Similar to all other positive-strand RNA viruses, enteroviruses reorganize host cellular membranes for efficient genome replication. A host lipid kinase, PI4KB, plays an important role in this membrane rearrangement. The exact mechanism of how enteroviruses recruit PI4KB was unclear. Here, we revealed a role of a Golgi-residing protein, ACBD3, as a mediator of PI4KB recruitment upon enterovirus replication. ACBD3 is responsible for proper localization of enteroviral 3A proteins in host cells, which is important for 3A to recruit PI4KB. By testing ACBD3 and PI4KB mutants that abrogate the ACBD3-PI4KB interaction, we showed that this interaction is crucial for enterovirus replication. The importance of specific domains of ACBD3 was evaluated for the first time, and the domains that are essential for enterovirus replication were identified. Our findings open up a possibility for targeting ACBD3 or its interaction with enteroviruses as a novel strategy for the development of broad-spectrum antienteroviral drugs.
KEYWORDS: acyl-coenzyme A binding domain containing 3 (ACBD3), phosphatidylinositol 4-kinase IIIβ (PI4KB), enterovirus, replication organelles, viral replication, virus-host interactions
ABSTRACT
The enterovirus genus of the picornavirus family includes a large number of important human pathogens such as poliovirus, coxsackievirus, enterovirus A71, and rhinoviruses. Like all other positive-strand RNA viruses, genome replication of enteroviruses occurs on rearranged membranous structures called replication organelles (ROs). Phosphatidylinositol 4-kinase IIIβ (PI4KB) is required by all enteroviruses for RO formation. The enteroviral 3A protein recruits PI4KB to ROs, but the exact mechanism remains elusive. Here, we investigated the role of acyl-coenzyme A binding domain containing 3 (ACBD3) in PI4KB recruitment upon enterovirus replication using ACBD3 knockout (ACBD3KO) cells. ACBD3 knockout impaired replication of representative viruses from four enterovirus species and two rhinovirus species. PI4KB recruitment was not observed in the absence of ACBD3. The lack of ACBD3 also affected the localization of individually expressed 3A, causing 3A to localize to the endoplasmic reticulum instead of the Golgi. Reconstitution of wild-type (wt) ACBD3 restored PI4KB recruitment and 3A localization, while an ACBD3 mutant that cannot bind to PI4KB restored 3A localization, but not virus replication. Consistently, reconstitution of a PI4KB mutant that cannot bind ACBD3 failed to restore virus replication in PI4KBKO cells. Finally, by reconstituting ACBD3 mutants lacking specific domains in ACBD3KO cells, we show that acyl-coenzyme A binding (ACB) and charged-amino-acid region (CAR) domains are dispensable for 3A-mediated PI4KB recruitment and efficient enterovirus replication. Altogether, our data provide new insight into the central role of ACBD3 in recruiting PI4KB by enterovirus 3A and reveal the minimal domains of ACBD3 involved in recruiting PI4KB and supporting enterovirus replication.
INTRODUCTION
The Picornaviridae family is a large group of viruses with a single-stranded, positive-sense RNA genome. Members of the Enterovirus genus, which includes poliovirus (PV), coxsackievirus (CV), enterovirus A71 (EV-A71), EV-D68, and rhinovirus (RV), can cause diverse human diseases such as poliomyelitis, meningitis, hand-foot-and-mouth disease, and respiratory illness (1). Even though enteroviruses are associated with a variety of clinical manifestations, there are currently no approved vaccines against most enteroviruses except for PV and EV-A71, and antiviral drugs are not available.
All positive-strand RNA viruses, including picornaviruses, induce reorganization of host cellular membranes (2–4) into so-called replication organelles (ROs). ROs are enriched with viral replication factors and coopted host factors, and serve several important purposes in virus replication (5), including facilitating genome replication. Among picornaviruses, enteroviruses and kobuviruses exploit a similar mechanism for RO formation. The host factor phosphatidylinositol 4-kinase type IIIβ (PI4KB) is recruited to the replication sites by viral 3A protein (6–8). PI4KB is a cytosolic lipid kinase that must be recruited to membranes to exert its function and to generate a phosphatidylinositol 4-phosphate (PI4P)-enriched environment (7, 9). PI4P recruits and concentrates cellular proteins, and possibly also viral proteins, to facilitate viral genome replication (10, 11). Among the cellular proteins that interact with PI4P are lipid transfer proteins, such as oxysterol binding protein (OSBP) (12). Under normal conditions, OSBP creates membrane contact sites between endoplasmic reticulum (ER) and PI4P-enriched trans-Golgi membranes and shuttles cholesterol in exchange for PI4P (13). In a similar manner, OSBP is recruited to RO membranes and mediates a PI4P-dependent flux of cholesterol from ER to ROs (14).
In uninfected cells, PI4KB is recruited to Golgi membranes among others by the small GTPase ADP-ribosylation factor 1 (Arf1) (15) or by acyl-CoA binding domain containing 3 (ACBD3) (7, 8, 16). Kobuviruses recruit PI4KB through ACBD3, which directly interacts with the viral protein 3A (7, 11). Recently, the crystal structure of the kobuvirus 3A-ACBD3 complex became available, which revealed the binding sites that are important for the 3A-ACBD3 interaction (6). Point mutations in 3A and ACBD3 at the binding interface inhibited the activation of PI4KB (17), suggesting that PI4KB recruitment to membranes via 3A-ACBD3-PI4KB interaction is necessary for kobuviruses to exploit PI4KB activity.
Enteroviruses also express a viral protein called 3A, but this differs from Kobuvirus 3A both in sequence and in structure. The 3A proteins of several enteroviruses (e.g., PV and coxsackievirus B3 [CVB3]) bind to brefeldin A resistance guanine nucleotide exchange factor 1 (GBF1), a guanine exchange factor that activates the small GTPase Arf1. Arf1 interacts with PI4KB in noninfected cells. However, PI4KB recruitment by CVB3 and RV 3A likely occurs independently of GBF1 and Arf1 (18, 19). A number of enterovirus 3A proteins have been shown to bind to ACBD3 (8, 18). Therefore, several studies have investigated whether enteroviruses depend on ACBD3 to recruit PI4KB. While in one study, knockdown of ACBD3 in HeLa cells inhibited poliovirus (PV) replication (8), another reported no inhibition of PV replication in ACBD3 knockdown HEK-293T, IMR5, and HeLa cells (20). In our previous work, we did not observe inhibition of CVB3 or RV replication and no effects on PI4KB recruitment upon ACBD3 knockdown (18, 19).
Here, we reevaluated the importance of ACBD3 for enterovirus replication using ACBD3 knockout (ACBD3KO) cells. We observed that ACBD3 supports replication of representative viruses of different human enterovirus species (EV-A/B/C/D, RV-A/B) by mediating PI4KB recruitment by 3A. For the first time, we showed that the interaction between ACBD3 and PI4KB is crucial for enterovirus replication. In addition, we dissected the different domains of ACBD3 and uncovered that the glutamine-rich region (Q) and Golgi dynamics domain (GOLD) together suffice to support enterovirus replication. Furthermore, our data suggest that ACBD3 is important for proper 3A localization. Overall, our findings implicate that ACBD3 is not just an intermediate through which 3A recruits PI4KB but may play a central role in RO formation by scaffolding viral proteins and host proteins.
RESULTS
ACBD3 knockout inhibits replication of enterovirus A to D and rhinovirus A and B species.
