Abstract
We investigated sex differences in the association between a measure of physical health, cardiorespiratory fitness (CRF), and brain function using resting-state functional connectivity fMRI. We examined these sex differences in the default, frontoparietal control, and cingulo-opercular networks, assemblies of functionally connected brain regions known to be impacted by both age and fitness level. Healthy older adults (n = 49; 29 women) were scanned to obtain measures of intrinsic connectivity within and across these 3 networks. We calculated global efficiency (a measure of network integration) and local efficiency (a measure of network specialization) using graph theoretical methods. Across all three networks combined, local efficiency was positively associated with CRF, and this was more robust in male versus female older adults. Furthermore, global efficiency was negatively associated with CRF, but only in males. Our findings suggest that in older adults, associations between brain network integrity and physical health are sex-dependent. These results underscore the importance of considering sex differences when examining associations between fitness and brain function in older adulthood.
NEW & NOTEWORTHY We examined the association between cardiorespiratory fitness and resting state functional connectivity in several brain networks known to be impacted by age and fitness level. We found significant associations between fitness and measures of network integration and network specialization, but in a sex-dependent manner, highlighting the interplay between sex differences, fitness, and aging brain health. Our findings underscore the importance of considering sex differences when examining associations between fitness and brain function in older adulthood.
Keywords: aging, brain networks, fMRI, network efficiency, physical fitness, resting-state functional connectivity
INTRODUCTION
Physical exercise improves brain physiology, structure, and function in older adulthood (14, 45). Exercise has been shown to influence neural growth factors and enhance processes such as angiogenesis, synaptogenesis, and neurogenesis (16; but see 73). These changes are also measurable at a systems level, impacting cortical structure and function (25, 54). Cardiorespiratory fitness (CRF), a measure of physical fitness indexing oxygen consumption and transport (41), has been associated with greater gray matter volume and density in frontal and parietal cortices as well as increased hippocampal volumes (12, 24, 36, 84). These changes are associated with higher cognitive functioning, particularly in domains most susceptible to age-related decline, including executive functioning and memory (14, 19, 84).
CRF is also related to changes in the functional architecture of the brain measured at rest (83). Estimates of resting-state functional connectivity (RSFC) characterize coherent patterns of intrinsic neural activity in the absence of explicit task demands. RSFC measures have been used to study brain health both in typical and atypical aging (20). RSFC is thought to reflect repeated patterns of coherent neural oscillatory activity reinforced across time and thus provides a stable, neurophysiological marker of brain function (6; also see Ref. 77 for a review). Thus, measures of RSFC are useful neural markers for assessing the impact of systemic lifestyle influences, such as CRF, on brain function. Furthermore, RSFC measures are readily obtained in older adult populations and have been shown to be both replicable (7, 42) and reliable (72, 87).
RSFC is altered in normal aging (e.g., 13, 30, 31), and these changes appear to target functional connectivity within and between networks associated with higher-order cognitive functioning (30, 31). The default, frontoparietal control, and cingulo-opercular networks have been particularly implicated (30, 31). The default network consists of the ventromedial prefrontal cortex (PFC), posterior cingulate and retrosplenial cortex, inferior parietal lobule, lateral temporal cortex, dorsomedial PFC, and the hippocampal formation, among others (9). This network is implicated in internally focused cognitive processes (8, 40). The frontoparietal control network is composed of anterior and dorsolateral PFC, anterior inferior parietal lobule, anterior cingulate, and insular cortices and is associated with intrinsic (i.e., top down) cognitive control processes (22, 79). Finally, the cingulo-opercular network (22), which encompasses anterior insula/operculum, thalamus, and the dorsal anterior cingulate cortex, is associated with sustaining cognitive set as well as external or salience-driven (i.e., bottom up) attentional processing. Common age-related changes across these networks include reduced within, or local, connectivity and increased between, or more global, connectivity (39). More broadly, aging is associated with greater overall network integration and reduced functional segregation, as well as reduced connectivity within networks (13, 30, 31, 74, 83).
Functional connectivity of the default, frontoparietal, and cingulo-opercular networks is also modulated by fitness and activity levels in older adulthood (80, 81, 83). CRF has been positively associated with global efficiency, a measure of network integration and distributed processing, and negatively associated with local efficiency, a measure of within-network segregation and regional specificity across the whole brain (47; see Ref. 67 for a review of network measures). These findings suggest that greater CRF is associated with increased network integration and reduced segregation between networks in older adults (47). However, the evidence remains equivocal. Local, or less-distributed, processing has also been positively associated with exercise levels (43, 44).
There is evidence for a relationship between RSFC and CRF in older adulthood and evidence of sex differences in functional brain aging, yet sex differences in the relationship between CRF and brain function in older adulthood have not been investigated. Older men and women show differential benefits in cognitive performance associated with exercise and fitness levels. Studies with a greater proportion of female participants report greater cognitive gains (14). A recent meta-analysis reported greater exercise-related cognitive benefits in women (4), yet the neural basis of sex differences in the relationship between fitness and brain function in older adults has not been directly explored.
To address this gap, here we investigate sex differences on the impacts of CRF on RSFC in older adults. We examine this relationship specifically focusing on three higher-order association networks (13) that have been most reliably associated with changes both in aging and fitness levels: the default network, frontoparietal control network, and cingulo-opercular network. We hypothesize that local, or greater within-network, connectivity would be associated with higher CRF levels in older adults (43, 44). As there are no studies investigating sex differences in the association between RSFC and CRF, we are unable to pose specific hypotheses. However, greater exercise-related cognitive benefits have been observed in older women (4). This suggests that patterns of functional brain activity associated with better cognitive performance, i.e., increased local efficiency, should be more reliably observed in women.
