, P < 0.05, post hoc comparison, P < 0.001 for rectal vs. colonic and rectal vs. intermediate). In addition, the opening angle at the intermediate segment is significantly higher than at the colonic segments (post hoc comparison, P < 0.02), collectively showing a trend of increased opening angle from proximal to distal direction.
Fig. 6.
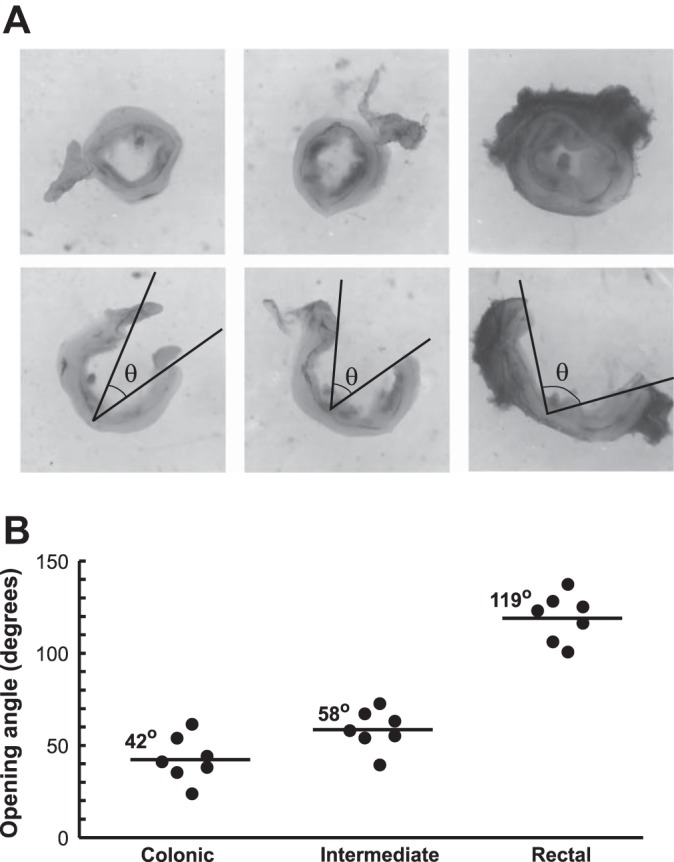
Residual stresses in the colorectum quantified by opening angle measurements. A: photographs of tissue rings (2 mm thick) harvested from rectal, intermediate, and colonic segments before and after cutting open. The opening angles are labeled as θ. B: average opening angles measured from seven colons are 42.4, 58.4, and 119.4°, gradually increasing from proximal to distal locations in the colorectum.
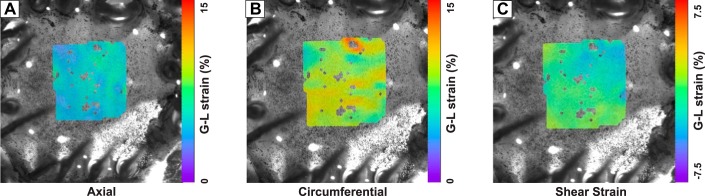
Homogeneous deformation during biaxial stretch.
Representative specimen in Fig. 7 shows average shear strain (−0.7480 ± 1.015, means ± SD) one order of magnitude smaller than average axial (5.010 ± 0.6725) and circumferential (9.041 ± 1.070) strains on the planar surface of a specimen during biaxial stretch. Further, axial and circumferential strains confirm reasonably homogeneous deformations at full extension with a SD ~10% of the mean.
Fig. 7.
Planer axial (A), circumferential (B), and shear strain (C) distribution during biaxial tissue testing on the 7 × 7 mm2 colorectal specimen verifies homogeneous strain distribution. Average shear strain is one order of magnitude lower than strain in axial and circumferential directions.