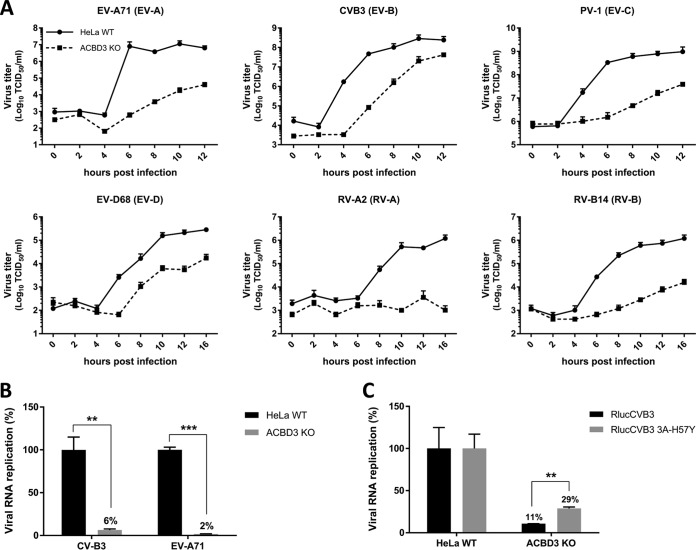
Previously, we observed no effects on CVB3 and RV replication in HeLa cells in which ACBD3 was knocked down more than 90% (18, 19). Here, we set out to study enterovirus replication in ACBD3KO HeLa cells. HeLa cells lacking ACBD3 were generated with CRISPR-Cas9 technology, and the knockout was confirmed by Western blot analysis (see Fig. S1A in the supplemental material). Next, we evaluated enterovirus replication kinetics in ACBD3KO cells using representative viruses of four different human enterovirus species (EV-A71 [EV-A], CVB3 [EV-B], PV-1 [EV-C], and EV-D68 [EV-D]) and two rhinovirus species (RV-A2 [RV-A] and RV-B14 [RV-B)]). RV-C was not tested, as HeLa R19 cells are not susceptible to RV-C because they lack the receptor, cadherin-related family member 3 (CDHR-3) (21). All of the viruses clearly showed deficient replication in ACBD3KO HeLa cells (Fig. 1A). Replication of enteroviruses was also impaired in another human cell line, haploid human cell line HAP1, in which ACBD3 was knocked out (Fig. S2).
FIG 1.
ACBD3 is crucial for enterovirus replication. (A) Growth curves of enteroviruses in HeLawt and ACBD3KO cells. After infection for 30 min at an MOI of 5, cells were incubated for the indicated times. Then, cells were freeze-thawed three times to harvest infectious virus particles. Total virus titers were determined by endpoint dilutions. (B) RNA replication of CVB3 and EV-A71 virus in HeLa ACBD3KO cells. HeLawt and ACBD3KO cells were transfected with in vitro-transcribed RNA of CVB3 or EV-A71 subgenomic replicons encoding firefly luciferase in place of the capsid region. After 7 h, cells were lysed to determine the intracellular luciferase activity. (C) Replication of the CVB3 3A-H57Y mutant in ACBD3KO cells. HeLawt and ACBD3KO cells were infected with wild-type (wt) or 3A-H57Y mutant CVB3 reporter viruses encoding Renilla luciferase (RlucCVB3) at an MOI of 0.1. After 8 h, cells were lysed to determine luciferase activity. Values are the means plus standard errors of the means (SEM) (error bars) for triplicate values. Values were statistically evaluated using a two-tailed paired t test. **, P < 0.01; ***, P < 0.001.
Generation of knockout cells using the CRISPR-Cas9 system. Download FIG S1, PDF file, 0.05 MB (49.7KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Enterovirus replication is inhibited in HAP1 ACBD3KO cells. Download FIG S2, PDF file, 0.02 MB (16.8KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To exclude the role of ACBD3 in virus entry, we assessed viral RNA replication of subgenomic replicons, which are widely used tools to study genome replication specifically and independently of virus entry. Replication of CVB3 and EV-A71 replicons transfected in HeLa ACBD3KO cells was reduced, which indicates a role for ACBD3 in the genome replication step (Fig. 1B). Next, to test whether ACBD3 functions in the same pathway as PI4KB in enterovirus replication, we employed a mutant virus that is less sensitive to PI4KB inhibition, CVB3 3A-H57Y (22). While the replication of wt CVB3 (RlucCVB3) was impaired in ACBD3KO cells, the replication of RlucCVB3 3A-H57Y was significantly increased (Fig. 1C). Encephalomyelitis virus (EMCV), which belongs to the genus Cardiovirus in the Picornaviridae family, depends on PI4KA but not on PI4KB for generating ROs (23). The replication of EMCV was not affected in ACBD3KO cells (Fig. S3), suggesting that the inhibition of CVB3 and EV-A71 replication in ACBD3KO cells is connected to the PI4KB pathway. Overall, these results indicate that ACBD3 is an important host factor for enterovirus replication.
EMCV replication is not sensitive to ACBD3 or PI4KB depletion. Download FIG S3, PDF file, 0.1 MB (79.1KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACBD3 is indispensable for PI4KB recruitment.
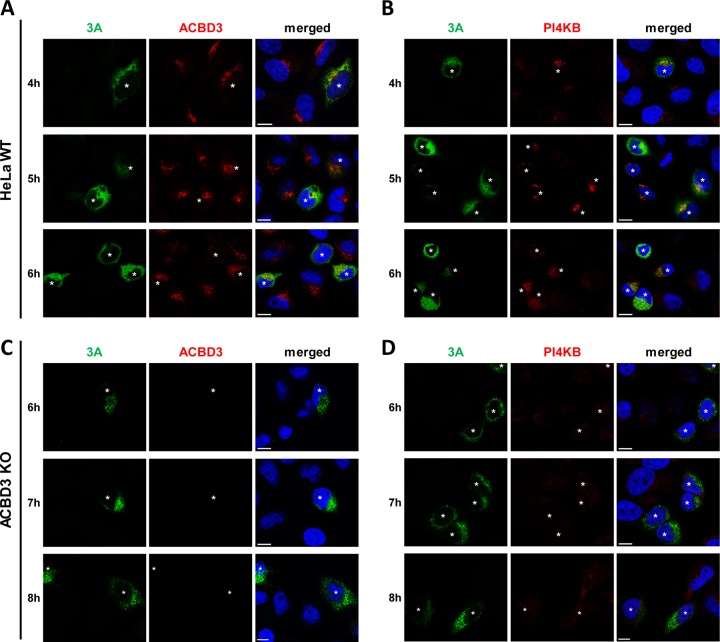
To determine the importance of ACBD3 for PI4KB recruitment during enterovirus replication, we investigated PI4KB localization in CVB3-infected ACBD3KO cells. Since we observed delayed virus replication in ACBD3KO cells (Fig. 1A), different time points were chosen for HeLawt cells and ACBD3KO cells to mitigate possible effects of different replication levels on PI4KB recruitment. As previously shown, the CVB3 3A protein colocalized with ACBD3 (Fig. 2A) and PI4KB (Fig. 2B) throughout infection in infected HeLawt cells, which implies that both ACBD3 and PI4KB localize to CVB3 ROs. PI4KB was more concentrated in 3A-positive cells than in 3A-negative cells, which suggests that it is actively recruited to virus replication sites. Notwithstanding the similar level of 3A expression compared to HeLawt cells (Fig. 2A and B), no recruitment of PI4KB was observed in infected ACBD3KO cells at any time point (Fig. 2D). These results indicate that ACBD3 mediates recruitment of PI4KB during enterovirus replication.
FIG 2.
PI4KB recruitment to virus replication sites depends on ACBD3. (A to D) HeLawt (A and B) and ACBD3KO (C and D) cells were infected with CVB3 wt at an MOI of 5. At the indicated time points, cells were fixed and stained with antibodies against CVB3 3A and ACBD3 (A and C) or CVB3 3A and PI4KB (B and D). Nuclei were stained with DAPI (blue). White asterisks indicate infected cells. Bars represent 10 µm.