METHODS
Ethical Approval (Human Subjects)
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Participants
Fifty-one older adults participated in this study and were recruited from the community in Ithaca, New York. All participants were healthy, over the age of 60, had normal or corrected-to-normal visual acuity, and had no history of psychiatric, neurological, or other medical illness that could compromise cognitive functions. In addition to the inclusion criteria noted above, participants were required to have Geriatric Depression Scores ≤9 (i.e., within the “normal” range; 86) as well as Mini-Mental State Exam scores of >25 (26) to be eligible. Two participants were excluded at this point because of elevated scores on the Geriatric Depression measure, resulting in a final sample of 49 older adults (age mean: 67.25 yr, SD = 5.44; years of education: 17.06 yr, SD = 2.77; 29 women). All procedures performed in the studies were approved by the Institutional Review Board at Cornell University and are in accordance with the ethical standards described in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Participants gave written informed consent in accordance with the Institutional Review Board of Cornell University.
CRF Assessment
Our CRF metric was derived using a formula developed by Jurca and colleagues (46). This formula takes a participant’s height, weight, age, sex, resting heart rate, and self-reported physical activity level to derive a CRF score in metabolic equivalents (1 metabolic equivalent = 3.5 ml O2 uptake·kg body mass−1·min−1). The metabolic equivalent values derived from the formula have been validated in a population of older adults (57) and significantly correlated with explicitly recorded metabolic equivalent values from the maximal graded exercise test and with CRF estimates derived from submaximal field testing. The formula was further validated by McAuley and colleagues (59) and significantly correlated with a physician-supervised maximal exercise test and a one-mile timed walk. The authors also reported that formula-derived CRF estimates were significantly correlated with cognitive function, hippocampal volume, and memory complaints consistent with the timed walk and exercise-derived fitness measures (59). Height and weight were obtained during the MRI safety protocol at time of scanning and were self-reported. In accordance with previous studies using this measure (46, 57, 59), participants self-reported their level of physical activity given a scale of 1–5 with predetermined descriptions as outlined in the original protocol by Jurca and colleagues (46). For example, an activity level of 3 requires participation in aerobic exercise (such as brisk walking, swimming, or jogging) at a comfortable pace for 20–60 min per week (46). Although this self-report component may impact the reliability of the measure (64), our approach is consistent with earlier validation studies (57, 59). Resting heart rate was obtained using Biopac Systems Software obtained during resting-state MRI scanning (Biopac Systems).
Neuroimaging
Structural imaging acquisition, preprocessing, and analysis.
Anatomical scans from the Cornell MRI facility were acquired on a GE750 Discovery series 3T scanner with a T1-weighted volumetric MRI magnetization-prepared rapid gradient echo [repetition time: 2,500 ms; echo time [TE]: 3.44 ms; flip angle: 7°; 1.0 mm isotropic voxels, 176 slices]. Anatomical scans were acquired during one 5-m, 25-s run with ×2 acceleration with sensitivity encoding. Structural data were corrected for nonuniform intensities, affine-registered to Montreal Neurological Institute atlas (15), and skull-stripped using Freesurfer (Athinoula A. Martinos Center for Biomedical Imaging, Harvard University, Cambridge, MA).
Functional imaging acquisition, preprocessing, and analysis.
Multi-echo functional images were acquired during two 10-m, 6-s resting-state scans. Participants were instructed to keep their eyes open, blinking and breathing normally. Multi-echo fMRI is a data acquisition sequence developed to enhance the blood oxygenation level contrast (49, 50). This method uses multiple echoes obtained at different TEs corresponding to different T2* weighted tissue relaxation rates (51). After recombining the echo times, independent components analysis is used to remove noise components (i.e., originating in white matter, CSF, movement, etc.) which are now more readily identifiable because of the greater signal contribution from the varying TEs. This procedure, known as multi-echo independent components analysis (ME-ICA), can render up to fourfold increases in the temporal signal-to-noise ratio (51). Resting-state functional scans were acquired using a multi-echo echo planar imaging sequence with online reconstruction (repetition time: 3,000 ms; TE: 13.7 ms, 30 ms, and 47 ms; flip angle: 83°; matrix size: 72 × 72; field of view: 210 mm; 46 axial slices; 3.0-mm isotropic voxels) with ×2.5 acceleration with sensitivity encoding.
Preprocessing was conducted with ME-ICA version 2.5 (49, 50) ((https://afni.nimh.nih.gov/pub/dist/src/pkundu/meica.py). The full ME-ICA preprocessing procedure has been described previously (75). Following ME-ICA, we identified nuisance components using a semiautomated procedure. This involved conducting a probabilistic independent components analysis (5) via multivariate exploratory linear decomposition into independent components (MELODIC) version 3.14, part of FSL (FMRIB Software Library, www.fmrib.ox.ac.uk/fsl), to isolate and extract remaining noise components following the ICA preprocessing.
Based on previous evidence of age-associated declines in RSFC (13, 30, 39, 83) or fitness-related modulation of network integrity (80, 81, 83), the default, cingulo-opercular, and frontoparietal control networks were selected a priori as our networks of interest. We used previously defined regions of interest (ROIs) based on a resting-state cortical parcellation (37). This parcellation was derived using resting-state data and has 333 ROIs, providing sufficient resolution to capture individual differences prominent in aging (27) and to avoid compromising sensitivity and blurring regional boundaries when networks are decomposed into simpler parcellations (63). Connectivity analyses were conducted using the Matlab-based Brain Connectivity Toolbox (67, 68; http://www.brain-connectivity-toolbox.net/). For a detailed description of this procedure, see Rubinov and Sporns (67, 68).
Preprocessed resting data were coregistered with the Montreal Neurological Institute-transformed anatomical scan within subjects. We identified the three a priori-selected networks corresponding to 105 ROIs (37; 41 default, 24 frontoparietal, and 40 cingulo-opercular nodes). Regions corresponding to these networks are illustrated in Fig. 1. Time courses were extracted for each ROI, and a node-wise correlation matrix was created. The matrices were thresholded using a cost density function (averaged over a range of 0.10–0.3, steps of 0.01). These values were used to calculate our topological parameters of interest.