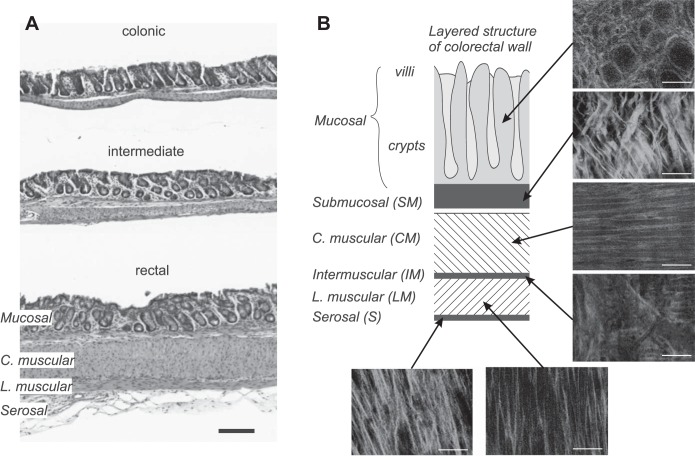
Qualitative collagen fiber orientation through the colorectal wall.
The integrity of the colorectal tissue patches after the mechanical biaxial stretch protocol was confirmed by the H&E staining in Fig. 8A, which showed no apparent tissue tearing or damage in either colonic, intermediate, or rectal segments. SHG imaging allowed us to resolve the individual layers through the thickness of the tissue, with representative images shown in Fig. 8B. The colorectal wall consists of the following layers from the external serosal side to the internal mucosal side: serosal (S), longitudinal muscular (LM), intermuscular (IM), circular muscular (CM), submucosal (SM), and mucosal (M) layers, each with a distinct collagen fiber orientation. Collagen fibers in the muscular layers (LM and CM) align with the direction of the muscle fibers. The S layer shows thicker fibers, aligned in axial direction, and the IM contains thin, isotropic fibers. The SM layer shows thick fiber bundles aligned about ± 30° to the axial direction. Fibers in M concentrate in the wall of the crypts.
Fig. 8.
Histological studies by hematoxylin-and-eosin (H&E) staining (A) and second harmonic generation (SHG) imaging (B), respectively. The H&E staining confirms the tissue integrity after the biaxial mechanical stretch protocol. Representative SHG images show different through-thickness fiber orientations and thicknesses for the corresponding layers. C. muscular, circular muscular; L. muscular, longitudinal muscular. Scale bars indicate 200 µm in A and 50 µm in B.
DISCUSSION
This is the first study to compare and contrast the colorectal regions with dominant innervations by the LSN (colonic segment) and PN (rectal segment), respectively. A handful of previous studies on the biomechanics of large intestine were conducted on colon segments proximal to the LSN and PN innervation (3, 4) and usually neglected the microscale mechanics (18, 36, 37). Biomechanics of more proximal portions of the gastrointestinal (GI) tract have been more extensively studied, including the small intestine (16, 27, 42, 47–49) and esophagus (25, 26, 28, 39, 44, 45). However, both the physiological function and anatomic structure of the distal colorectum differ significantly from their proximal counterparts in the GI tract (24), preventing direct translation of knowledge to the distal colon and rectum. Because the LSN and PN innervations in mice are clustered in short segments of the colorectum (~10 mm), we were prohibited from using the conventional approach of inflating the tubular colorectum to determine the stress-strain relationship, as used previously on longer intestinal tissues, e.g., rat large (18) and small intestines (6) of more than 30 mm in length. To address this issue, we developed this novel biaxial testing setup and determined the stress-strain behaviors from small square specimens (7×7 mm) harvested from different regions in the colorectum. We implemented a force actuator with force precision of 0.1 mN and displacement precision of 0.1 mm (model 300D; Aurora Scientific, Aurora, CA) to deliver the biaxial stretch force. We did not attempt to study specimens smaller than 7×7 mm, which will require more accurate recordings of stretch force and displacement beyond the capacity of our current equipment. To allow free lateral movement of the tissue, we developed custom-built adaptors consisting of 30-mm-long