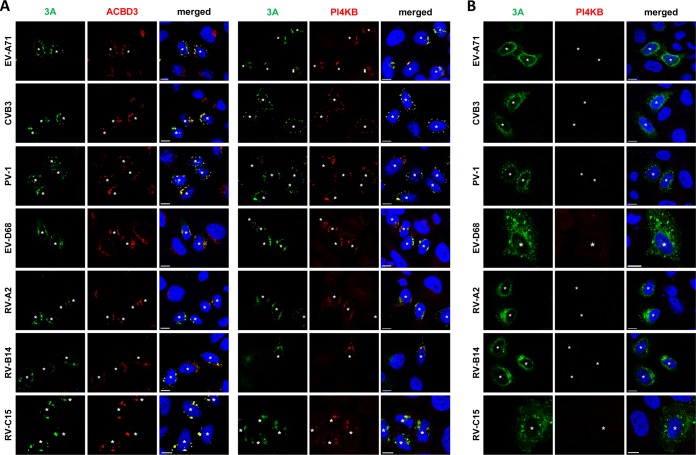
Enterovirus 3A expression alone is sufficient to recruit PI4KB to membranes (9, 18, 19). To further investigate whether PI4KB recruitment by 3A is mediated by ACBD3, we transiently expressed the 3A proteins from representative human enteroviruses from seven different species (EV-A/B/C/D and RV-A/B/C) with either a C-terminal myc tag or an N-terminal GFP tag and examined the localization of ACBD3 and PI4KB (Fig. 3 and Fig. S4). In HeLawt cells, all 3A proteins colocalized with ACBD3 (Fig. 3A). PI4KB was more concentrated in cells expressing 3A than in cells that did not express 3A, and it colocalized with 3A (Fig. 3A), which indicates that PI4KB is actively recruited by enterovirus 3A proteins. In contrast, no PI4KB recruitment was observed in ACBD3KO cells expressing any of the enterovirus 3A proteins (Fig. 3B). These results imply that all enteroviruses utilize a shared mechanism to recruit PI4KB to replication sites, which is via a 3A-ACBD3-PI4KB interaction. Interestingly, we noticed that the localization of 3A differs from HeLawt cells to ACBD3KO cells (Fig. 3 and Fig. S5). Unlike the punctate localization in HeLawt cells (Fig. 3A), 3A proteins were dispersed throughout the cytoplasm in ACBD3KO cells into a more reticular pattern (Fig. 3B and Fig. S5), suggesting that ACBD3 is important for proper localization of 3A.
FIG 3.
Effects of ACBD3 knockout on the localization of enterovirus 3A proteins and the recruitment of PI4KB. (A and B) HeLawt (A) and ACBD3KO (B) cells were transfected with plasmids encoding myc-tagged EV-A71 3A, CVB3 3A, or PV-1 3A or EGFP-tagged EV-D68 3A, RV-2 3A, RV-14 3A. The next day, cells were fixed and stained with antibodies against the myc tag to detect 3A (in the case of myc-3A), ACBD3, or PI4KB. White asterisks indicate 3A-expressing cells. Nuclei were stained with DAPI (blue). Bars represent 10 µm.
Localization of PI4KB in HeLawt and ACBD3KO cells. Download FIG S4, PDF file, 0.1 MB (75.6KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Localization of enterovirus 3A proteins in ACBD3KO cells. Download FIG S5, PDF file, 0.1 MB (127.9KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACBD3 is crucial for 3A localization to the Golgi.
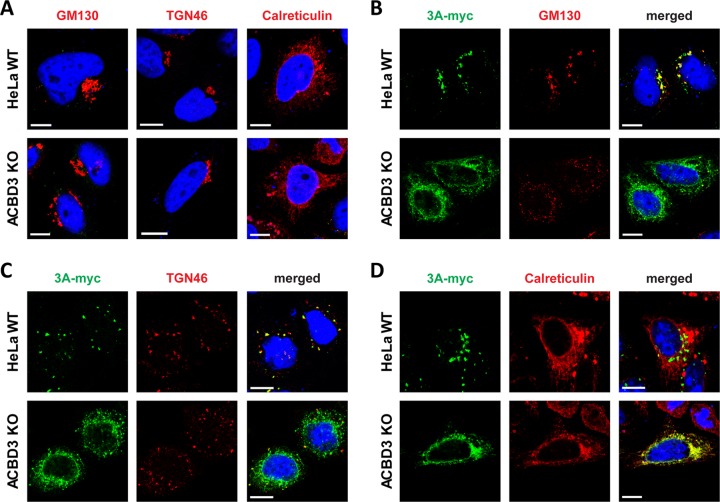
Because the typical punctate localization of 3A on Golgi-derived membranes is lost in ACBD3KO cells, we assessed the overall structure of the Golgi and ER as well as the colocalization between 3A and markers for the Golgi (GM130 and TGN46) and ER (calreticulin). In mock-transfected cells, no gross differences in localization of any of the above markers were observed between HeLawt cells and ACBD3KO cells, although in some ACBD3KO cells, the Golgi appeared to be slightly more scattered than in HeLawt cells (Fig. 4). Importantly, this slight Golgi scattering clearly differs from the massive Golgi scattering that can be observed upon knockout of structural Golgi proteins GRASP55 and GRASP65 (24). Nevertheless, smaller disruptions of Golgi structure that cannot be readily visualized at the light microscopy level cannot be excluded. Indeed, others have reported fragmentation of Golgi cisternae when they studied ACBD3 knockdown cells by electron microscopy (25). As previously described, the disintegration of the Golgi in enterovirus-infected cells and in 3A-expressing cells is likely a consequence of the blockage of ER-to-Golgi transport that depends on the interaction between 3A and GBF1/Arf1 (26, 27). In agreement with this, overexpression of 3A caused disassembly of the Golgi apparatus in both wt and ACBD3KO HeLa cells (Fig. 4B and C), pointing out that the disruption of the Golgi by 3A occurs independently of ACBD3. 3A partially colocalized with the Golgi markers but not the ER marker in HeLawt cells, whereas 3A was localized to the ER, as labeled by calreticulin, in ACBD3KO cells (Fig. 4B to D). This suggests that 3A cannot localize to the Golgi without ACBD3, which may contribute to the lack of PI4KB recruitment. Collectively, our results suggest that ACBD3 is not merely a mediator between 3A and PI4KB but that it plays a central role in recruiting 3A and PI4KB to facilitate virus replication.
FIG 4.
The localization of 3A differs between HeLaWT cells and ACBD3KO cells. (A) Golgi and ER integrity in ACBD3KO cells. HeLawt and ACBD3KO cells were fixed and stained with antibodies against the Golgi markers GM130 and TGN46 or the ER marker calreticulin. (B to D) HeLawt and ACBD3KO cells were transfected with plasmid encoding myc-tagged CVB3 3A. The next day, cells were fixed and stained with an antibody against the myc tag to detect 3A and with antibodies against GM130 (B), TGN46 (C), or calreticulin (D). Nuclei were stained with DAPI (blue). Bars represent 10 µm.
Exogenous expression of wt ACBD3 in ACBD3KO cells restores 3A localization, PI4KB recruitment, and enterovirus replication.
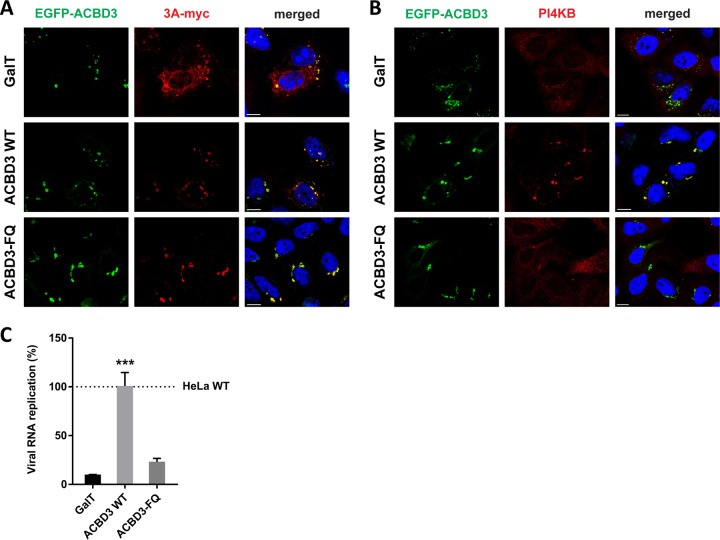
To confirm that ACBD3 recruits 3A to the Golgi and mediates the interaction between 3A and PI4KB, we tested whether reconstitution of GFP-tagged ACBD3 in ACBD3KO cells can restore 3A localization and PI4KB recruitment. For a negative control, we used a Golgi-localized GFP (i.e., GFP coupled to amino acids 1 to 60 of galactosyltransferase [GalT]), which failed to restore 3A localization (Fig. 5A, top panel) and PI4KB recruitment (Fig. 5B, top panel). When wt ACBD3 was reconstituted, 3A regained its punctate localization (Fig. 5A, middle panel), and PI4KB was recruited to the same sites (Fig. 5B, middle panel). ACBD3 expressed without 3A was found in the Golgi, where it colocalized with giantin, but no concentrated PI4KB was observed (Fig. S6). These results indicate that proper 3A localization and PI4KB recruitment by 3A depend on ACBD3.
FIG 5.