Fig. 1.
Visualization of our networks of interest. Regions of interest for the default (DN), cingulo-opercular (CO), and frontoparietal (FPCN) control networks were taken from a resting-state parcellation by Gordon and colleagues (37) and are represented as spheres. For illustrative purposes, regions of interest were superimposed on an overlay (85) to validate functional network assignment. Figure was created using Connectome Workbench (58).
Functional connectivity metrics.
To measure the integrity of functional brain networks, we used graph theoretical measures. Graph theory depicts the brain as a set of interacting nodes and edges. In a functional data set, “nodes” represent brain regions and “edges” represent the strength of functional coupling between those regions (10, 67). Examining the temporal nature of cross-correlations in the blood oxygenation level signal between nodes allows us to index the intrinsic functional architecture of the brain (67).
To capture changes in overall network connectivity, we derived estimates of global efficiency (to assess network integration or distributed processing) and local efficiency (to assess network segregation or more regional processing specificity). Global efficiency is the average inverse shortest path length in the network (52). In other words, it is derived by examining the connectivity between each node and every other node and averaging the inverse of this measure for all nodes in the network. Global efficiency was calculated using the Brain Connectivity Toolbox (67, 68) and is represented in equation form below:
where dij is the shortest path (smallest number of edges) between nodes i and j (21).
Local efficiency is a measure of functional segregation. Unlike global efficiency, local efficiency measures only the edges connecting direct neighbor nodes and thus quantifies the average efficiency of local subgraphs (52). A network with high local efficiency then describes a topological organization with notable segregated neural processing, which is believed to underlie functional specialization (67). Local efficiency was also calculated in the Brain Connectivity Toolbox and is represented here:
where is the number of nodes in the subgraph Gi. Local efficiencies for each node can be averaged over all nodes to estimate the mean local efficiency of the graph.
Statistical Analyses
Statistical analyses were conducted in R (version 3.3.2) using the RStanArm package and default settings (76). This software was used to fit two linear Bayesian models using the Markov Chain Monte Carlo algorithm to the data evaluating the impact of CRF of brain network metrics (global and local efficiency were fit separately) for men and women.
For each model, the outcome variable of interest was the network connectivity metric (either local or global efficiency), and the predictors were CRF, sex, and the interaction between CRF and sex. Age and education were included as covariates of no interest. Log10 transformations were used to correct variables that did not meet the assumptions for normality before analysis. Evidence for the role of sex was assessed using posterior distributions from each model along with 95% credible intervals and posterior probabilities. This information allows us to determine whether we can reasonably expect to exclude a null finding from our data (i.e., if the 95% credible interval includes 0, we cannot preclude the possibility of no difference). More useful, however, is that posterior distributions can determine exact probabilities for the effect of interest (e.g., “there was an 85% chance that the effect was greater than 0”). Such information is useful, as it helps to quantify the degree of uncertainty in the data.
RESULTS
Behavior
A summary of the demographic and behavioral data can be found in Table 1. We report Bayesian estimates of the posterior difference between groups (i.e., a Bayesian t-test) along with 95% credible highest density intervals (HDIs). Posterior credible intervals excluded zero for only one estimate. First, men had higher CRF levels than women (μ difference: −2.97; 95% HDI: −3.77, −2.15), corresponding to an effect size of d = −2.2. There were no sex differences in self-reported physical activity levels, education levels, or intelligence quotient (credible intervals included 0, and all effect sizes < ±0.45). Descriptive statistics for network efficiencies are available in Table 2. Men and women did not reliably differ on any of these network metrics (all 95% HDIs include 0).
Table 1.
Descriptive statistics
| Variable | Female Mean | Female SD | Male Mean | Male SD | μ Difference | SD Difference | HDI Lower | HDI Upper |
|---|---|---|---|---|---|---|---|---|
| CRF | 6.09 | 0.27 | 9.06 | 0.31 | −2.97 | 0.41 | −3.77 | −2.15 |
| Education | 16.86 | 0.49 | 17.29 | 0.78 | −0.43 | 0.93 | −2.21 | 1.42 |
| Age | 66.28 | 0.94 | 68.37 | 1.52 | −2.08 | 1.79 | −5.57 | 1.44 |
| MMSE | 28.05 | 0.28 | 28.18 | 0.36 | −0.13 | 0.46 | −1.02 | 0.76 |
Difference scores and HDIs refer to Bayesian posterior density estimates of the difference between groups. For mean and SD, CRF values are in metabolic equivalents, and age and education values are in years. CRF, cardiorespiratory fitness; HDI, highest density interval; MMSE, Mini-Mental Status Exam.
Table 2.
Global and local efficiency across networks
| Variable | Female Mean | Female SD | Male Mean | Male SD | μ Difference | SD Difference | HDI Lower | HDI Upper |
|---|---|---|---|---|---|---|---|---|
| LE All | 0.750 | 0.005 | 0.749 | 0.006 | 0.001 | 0.008 | −0.013 | 0.017 |
| FPCN LE | 0.693 | 0.013 | 0.718 | 0.019 | −0.025 | 0.023 | −0.071 | 0.020 |
| DN LE | 0.711 | 0.008 | 0.721 | 0.006 | −0.010 | 0.010 | −0.030 | 0.008 |
| CO LE | 0.693 | 0.008 | 0.708 | 0.012 | −0.015 | 0.014 | −0.042 | 0.014 |
| GE All | 0.533 | 0.003 | 0.531 | 0.004 | 0.002 | 0.005 | −0.007 | 0.011 |
| FPCN GE | 0.425 | 0.007 | 0.404 | 0.009 | 0.022 | 0.012 | −0.001 | 0.045 |
| DN GE | 0.477 | 0.007 | 0.470 | 0.006 | 0.007 | 0.009 | −0.011 | 0.025 |
| CO GE | 0.477 | 0.006 | 0.479 | 0.007 | −0.003 | 0.009 | −0.020 | 0.016 |
Global and local network statistics are presented by group within and across the networks of interest. Difference scores and HDIs refer to Bayesian posterior density estimates of the difference between groups. CO, cingulo-opercular network; DN, default network; FPCN, frontoparietal control network; GE, global efficiency; GE All, global efficiency for all networks; HDI, highest density interval; LE, local efficiency; LE All, local efficiency for all networks.