cantilevers for mounting the tissue. The long cantilevers were designed and verified to have a low elastic modulus laterally (0.5 mN/mm) to allow minimal resistance to the lateral deformation of the tissue. The stress-stretch ratio properties determined from our biaxial device is qualitatively comparable to the stress-strain data reported previously from rat large intestine (18, 46). In addition, our local strain measurements via speckle tracking of the sprayed carbon dots validate homogeneous biaxial deformations in our testing (see Fig. 7). The limitation of our biaxial tests is the stress concentration at the specimen mounting sites on the cantilever tips (by minute metal pins) that causes the specimen to fail at the mounting sites before reaching the rupture stress of the colorectum. However, the maximum stretch force of 80 mN implemented throughout this study correlates to ~40 mmHg intraluminal colorectal pressure, which covers most of the physiological pressure range of mouse colorectum and is beyond the noxious threshold of 20 mmHg for mice (7, 21). Thus, our novel approach of biaxial mechanical tissue testing adequately determines the local physiological stress-strain properties of small tissue samples like mouse colorectum with typical circumference of less than 10 mm.
Our biaxial tests reveal apparent tissue anisotropy in mouse colorectum with stiffer stress-strain curves in the axial direction than the circumferential direction, consistent with prior studies on small and large intestines (3, 18, 34, 46). Biological tissues generally show some anisotropy, such as esophageal tissue (39), the myocardium (38), and the aorta (29), which likely results from the distributions and orientations of collagen fibers, the load-carrying and reinforcing proteins in soft tissues (19). The colorectum is about twice as stiff in the axial direction than the circumferential direction. Our SHG images suggest thick axially aligned collagen fibers in the submucosa layer, consistent with prior findings via electron microscopy in rat small intestine (17, 30, 31). The serosal layer as a connective tissue membrane is also rich in collagen, but its contribution to the bulk colorectal mechanical strength is limited by its thinness. It might have a similar protective role to prevent overstretching like the outermost adventitia layer of the arterial wall (20). Our findings of a collagen-rich network in mouse colorectal submucosa together with similar findings by others in rat small intestine collectively suggest that the submucosa is the load-carrying skeleton for the gastrointestinal tract (17, 30, 31). In addition, prior electron microscopy studies in rat small intestine determined that the collagen fibers are oriented ± 30° to the axial direction (31), in good agreement with our current findings that colorectum is almost twice as stiff in the axial direction as in the circumferential direction. Submucosa, as the skeleton of the gut, proves its protective role in high-intensity colorectal distension and probing, which logically suggests the presence of nociceptive afferent endings in the submucosa for detecting noxious and tissue-injurious mechanical stimuli. This is further supported by a neural afferent tracing study, which showed the highest concentration of nerve endings in the submucosa (32% of total afferent endings), even higher than in the myenteric ganglion (22%) and circular muscle layer (25%) (41).