Reconstitution of wt ACBD3 but not ACBD3-FQ mutant rescues PI4KB recruitment and CVB3 replication. (A and B) HeLa ACBD3KO cells were cotransfected with plasmids encoding myc-tagged CVB3 3A and EGFP-tagged GalT, ACBD3 wt, or ACBD3-FQ mutant. The next day, cells were fixed and stained with antibodies against the myc tag to detect 3A (A) or PI4KB (B). Nuclei were stained with DAPI (blue). Bars represent 10 µm. (C) HeLawt and ACBD3KO cells were transfected with plasmids encoding EGFP-tagged GalT, ACBD3 wt, or ACBD3-FQ mutant. At 24 h posttransfection (p.t.), cells were infected with RlucCVB3 at an MOI of 0.1. After 8 h, cells were lysed to determine luciferase activity. Values are the means plus SEM of triplicate values. Values were statistically evaluated compared to the EGFP-GalT control using a one-way ANOVA. ***, P < 0.001.
Effects of ACBD3 reconstitution on PI4KB localization in ACBD3KO cells. Download FIG S6, PDF file, 0.2 MB (219.6KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACBD3 forms a tight complex with PI4KB (16). Recently, it was reported that one or two amino substitution(s) in the Q domain of ACBD3 (F258A or F258A/Q259A) can abrogate binding between purified recombinant ACBD3 and PI4KB in pulldown experiments (16, 17). We confirmed that the F258A/Q259A mutant (hereafter called the FQ mutant) lost its interaction with PI4KB by coimmunoprecipitation (Fig. S7) and employed this mutant to test whether the ACBD3-PI4KB interaction is required for PI4KB recruitment and efficient enterovirus replication. Expression of the ACBD3-FQ mutant restored the punctate localization of 3A (Fig. 5A, bottom panel) but did not support PI4KB recruitment (Fig. 5B, bottom panel). Furthermore, exogenous expression of wt ACBD3 in ACBD3KO cells restored replication of CVB3 to a level comparable to that in HeLawt cells, while the negative control (GalT) and ACBD3-FQ mutant could not restore virus replication in ACBD3KO cells (Fig. 5C). Taken together, we showed that 3A localization to the Golgi-derived membranes occurs in an ACBD3-dependent manner and that the interaction between ACBD3-PI4KB is crucial for PI4KB recruitment and efficient virus replication.
Coimmunoprecipitation of PI4KB with ACBD3 and enterovirus 3A protein. Download FIG S7, PDF file, 0.05 MB (52.3KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Reconstituted wt PI4KB in PI4KBKO cells can be recruited to membranes through the 3A-ACBD3-PI4KB interaction, thereby restoring enterovirus replication.
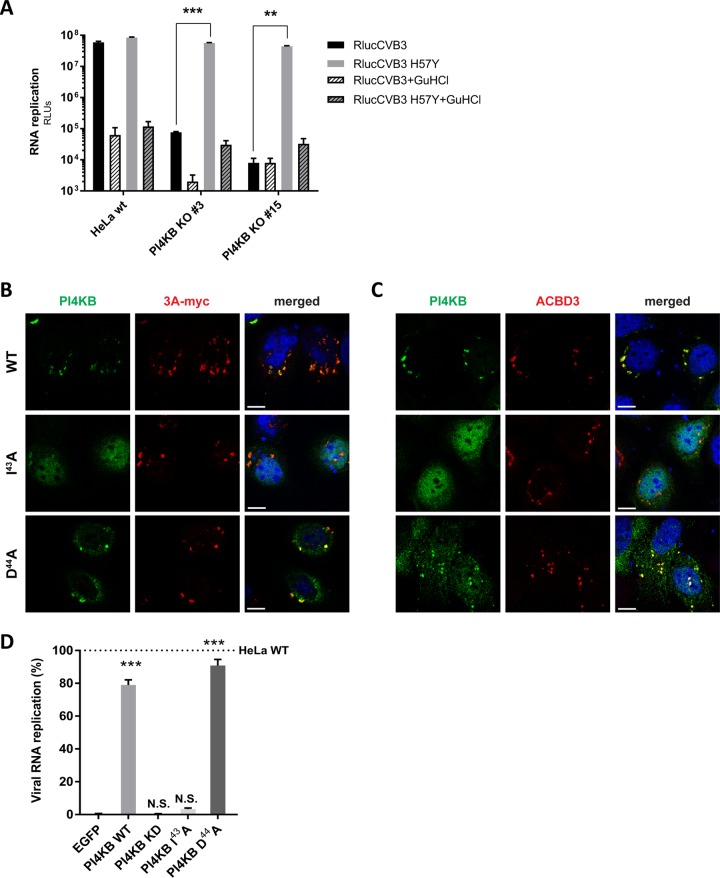
PI4KB is recruited by 3A to ROs during enterovirus replication (8, 9), and depletion of PI4KB by RNAi (9) or pharmacologic inhibition (reviewed in reference 28) have been shown to suppress virus replication. Enterovirus mutants resistant to inhibitors of PI4KB contain single amino acid substitutions in the 3A protein (e.g., H57Y for CVB3). CVB3 replication is severely impaired in PI4KBKO cells that we generated by CRISPR/Cas9 technology (Fig. S1B), while the resistant mutant virus (3A-H57Y) replicated well in PI4KBKO cells (Fig. 6A).
FIG 6.
A PI4KB mutant, which does not interact with ACBD3, cannot be recruited by 3A and cannot restore virus replication in PI4KBKO cells. (A) RNA replication of CVB3 mutant in PI4KBKO cells. HeLawt cells and two PI4KBKO cell clones were infected with wt or 3A-H57Y mutant CVB3 reporter viruses carrying a Renilla luciferase (RlucCVB3) at an MOI of 0.1. Guanidine hydrochloride (GuHCl), a replication inhibitor, was included as a control. After 8 h, cells were lysed to determine luciferase activity (in relative light units [RLUs]). Values are means plus SEM of triplicate values. Values were statistically evaluated using a two-tailed paired t test. **, P < 0.01; ***, P < 0.001. (B and C) HeLa PI4KBKO cells were cotransfected with plasmids encoding myc-tagged CVB3 3A and FLAG-tagged PI4KB wt and PI4KB-I43A or -D44A mutants. The next day, cells were fixed and stained with antibodies against the FLAG tag to detect PI4KB and against the myc tag to detect 3A (B) or ACBD3 (C). Nuclei were stained with DAPI (blue). Bars represent 10 µm. (D) HeLawt and PI4KBKO cells were transfected with plasmids encoding EGFP-tagged GalT, FLAG-tagged PI4KB wt, PI4KB-I43A or -D44A mutant. At 24 p.t., cells were infected with RlucCVB3 at an MOI of 0.1. After 8 h, cells were lysed to determine luciferase activity. Values are means plus SEM of triplicate values. Values were statistically evaluated compared to the EGFP-GalT control using a one-way ANOVA. ***, P < 0.001; N.S., not significant.
Two PI4KB mutants (I43A and D44A) were previously shown to reduce binding between recombinant PI4KB and ACBD3 in vitro (16, 17). In agreement with this, the I43A mutant failed to coimmunoprecipitate ACBD3 from cells (Fig. S7). While wt PI4KB reconstituted in PI4KBKO cells colocalized with 3A and ACBD3 (Fig. 6B and C, top panels), the PI4KB-I43A mutant did not colocalize with 3A and ACBD3, and instead mostly localized to the nucleus (middle panels). Unexpectedly, the D44A mutant did colocalize with 3A and ACBD3 (bottom panels). In line with this, wt PI4KB and the D44A mutant could restore enterovirus replication in PI4KBKO cells, while the I43A mutant and the negative controls, EGFP and a PI4KB kinase-dead mutant that lacks catalytic activity (PI4KB-KD), could not (Fig. 6D). Why the D44A mutant behaves differently from the I43A mutant is presently unclear. Possibly, the D44A mutant has residual interaction with ACBD3 that could not be detected in vitro. Nevertheless, these results imply that the interaction between ACBD3 and PI4KB is important for enterovirus replication.