CRF and Network Connectivity
Global efficiency and local efficiency were computed for nodes within the three networks of interest (the default, frontoparietal control, and cingulo-opercular networks). Age and education were included as covariates in all graph theory analyses.
Local efficiency.
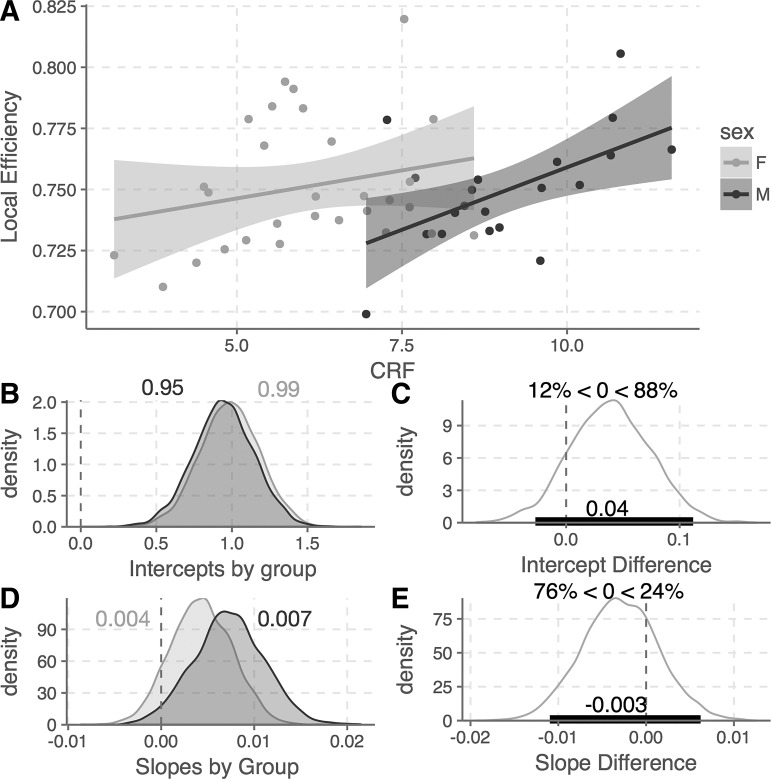
As described earlier, we first ran a Bayesian linear model predicting local efficiency with sex as the between-groups variable, CRF as a continuous predictor variable, and age and education as controls (Fig. 2). The main effect of CRF was not reliably different from zero [b = 0.004, 95% HDI (−0.002, 0.010)], nor was the main effect of sex [b = −0.04, 95% HDI (−0.11, 0.03)], though in this case there was an 88% posterior probability that women had higher local efficiency values than men (Fig. 2B). The interaction between sex and CRF (i.e., the difference of slopes) did not reliably differ by sex [b = 0.003, 95% HDI (−0.005, 0.01)], though again, posterior probabilities suggest that men are 76% more likely than women to have a stronger positive relationship between CRF and local efficiency.
Fig. 2.
Bayesian linear model of the relationship between CRF and local efficiency by sex. A: linear relationship between CRF and local efficiency by group (shaded regions represent standard errors). B: posterior distributions of intercepts for each group (F, M). Numbers appended to the plot are the median posterior density values. C: posterior difference between the group intercepts, along with a 95% credible interval and posterior probabilities (i.e., there is an 88% probability that women have higher local efficiency values than men). D: posterior distributions for the model slopes by group. E: posterior difference as described above for the intercepts. CRF, cardiorespiratory fitness; F, female; M, male.
As posterior values for each group were included as part of the specification for the original model, these distributions could be extracted and examined separately without the need for post hoc tests. The median posterior slope value for men was 0.007, with a 95% HDI that excluded 0 (0.0003, 0.014); indeed, posterior probabilities suggest a 97% likelihood that the slope value for men is greater than 0, and the posterior R2 value for this group was 0.34. The median posterior slope value for women was lower, 0.004, and had a 95% HDI that included 0 (−0.002, 0.011). For women, the posterior probability that their slope is greater than 0 is 90%, and the posterior R2 value for this group was 0.21. The posterior probability distributions suggest that there is a 75% probability that men had steeper slope values than women, indicating that CRF has a similar positive effect on local efficiency across networks for both sexes but that this association is more robust in men than in women. Critically, the relationship across the groups is weaker than the relationships observed within the groups, resulting in a failure to observe a reliable relationship between CRF and local network efficiency across the full sample (Fig. 3).
Fig. 3.
Illustration of the relationship between CRF and network efficiencies demonstrating Simpson’s paradox. A: relationship between CRF and local efficiency which, when combined across sexes, is not reliably different from zero (shaded regions represent standard errors). B: same relationship between CRF and local efficiency which, when stratified, is reliably different from zero in males. C and D: same effect as applied to global efficiency. CRF, cardiorespiratory fitness; F, female; M, male.
Global efficiency.