Prior studies of colorectal mechanotransduction have largely focused on the neural encoding function of sensory afferents via electrophysiological recordings from lumbar splanchnic (LSN) and pelvic nerves (PN), two major innervation pathways of the distal colon and rectum (1, 2, 8). The LSN and PN innervations appear to dominate in the proximal colonic and distal rectal regions of the colorectum, respectively (7). We need to emphasize that there is no clear separation of the LSN and PN innervation regions in the colorectum, as anterograde tracing studies have shown that some PN afferents can traverse considerable distances to the proximal region generally innervated by the LSN afferents (41). The biomechanics of the colorectal wall could play critical roles in the initiation of mechanotransduction. Consistent with the different neural encoding characteristics between the LSN and PN pathways, we discovered apparent differences in biomechanical properties between the colonic and rectal segments in 1) tissue stiffness, 2) viscoelasticity, 3) residual stress, and 4) anatomic thickness. The circumferential stiffness is significantly lower in the rectum than in the colon, which is consistent with the dominant PN innervation of the rectum with the majority of the afferents responding to circumferential colorectal stretch (8, 12). LSN afferents that predominantly innervate the colonic region generally do not respond to circumferential colorectal stretch. The lower rectal stiffness in circumferential direction also agrees with electrophysiological findings that PN afferents with receptive fields in the rectal region have higher firing rates to circumferential stretch than afferents in the colonic region (7). The colorectum tissue is viscoelastic and dissipates more energy under deformation in the circumferential direction than in the axial, which could underlie the adaptation of afferent activities to circumferential colorectal stretch (7, 8). The range of opening angles in this report is consistent with a previous study on mouse large intestine (18). Unlike the small intestine that folds toward the outer surface when cut open (6), the opening angle of the colorectum is comparatively small, indicating modest compression of the inner mucosa layer and extension of the outer muscular layers in the physiological condition to mitigate stress concentrations in the inner layer during intraluminal distension. The increasing opening angle toward the rectum indicates that the rectal afferents in the muscular layers are prestretched in the physiological conditions, which might contribute to their higher firing rate than afferents in the colonic segments (7). Last, the rectum is significantly thicker than the colon (7), which implies more severe stress concentration in the inner layer of the rectum during colorectal distension than in the colon. This is also consistent with the current report of larger opening angle (i.e., higher prestress) in the rectum than in the colon to attenuate stress concentration in the inner layer.
In summary, we systematically conducted mechanical tissue testing on the distal 30 mm of mouse large intestine, i.e., the colorectum consisting of colonic, intermediate, and rectal segments that are differentially innervated by sensory afferents in the LSN and PN. Biaxial stretch testing reveals that the colorectum is almost twice as stiff in the axial direction as in the circumferential direction, consistent with the finding of a rich network of collagen fibers in the submucosa that are aligned about ±30° to the axial direction. The rectal region is more compliant than the proximal region in the circumferential direction. The colorectum is significantly more viscoelastic in the circumferential direction than in the axial direction. The rectum is thicker than the proximal regions and subjected to more precompression in the mucosa and pretension in the muscular layers, which could contribute to the higher firing rate of afferents with endings in the rectal muscular layers. The current study reveals the distinct mechanical properties between the colonic and rectal regions in the colorectum, consistent with the differential neural encoding functions of LSN and PN afferents that dominate the colonic and rectal innervations, respectively. Further biomechanical studies of the colorectum are required to complement the existing electrophysiological findings to synergistically advance our mechanistic understanding of colorectal mechanotransduction, which is crucial in IBS-related visceral pain and hyperalgesia.
GRANTS
This article was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grant K01-DK-100460 (to B. Feng) and National Science Foundation Grant CMMI-1727185 (to B. Feng and D. Pierce).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S. Siri, D.M.P., and B.F. conceived and designed research; S. Siri, F.M., L.C., S. Santos, and B.F. performed experiments; S. Siri, F.M., L.C., S. Santos, D.M.P., and B.F. analyzed data; S. Siri, D.M.P., and B.F. interpreted results of experiments; S. Siri, F.M., and B.F. prepared figures; S. Siri, F.M., and B.F. drafted manuscript; S. Siri, F.M., L.C., S. Santos, D.M.P., and B.F. edited and revised manuscript; L.C., S. Santos, D.M.P., and B.F. approved final version of manuscript.