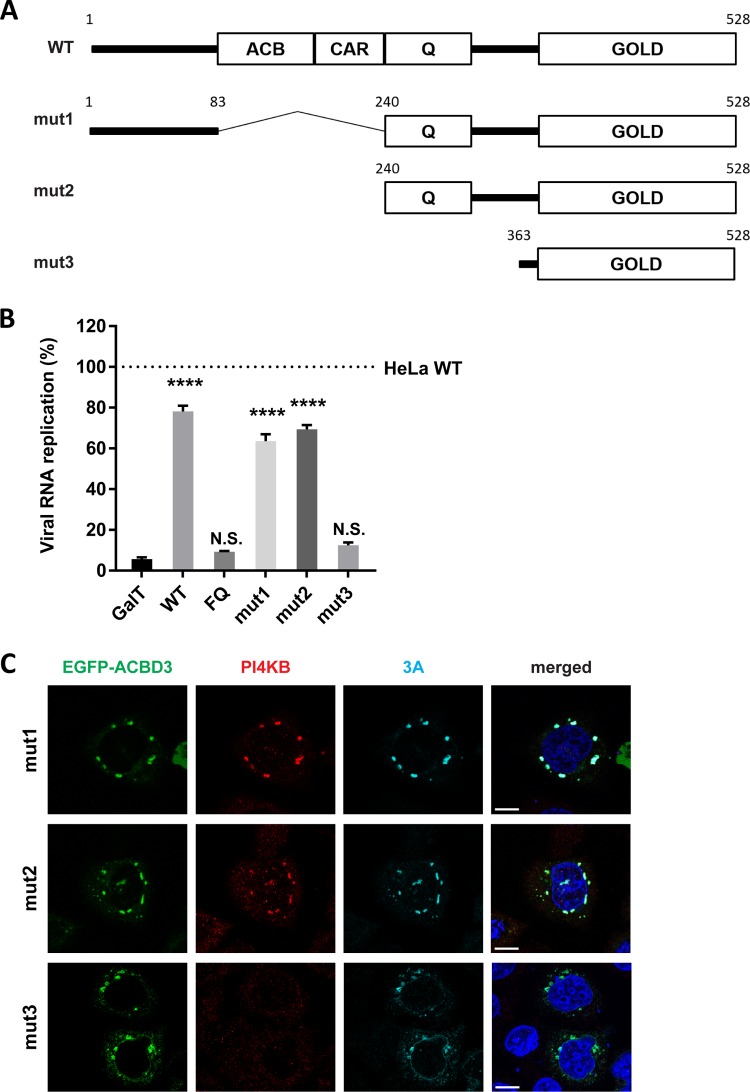
Enterovirus replication does not require the ACB and CAR domains of ACBD3.
Four domains are recognized in ACBD3; the acyl-CoA binding (ACB) domain, the charged-amino-acid region (CAR), the glutamine-rich region (Q), and the Golgi dynamics domain (GOLD) (Fig. 7A). The ACB domain, which is relatively conserved among all known ACBD proteins (ACBD1 to ACBD7), has been suggested to be important for binding to long-chain acyl-CoA (29) and for binding to sterol regulatory element binding protein 1 (SREBP1), causing reduction of de novo palmitate synthesis (30). The CAR domain contains a nuclear localization signal (31), yet the function of the CAR domain is unknown. The Q domain interacts with the N-terminal helix of PI4KB (12, 18). The GOLD domain interacts with giantin, and by doing so, tethers ACBD3 to the Golgi membrane (31). Enterovirus and kobuvirus 3A proteins bind to the GOLD domain, most probably at the same site as giantin (7, 18). To investigate the importance of the ACB and CAR domains for enterovirus replication, we tested whether N-terminal deletion mutants of ACBD3 could restore enterovirus replication in ACBD3KO cells. mut1 and mut2, which contain intact Q and GOLD domains, could restore virus replication to a level comparable to cells reconstituted with wt ACBD3 (Fig. 7B). This is in alignment with our observation that these mutants colocalize with 3A and PI4KB in ACBD3KO cells (Fig. 7C). In contrast, mut3, which contains only the GOLD domain, could not rescue virus replication (Fig. 7B) or PI4KB recruitment (Fig. 7C), like the negative controls (GalT and the FQ mutant) (Fig. 7B), even though all mutants colocalized with 3A (Fig. 7C). Of note, although mut3 and 3A colocalized in punctate structures, they also partly colocalized to reticular and nuclear envelope-like structures, which may indicate that PI4KB also plays a role in firmly localizing 3A and ACBD3 to Golgi-derived membranes. These results indicate that enterovirus replication requires the Q and GOLD domains of ACBD3 for localization of viral protein 3A to the Golgi and for hijacking PI4KB.
FIG 7.
The Q and GOLD domains of ACBD3 are sufficient to support proper 3A localization, PI4KB recruitment, and enterovirus replication. (A) Schematic representation of full-length ACBD3 and its N-terminal deletion mutants (mut1 to mut3). ACBD3 contains the acyl-CoA binding (ACB) domain, the charged-amino-acid region (CAR), the glutamine-rich (Q) domain, and the Golgi dynamics domain (GOLD). The numbers indicate the amino acid positions. (B) HeLawt and ACBD3KO cells were transfected with plasmids encoding EGFP-tagged GalT, ACBD3 wt, ACBD3-FQ mutant, or ACBD3 N-terminal deletion mutants (mut1 to mut3). At 24 h p.t., cells were infected with RlucCVB3 at an MOI of 0.1. After 8 h, cells were lysed to determine luciferase activity. Values are means plus SEM of triplicate values. Values were statistically evaluated compared to the EGFP control using a one-way ANOVA. ****, P < 0.0001; N.S., not significant. (C) HeLa ACBD3KO cells were cotransfected with plasmids encoding myc-tagged CVB3 3A and EGFP-tagged ACBD3 wt, or ACBD3 N-terminal deletion mutants (mut1 to -3). The next day, the cells were fixed and stained with antibodies against PI4KB (red) and the myc tag to detect 3A (light blue). Nuclei were stained with DAPI (blue). Bars represent 10 µm.
DISCUSSION
Both enteroviruses and kobuviruses of the Picornaviridae family coopt PI4KB to build up ROs. Viral protein 3A is responsible for PI4KB recruitment to enterovirus replication sites, yet the underlying mechanism has remained elusive. Despite the direct interaction between ACBD3 and enterovirus 3A proteins (8, 18), there has not yet been a consensus about the importance of ACBD3 for enterovirus replication and PI4KB recruitment. Previously, we observed no inhibition of CVB3 and RV replication and no effects on PI4KB recruitment, even though more than 90% of ACBD3 knockdown was achieved by siRNA (18, 19). In the present study in which we use ACBD3KO cells, we showed that ACBD3 is an important host factor for replication of four different human enterovirus species (EV-A/B/C/D) and two rhinovirus species (RV-A/B). All viruses showed impaired growth in ACBD3KO cells (Fig. 1 and Fig. S2). In addition, neither virus infection (Fig. 2) nor the expression of enterovirus 3A proteins alone (Fig. 3) elicited PI4KB recruitment in the absence of ACBD3. In agreement with our data, the inhibition of EV-A71 and CVB3 was recently reported in ACBD3KO cells (32–34). The discrepancy in the role of ACBD3 from KD to KO condition could result from insufficient suppression of ACBD3 function by RNA interference. In fact, this implies that the small amounts (∼10%) of ACBD3 that remained after knockdown are sufficient to support enterovirus replication and PI4KB recruitment. Similar issues on the differences between knockdown and knockout have been raised by others (35). For instance, the importance of cyclophilin A (CypA) in nidovirus replication was prominent only in CypA knockout cells but not in knockdown cells, even though CypA protein was undetectable after knockdown (36).
We observed that the lack of ACBD3 has a profound effect on enterovirus 3A protein localization. 3A proteins were found almost exclusively at the ER in ACBD3KO cells (Fig. 4D), whereas in HeLawt cells, they showed a punctate localization mostly on Golgi-derived membranes (Fig. 4B and C). Upon reconstitution of wt ACBD3 in ACBD3KO cells, the localization of 3A was restored to a punctate pattern (Fig. 5A). These findings hint at a new role of ACBD3 for enterovirus replication, which is more than merely being a connector between 3A proteins and PI4KB.
Considering that ACBD3 is involved in several different protein complexes, enteroviruses may take advantage of ACBD3 in several ways, more than just for PI4KB recruitment. ACBD3 may be a scaffold responsible for positioning 3A near cellular factors, including other ACBD3-interacting proteins required for RO formation. For example, ACBD3 and PV1 3A were found in a protein complex together with the putative Rab33 GTPase-activating proteins TBC1D22A/B (37). In addition, several Golgi stacking proteins such as Golgin45 and Golgi reassembly stacking protein 2 (GORASP2) were recently identified as novel interaction partners of ACBD3, and ACBD3 was proposed as a scaffold tethering Golgin45, GRASP55, and TBC1D22 for the formation of a Golgi cisternal adhesion complex at the medial Golgi (38). It is largely unknown which domains of ACBD3 are responsible for the interaction with the above-mentioned interacting partners and whether these proteins are recruited to enterovirus ROs also remains to be investigated.