Next, we ran a similar Bayesian linear model predicting global efficiency. The model was defined as above, but with global efficiency as the outcome. As with local efficiency, the main effect of CRF was not reliably different from 0 [b = −0.002, 95% HDI (−0.006, 0.001)]. There was a marginal main effect of sex [b = 0.031, 95% HDI (−0.007, 0.07)]. This was associated with a 94% likelihood that men have higher global efficiency values than women (Fig. 4). The interaction between CRF and sex again was marginally reliable [b = −0.003, 95% HDI (−0.008, 0.002]; however, the posterior probability that men had a steeper negative slope than women was 90%. Given the posterior probability values, we again examined the posterior slope distributions separately by sex. Men had a median posterior slope value of b = −0.005 with a 95% HDI of (−0.0092, −0.00094) and a posterior R2 value of 0.35, suggesting that for this group the slope was reliably different from 0. By contrast, women had a median posterior slope value of b = −0.002 with a 95% HDI of (−0.006, 0.001) and a posterior R2 value of 0.27, suggesting a weaker, nonreliable relationship for this group.
Fig. 4.
Bayesian linear model of the relationship between CRF and global efficiency by sex. A: linear relationship between CRF and global efficiency by group (shaded regions represent standard errors). B: posterior distributions of intercepts for each group (F, M). Numbers appended to the plot are the median posterior density values. C: posterior difference between the group intercepts, along with a 95% credible interval and posterior probabilities (i.e., there is a 94% probability that men have higher global efficiency values than women). D: posterior distributions for the model slopes by group. E: posterior difference as described above for the intercepts. CRF, cardiorespiratory fitness; F, female; M, male.
DISCUSSION
CRF is frequently cited as a modifiable lifestyle factor that is associated with brain health in older adulthood (11, 41, 80, 82). This study investigated the relationship between CRF and RSFC and how these associations differ for men and women. Across the default, frontoparietal control, and cingulo-opercular networks, CRF levels were positively associated with local network efficiency, a measure of regional connectedness, and negatively associated with global efficiency, a measure of overall network integration. However, these associations were less reliable across the entire participant sample (Fig. 3). These findings reflect the Simpson’s paradox (34), wherein associations within groups are lost when combined into a single sample. This result speaks directly to the importance of considering sex in research that examines relationships between exercise and brain function. Analyses by sex revealed a positive relationship between CRF and local network efficiency and a negative relationship with global efficiency, but these associations were only reliably observed for men. Women showed a similar overall pattern, with positive associations between CRF and local efficiency and negative associations with global efficiency; however, the associations were weaker and were not reliably different from zero. The results show that physical fitness is related to functional connectivity of the brain in older adults during the resting state; however, these associations are sex-specific.
Older adults who are more physically fit have greater local efficiency among functionally connected brain regions and show stronger connections within discrete brain networks (83). This trend toward greater local efficiency in fit older adults contrasts with typical age-related shifts from local to more global efficiency, signaling increasingly dedifferentiated network connections with age (13, 20, 30, 39, 60, 75). In this context, the findings of the current study and others (e.g., 43) suggest that remaining physically fit may help to sustain a “younger” network architecture into later life. Furthermore, these associations may be neuroprotective, as greater local processing has been associated with better executive functioning (3, 43) in older adults and is positively predictive of cognitive gains following both cognitive training (2, 29) and exercise interventions (3).
Our findings of an association between CRF and increased local efficiency differ from that of a recent report by Kawagoe and colleagues (47). In their study, lower local efficiency and greater global efficiency was observed for more fit older adults. This efficiency pattern was also associated with better cognitive functioning, which the authors interpreted as a fitness-related pattern of compensatory network changes. Although they did not stratify their sample by sex, potentially masking the sex differences we report here, other methodological differences may have contributed to these divergent findings. We examined network efficiencies within and among three a priori-identified associative networks with a denser array of functionally defined nodes in contrast to a whole brain, structurally defined node array (47). These differences in network identification may have enhanced our capacity to identify network-specific associations between RSFC and fitness levels. Furthermore, unlike a network compensation account (47), our findings are consistent with studies suggesting that decreased local, or segregated, network organization and increased global, or dedifferentiated, networks are associated with age-related decline (20, 38, 74). However, given the correlational nature of the study, further work will be necessary to determine the causal impact of CRF in later life. Specifically, it will be important to investigate whether CRF promotes a more “young-like” functional architecture or a compensatory pattern of dedifferentiated network connectivity. Furthermore, although the focus of this study was to elucidate sex differences in the impact of CRF on brain function specifically, the role of network efficiency as a mediator between CRF and cognitive functioning is an important future direction.
Future research will also be necessary to more fully elucidate sex differences in the relationship between CRF and brain function. As we observed here, sex-dependent associations exist between CRF and RSFC in brain networks that are most susceptible to change with age and fitness levels. It is well established that brain structure and function are sexually dimorphic (1, 18, 32, 33, 55, 56, 78). These sex differences persist into older age and have been observed during the resting state. In this context, sex differences might also be expected in the relationship between RSFC and CRF in later life. Our findings suggest that this is indeed the case. Men, but not women, showed reliable and robust associations between CRF and measures of network connectivity in older adults. This sex difference is consistent with reports of sex-dependent associations, favoring men, in the relationship between fitness levels and peripheral physical and central nervous system function in older adults (65, 66, 71; also see Refs. 28 and 61 for reviews). However, to our knowledge, these sex differences have not previously been investigated at the level of large-scale cortical networks.