REFERENCES
- 1.Brierley SM, Carter R, Jones W 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol 567: 267–281, 2005. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brierley SM, Jones RC 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Carniel EL, Gramigna V, Fontanella CG, Frigo A, Stefanini C, Rubini A, Natali AN. Characterization of the anisotropic mechanical behaviour of colonic tissues: experimental activity and constitutive formulation. Exp Physiol 99: 759–771, 2014. doi: 10.1113/expphysiol.2013.076091. [DOI] [PubMed] [Google Scholar]
- 4.Carniel EL, Gramigna V, Fontanella CG, Stefanini C, Natali AN. Constitutive formulations for the mechanical investigation of colonic tissues. J Biomed Mater Res A 102: 1243–1254, 2014. doi: 10.1002/jbm.a.34787. [DOI] [PubMed] [Google Scholar]
- 5.Clarke G, Quigley EM, Cryan JF, Dinan TG. Irritable bowel syndrome: towards biomarker identification. Trends Mol Med 15: 478–489, 2009. doi: 10.1016/j.molmed.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Dou Y, Fan Y, Zhao J, Gregersen H. Longitudinal residual strain and stress-strain relationship in rat small intestine. Biomed Eng Online 5: 37, 2006. doi: 10.1186/1475-925X-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng B, Brumovsky PR, Gebhart GF. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol 298: G402–G409, 2010. doi: 10.1152/ajpgi.00487.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol 300: G170–G180, 2011. doi: 10.1152/ajpgi.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng B, Gebhart GF. In vitro functional characterization of mouse colorectal afferent endings. J Vis Exp 95: 52310, 2015. doi: 10.3791/52310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng B, Joyce SC, Gebhart GF. Optogenetic activation of mechanically insensitive afferents in mouse colorectum reveals chemosensitivity. Am J Physiol Gastrointest Liver Physiol 310: G790–G798, 2016. doi: 10.1152/ajpgi.00430.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng B, Kiyatkin ME, La JH, Ge P, Solinga R, Silos-Santiago I, Gebhart GF. Activation of guanylate cyclase-C attenuates stretch responses and sensitization of mouse colorectal afferents. J Neurosci 33: 9831–9839, 2013. doi: 10.1523/JNEUROSCI.5114-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng B, La JH, Schwartz ES, Gebhart GF. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 302: G1085–G1098, 2012. doi: 10.1152/ajpgi.00542.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng B, La JH, Schwartz ES, Tanaka T, McMurray TP, Gebhart GF. Long-term sensitization of mechanosensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. Am J Physiol Gastrointest Liver Physiol 302: G676–G683, 2012. doi: 10.1152/ajpgi.00490.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng B, La JH, Tanaka T, Schwartz ES, McMurray TP, Gebhart GF. Altered colorectal afferent function associated with TNBS-induced visceral hypersensitivity in mice. Am J Physiol Gastrointest Liver Physiol 303: G817–G824, 2012. doi: 10.1152/ajpgi.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng B, Zhu Y, La JH, Wills ZP, Gebhart GF. Experimental and computational evidence for an essential role of NaV1.6 in spike initiation at stretch-sensitive colorectal afferent endings. J Neurophysiol 113: 2618–2634, 2015. doi: 10.1152/jn.00717.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frøkjær JB, Andersen SD, Drewes AM, Gregersen H. Ultrasound-determined geometric and biomechanical properties of the human duodenum. Dig Dis Sci 51: 1662–1669, 2006. doi: 10.1007/s10620-005-9015-y. [DOI] [PubMed] [Google Scholar]
- 17.Gabella G. The collagen fibrils in the collapsed and the chronically stretched intestinal wall. J Ultrastruct Res 85: 127–138, 1983. doi: 10.1016/S0022-5320(83)90102-8. [DOI] [PubMed] [Google Scholar]