The GOLD domain of ACBD3 is responsible for the interaction with enterovirus 3A protein (18, 37), while the Q domain interacts with PI4KB (16, 17). By utilizing mutants of ACBD3 or PI4KB which disturb the interaction with each other (Fig. 5 and 7), we show for the first time that the interaction between ACBD3 and PI4KB is crucial for enterovirus replication. Aside from the Q and GOLD domains, other domains (i.e., ACB and CAR) of ACBD3 seem to be not involved in enterovirus replication (Fig. 7). This indicates that the functions of the ACB and CAR domains, as well as the cellular proteins and/or lipids that interact with these domains, are unlikely required for enterovirus replication. Although we cannot exclude the possibility that additional or unidentified proteins that bind to the Q or GOLD domain of ACBD3 might also be important for enterovirus replication, our findings suggest that ACBD3 mainly serves to coordinate 3A and PI4KB recruitment at RO membranes, involving the Q and GOLD domains.
How exactly enterovirus 3A protein interacts with ACBD3 needs to be further investigated. The GOLD domain of ACBD3 interacts with enterovirus 3A proteins (18). Similarly, kobuvirus 3A interacts with the ACBD3 GOLD domain, and the crystal structure of kobuvirus 3A in complex with the ACBD3 GOLD domain was revealed recently (6). According to this structure, kobuvirus 3A wraps ACBD3 and stabilizes ACBD3 on membranes through the membrane binding features at the myristoylated N-terminal and hydrophobic C-terminal ends of 3A. However, enterovirus 3A proteins can bind to membranes only through the hydrophobic C terminus, and the 3A proteins of enteroviruses differ greatly in sequence from kobuvirus 3A. Therefore, the way by which enterovirus 3A interacts with ACBD3 could be different from that of kobuvirus. Thus, structural insight into the enterovirus 3A- ACBD3 GOLD complex is urgently required to understand how enterovirus 3A interacts with ACBD3.
In conclusion, our study reveals that enteroviruses employ a conserved mechanism to recruit PI4KB, which depends on the Golgi-residing protein ACBD3. Furthermore, we suggest that ACBD3 tethers viral and host proteins to form ROs. Considering the pan-enteroviral dependency on ACBD3, targeting ACBD3 or the 3A-ACBD3 interaction presents a novel strategy for broad-spectrum antiviral drug development.
MATERIALS AND METHODS
Cells and culture conditions.
HAP1wt cells and HAP1 ACBD3KO cells were obtained from Horizon Discovery. HeLa R19 cells were obtained from G. Belov (University of Maryland and Virginia-Maryland Regional College of Veterinary Medicine, US). HAP1 cells were cultured in IMDM (Thermo Fisher Scientific) supplemented with 10% fetal calf serum (FCS) and penicillin-streptomycin. HeLa cells and HEK 293T cells (ATCC CRL-3216) were cultured in DMEM (Lonza) supplemented with 10% FCS and penicillin-streptomycin. All cells were grown at 37°C in 5% CO2.
Generation of CRISPR-Cas9 knockout cell line.
HeLa ACBD3KO and PI4KBKO cells were generated with CRISPR/Cas9 technology as described previously (39). In brief, gRNA encoding oligonucleotide cassettes (gRNA1 [5′-GCTGAACGCAGAGCGACTCG-3′] and gRNA2 [5′-TCGCCACCTGGATCCGGTCG-3′] for ACBD3; gRNA1 [5′-GTGTGGGGTACACGGACCACG-3′] and gRNA2 [5′-GAGACTCGGGCAGGGAGCTTA-3′] for PI4KB) were cloned into the SapI restriction sites of the pCRISPR-hCas9-2xgRNA-Puro plasmid. HeLa R19 cells were transfected with the resulting plasmid. Single-cell clones were generated using endpoint dilutions. Knockout was verified by sequence analysis of the genomic DNA and by Western blot analysis (see Fig. S1A and S1B in the supplemental material).
Viruses.
The following enteroviruses were used: EV-A71 (strain BrCr, obtained from the National Institute for Public Health and Environment; RIVM, The Netherlands), CVB3 (strain Nancy, obtained by transfection of the infectious clone p53CB3/T7 as described previously [40]), RlucCVB3, RlucCVB3 3A-H57Y (obtained by transfection of infectious clones pRLuc-53CB3/T7 as described previously [22]), RlucEMCV (strain Mengovirus, obtained by transfection of the infectious clone pRLuc‐QG‐M16.1 as described previously [41]), PV1 (strain Sabin, ATCC), EV-D68 (strain Fermon, obtained from RIVM, The Netherlands), and RV-2 and RV-14 (obtained from Joachim Seipelt, Medical University of Vienna, Austria). Virus titers were determined by endpoint titration analysis and expressed as 50% tissue culture infectious dose (TCID50).
Virus infection.
Virus infections were carried out by incubating subconfluent HAP1 or HeLa cells for 30 min with virus. Following virus removal, fresh medium or medium containing the control inhibitors guanidine hydrochloride (2 mM) or dipyridamole (100 μM) was added to the cells. To determine one-step growth kinetics for each virus, infected cells were frozen from 2 to 16 h postinfection (p.i.). Virus titers were determined by endpoint titration analysis and expressed as 50% tissue culture infectious dose (TCID50). To check for the recruitment of PI4KB upon virus replication, cells were fixed for immunofluorescence staining as described below separately. To check genome replication by measuring intracellular Renilla luciferase activity, cells were lysed at 8 h p.i. and followed the manufacturer’s protocol (Renilla luciferase assay system; Promega).
RNA transfection.
The subgenomic replicons of CVB3 (10) and EV-A71 (42) were described previously. HeLa cells were transfected with RNA transcripts of replicon constructs. After 7 h, cells were lysed to determine intracellular firefly luciferase activity.
Plasmids.
p3A(CVB3)-myc (27), pEGFP-3A(RV-2), and pEGFP-3A(RV-14) were described previously (19). p3A(EV-A71)-myc, p3A(PV1)-myc, pEGFP-3A(EV-D68), and pEGFP-3A(RV-C15) were prepared by cloning cDNA encoding EV-A71 and PV1 3A into p3A(CVB3)-myc vectors from which CVB3 3A was excised using restriction enzyme sites SalI and BamHI, and EV-D68 and RV-C15 3A into pEGFP vectors using restriction enzyme sites BglII and BamHI. pEGFP-GalT was a gift from Jennifer Lippincott-Schwartz (Addgene plasmid 11929). pEGFP-ACBD3 was a gift from Carolyn E. Machamer (Johns Hopkins University, USA). pEGFP-ACBD3-FQ and pEGFP-ACBD3-mut1/mut2/mut3 were generated by using a Q5 site-directed mutagenesis kit (New England BioLabs). pCDNA3-FLAG-PI4KB(wt) was a gift from Tamas Balla (NIH, USA). pCDNA3-FLAG-PI4KB(D671A) (kinase activity dead [KD] mutant), pCDNA3-FLAG-PI4KB(I43A), and pCDNA3-FLAG-PI4KB(D44A) were generated by using a Q5 site-directed mutagenesis kit (New England BioLabs).
Replication rescue assay.
HeLa cells were transfected with plasmids carrying wild-type (wt) or mutant ACBD3 (FQ, mut1, mut2, mut3), wt or mutant PI4KB (I43A, D44A), Golgi-targeting EGFP (pEGFP-GalT), or kinase-dead PI4KB (PI4KB-KD) as a negative control. At 24 h posttransfection, the cells were infected with RlucCVB3. At 8 h p.i., the intracellular Renilla luciferase activity was determined by using the Renilla luciferase assay system (Promega).
Antibodies.