An obvious next question is why this CRF and RSFC association was only reliable for men in our sample. We hypothesized effects favoring women given research demonstrating stronger associations between fitness and cognition in women (4). However, it is important to reiterate that previous studies have not examined sex differences on the impact of fitness at the level of brain function (but at the level of overt cognition). Thus, we are the first to investigate (and interpret) sex differences in this domain. Based on our results, we suggest that the stronger association observed in men is the result of a more rapid shift toward global efficiency among these associative brain networks in men versus women. Age-related declines in brain structure and function are known to occur more rapidly in men, particularly among association regions and related brain networks, which were the focus of the current study (62, 88). Consistent with this interpretation, we observed tendencies for lower measures of local efficiency (88% likelihood) and greater global efficiency (94% likelihood) in men versus women in our sample. Sex differences in the trajectory of age-related changes, with men showing a more rapid shift toward less localized network organization, suggest that lifestyle factors such as physical fitness levels may have a relatively greater impact on the preservation of more differentiated brain networks in older men. Although much research has investigated age and sex as factors in network neuroscience research, these have rarely been investigated within a single study (70). The current findings argue for careful consideration of sex as a factor in future research investigating the determinants and implications of changes in the organization of functional brain networks in older adulthood.
Notably, we did not see sex differences in reported physical activity level in this study, providing greater evidence that the differences seen between sexes are attributable to physiological attributes associated with CRF (i.e., the sum of other components of the CRF equation, including resting heart rate and body mass index) as opposed to systematic differences in physical activity reporting (which are known to occur between the sexes; 48). It should also be noted that we cannot exclude the possibility of a sex-specific reporting bias in our CRF metric. The formula utilizes self-reported height and weight information to calculate body mass index. Although the information was gathered as part of the MRI safety protocol, in which it would be in the participant’s interest to provide an accurate report, a sex-dependent bias in reporting these measures is possible (15a).
Furthermore, although our total sample size is generally consistent with similar studies, individual difference studies typically require large cohorts. To help mitigate this limitation, the neuroimaging methods employed in the study (e.g., the use of ME-ICA) served to ensure stronger signal-to-noise ratio for obtained neuroimaging data (i.e., a fourfold increase in signal-to-noise ratio). Furthermore, our use of Bayesian statistics served to minimize the influence of statistical outliers and enabled us to report probabilities (and thereby quantify uncertainties) in the data.
Although our findings identified sex differences in the association between fitness level and brain function in older adulthood, further research will be necessary to reconcile these findings with previous cognitive neuroscience investigations (47) as well as neurocognitive studies identifying stronger associations between CRF and cognitive functioning in women (4). Unfortunately, myriad methodological differences often preclude direct comparisons among studies in the field. Perhaps the most limiting of these involves discrepancies in the measurement of physical fitness. Differences include the use of self-report versus objective measures (69), as well as the operationalization of physical activity and physical fitness (53, 83). Furthermore, investigating sex differences in this functional domain is complicated by other sex-based differences such as the impact of hormonal replacement therapy (16, 17, 23). Although these challenges are endemic to the field, our findings that CRF is associated with brain function in a sex-dependent manner underscores the importance of considering sex as a factor when studying associations between exercise and brain health in older adulthood. Rapid increases in the popularity of exercise as an intervention to promote brain health in later life presents an urgent need to overcome these methodological challenges toward the goal of building a coherent body of research to inform evidence-based public health initiatives.
GRANTS
This work was supported by grants from the Canadian Institute of Health Research to G. R. Turner and R. N. Spreng. C. J. Dimech was supported by a doctoral award from the Canadian Institute of Health Research and as an Ontario Women’s Health Scholar, funded by the Ministry of Health and Long-Term Care. This project was supported in part by NIH Grant 1-S10-RR-025145. Funding sources were not involved in the design, implementation, or interpretation of the research conducted.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.D. and G.R.T. conceived and designed research; C.J.D. and A.W.L. performed experiments; C.J.D. and J.A.E.A. analyzed data; C.J.D., J.A.E.A., R.N.S., and G.R.T. interpreted results of experiments; C.J.D. and J.A.E.A. prepared figures; C.J.D., J.A.E.A., and G.R.T. drafted manuscript; C.J.D., J.A.E.A., A.W.L., R.N.S., and G.R.T. edited and revised manuscript; C.J.D., J.A.E.A., A.W.L., R.N.S., and G.R.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Udi Alter for assistance in data collection and Elizabeth DuPre for neuroimaging data collection.
REFERENCES
- 1.Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage 18: 880–894, 2003. doi: 10.1016/S1053-8119(03)00034-X. [DOI] [PubMed] [Google Scholar]
- 2.Arnemann KL, Chen AJ, Novakovic-Agopian T, Gratton C, Nomura EM, D’Esposito M. Functional brain network modularity predicts response to cognitive training after brain injury. Neurology 84: 1568–1574, 2015. doi: 10.1212/WNL.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baniqued PL, Gallen CL, Voss MW, Burzynska AZ, Wong CN, Cooke GE, Duffy K, Fanning J, Ehlers DK, Salerno EA, Aguiñaga S. Brain network modularity predicts exercise-related executive function gains in older adults. Front Aging Neurosci 9: 426, 2018. doi: 10.3389/fnagi.2017.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barha CK, Davis JC, Falck RS, Nagamatsu LS, Liu-Ambrose T. Sex differences in exercise efficacy to improve cognition: a systematic review and meta-analysis of randomized controlled trials in older humans. Front Neuroendocrinol 46: 71–85, 2017. doi: 10.1016/j.yfrne.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23: 137–152, 2004. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 6.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541, 1995. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 7.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci USA 107: 4734–4739, 2010. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 44: 195–208, 2004. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38, 2008. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 10.Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci 13: 336–349, 2012. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 11.Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Hayasaka S, Jennings JM, Katula JA, Kraft RA, Rejeski WJ. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci 2: 23, 2010. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology 71: 210–216, 2008. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci USA 111: E4997–E5006, 2014. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 14: 125–130, 2003. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 15.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205, 1994. doi: 10.1097/00004728-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 15a.Connor Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev 8: 307–326, 2007. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 16.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30: 464–472, 2007. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 25: 295–301, 2002. doi: 10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 18.Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes. J Neurosci 14: 4748–4755, 1994. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly M, McMinn D, Allan JL. A bidirectional relationship between physical activity and executive function in older adults. Front Hum Neurosci 8: 1044, 2015. doi: 10.3389/fnhum.2014.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damoiseaux JS. Effects of aging on functional and structural brain connectivity. Neuroimage 160: 32–40, 2017. doi: 10.1016/j.neuroimage.2017.01.077. [DOI] [PubMed] [Google Scholar]
- 21.Dijkstra EW. A note on two problems in connexion with graphs. Numer Math 1: 269–271, 1959. doi: 10.1007/BF01386390. [DOI] [Google Scholar]
- 22.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104: 11073–11078, 2007. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, Kramer AF. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging 28: 179–185, 2007. doi: 10.1016/j.neurobiolaging.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wójcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 19: 1030–1039, 2009. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108: 3017–3022, 2011. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198, 1975. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Fornito A, Zalesky A, Bullmore ET. Network scaling effects in graph analytic studies of human resting-state FMRI data. Front Syst Neurosci 4: 22, 2010. doi: 10.3389/fnsys.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Q, Vongpatanasin W, Levine BD. Neural and nonneural mechanisms for sex differences in elderly hypertension: can exercise training help? Hypertension 52: 787–794, 2008. doi: 10.1161/HYPERTENSIONAHA.108.118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallen CL, Baniqued PL, Chapman SB, Aslan S, Keebler M, Didehbani N, D’Esposito M. Modular brain network organization predicts response to cognitive training in older adults. PLoS One 11: e0169015, 2016. [Erratum in: PLoS One 12: e0174570, 2017. 10.1371/journal.pone.0174570. 28319192] doi: 10.1371/journal.pone.0169015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geerligs L, Maurits NM, Renken RJ, Lorist MM. Reduced specificity of functional connectivity in the aging brain during task performance. Hum Brain Mapp 35: 319–330, 2014b. doi: 10.1002/hbm.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A brain-wide study of age-related changes in functional connectivity. Cereb Cortex 25: 1987–1999, 2015a. doi: 10.1093/cercor/bhu012. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex 11: 490–497, 2001. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 33.Gong G, Rosa-Neto P, Carbonell F, Chen ZJ, He Y, Evans AC. Age- and gender-related differences in the cortical anatomical network. J Neurosci 29: 15684–15693, 2009. doi: 10.1523/JNEUROSCI.2308-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Good IJ, Mittal Y. The amalgamation and geometry of two-by-two contingency tables. Ann Stat 15: 694–711, 1987. doi: 10.1214/aos/1176350369. [DOI] [Google Scholar]
- 36.Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, McAuley E, Kramer AF, Colcombe S, Gratton G, Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology 45: 825–838, 2008. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex 26: 288–303, 2016. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grady C, Sarraf S, Saverino C, Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging 41: 159–172, 2016. doi: 10.1016/j.neurobiolaging.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci 13: 491–505, 2012. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264, 2001. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes SM, Hayes JP, Cadden M, Verfaellie M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Front Aging Neurosci 5: 31, 2013. doi: 10.3389/fnagi.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, Tang H, Zhu C, Gong Q, Zang Y, Evans AC. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One 4: e5226, 2009. doi: 10.1371/journal.pone.0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heisz JJ, Gould M, McIntosh AR. Age-related shift in neural complexity related to task performance and physical activity. J Cogn Neurosci 27: 605–613, 2015. doi: 10.1162/jocn_a_00725. [DOI] [PubMed] [Google Scholar]
- 44.Heisz JJ, Kovacevic A. Exercise impacts age-related changes in cognitive function and neural complexity. Kinesiol Rev 5: 30–38, 2016. doi: 10.1123/kr.2015-0050. [DOI] [Google Scholar]
- 45.Hötting K, Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev 37: 2243–2257, 2013. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Jurca R, Jackson AS, LaMonte MJ, Morrow JR Jr, Blair SN, Wareham NJ, Haskell WL, van Mechelen W, Church TS, Jakicic JM, Laukkanen R. Assessing cardiorespiratory fitness without performing exercise testing. Am J Prev Med 29: 185–193, 2005. doi: 10.1016/j.amepre.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Kawagoe T, Onoda K, Yamaguchi S. Associations among executive function, cardiorespiratory fitness, and brain network properties in older adults. Sci Rep 7: 40107, 2017. doi: 10.1038/srep40107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuk JL, Ardern CI. The influence of ethnicity and gender on the association between measured obesity and cardiorespiratory fitness with self-rated overweight, physical activity and health. Perspect Public Health 134: 38–43, 2014. doi: 10.1177/1757913913480751. [DOI] [PubMed] [Google Scholar]