- 18.Gao C, Gregersen H. Biomechanical and morphological properties in rat large intestine. J Biomech 33: 1089–1097, 2000. doi: 10.1016/S0021-9290(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 19.Holzapfel GA. Biomechanics of soft tissue. Handb Mater Behav Models 3: 1049–1063, 2001. [Google Scholar]
- 20.Holzapfel GA, Gasser TC, Ogden RW. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elasticity Phys Sci Solids 61: 1–48, 2000. [Google Scholar]
- 21.Kamp EH, Jones RC 3rd, Tillman SR, Gebhart GF. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am J Physiol Gastrointest Liver Physiol 284: G434–G444, 2003. doi: 10.1152/ajpgi.00324.2002. [DOI] [PubMed] [Google Scholar]
- 22.Kyloh M, Nicholas S, Zagorodnyuk VP, Brookes SJ, Spencer NJ. Identification of the visceral pain pathway activated by noxious colorectal distension in mice. Front Neurosci 5: 16, 2011. doi: 10.3389/fnins.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La JH, Feng B, Schwartz ES, Brumovsky PR, Gebhart GF. Luminal hypertonicity and acidity modulate colorectal afferents and induce persistent visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 303: G802–G809, 2012. doi: 10.1152/ajpgi.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung PS. (). The gastrointestinal system: gastrointestinal, nutritional, and hepatobiliary physiology. Dordrecht, The Netherlands: Springer, 2014. [Google Scholar]
- 25.Liao D, Fan Y, Zeng Y, Gregersen H. Stress distribution in the layered wall of the rat oesophagus. Med Eng Phys 25: 731–738, 2003. doi: 10.1016/S1350-4533(03)00122-X. [DOI] [PubMed] [Google Scholar]
- 26.Liao D, Zhao J, Fan Y, Gregersen H. Two-layered quasi-3D finite element model of the oesophagus. Med Eng Phys 26: 535–543, 2004. doi: 10.1016/j.medengphy.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Lu X, Zhao J, Gregersen H. Small intestinal morphometric and biomechanical changes during physiological growth in rats. J Biomech 38: 417–426, 2005. doi: 10.1016/j.jbiomech.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Natali AN, Carniel EL, Gregersen H. Biomechanical behaviour of oesophageal tissues: material and structural configuration, experimental data and constitutive analysis. Med Eng Phys 31: 1056–1062, 2009. doi: 10.1016/j.medengphy.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Niestrawska JA, Viertler C, Regitnig P, Cohnert TU, Sommer G, Holzapfel GA. Microstructure and mechanics of healthy and aneurysmatic abdominal aortas: experimental analysis and modelling. J R Soc Interface 13: 20160620, 2016. doi: 10.1098/rsif.2016.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orberg J, Baer E, Hiltner A. Organization of collagen fibers in the intestine. Connect Tissue Res 11: 285–297, 1983. doi: 10.3109/03008208309004861. [DOI] [PubMed] [Google Scholar]
- 31.Orberg JW, Klein L, Hiltner A. Scanning electron microscopy of collagen fibers in intestine. Connect Tissue Res 9: 187–193, 1982. doi: 10.3109/03008208209160260. [DOI] [PubMed] [Google Scholar]
- 32.Pasricha PJ, Willis WD, Gebhart GF. Chronic Abdominal and Visceral Pain: Theory and Practice. Boca Raton, FL: CRC Press, 2006. [Google Scholar]
- 33.Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain 7: 529–535, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Sacks MS, Gloeckner DC. Quantification of the fiber architecture and biaxial mechanical behavior of porcine intestinal submucosa. J Biomed Mater Res 46: 1–10, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 35.Schriefl AJ, Zeindlinger G, Pierce DM, Regitnig P, Holzapfel GA. Determination of the layer-specific distributed collagen fibre orientations in human thoracic and abdominal aortas and common iliac arteries. J R Soc Interface 9: 1275–1286, 2012. doi: 10.1098/rsif.2011.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokolis DP, Orfanidis IK, Peroulis M. Biomechanical testing and material characterization for the rat large intestine: regional dependence of material parameters. Physiol Meas 32: 1969–1982, 2011. doi: 10.1088/0967-3334/32/12/007. [DOI] [PubMed] [Google Scholar]