The rabbit antiserum and the mouse monoclonal antibody against CVB3 3A were described previously (18, 26). Mouse monoclonal antibodies included anti-ACBD3 (Sigma), anti-myc (Sigma), anti-GM130 (BD Biosciences), and antigiantin (Enzo Life Science). Rabbit polyclonal antibodies included anti-PI4KB (Millipore), anti-myc (Thermo Fisher Scientific), anti-TGN46 (Novus Biologicals), anticalreticulin (Sigma), anti-FLAG (Sigma), and anti-EGFP (a gift from J. Fransen, NCMLS, Nijmegen, The Netherlands). Goat anti-rabbit and goat anti-mouse antibodies conjugated to Alexa Fluor 488, 596, or 647 (Molecular Probes) were used as secondary antibodies for immunofluorescence analysis. For Western blot analysis, IRDye goat anti-mouse or anti-rabbit (LI-COR) were used.
Immunofluorescence microscopy.
HeLa cells were grown on coverslips in 24-well plates. Subconfluent cells were transfected with 200 ng of plasmids using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol or infected with CVB3 at an MOI of 5. At 16 h posttransfection (p.t.) or 5 to 9 h p.i., cells were fixed with 4% paraformaldehyde for 15 min at room temperature. After permeabilization with 0.1% Triton X-100 in PBS for 5 min, cells were incubated with primary and secondary antibodies diluted in 2% normal goat serum in PBS. Nuclei were stained with DAPI. Coverslips were mounted with FluorSave (Calbiochem), and confocal imaging was performed with a Leica SpeII confocal microscope.
Western blot analysis.
HAP1 and HeLa cells were harvested and lysed by TEN lysis buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.05% SDS). After 30-min incubation on ice, lysates were centrifuged for 20 min at 10,000 × g. Supernatants were boiled in Laemmli sample buffer for 5 min at 95°C. Samples were run on polyacrylamide gels and transferred to PVDF membranes (Bio-Rad). The membranes were incubated with primary antibody against ACBD3 or PI4KB at 4°C overnight and then with secondary antibodies against mouse IgG or rabbit IgG for 1 h at room temperature. Images were acquired with an Odyssey imaging system (LI-COR).
Details on immunoprecipitation. Download Text S1, PDF file, 0.1 MB (73.8KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplemental references. Download Text S2, PDF file, 0.04 MB (37.9KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
We thank the Center for Cell Imaging (Faculty of Veterinary Medicine, Utrecht University) for support with microscopy experiments. This work was supported by research grants from the Netherlands Organisation for Scientific Research (NWO-VENI-863.12.005 to H.M.V.D.S, NWO-VENI-722.012.066 to J.R.P.M.S., NWO-VICI-91812628, NWO-ECHO-711.017.002, and ERASysApp project “SysVirDrug” ALW project 832.14.003 to F.J.M.V.K.) and from the European Union (Horizon 2020 Marie Skłodowska-Curie ETN “EUVIRNA”, grant agreement 264286 and “ANTIVIRALS,” grant agreement 642434 to F.J.M.V.K.).
We declare that we have no conflicts of interest. The sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, and in the decision to publish the results.
Footnotes
Citation Lyoo H, van der Schaar HM, Dorobantu CM, Rabouw HH, Strating JRPM, van Kuppeveld FJM. 2019. ACBD3 is an essential pan-enterovirus host factor that mediates the interaction between viral 3A protein and cellular protein PI4KB. mBio 10:e02742-18. https://doi.org/10.1128/mBio.02742-18.
REFERENCES
- 1.Tapparel C, Siegrist F, Petty TJ, Kaiser L. 2013. Picornavirus and enterovirus diversity with associated human diseases. Infect Genet Evol 14:282–293. doi: 10.1016/j.meegid.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Belov GA, van Kuppeveld FJM. 2012. (+)RNA viruses rewire cellular pathways to build replication organelles. Curr Opin Virol 2:740–747. doi: 10.1016/j.coviro.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller S, Krijnse-Locker J. 2008. Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol 6:363. doi: 10.1038/nrmicro1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagy PD, Pogany J. 2011. The dependence of viral RNA replication on co-opted host factors. Nat Rev Microbiol 10:137. doi: 10.1038/nrmicro2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul D, Bartenschlager R. 2013. Architecture and biogenesis of plus-strand RNA virus replication factories. World J Virol 2:32–48. doi: 10.5501/wjv.v2.i2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klima M, Chalupska D, Różycki B, Humpolickova J, Rezabkova L, Silhan J, Baumlova A, Dubankova A, Boura E. 2017. Kobuviral non-structural 3A proteins act as molecular harnesses to hijack the host ACBD3 protein. Structure 25:219–230. doi: 10.1016/j.str.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki J, Ishikawa K, Arita M, Taniguchi K. 2012. ACBD3-mediated recruitment of PI4KB to picornavirus RNA replication sites. EMBO J 31:754–766. doi: 10.1038/emboj.2011.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greninger AL, Knudsen GM, Betegon M, Burlingame AL, Derisi JL. 2012. The 3A protein from multiple picornaviruses utilizes the Golgi adaptor protein ACBD3 to recruit PI4KIIIbeta. J Virol 86:3605–3616. doi: 10.1128/JVI.06778-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu NY, Ilnytska O, Belov G, Santiana M, Chen YH, Takvorian PM, Pau C, van der Schaar H, Kaushik-Basu N, Balla T, Cameron CE, Ehrenfeld E, van Kuppeveld FJ, Altan-Bonnet N. 2010. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanke KH, van der Schaar HM, Belov GA, Feng Q, Duijsings D, Jackson CL, Ehrenfeld E, van Kuppeveld FJ. 2009. GBF1, a guanine nucleotide exchange factor for Arf, is crucial for coxsackievirus B3 RNA replication. J Virol 83:11940–11949. doi: 10.1128/JVI.01244-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa-Sasaki K, Sasaki J, Taniguchi K. 2014. A complex comprising phosphatidylinositol 4-kinase IIIβ, ACBD3, and Aichi virus proteins enhances phosphatidylinositol 4-phosphate synthesis and is critical for formation of the viral replication complex. J Virol 88:6586–6598. doi: 10.1128/JVI.00208-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine TP, Munro S. 2002. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr Biol 12:695–704. [DOI] [PubMed] [Google Scholar]
- 13.Mesmin B, Bigay J, Moser von Filseck J, Lacas-Gervais S, Drin G, Antonny B. 2013. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 155:830–843. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 14.Strating JR, van der Linden L, Albulescu L, Bigay J, Arita M, Delang L, Leyssen P, van der Schaar HM, Lanke KH, Thibaut HJ, Ulferts R, Drin G, Schlinck N, Wubbolts RW, Sever N, Head SA, Liu JO, Beachy PA, De Matteis MA, Shair MD, Olkkonen VM, Neyts J, van Kuppeveld FJ. 2015. Itraconazole inhibits enterovirus replication by targeting the oxysterol-binding protein. Cell Rep 10:600–615. doi: 10.1016/j.celrep.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. 1999. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol 1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- 16.Klima M, Toth DJ, Hexnerova R, Baumlova A, Chalupska D, Tykvart J, Rezabkova L, Sengupta N, Man P, Dubankova A, Humpolickova J, Nencka R, Veverka V, Balla T, Boura E. 2016. Structural insights and in vitro reconstitution of membrane targeting and activation of human PI4KB by the ACBD3 protein. Sci Rep 6:23641. doi: 10.1038/srep23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPhail JA, Ottosen EH, Jenkins ML, Burke JE. 2017. The molecular basis of Aichi virus 3A protein activation of phosphatidylinositol 4 kinase IIIbeta, PI4KB, through ACBD3. Structure 25:121–131. doi: 10.1016/j.str.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Dorobantu CM, van der Schaar HM, Ford LA, Strating JR, Ulferts R, Fang Y, Belov G, van Kuppeveld FJ. 2014. Recruitment of PI4KIIIbeta to coxsackievirus B3 replication organelles is independent of ACBD3, GBF1, and Arf1. J Virol 88:2725–2736. doi: 10.1128/JVI.03650-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorobantu CM, Ford-Siltz LA, Sittig SP, Lanke KH, Belov GA, van Kuppeveld FJ, van der Schaar HM. 2015. GBF1- and ACBD3-independent recruitment of PI4KIIIbeta to replication sites by rhinovirus 3A proteins. J Virol 89:1913–1918. doi: 10.1128/JVI.02830-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teoule F, Brisac C, Pelletier I, Vidalain PO, Jegouic S, Mirabelli C, Bessaud M, Combelas N, Autret A, Tangy F, Delpeyroux F, Blondel B. 2013. The Golgi protein ACBD3, an interactor for poliovirus protein 3A, modulates poliovirus replication. J Virol 87:11031–11046. doi: 10.1128/JVI.00304-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, Palmenberg AC, Gern JE. 2015. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A 112:5485–5490. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Schaar HM, van der Linden L, Lanke KH, Strating JR, Purstinger G, de Vries E, de Haan CA, Neyts J, van Kuppeveld FJ. 2012. Coxsackievirus mutants that can bypass host factor PI4KIIIbeta and the need for high levels of PI4P lipids for replication. Cell Res 22:1576–1592. doi: 10.1038/cr.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorobantu CM, Albulescu L, Harak C, Feng Q, van Kampen M, Strating JR, Gorbalenya AE, Lohmann V, van der Schaar HM, van Kuppeveld FJ. 2015. Modulation of the host lipid landscape to promote RNA virus replication: the picornavirus encephalomyocarditis virus converges on the pathway used by hepatitis C virus. PLoS Pathog 11:e1005185. doi: 10.1371/journal.ppat.1005185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bekier ME, Wang L, Li J, Huang H, Tang D, Zhang X, Wang Y. 2017. Knockout of the Golgi stacking proteins GRASP55 and GRASP65 impairs Golgi structure and function. Mol Biol Cell 28:2833–2842. doi: 10.1091/mbc.E17-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao J, Guan Y, Chen W, Shi C, Yao D, Wang F, Lam SM, Shui G, Cao X. 10 May 2018. ACBD3 is required for FAPP2 transferring glucosylceramide through maintaining the Golgi integrity. J Mol Cell Biol doi: 10.1093/jmcb/mjy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wessels E, Duijsings D, Niu TK, Neumann S, Oorschot VM, de Lange F, Lanke KH, Klumperman J, Henke A, Jackson CL, Melchers WJ, van Kuppeveld FJ. 2006. A viral protein that blocks Arf1-mediated COP-I assembly by inhibiting the guanine nucleotide exchange factor GBF1. Dev Cell 11:191–201. doi: 10.1016/j.devcel.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Wessels E, Duijsings D, Lanke KH, Melchers WJ, Jackson CL, van Kuppeveld FJ. 2007. Molecular determinants of the interaction between coxsackievirus protein 3A and guanine nucleotide exchange factor GBF1. J Virol 81:5238–5245. doi: 10.1128/JVI.02680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer L, Lyoo H, van der Schaar HM, Strating JR, van Kuppeveld FJ. 2017. Direct-acting antivirals and host-targeting strategies to combat enterovirus infections. Curr Opin Virol 24:1–8. doi: 10.1016/j.coviro.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan J, Liu J, Culty M, Papadopoulos V. 2010. Acyl-coenzyme A binding domain containing 3 (ACBD3; PAP7; GCP60): an emerging signaling molecule. Prog Lipid Res 49:218–234. doi: 10.1016/j.plipres.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Patel V, Bang S, Cohen N, Millar J, Kim SF. 2012. Maturation and activity of sterol regulatory element binding protein 1 is inhibited by acyl-CoA binding domain containing 3. PLoS One 7:e49906. doi: 10.1371/journal.pone.0049906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohda M, Misumi Y, Yamamoto A, Yano A, Nakamura N, Ikehara Y. 2001. Identification and characterization of a novel Golgi protein, GCP60, that interacts with the integral membrane protein giantin. J Biol Chem 276:45298–45306. doi: 10.1074/jbc.M108961200. [DOI] [PubMed] [Google Scholar]
- 32.Lei X, Xiao X, Zhang Z, Ma Y, Qi J, Wu C, Xiao Y, Zhou Z, He B, Wang J. 2017. The Golgi protein ACBD3 facilitates Enterovirus 71 replication by interacting with 3A. Sci Rep 7:44592. doi: 10.1038/srep44592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao X, Lei X, Zhang Z, Ma Y, Qi J, Wu C, Xiao Y, Li L, He B, Wang J. 2017. Enterovirus 3A facilitates viral replication by promoting phosphatidylinositol 4-kinase IIIβ-ACBD3 interaction. J Virol 91:e00791-17. doi: 10.1128/JVI.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HS, Lee K, Kim SJ, Cho S, Shin HJ, Kim C, Kim JS. 2018. Arrayed CRISPR screen with image-based assay reliably uncovers host genes required for coxsackievirus infection. Genome Res 28:859–868. doi: 10.1101/gr.230250.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgens DW, Deans RM, Li A, Bassik MC. 2016. Systematic comparison of CRISPR/Cas9 and RNAi screens for essential genes. Nat Biotechnol 34:634. doi: 10.1038/nbt.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Wilde AH, Zevenhoven-Dobbe JC, Beugeling C, Chatterji U, de Jong D, Gallay P, Szuhai K, Posthuma CC, Snijder EJ. 2018. Coronaviruses and arteriviruses display striking differences in their cyclophilin A-dependence during replication in cell culture. Virology 517:148–156. doi: 10.1016/j.virol.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greninger AL, Knudsen GM, Betegon M, Burlingame AL, DeRisi JL. 2013. ACBD3 interaction with TBC1 domain 22 protein is differentially affected by enteroviral and kobuviral 3A protein binding. mBio 4:e00098-13. doi: 10.1128/mBio.00098-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yue X, Bao M, Christiano R, Li S, Mei J, Zhu L, Mao F, Yue Q, Zhang P, Jing S, Rothman JE, Qian Y, Lee I. 2017. ACBD3 functions as a scaffold to organize the Golgi stacking proteins and a Rab33b-GAP. FEBS Lett 591:2793–2802. doi: 10.1002/1873-3468.12780. [DOI] [PubMed] [Google Scholar]
- 39.Langereis MA, Rabouw HH, Holwerda M, Visser LJ, van Kuppeveld FJ. 2015. Knockout of cGAS and STING rescues virus infection of plasmid DNA-transfected cells. J Virol 89:11169–11173. doi: 10.1128/JVI.01781-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wessels E, Duijsings D, Notebaart RA, Melchers WJ, van Kuppeveld FJ. 2005. A proline-rich region in the coxsackievirus 3A protein is required for the protein to inhibit endoplasmic reticulum-to-Golgi transport. J Virol 79:5163–5173. doi: 10.1128/JVI.79.8.5163-5173.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albulescu L, Wubbolts R, van Kuppeveld FJ, Strating JR. 2015. Cholesterol shuttling is important for RNA replication of coxsackievirus B3 and encephalomyocarditis virus. Cell Microbiol 17:1144–1156. doi: 10.1111/cmi.12425. [DOI] [PubMed] [Google Scholar]
- 42.van der Schaar HM, Leyssen P, Thibaut HJ, de Palma A, van der Linden L, Lanke KHW, Lacroix C, Verbeken E, Conrath K, Macleod AM, Mitchell DR, Palmer NJ, van de Poël H, Andrews M, Neyts J, van Kuppeveld FJM. 2013. A novel, broad-spectrum inhibitor of enterovirus replication that targets host cell factor phosphatidylinositol 4-kinase IIIbeta. Antimicrob Agents Chemother 57:4971–4981. doi: 10.1128/AAC.01175-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation of knockout cells using the CRISPR-Cas9 system. Download FIG S1, PDF file, 0.05 MB (49.7KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Enterovirus replication is inhibited in HAP1 ACBD3KO cells. Download FIG S2, PDF file, 0.02 MB (16.8KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
EMCV replication is not sensitive to ACBD3 or PI4KB depletion. Download FIG S3, PDF file, 0.1 MB (79.1KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Localization of PI4KB in HeLawt and ACBD3KO cells. Download FIG S4, PDF file, 0.1 MB (75.6KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Localization of enterovirus 3A proteins in ACBD3KO cells. Download FIG S5, PDF file, 0.1 MB (127.9KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effects of ACBD3 reconstitution on PI4KB localization in ACBD3KO cells. Download FIG S6, PDF file, 0.2 MB (219.6KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Coimmunoprecipitation of PI4KB with ACBD3 and enterovirus 3A protein. Download FIG S7, PDF file, 0.05 MB (52.3KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Details on immunoprecipitation. Download Text S1, PDF file, 0.1 MB (73.8KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplemental references. Download Text S2, PDF file, 0.04 MB (37.9KB, pdf) .
Copyright © 2019 Lyoo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.