- 49.Kundu P, Benson BE, Baldwin KL, Rosen D, Luh WM, Bandettini PA, Pine DS, Ernst M. Robust resting state fMRI processing for studies on typical brain development based on multi-echo EPI acquisition. Brain Imaging Behav 9: 56–73, 2015. doi: 10.1007/s11682-014-9346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kundu P, Brenowitz ND, Voon V, Worbe Y, Vértes PE, Inati SJ, Saad ZS, Bandettini PA, Bullmore ET. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc Natl Acad Sci USA 110: 16187–16192, 2013. doi: 10.1073/pnas.1301725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kundu P, Inati SJ, Evans JW, Luh WM, Bandettini PA. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage 60: 1759–1770, 2012. doi: 10.1016/j.neuroimage.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett 87: 198701, 2001. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- 53.Laye MJ, Nielsen MB, Hansen LS, Knudsen T, Pedersen BK. Physical activity enhances metabolic fitness independently of cardiorespiratory fitness in marathon runners. Dis Markers 2015: 1–11, 2015. doi: 10.1155/2015/806418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, Phillips SM, Gothe NP, Mailey E, Vieira-Potter VJ, Martin SA, Pence BD, Lin M, Parasuraman R, Greenwood PM, Fryxell KJ, Woods JA, McAuley E, Kramer AF, Erickson KI. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci 8: 985, 2014. doi: 10.3389/fnhum.2014.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luders E, Gaser C, Narr KL, Toga AW. Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci 29: 14265–14270, 2009. doi: 10.1523/JNEUROSCI.2261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luders E, Narr KL, Thompson PM, Rex DE, Woods RP, Deluca H, Jancke L, Toga AW. Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp 27: 314–324, 2006. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mailey EL, White SM, Wójcicki TR, Szabo AN, Kramer AF, McAuley E. Construct validation of a non-exercise measure of cardiorespiratory fitness in older adults. BMC Public Health 10: 59, 2010. doi: 10.1186/1471-2458-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marcus DS, Harwell J, Olsen T, Hodge M, Glasser MF, Prior F, Jenkinson M, Laumann T, Curtiss SW, Van Essen DC. Informatics and data mining tools and strategies for the human connectome project. Front Neuroinform 5: 4, 2011. doi: 10.3389/fninf.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McAuley E, Szabo AN, Mailey EL, Erickson KI, Voss M, White SM, Wójcicki TR, Gothe N, Olson EA, Mullen SP, Kramer AF. Non-exercise estimated cardiorespiratory fitness: associations with brain structure, cognition, and memory complaints in older adults. Ment Health Phys Act 4: 5–11, 2011. doi: 10.1016/j.mhpa.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meunier D, Achard S, Morcom A, Bullmore E. Age-related changes in modular organization of human brain functional networks. Neuroimage 44: 715–723, 2009. doi: 10.1016/j.neuroimage.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 61.Parker BA, Kalasky MJ, Proctor DN. Evidence for sex differences in cardiovascular aging and adaptive responses to physical activity. Eur J Appl Physiol 110: 235–246, 2010. doi: 10.1007/s00421-010-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage 65: 176–193, 2013. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron 72: 665–678, 2011. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 5: 56, 2008. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Proctor DN, Le KU, Ridout SJ. Age and regional specificity of peak limb vascular conductance in men. J Appl Physiol (1985) 98: 193–202, 2005. doi: 10.1152/japplphysiol.00704.2004. [DOI] [PubMed] [Google Scholar]
- 66.Ridout SJ, Parker BA, Smithmyer SL, Gonzales JU, Beck KC, Proctor DN. Age and sex influence the balance between maximal cardiac output and peripheral vascular reserve. J Appl Physiol (1985) 108: 483–489, 2010. doi: 10.1152/japplphysiol.00985.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52: 1059–1069, 2010. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Rubinov M, Sporns O. Weight-conserving characterization of complex functional brain networks. Neuroimage 56: 2068–2079, 2011. doi: 10.1016/j.neuroimage.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 69.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport 71, Suppl 2: 1–14, 2000. doi: 10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]
- 70.Scheinost D, Finn ES, Tokoglu F, Shen X, Papademetris X, Hampson M, Constable RT. Sex differences in normal age trajectories of functional brain networks. Hum Brain Mapp 36: 1524–1535, 2015. doi: 10.1002/hbm.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sen A, Gider P, Cavalieri M, Freudenberger P, Farzi A, Schallert M, Reichmann F, Watzinger N, Zweiker R, Schmidt R, Schmidt H. Association of cardiorespiratory fitness and morphological brain changes in the elderly: results of the Austrian Stroke Prevention Study. Neurodegener Dis 10: 135–137, 2012. doi: 10.1159/000334760. [DOI] [PubMed] [Google Scholar]
- 72.Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: unconstrained yet reliable. Cereb Cortex 19: 2209–2229, 2009. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555: 377–381, 2018. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spreng RN, Stevens WD, Viviano JD, Schacter DL. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol Aging 45: 149–160, 2016. doi: 10.1016/j.neurobiolaging.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spreng RN, Cassidy BN, Darboh BS, DuPre E, Lockrow AW, Setton R, Turner GR. Financial exploitation is associated with structural and functional brain differences in healthy older adults. J Gerontol A Biol Sci Med Sci 72: 1365–1368, 2017. doi: 10.1093/gerona/glx051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stan Development Team rstanarm : Bayesian applied regression modeling via Stan. R package version 2.13.1 (Online) http://mc-stan.org/, [October 2018].
- 77.Stevens WD, Spreng RN. Resting-state functional connectivity MRI reveals active processes central to cognition. Wiley Interdiscip Rev Cogn Sci 5: 233–245, 2014. doi: 10.1002/wcs.1275. [DOI] [PubMed] [Google Scholar]
- 78.Tomasi D, Volkow ND. Gender differences in brain functional connectivity density. Hum Brain Mapp 33: 849–860, 2012. doi: 10.1002/hbm.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100: 3328–3342, 2008. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wójcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci 2: 32, 2010a. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wójcicki TR, Hu L, Szabo A, Klamm E, McAuley E, Kramer AF. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia 48: 1394–1406, 2010b. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wójcicki TR, White SM, Gothe N, McAuley E, Sutton BP, Kramer AF. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp 34: 2972–2985, 2013. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voss MW, Weng TB, Burzynska AZ, Wong CN, Cooke GE, Clark R, Fanning J, Awick E, Gothe NP, Olson EA, McAuley E, Kramer AF. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. Neuroimage 131: 113–125, 2016. doi: 10.1016/j.neuroimage.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weinstein AM, Voss MW, Prakash RS, Chaddock L, Szabo A, White SM, Wojcicki TR, Mailey E, McAuley E, Kramer AF, Erickson KI. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun 26: 811–819, 2012. doi: 10.1016/j.bbi.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106: 1125–1165, 2011. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17: 37–49, 1982–1983. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 87.Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage 49: 2163–2177, 2010. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, Milham MP. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 30: 15034–15043, 2010. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]