- 37.Sokolis DP, Sassani SG. Microstructure-based constitutive modeling for the large intestine validated by histological observations. J Mech Behav Biomed Mater 21: 149–166, 2013. doi: 10.1016/j.jmbbm.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Sommer G, Haspinger DC, Andrä M, Sacherer M, Viertler C, Regitnig P, Holzapfel GA. Quantification of shear deformations and corresponding stresses in the biaxially tested human myocardium. Ann Biomed Eng 43: 2334–2348, 2015. doi: 10.1007/s10439-015-1281-z. [DOI] [PubMed] [Google Scholar]
- 39.Sommer G, Schriefl A, Zeindlinger G, Katzensteiner A, Ainödhofer H, Saxena A, Holzapfel GA. Multiaxial mechanical response and constitutive modeling of esophageal tissues: Impact on esophageal tissue engineering. Acta Biomater 9: 9379–9391, 2013. doi: 10.1016/j.actbio.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 40.Spencer NJ, Kerrin A, Zagorodnyuk VP, Hennig GW, Muto M, Brookes SJ, McDonnell O. Identification of functional intramuscular rectal mechanoreceptors in aganglionic rectal smooth muscle from piebald lethal mice. Am J Physiol Gastrointest Liver Physiol 294: G855–G867, 2008. doi: 10.1152/ajpgi.00502.2007. [DOI] [PubMed] [Google Scholar]
- 41.Spencer NJ, Kyloh M, Duffield M. Identification of different types of spinal afferent nerve endings that encode noxious and innocuous stimuli in the large intestine using a novel anterograde tracing technique. PLoS One 9: e112466, 2014. doi: 10.1371/journal.pone.0112466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storkholm JH, Villadsen GE, Jensen SL, Gregersen H. Mechanical properties and collagen content differ between isolated guinea pig duodenum, jejunum, and distal ileum. Dig Dis Sci 43: 2034–2041, 1998. doi: 10.1023/A:1018855113849. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, Shinoda M, Feng B, Albers KM, Gebhart GF. Modulation of visceral hypersensitivity by glial cell line-derived neurotrophic factor family receptor α-3 in colorectal afferents. Am J Physiol Gastrointest Liver Physiol 300: G418–G424, 2011. doi: 10.1152/ajpgi.00456.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J, Liao D, Zhao J, Gregersen H. Shear modulus of elasticity of the esophagus. Ann Biomed Eng 32: 1223–1230, 2004. doi: 10.1114/B:ABME.0000039356.24821.6c. [DOI] [PubMed] [Google Scholar]
- 45.Yang J, Zhao J, Liao D, Gregersen H. Biomechanical properties of the layered oesophagus and its remodelling in experimental type-1 diabetes. J Biomech 39: 894–904, 2006. doi: 10.1016/j.jbiomech.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Zhao J, Nakaguchi T, Gregersen H. Biomechanical changes in oxazolone-induced colitis in BALB/C mice. J Biomech 42: 811–817, 2009. doi: 10.1016/j.jbiomech.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Zeng Y, Zhao J, Liao D, Gregersen H. Quantitative analysis of collagen fiber angle in the submucosa of small intestine. Comput Biol Med 34: 539–550, 2004. doi: 10.1016/j.compbiomed.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Zeng YJ, Qiao AK, Yu JD, Zhao JB, Liao DH, Xu XH, Hans G. Collagen fiber angle in the submucosa of small intestine and its application in gastroenterology. World J Gastroenterol 9: 804–807, 2003. doi: 10.3748/wjg.v9.i4.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao JB, Sha H, Zhuang FY, Gregersen H. Morphological properties and residual strain along the small intestine in rats. World J Gastroenterol 8: 312–317, 2002. doi: 10.3748/wjg.v8.i2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Q, Verne GN. New insights into visceral hypersensitivity—clinical implications in IBS. Nat Rev Gastroenterol Hepatol 8: 349–355, 2011. doi: 10.1038/nrgastro.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]